Abstract
Transient receptor potential ankyrin type 1 (TRPA1) and vanilloid type 1 (TRPV1) receptors are co-expressed in vagal pulmonary C-fiber sensory nerves. Because both these ligand-gated non-selective cation channels are sensitive to a number of endogenous inflammatory mediators, it is highly probable that they can be activated simultaneously during airway inflammation. Studies were carried out to investigate whether there is an interaction between these two polymodal transducers upon simultaneous activation, and how it modulates the activity of vagal pulmonary C-fiber sensory nerves. Our studies showed a distinct potentiating effect induced abruptly by simultaneous activations of TRPA1 and TRPV1 by their respective selective agonists, allyl isothiocyanate (AITC) and capsaicin (Cap), at near-threshold concentrations. This synergistic effect was demonstrated in the studies of single-unit recording of vagal bronchopulmonary C-fiber afferents and the reflex responses elicited by activation of these afferents in intact animals, as well as in the isolated nodose and jugular bronchopulmonary sensory neurons. This potentiating effect was absent when either AITC or Cap was replaced by non-TRPA1 and non-TRPV1 chemical activators of these neurons, demonstrating the selectivity of the interaction between these two TRP channels. Furthermore, the synergism was dependent upon the extracellular Ca2+, and the rapid onset of the action further suggests that the interaction probably occurred locally at the sites of these channels. These findings suggest that the TRPA1-TRPV1 interaction may play an important role in regulating the function and excitability of pulmonary sensory neurons during airway inflammation, but the mechanism underlying this positive interaction is not yet fully understood.
Keywords: airway, C fiber, inflammation, TRPA1, TRPV1
Introduction
Transient receptor potential ankyrin type 1 (TRPA1) and vanilloid type 1 (TRPV1) receptors are tetrameric membrane protein with four identical subunits, each containing six transmembrane-spaning domains, which form a ligand-gated non-selective cation channel with a high permeability to calcium (Ca2+) [1, 2]. TRPA1 and TRPV1 have been described as “gatekeeper of inflammation” and “molecular gateway to the pain pathway”, respectively, for their pivotal roles as the nociceptive transducers in generating the pain sensation during tissue damage and inflammation [3, 4]. In dorsal root ganglion (DRG), TRPA1 is found in a subset of the neurons that express TRPV1 [1, 2, 5]. Interestingly, the percentage of TRPV1-expressing neurons that also express TRPA1 can change under the influence of various conditions; for example, it increased from 55% to 80% in trigeminal ganglion neurons after treatment of nerve growth factor [6].
In the respiratory system, both these channels are abundantly and selectively expressed in small-diameter, slowly-conducting unmyelinated (C-fiber) neurons [7]. These C-fiber are the dominant type of vagal bronchopulmonary afferents [8], and are primarily responsible for eliciting pulmonary defense reflex responses against inhaled irritants such as acid aerosol, cigarette smoke, ozone, etc. [9–11]. Hypersensitivity of these afferents is involved in the manifestation of various symptoms associated with airway inflammation, which include bronchoconstriction and mucus hypersecretion via the cholinergic pathway, accompanied by the sensation of airway irritation and urge to cough [9, 10]. Furthermore, sustained and/or intense stimulation of these C-fiber afferents triggers the release of tachykinins and calcitonin gene-related peptide (CGRP) from the sensory terminals. These sensory neuropeptides can act on a number of effector cells in the respiratory tract (e.g., smooth muscles, cholinergic ganglia, mucous glands, immune cells), and elicit the local “axon reflexes” such as bronchoconstriction, protein extravasation and inflammatory cell chemotaxis [12, 13].
Both TRPA1 and TRPV1 can be activated by a number of chemical mediators and substances that are known to be released endogenously in the airways during inflammatory reactions; for example, TRPV1 by proton, certain lipoxygenase products (LO), etc. [2, 14–16], and TRPA1 by reactive oxygen species, bradykinin, 4-oxononenal, etc. [3, 17–19]. Furthermore, certain endogenous inflammatory mediators (e.g., prostaglandin E2, protease, etc.), though not a TRPV1 or TRPA1 activator themselves, can enhance the sensitivity of these channels [13, 20–22]. Hence, there is a distinct possibility that both these channels are activated simultaneously during airway inflammation. More importantly, because these two channels are co-localized in the same C-fiber sensory neurons, an interaction between them may play an important role in regulating the sensitivity and function of these neurons. This hypothesis is supported by the observation that certain functional properties of TRPA1 in native nociceptive neurons are not present in heterologously expressed TRPA1, but they can be restored when both TRPA1 and TRPV1 channels are co-expressed [23, 24].
Synergy between TRPA1 and TRPV1 in Vagal Bronchopulmonary C-fibers
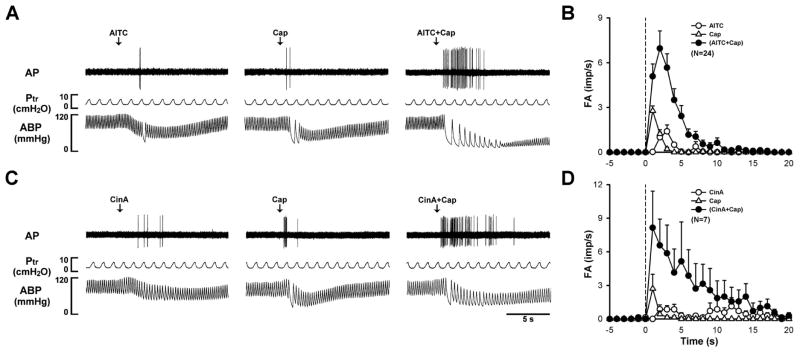
To investigate the potential interaction between TRPA1 and TRPV1, we studied the vagal pulmonary C-fiber responses to intravenous injections of allyl isothiocyanate (AITC, a TRPA1 activator) and capsaicin (Cap, a TRPV1 activator) using the single-fiber recording technique in anesthetized rats. To avoid non-selective activation of TRPA1 and TRPV1 receptors [1, 25–27], only low (slightly above threshold) doses of AITC (0.5–0.75 mg/kg) and Cap (0.35–0.75 μg/kg) were chose for the study. Thus, intravenous bolus injections of AITC and Cap evoked very mild responses when they were administered individually. However, when the same doses of AITC and Cap were injected in combination, the afferent activity was strikingly amplified (Fig. 1A & 1B), accompanied by pronounced bradycardia and hypotension. Group data showed that the peak fiber activity evoked by an injection of AITC and Cap in combination was ~406% (n=24) greater than the mathematical sum of the responses to AITC and Cap when they were administered individually [28]. In addition, the fiber discharge lasted for a substantially longer duration following the combined injection of AITC and Cap (Fig. 1A & 1B). In a separate group of animals, a strong synergistic effect was also evoked when cinnamaldehyde (CinA), another selective agonist of TRPA1, was administered in combination with Cap, as shown by a pronounced potentiation of both the peak activity and duration of the C-fiber discharge (Fig. 1C & 1D). However, the potentiation was completely absent when either AITC or Cap was replaced by one of the non-TRPA1 and non-TRPV1 chemical activators of pulmonary C-fiber afferents, such as phenylbiguanide (PBG), a 5-HT3 receptor agonist; adenosine 5′-triphosphate (ATP), an agonist of P2X2 and P2X3 receptors; and adenosine, an A1 adenosine receptor agonist, indicating that the positive interaction occurred specifically between TRPA1 and TRPV1 [28].
Figure 1.
Synergistic effects of TRPA1 and TRPV1 agonists on vagal pulmonary C-fibers. A: experimental records illustrating responses of a pulmonary C-fiber arising from the lower lobe of right lung to intravenous bolus injections of allyl isothiocyanate (AITC; 0.75 mg/kg), capsaicin (Cap; 0.75 μg/kg), and a combination of AITC and Cap at the same doses in an anesthetized and artificially ventilated rat (body weight: 310 g). The same volume (0.15 ml) was delivered in all three injections, first into the catheter (dead space volume 0.2 ml) and then flushed (at arrow) into the right atrium by a bolus of 0.3 ml saline. The interval between injections was 20 min. AP, action potential; Ptr, tracheal pressure; ABP, arterial blood pressure. B: histograms of pulmonary C-fibers activity (FA) to intravenous injections (dashed line) of AITC (0.50–0.75 mg/kg), Cap (0.35–0.75 μg/kg), and a combination of AITC and Cap at the same doses. Data are means ± SEM. Imp/s, impulses per second. C: responses of a pulmonary C-fiber arising from the lower lobe of right lung to intravenous bolus injections of cinnamaldehyde (CinA, 1.8 mg/kg), Cap (0.5 μg/kg), and a combination of CinA and Cap at the same doses (body weight: 280 g). Descriptions in D are similar to that of B, except that AITC was replaced by CinA (1.5–2.0 mg/kg). (Modified from reference 28)
Furthermore, this distinct synergistic effect was also observed in the pulmonary chemoreflex responses, elicited by the activation of pulmonary C-fiber afferents, in anesthetized, spontaneously breathing rats; the apneic response to intravenous bolus injection of a combined low doses of AITC and Cap was ~202% greater than the mathematical sum of the responses to AITC and Cap when they were administered individually [28]. In addition, the synergism is not species-dependent as a similar potentiating effect was also observed in the pulmonary chemoreflex responses in mice [28].
It is well documented that TRPA1 and TRPV1 are also expressed in the non-neuronal cells [29], though the levels of protein expression, particularly for TRPV1, are considerably lower in other cell types. TRPA1 expression was found in airway epithelial cells, fibroblasts, lymphocytes, etc; and the TRPV1 expression detected in bronchial epithelial cells [30]. Therefore, it seems conceivable that activation of TRPA1 may have triggered the release of intermediate mediator(s) from other target cells in the airways and pulmonary vessels, which can in turn lead to the sensitization of C-fiber afferents to Cap. Indeed, recent studies have shown that TRPA1 or TRPV1 activation on bronchial epithelial cells promoted the secretion of pro-inflammatory mediators and cytokines [31, 32]. Therefore, although this in-vivo study demonstrated a distinct synergistic effect on vagal pulmonary C-fiber sensory nerves generated by a simultaneous activation of both TRPA1 and TRPV1, whether a positive interaction between these two channels occurred directly in the sensory neurons lacks the support of definitive evidence.
Positive Interaction between TRPA1 and TRPV1 in Isolated Airway Sensory Neurons
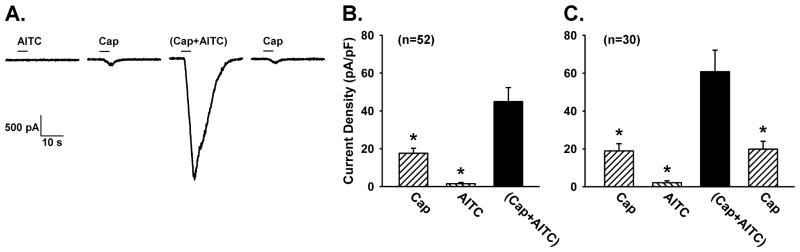
To avoid possible effects of chemical mediator(s) released from other target cells resulting from the TRPA1 activation, we studied the interaction between TRPA1 and TRPV1 in isolated rat vagal pulmonary sensory neurons using the whole-cell perforated patch-clamp recording technique [22, 33]. Pulmonary sensory neurons (n=168) were identified by retrograde labeling with DiI, a fluorescent dye, and isolated from nodose and jugular ganglia [22]. After the concentration-response curves to both AITC and Cap were established first, we purposely chose relatively low (near-threshold) concentrations of AITC (30 μM; 4 sec) and Cap (0.1 or 0.3 μM; 4 sec) in order to avoid desensitization and to evaluate the responses of these neurons to TRPA1 and TRPV1 activations near physiological and pathophysiological conditions [34]. Results of this study demonstrated a distinct positive interaction occurring abruptly immediately upon simultaneous TRPA1 and TRPV1 activations in these neurons (Fig. 2A). The peak current density evoked by a combination of these low concentrations of AITC and Cap was more than two-fold greater than the mathematical sum of the responses to AITC and Cap when they were administered separately at the same concentrations in the same neurons (Fig. 2B). In addition, the duration of current evoked by the combined challenge of AITC and Cap was also distinctly longer than that of AITC or Cap when they were applied individually (e.g., Fig. 2A) in the same neurons. A similar pattern of this synergistic effect of simultaneous TRPA1 and TRPV1 activations was also found when AITC was replaced by CinA [34]. However, this potentiating effect was eliminated when either AITC or Cap was replaced by non-TRPA1 and non-TRPV1 chemical activators of these neurons (ATP and PBG), demonstrating the selectivity of the interaction between these two TRP channels [34].
Figure 2.
Synergistic effect generated by a simultaneous application of Cap and AITC in isolated vagal pulmonary sensory neurons. A: experimental records illustrating the inward currents evoked by AITC (30 μM), Cap (0.3 μM), (Cap+AITC), a combination of Cap and AITC at the same concentrations, and Cap (repeated after washout) in a nodose neuron (19.5 pF). The duration of each chemical application was 4 s, as depicted by the horizontal bar. An interval of 20 min elapsed between two challenges for recovery. B: group data of current densities evoked by Cap (0.1 or 0.3 μM), AITC (30 μM) and (Cap+AITC); n = 52 (including 24 nodose and 28 jugular neurons). C: to test if the effect was reversible, the response to Cap was measured again after washout in a majority (30 out of 52) of these neurons shown in B. Data are means ± SEM. *, significantly (P < 0.05) different from the response to (Cap+AITC). (Modified from reference 34)
In view of the high permeability to Ca2+ in both these channels [16, 35] and the important regulatory function of intracellular Ca2+ on the activity and sensitivity of these channels, we further studied the potential role of Ca2+ as an intracellular signaling molecule of the TRPA1-TRPV1 interaction [36–40]. After the positive interaction of a combined application of Cap and AITC was confirmed (e.g., Fig. 3A), the same experimental protocol was repeated in the same neurons after they had been perfused with the Ca2+-free ECS for 10 min (e.g., Fig. 3B). It was evident that the positive interaction was completely abolished when Ca2+ was removed from the extracellular solution. The current density evoked by a combination of Cap and AITC (both prepared in Ca2+-free ECS) was not different from the mathematical sum of the responses to Cap and AITC when they were perfused individually (Fig. 3B and 3D). The absence of a positive interaction in the Ca2+-free ECS (Fig. 3) was not due to a loss of the charge carrier for the current because the concentration-current relationships of Cap and AITC were still clearly present under the Ca2+-free condition [34].
Figure 3.
Role of extracellular Ca2+ in the positive interaction of TRPA1 and TRPV1 channels. A: experimental records illustrating the positive interaction of TRPA1 and TRPV1 channels during control in a jugular neuron (27.4 pF). B: the positive interaction was completely abolished after the same neuron was perfused by Ca2+-free ECS for 10 min. C: the positive interaction returned after the same neuron was perfused by regular ECS again for 20 min. D: group data of the responses (n=16) when neurons were perfused with regular ECS (Control) and Ca2+-free ECS, respectively. E: in 4 of these 16 neurons, group data were also obtained after the Ca2+-free ECS was washed out. Data are means ± SEM. *, significantly (P < 0.05) different from the response to (Cap+AITC). (From reference 34)
In summary, these results have provided convincing evidence that a positive interaction between these two TRP channels occurs at the pulmonary sensory neurons, and is not mediated through the release of intermediators from other cell types in the airways. Furthermore, when Ca2+ was removed from the extracellular solution, the synergistic effect of Cap and AITC on pulmonary sensory neurons was completely abrogated, clearly indicating a critical role of Ca2+ in mediating the action.
Mechanisms Possibly Involved in the TRPA1-TRPV1 Interaction
Functional Interaction between TRPA1 and TRPV1
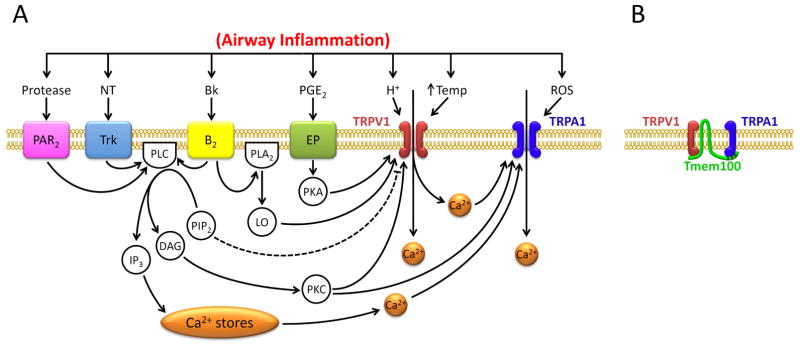
Several mechanisms are possibly involved in the positive interaction between TRPA1 and TRPV1 in pulmonary sensory neurons. The fact that this positive interaction was completely abrogated when Ca2+ was removed from the extracellular solution (Fig. 3) suggests that activation of certain intracellular signaling pathway(s) and molecule(s) initiated by the Ca2+ influx conducted through these channels is probably involved. TRPA1 has been shown to be directly gated by Ca2+ that activates the channel by binding the EF-hand domain in its N-terminus [38, 39], and activation of both TRPA1 and TRPV1 can trigger an abrupt influx of Ca2+ and a rapid rise of intracellular Ca2+ concentration [1, 16, 35]. Wang and coworkers have shown that the entry of extracellular Ca2+ via TRPA1 into the cell further potentiates the TRPA1 channel activity because its effect was abolished when the Ca2+ permeability was blocked by a selective mutation of Asp918 near the pore region of TRPA1 [40]. This proposition is further supported by the observation that TRPA1 channels are activated when the solution containing Ca2+ was applied to the cytosolic face in the excised patch preparation or when Ca2+ was dialyzed into cells [37–39]. Therefore, it seems plausible that the Ca2+ influx through the opening of TRPV1 may in turn potentiate the sensitivity of TRPA1 located in the close vicinity, resulting in a positive interaction between these two channels [23, 39] (Fig. 4A).
Figure 4.
Hypothesized mechanisms involved in the TRPA1-TRPV1 interaction in pulmonary sensory neurons. A: functional interaction between TRPA1 and TRPV1 during airway inflammation. Dashed line depicts an inhibitory pathway. Bk, bradykinin; B2, bradykinin B2 receptor; DAG, diacyglycerol; EP, prostanoid EP receptors; IP3, inositol 1,4,5-triphosphate; LO, lipoxygenase products; NT, neurotrophins; PAR2, protease-activated receptor-2; PGE2, prostaglandin E2 ; PLC, phospholipase C; PLA2, phospholipase A2; PIP2, phosphatidylinositol 4,5-bisphosphate; PKA, protein kinase A; PKC, protein kinase C; ROS, reactive oxygen species; Temp, temperature; Trk receptors, tryosine receptor kinase receptors A and B. B: potential physical interaction between TRPA1 and TRPV1 subunits in a heteromeric A1/V1 channel complex (Modified from reference 53). Tmem100, a two-transmembrane adaptor protein. See text for details.
The role of intracellular Ca2+ as a mediator regulating the interaction between the TRPA1 and TRPV1 channels has been described in the nociceptor neurons during acute inflammatory hyperalgesia [17, 36, 41]. For example, autacoids such as bradykinin can bind to the B2 receptor, a G protein-coupled receptor, located on the nociceptor neuronal membrane and activates phospholipase C (PLC), which in turn cleaves the phospholipid phosphatidylinositol 4,5-bisphosphate (PIP2) into inositol 1,4,5-trisphosphate (IP3), leading to the release of Ca2+ from intracellular store (Fig. 4A). PLC can also activate protein kinase C, increasing the sensitivity of TRPV1 [42, 43]. In addition, bradykinin can generate lipoxygenase products (LO) by activation of the B2 receptor and phospholipase A2 (PLA2); some of the LO products can in turn activate TRPV1 [44] (Fig. 4A). Both the Ca2+ influx through TRPV1 and the internal Ca2+ release can then activate TRPA1 [36]. Spahn and coworkers recently reported that TRPV1 can be sensitized after sustained stimulation of TRPA1 (for 2 min) by CinA or mustard oil in DRG nociceptor neurons [45]. Their study suggested that the TRPA1 activation triggers the signaling cascade involving activation of Ca2+-sensitive adenylyl cyclase, increase in cAMP, activation of protein kinase A (PKA) and phosphorylation of TRPV1 [45]. The cAMP/PKA signaling cascade has also been shown to be involved in the inflammatory mediators- (e.g., PGE2-) induced TRPV1 hypersensitivity [21, 22]. These intracellular second-messenger signaling cascades are attractive hypothesis for explaining the TRPA1-TRPV1 interaction. However, the rapidity of the onset of this positive interaction observed in our study seems to argue against a major role of these signaling pathways.
An increase in the intracellular Ca2+ concentration via TRPV1 activation has also been shown to lead to fast trafficking of the TRPA1 protein from cytoplasma to plasma membrane, and thereby increases the TRPA1 sensitivity in both heterologous expression systems and native nociceptive neurons [46]. Again, it seems questionable that the translocation of TRPA1 to the membrane can occur so rapidly as a contributing factor to the positive interaction that occurred almost immediately between these two channels. In fact, in a vast majority (82%) of neurons, the combined application of AITC and Cap evoked an abrupt and marked increase in the slope of the initial current trajectory, as compared to that evoked by Cap alone. This increase in the slope of the evoked current occurred so rapidly after the cell was exposed to the combined application of AITC and Cap, indicating that an increasing number of TRPV1 and/or TPRA1 channels were opened from the very outset of the response [34].
Physical Interaction between TRPA1 and TRPV1
TRP channels from members of different subfamilies can form heteromultimeric complexes [47, 48]. Recent studies have shown that TRPA1 and TRPV1 can form physical as well as functional heteromeric channel complex that was identified on the cytoplasmic membrane of native DRG neurons and heterologously expressed cells; and these channel complexes exhibit pharmacological and electrophysiological properties that are different from these individual channels [24, 49–51]. Furthermore, the physical interaction between these two channels may change the conformation of these channels and alter their channel properties and excitabilities. The agonist-induced conformational changes may also lead to alteration of gating properties of these channels and modulate their activities [24]. Thus, activation of TRPV1 can modulate the intrinsic properties of the TRPA1 channel, probably through direct interaction of the channels within the heteromeric TRPA1-TRPV1 complex. Indeed, their study indicated that single channel properties of TRPA1 are modulated by TRPV1 activity, independent of the change of intracellular Ca2+ [50]. A recent study by Fischer et al. (2014) has also shown that a constructed TRPV1::TRPA1 concatemer exhibited sensitivity to TRPV1 agonists and was inhibited by TRPV1 anatogonists, but was not activated by TRPA1 agonists [52]. To validate the potential contribution of a TRPA1-TRPV1 interaction occurring within the heteromeric complex to the synergistic effect observed in our study, the abundance of the expression of these channel complexes in pulmonary sensory neurons needs to be determined. Unfortunately, to our knowledge, the selective antagonist that specifically recognizes the TRPA1-TRPV1 complex (and not TRPA1 homomer) is not yet available (personal communication with Dr. Armen Akopian).
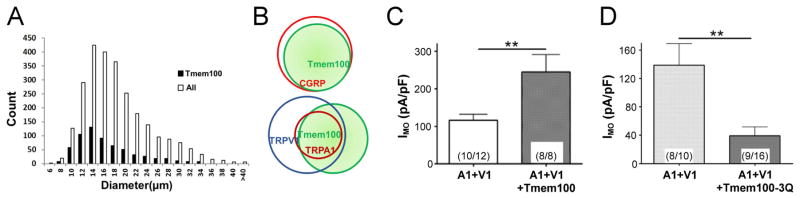
A recent study by Weng and coworkers has shown that Tmem100, a two-transmembrane adaptor protein with a putative TRPA1 binding site (KRR) at its C-terminus, can alter the physical association between TRPA1 and TRPV1, and thereby regulate the functionality and excitability of the TRPA1-TRPV1 channel complex [53]. In L4–L6 DRG neurons, Tmem100 was found predominantly in the small-diameter neurons, and co-expressed with 88.4%, 89.9% and 52.2% of the CGRP-, TRPA1-, and TRPV1-expressing neurons, respectively (Fig. 5A & 5B) [53]. In the presence of Tmem100, the physical association between TRPA1 and TRPV1 was weakened, which promoted the single-channel open probability of the TRPA1-TRPV1 complex and enhanced its sensitivity to TRPA1 agonist (mustard oil) in a TRPV1-dependent manner, but did not alter the sensitivity to TRPV1 agonist (capsaicin) (Fig. 5C). Tmem100-3Q, a mutant version of Tmem100 by replacing the positively charged K-R-R sequence at its C-terminus with un-charged Q-Q-Q sequence, exerted opposite effects (Fig. 5D). Thus, Tmem100-deficient mice showed a significant reduction in mechanical pain sensitivity following inflammatory injury. More importantly, the number of Tmem100-expressing neurons in the DRG was significantly increased after tissue inflammation was induced [53], seemingly pointing to a possible involvement of Tmem100 in the modulation of the sensitivity of these neurons during airway inflammation.
Figure 5.
Expression and function of a transmembrane adaptor protein Tmem100 in regulating the TRPA1-TRPV1 complex channel activity. A: Tmem100 is expressed in 24% of L4–L6 doral root ganglion (DRG) neurons, predominantly small-diameter cells with an average diameter of 15.7 μm. B: Diagrams showing the relationship of Tmem100 with other DRG markers based upon the data obtained by the double staining technique. C: Mustard oil (MO; 10 μM)-gated whole-cell voltage clamp (holding potential at −60 mV) current densities (IMO) in TRPA1+TRPV1 (1:1)- and TRPA1+TRPV1+Tmem100 (1:1:4)-expressing CHO cells. D: MO (10 μM)-gated whole-cell voltage clamp current densities (IMO) in TRPA1+TRPV1 (1:1)- and TRPA1+TRPV1+Tmem100-3Q (1:1:4)-expressing CHO cells; Tmem100-3Q is a Tmem100 mutant. In both C and D, the number of cells analyzed and those that responded are shown in the parentheses within bars. Data are means ±SEM; ** P < 0.01 (unpaired t-test). (Modified from reference 53)
Conclusion
These studies have clearly demonstrated a synergistic effect induced abruptly by simultaneous activations of TRPA1 and TRPV1 by their respective agonists at near-threshold concentrations. The synergy was found in the studies of single-unit recording of bronchopulmonary C-fiber afferents and reflex responses elicited by activation of these afferents in intact animals, as well as in isolated bronchopulmonary sensory neurons. This potentiating effect was critically dependent upon the action of Ca2+ as a signaling molecule. The rapid onset of the action further suggests that the interaction probably occurred locally at the sites of these channels. The underlying mechanism(s) of this positive interaction is not yet fully understood.
Many important questions remain unanswered and will require further investigations. For example, what are the relative roles and contributions of TRPA1 and TRPV1 to this synergistic effect? When AITC and Cap were administered in combination, did it enhance the current through TRPA1, TRPV1, both these channels, or their heteromeric channel complexes? In 85% of the neurons, the current evoked by the combined (Cap+AITC) application reached the peak precisely at the same time as that by Cap alone [34], which appears to suggest that the primary current was conducted through TRPV1 channels during the combined application. Obviously, direct evidence is required to validate this postulation. Whether and to what extent the heteromeric TRPA1-TRPV1 channel complex is involved in regulating the excitability of pulmonary sensory neurons also merits further investigation, especially during airway inflammation. Another interesting question concerns the role of Tmem100 in the positive interaction between TRPA1 and TRPV1 observed in our study.
The functions and properties of TPRA1 and the wide variety of identified chemical activators of TRPA1 have been well documented and supported by extensive and convincing evidence, but the weight of its role in regulating airway defense function will always be challenged on the ground that relatively high concentrations of selective agonists are required for its activation [7, 54–56]. As shown in these studies, although AITC applied alone at the near- or below-threshold concentration evoked only minimal or undetectable responses in isolated pulmonary sensory neurons, it markedly amplified the current evoked by the low-level activation of TRPV1 in the same neurons. Taken together, these findings suggest that the TRPA1-TRPV1 interaction may play an important role in regulating the excitability and function of bronchopulmonary C-fiber sensory nerves during airway inflammatory reaction.
Acknowledgments
This study was supported in part by National Institutes of Health (NIH) grants HL-96914 and UL1TR0000117.
Footnotes
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gees M, Owsianik G, Nilius B, Voets T. TRP channels. Comprehensive Physiology. 2012;2:563–608. doi: 10.1002/cphy.c110026. [DOI] [PubMed] [Google Scholar]
- 2.Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiological reviews. 2007;87:165–217. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- 3.Bautista DM, Pellegrino M, Tsunozaki M. TRPA1: A gatekeeper for inflammation. Annual review of physiology. 2013;75:181–200. doi: 10.1146/annurev-physiol-030212-183811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi K, Fukuoka T, Obata K, Yamanaka H, Dai Y, Tokunaga A, et al. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J Comp Neurol. 2005;493:596–606. doi: 10.1002/cne.20794. [DOI] [PubMed] [Google Scholar]
- 6.Diogenes A, Akopian AN, Hargreaves KM. NGF up-regulates TRPA1: implications for orofacial pain. J Dent Res. 2007;86:550–5. doi: 10.1177/154405910708600612. [DOI] [PubMed] [Google Scholar]
- 7.Nassenstein C, Kwong K, Taylor-Clark T, Kollarik M, Macglashan DM, Braun A, et al. Expression and function of the ion channel TRPA1 in vagal afferent nerves innervating mouse lungs. The Journal of physiology. 2008;586:1595–604. doi: 10.1113/jphysiol.2007.148379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jammes Y, Fornaris E, Mei N, Barrat E. Afferent and efferent components of the bronchial vagal branches in cats. Journal of the autonomic nervous system. 1982;5:165–76. doi: 10.1016/0165-1838(82)90037-6. [DOI] [PubMed] [Google Scholar]
- 9.Coleridge JC, Coleridge HM. Afferent vagal C fibre innervation of the lungs and airways and its functional significance. Reviews of physiology, biochemistry and pharmacology. 1984;99:1–110. doi: 10.1007/BFb0027715. [DOI] [PubMed] [Google Scholar]
- 10.Lee LY, Yu J. Sensory nerves in lung and airways. Comprehensive Physiology. 2014;4:287–324. doi: 10.1002/cphy.c130020. [DOI] [PubMed] [Google Scholar]
- 11.Bessac BF, Jordt SE. Breathtaking TRP channels: TRPA1 and TRPV1 in airway chemosensation and reflex control. Physiology (Bethesda, Md) 2008;23:360–70. doi: 10.1152/physiol.00026.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Swert KO, Joos GF. Extending the understanding of sensory neuropeptides. European journal of pharmacology. 2006;533:171–81. doi: 10.1016/j.ejphar.2005.12.066. [DOI] [PubMed] [Google Scholar]
- 13.Lee LY, Gu Q. Role of TRPV1 in inflammation-induced airway hypersensitivity. Current opinion in pharmacology. 2009;9:243–9. doi: 10.1016/j.coph.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geppetti P, Materazzi S, Nicoletti P. The transient receptor potential vanilloid 1: role in airway inflammation and disease. European journal of pharmacology. 2006;533:207–14. doi: 10.1016/j.ejphar.2005.12.063. [DOI] [PubMed] [Google Scholar]
- 15.Gu Q, Lee LY. Characterization of acid signaling in rat vagal pulmonary sensory neurons. American journal of physiology Lung cellular and molecular physiology. 2006;291:L58–65. doi: 10.1152/ajplung.00517.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–24. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 17.Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, et al. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–57. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- 18.Taylor-Clark TE, McAlexander MA, Nassenstein C, Sheardown SA, Wilson S, Thornton J, et al. Relative contributions of TRPA1 and TRPV1 channels in the activation of vagal bronchopulmonary C-fibres by the endogenous autacoid 4-oxononenal. The Journal of physiology. 2008;586:3447–59. doi: 10.1113/jphysiol.2008.153585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nilius B, Appendino G, Owsianik G. The transient receptor potential channel TRPA1: from gene to pathophysiology. Pflugers Archiv : European journal of physiology. 2012;464:425–58. doi: 10.1007/s00424-012-1158-z. [DOI] [PubMed] [Google Scholar]
- 20.Dai Y, Wang S, Tominaga M, Yamamoto S, Fukuoka T, Higashi T, et al. Sensitization of TRPA1 by PAR2 contributes to the sensation of inflammatory pain. J Clin Invest. 2007;117:1979–87. doi: 10.1172/JCI30951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gu Q, Kwong K, Lee LY. Ca2+ transient evoked by chemical stimulation is enhanced by PGE2 in vagal sensory neurons: role of cAMP/PKA signaling pathway. Journal of neurophysiology. 2003;89:1985–93. doi: 10.1152/jn.00748.2002. [DOI] [PubMed] [Google Scholar]
- 22.Kwong K, Lee LY. PGE(2) sensitizes cultured pulmonary vagal sensory neurons to chemical and electrical stimuli. Journal of applied physiology. 2002;93:1419–28. doi: 10.1152/japplphysiol.00382.2002. [DOI] [PubMed] [Google Scholar]
- 23.Patil MJ, Jeske NA, Akopian AN. Transient receptor potential V1 regulates activation and modulation of transient receptor potential A1 by Ca2+ Neuroscience. 2010;171:1109–19. doi: 10.1016/j.neuroscience.2010.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salas MM, Hargreaves KM, Akopian AN. TRPA1-mediated responses in trigeminal sensory neurons: interaction between TRPA1 and TRPV1. The European journal of neuroscience. 2009;29:1568–78. doi: 10.1111/j.1460-9568.2009.06702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Everaerts W, Gees M, Alpizar YA, Farre R, Leten C, Apetrei A, et al. The capsaicin receptor TRPV1 is a crucial mediator of the noxious effects of mustard oil. Curr Biol. 2011;21:316–21. doi: 10.1016/j.cub.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 26.Alpizar YA, Boonen B, Gees M, Sanchez A, Nilius B, Voets T, et al. Allyl isothiocyanate sensitizes TRPV1 to heat stimulation. Pflugers Archiv : European journal of physiology. 2014;466:507–15. doi: 10.1007/s00424-013-1334-9. [DOI] [PubMed] [Google Scholar]
- 27.Gees M, Alpizar YA, Boonen B, Sanchez A, Everaerts W, Segal A, et al. Mechanisms of transient receptor potential vanilloid 1 activation and sensitization by allyl isothiocyanate. Molecular pharmacology. 2013;84:325–34. doi: 10.1124/mol.113.085548. [DOI] [PubMed] [Google Scholar]
- 28.Lin YJ, Lin RL, Ruan T, Khosravi M, Lee LY. A synergistic effect of simultaneous TRPA1 and TRPV1 activations on vagal pulmonary C-fiber afferents. Journal of applied physiology. 2015;118:273–81. doi: 10.1152/japplphysiol.00805.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fernandes ES, Fernandes MA, Keeble JE. The functions of TRPA1 and TRPV1: moving away from sensory nerves. British journal of pharmacology. 2012;166:510–21. doi: 10.1111/j.1476-5381.2012.01851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGarvey LP, Butler CA, Stokesberry S, Polley L, McQuaid S, Abdullah H, et al. Increased expression of bronchial epithelial transient receptor potential vanilloid 1 channels in patients with severe asthma. The Journal of allergy and clinical immunology. 2014;133:704–12. e4. doi: 10.1016/j.jaci.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 31.Nassini R, Pedretti P, Moretto N, Fusi C, Carnini C, Facchinetti F, et al. Transient receptor potential ankyrin 1 channel localized to non-neuronal airway cells promotes non-neurogenic inflammation. PloS one. 2012;7:e42454. doi: 10.1371/journal.pone.0042454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reilly CA, Johansen ME, Lanza DL, Lee J, Lim JO, Yost GS. Calcium-dependent and independent mechanisms of capsaicin receptor (TRPV1)-mediated cytokine production and cell death in human bronchial epithelial cells. Journal of biochemical and molecular toxicology. 2005;19:266–75. doi: 10.1002/jbt.20084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ni D, Gu Q, Hu HZ, Gao N, Zhu MX, Lee LY. Thermal sensitivity of isolated vagal pulmonary sensory neurons: role of transient receptor potential vanilloid receptors. American journal of physiology Regulatory, integrative and comparative physiology. 2006;291:R541–50. doi: 10.1152/ajpregu.00016.2006. [DOI] [PubMed] [Google Scholar]
- 34.Hsu CC, Lee LY. Role of calcium ions in the positive interaction between TRPA1 and TRPV1 channels in bronchopulmonary sensory neurons. Journal of applied physiology. 2015;118:1533–43. doi: 10.1152/japplphysiol.00043.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–29. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 36.Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–82. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 37.Cavanaugh EJ, Simkin D, Kim D. Activation of transient receptor potential A1 channels by mustard oil, tetrahydrocannabinol and Ca2+ reveals different functional channel states. Neuroscience. 2008;154:1467–76. doi: 10.1016/j.neuroscience.2008.04.048. [DOI] [PubMed] [Google Scholar]
- 38.Doerner JF, Gisselmann G, Hatt H, Wetzel CH. Transient receptor potential channel A1 is directly gated by calcium ions. The Journal of biological chemistry. 2007;282:13180–9. doi: 10.1074/jbc.M607849200. [DOI] [PubMed] [Google Scholar]
- 39.Zurborg S, Yurgionas B, Jira JA, Caspani O, Heppenstall PA. Direct activation of the ion channel TRPA1 by Ca2+ Nature neuroscience. 2007;10:277–9. doi: 10.1038/nn1843. [DOI] [PubMed] [Google Scholar]
- 40.Wang YY, Chang RB, Waters HN, McKemy DD, Liman ER. The nociceptor ion channel TRPA1 is potentiated and inactivated by permeating calcium ions. The Journal of biological chemistry. 2008;283:32691–703. doi: 10.1074/jbc.M803568200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McMahon SB, Wood JN. Increasingly irritable and close to tears: TRPA1 in inflammatory pain. Cell. 2006;124:1123–5. doi: 10.1016/j.cell.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 42.Tominaga M, Tominaga T. Structure and function of TRPV1. Pflugers Archiv : European journal of physiology. 2005;451:143–50. doi: 10.1007/s00424-005-1457-8. [DOI] [PubMed] [Google Scholar]
- 43.Vellani V, Mapplebeck S, Moriondo A, Davis JB, McNaughton PA. Protein kinase C activation potentiates gating of the vanilloid receptor VR1 by capsaicin, protons, heat and anandamide. The Journal of physiology. 2001;534:813–25. doi: 10.1111/j.1469-7793.2001.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carr MJ, Kollarik M, Meeker SN, Undem BJ. A role for TRPV1 in bradykinin-induced excitation of vagal airway afferent nerve terminals. J Pharmacol Exp Ther. 2003;304:1275–9. doi: 10.1124/jpet.102.043422. [DOI] [PubMed] [Google Scholar]
- 45.Spahn V, Stein C, Zollner C. Modulation of transient receptor vanilloid 1 activity by transient receptor potential ankyrin 1. Molecular pharmacology. 2014;85:335–44. doi: 10.1124/mol.113.088997. [DOI] [PubMed] [Google Scholar]
- 46.Schmidt M, Dubin AE, Petrus MJ, Earley TJ, Patapoutian A. Nociceptive signals induce trafficking of TRPA1 to the plasma membrane. Neuron. 2009;64:498–509. doi: 10.1016/j.neuron.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hellwig N, Albrecht N, Harteneck C, Schultz G, Schaefer M. Homo- and heteromeric assembly of TRPV channel subunits. Journal of cell science. 2005;118:917–28. doi: 10.1242/jcs.01675. [DOI] [PubMed] [Google Scholar]
- 48.Park JY, Hwang EM, Yarishkin O, Seo JH, Kim E, Yoo J, et al. TRPM4b channel suppresses store-operated Ca2+ entry by a novel protein-protein interaction with the TRPC3 channel. Biochemical and biophysical research communications. 2008;368:677–83. doi: 10.1016/j.bbrc.2008.01.153. [DOI] [PubMed] [Google Scholar]
- 49.Ruparel NB, Patwardhan AM, Akopian AN, Hargreaves KM. Desensitization of transient receptor potential ankyrin 1 (TRPA1) by the TRP vanilloid 1-selective cannabinoid arachidonoyl-2 chloroethanolamine. Molecular pharmacology. 2011;80:117–23. doi: 10.1124/mol.110.068940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Staruschenko A, Jeske NA, Akopian AN. Contribution of TRPV1-TRPA1 interaction to the single channel properties of the TRPA1 channel. The Journal of biological chemistry. 2010;285:15167–77. doi: 10.1074/jbc.M110.106153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Akopian AN. Regulation of nociceptive transmission at the periphery via TRPA1-TRPV1 interactions. Current pharmaceutical biotechnology. 2011;12:89–94. doi: 10.2174/138920111793937952. [DOI] [PubMed] [Google Scholar]
- 52.Fischer MJ, Balasuriya D, Jeggle P, Goetze TA, McNaughton PA, Reeh PW, et al. Direct evidence for functional TRPV1/TRPA1 heteromers. Pflugers Archiv : European journal of physiology. 2014;466:2229–41. doi: 10.1007/s00424-014-1497-z. [DOI] [PubMed] [Google Scholar]
- 53.Weng HJ, Patel KN, Jeske NA, Bierbower SM, Zou W, Tiwari V, et al. Tmem100 is a regulator of TRPA1-TRPV1 complex and contributes to persistent pain. Neuron. 2015;85:833–46. doi: 10.1016/j.neuron.2014.12.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Birrell MA, Belvisi MG, Grace M, Sadofsky L, Faruqi S, Hele DJ, et al. TRPA1 agonists evoke coughing in guinea pig and human volunteers. Am J Respir Crit Care Med. 2009;180:1042–7. doi: 10.1164/rccm.200905-0665OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brozmanova M, Mazurova L, Ru F, Tatar M, Kollarik M. Comparison of TRPA1-versus TRPV1-mediated cough in guinea pigs. European journal of pharmacology. 2012;689:211–8. doi: 10.1016/j.ejphar.2012.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Talavera K, Gees M, Karashima Y, Meseguer VM, Vanoirbeek JA, Damann N, et al. Nicotine activates the chemosensory cation channel TRPA1. Nature neuroscience. 2009;12:1293–9. doi: 10.1038/nn.2379. [DOI] [PubMed] [Google Scholar]