Abstract
The diseases of the esophagus and nose are among the major factors contributing to chronic cough although their role in different patient populations is debated. Studies in animal models and in humans show that afferent C-fiber activators applied on esophageal or nasal mucosa do not initiate cough, but enhance cough induced by inhaled irritants. These results are consistent with the hypothesis that activation of esophageal and nasal C-fibers contribute to cough reflex hypersensitivity observed in chronic cough patients with gastroesophageal reflux disease (GERD) and chronic rhinitis, respectively. The afferent nerves mediating cough sensitization from the esophagus are probably the neural crest-derived vagal jugular C-fibers. In addition to their responsiveness to high concentration of acid typical for gastroesophageal reflux (pH<5), esophageal C-fibers also express receptors for activation by weakly acidic reflux such as receptors highly sensitive to acid and receptors for bile acids. The nature of sensory pathways from the nose and their activators relevant for cough sensitization are less understood. Increased cough reflex sensitivity was also reported in many patients with GERD or rhinitis who do not complain of cough indicating that additional endogenous or exogenous factors may be required to develop chronic coughing in these diseases.
Keywords: cough, vagus nerve, capsaicin, nociceptor, postnasal drip, rhinosinusitis
1. Introduction
The diseases of the nose and esophagus are considered to be among the major factors contributing to chronic cough [1], although their contribution vary in different patient populations and may not be as prominent as concluded by the pioneering studies in the chronic cough research field [2, 3]. Several mechanisms by which the diseases of the nose and esophagus may contribute to chronic cough have been proposed. Direct or circumstantial supporting evidence has been provided in clinical studies for some of these mechanisms. However, mechanistic studies occasionally arrived at conflicting conclusions (e.g.[2, 4]). This is probably because different mechanisms may operate in different patients, and/or because a combination of two or more mechanisms may operate in an individual patient. Here we focus on the mechanism that is the most often observed in patients with chronic cough – the sensitization of cough reflex also termed cough hypersensitivity. Specifically, we will discuss neurally-mediated sensitization of cough reflex from nose and esophagus.
Sensitization of the cough reflex refers to a condition in which the cough reflex is more readily induced. Cough reflex sensitization can be demonstrated as decreased intensity of a stimulus required to trigger cough or increased coughing in response to a stimulus with constant intensity. Experimentally, the changes in cough reflex sensitivity are evaluated by measuring the cough threshold to inhaled irritant or by counting the number of coughs evoked by an inhaled irritant with defined intensity. The cough threshold is most often measured by a controlled inhalation of increasing concentrations of an aerosolized tussigen, commonly capsaicin or acidic solutions [5–7]. The cough threshold is defined as the lowest concentration of an irritant required to evoke a predetermined number of coughs (2 and 5 coughs are commonly used, denoted as C2 and C5, respectively).
The cough reflex hypersensitivity is often reported in patients with chronic cough attributed to disparate causes including the diseases of nose and esophagus [8–10]. It seems to be a straightforward conclusion that the sensitization of the cough reflex contributes to coughing. In patients with sensitized cough reflex, the endogenous and environmental stimuli are expected to be more effective to initiate coughing and thus these patients cough in the situations when their healthy counterparts do not. By analogy, the state of increased cough reflex sensitivity is often compared to the state of hypersensitivity to stimuli that cause pain in somatosensory system (hyperalgesia) and referred to as hypertussive state. Clinical observations that the cough hypersensitivity normalizes in patients in with successful treatment of cough are also consistent with this notion.
Recent reviews indicate consensus that cough hypersensitivity is an important mechanistic concept in chronic cough [10, 11]. Indeed, the cough hypersensitivity syndrome is defined as a disorder characterized by troublesome coughing often triggered by low levels of thermal, mechanical or chemical exposure and is critical for chronic cough. Common feature of the diseases of the nose (exemplified by chronic rhinitis) and esophagus (exemplified by GERD) that are thought to contribute to chronic cough is that they often cause increase in the cough reflex sensitivity. We will therefore discuss them together in this review.
Experimental studies in humans and animal models provided evidence that the cough reflex is sensitized in certain diseases of the nose and esophagus that are implicated in chronic cough. Cough reflex sensitivity to inhaled capsaicin was increased in adult patients with allergic rhinitis who did not complain of cough and had a normal lung function [12]. Moreover, cough sensitivity to capsaicin was further increased during the pollen season in pollen-sensitive patients [13]. Cough reflex sensitivity in patients with allergic rhinitis was also increased in response to humidified hot air [14]. Patients with chronic rhinitis/sinusitis of etiology other than allergic had also increased cough reflex sensitivity to capsaicin [15]. Similarly, increased cough reflex sensitivity has been described in GERD [16, 17]. Furthermore, cough reflex sensitivity in GERD decreased with effective treatment of gastroesophageal reflux [8, 17, 18, 19].
Nonetheless, it should be emphasized that while many patients with rhinitis or GERD have increased cough reflex sensitivity, only a small fraction of these patients will develop chronic cough. This observation argues that the increased cough reflex sensitivity alone is unlikely solely responsible for chronic cough observed in patients with chronic cough attributed to chronic rhinitis or GERD. Other factors, either environmental or more probably endogenous, may be required in addition to increased cough reflex sensitivity to cause chronic cough in these patients. For example, an airway involvement that may be considered insignificant by itself (e.g. modest allergic airway inflammation in patients with allergic rhinitis[20]) combined with increased cough sensitivity may result in chronic coughing. This problem has been discussed in more details in our previous review [21].
2. Sensitization of cough reflex due to stimulation and/or inflammation of airway mucosa
In the diseases of the nose and esophagus it is possible that cough reflex sensitization is due to stimulation (“irritation”) and/or inflammation in the areas from which the cough reflex can be normally evoked, i.e. the larynx and large airways. For example, gastroesophageal reflux can cause irritation and/or inflammation of the larynx in some patients whether due to acid or other components of the refluxed fluid or aerosol. The stimulation of cough-mediating nerves in the larynx and/or the inflammation in laryngeal mucosa can directly contribute to increase cough reflex sensitivity in these patients. We even speculate that repeated stimulation of laryngeal nerves (for example by low amounts of refluxed acid) may not be sufficient to induce appreciable laryngeal inflammation, but may cause central sensitization of laryngeal afferent pathways by a mechanisms analogous to central sensitization described in somatosensory system[22].
The causal link between gastroesophageal reflux, inflammatory changes in the larynx and chronic cough is difficult to establish because the detection of refluxed content in the larynx with currently available technology is challenging [23, 24]. Nonetheless, the cough sensitization due to irritation/inflammation of the larynx and possibly other parts of airways is a plausible mechanism that should be rigorously addressed as technology improves. Similarly, in the diseases of nose the possibility exists that the inflammation is not limited to the nasal mucosa, but affects other parts of the airways. For example, it should be noted that patients with allergic rhinitis often have extensive inflammation in the lungs and airways although they do not suffer from asthma (e.g. [20]).
3. Sensitization of cough reflex by activation of sensory nerves in the nose and esophagus
In theory chronic coughing in patients with the diseases of nose or esophagus can be due to direct initiation of cough by the activation of sensory nerves in these organs. This possibility is not supported by clinical and experimental studies. Infusion of sensory stimulus acid (pH=1.0) into the esophagus does not consistently initiate cough in patients with chronic cough and GERD. Cough is occasionally encountered during prolonged infusion of acid into the esophagus [4], but this is a rare finding [2, 25]. Modest variable association between reflux and cough also does not provide support for the notion that cough is directly initiated from the esophagus in most patients [26–33]. Similarly to the observations in the esophagus, sensory activators applied to nasal mucosa also failed to initiate cough from the nose [34–38]. Thus, available data indicate that direct initiation of cough by stimulation of esophageal or nasal nerves is not a major mechanism of chough associated with the diseases of these organs.
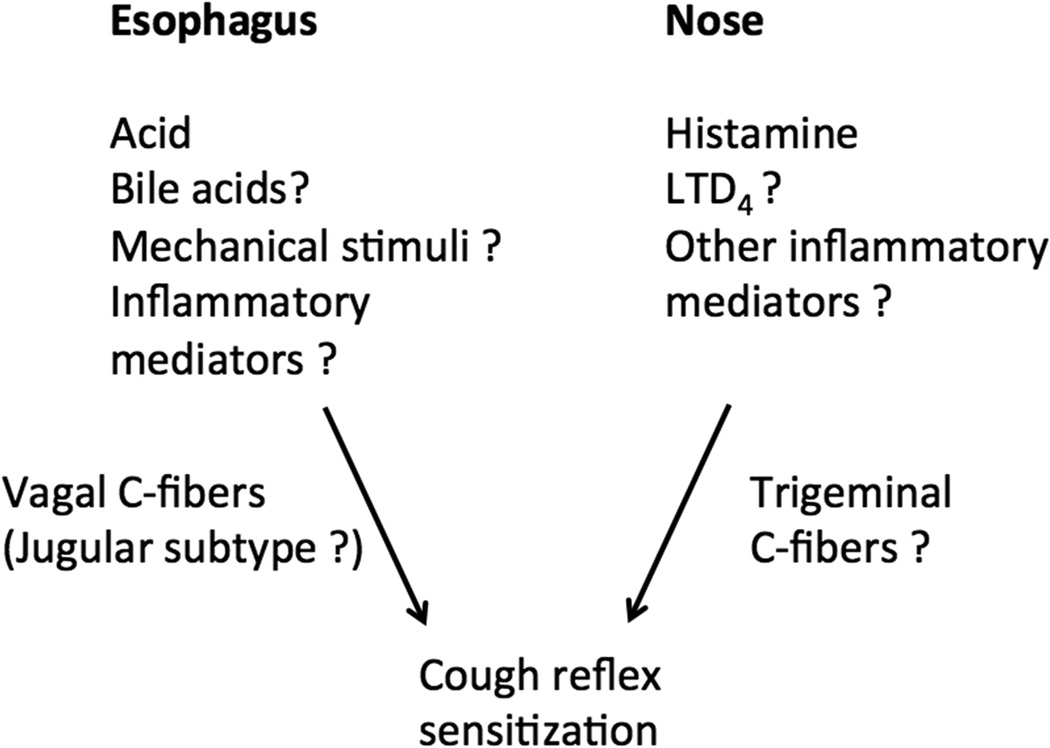
Experimental and clinical evidence supports the notion that the activation of sensory nerves in the nose and esophagus leads to increase in cough reflex sensitivity (Fig. 1). When applied to nasal mucosa in healthy humans the afferent C-fiber activators histamine and capsaicin that efficiently stimulate nasal sensory neurons[39] did not trigger cough, but sensitized the cough reflex [37, 38]. Following the intranasal capsaicin or histamine, the number of coughs evoked by inhalation of a single dose of capsaicin was increased by 60–80%. Similarly, intranasal histamine caused the cough reflex sensitization in patients with allergic rhinitis [36]. Similar to human studies intranasal capsaicin sensitized the cough reflex in awake guinea pigs by approximately 70% [40]. Intranasal application of allergen ovalbumin in the ovalbumin-sensitized guinea pigs also induced sensitization of the cough reflex possibly by a mechanism involving leukotriene cys-LT1 receptor [41]. These studies were previously reviewed in details in [21].
Figure 1.
Schematic drawing of cough sensitization from the nose and esophagus.
It is important to note, that not every activator of nasal sensory nerves will enhance cough reflex. For example, nasal stimulation with water was reported to inhibit cough in anesthetized rabbits [42]. Similarly, intranasal application of the TRPM8 activator (-)-menthol inhibited cough in guinea pigs[43]. The data on the effect of intranasal TRPA1 agonists are thus far inconclusive. The observations that some intranasal stimuli sensitize and others inhibit coughing suggests that there are at least two separate afferent nerve pathways that have opposite regulatory effects on cough or a different pattern of activation of the same pathway have opposite effects.
Stimulation of nasal sensory nerves also affected the urge-to-cough. The urge-to-cough is a sensation that has a strong representation in the laryngeal airways of patients across a diversity of airways diseases and drives behavioral coughing to satiate the unpleasant sensation [44]. The urge-to-cough is most commonly described as an irritation or tickle in the back of the neck and is associated with >90% of coughs in patients with chronic cough[45]. Capsaicin inhalation evokes an urge-to-cough, which was associated with activations in a variety of brain regions relevant for cough [46]. This approach was used to investigate the effects of nasal sensory activators on the urge to cough. Studies in healthy volunteers revealed that nasal administration of the TRPA1 agonist AITC increased the urge to cough to capsaicin (measured as the lowest concentration of capsaicin that evoked the urge to cough during the capsaicin cough challenge) [47]. Interestingly, the cough threshold to capsaicin (C2) was not affected. In contrast, intranasal administration of TRPM8 agonist menthol decreased both the urge to cough and capsaicin cough threshold in healthy subjects [47].
Cough reflex can be also sensitized by the afferent C-fiber activator acid in the esophagus. Infusion of acid(pH=1.0) into the distal esophagus of patients with GERD and chronic cough induced cough reflex hypersensitivity (decreased the C2 cough threshold to inhaled capsaicin) [25]. Sensitization of cough to capsaicin was also observed after esophageal acid infusion in asthmatics [48]. To our knowledge the urge-to-cough has not been evaluated after esophageal stimulation. Inclusion of the measurement of urge-to-cough into this type of studies could provide additional valuable information.
Importantly, the sensitization of cough reflex following the application of sensory stimuli into the nose or esophagus develops relatively quickly arguing for a neutrally-mediated effect. The cough reflex sensitivity was increased at the earliest time points evaluated within 10 min following the intranasal application of capsaicin or histamine [37, 38]. After acid infusion into the esophagus the increase in the cough reflex sensitivity was observed also at the earliest time points evaluated within 10–15 min [25, 48].
Central mechanisms of cough sensitization from the esophagus and nose are poorly understood. A convergent input from nasal and laryngeal sensory nerves to the rostral sensory trigeminal nuclei has been reported previously [49]. Recent elegant tracing studies using a novel conditional anterograde transneuronal viral tracing system have shown that the neurons derived from the jugular vagal ganglia favor trigeminal nucleus terminations [50, 51]. This may provide anatomical pathways for interaction between the airway sensory nerves initiating cough due to inhaled irritants (jugular C-fibers) and trigeminal capsaicin-sensitive afferent nerves (presumed C-fibers) innervating nasal mucosa [39]. As discussed below, the nerve fiber type implicated in cough sensitization from the esophagus is esophageal jugular C-fiber that may interact with the input from airway jugular C-fibers at multiple levels.
It is unknown what afferent nerves mediate the cough reflex sensitization from the nose. The cell bodies of these nerves are most likely the primary afferent neurons located in the trigeminal ganglia that project into nasal mucosa[52, 53], although the role for olfactory nerves cannot be ruled out. It has been established that trigeminal neurons can be divided into several types based on their responsiveness to mediators and neuropeptide content [39, 54, 55]. While it is probable that the cough reflex sensitization from the nose is mediated by capsaicin- and histamine-sensitive trigeminal C-fibers containing neuropeptides, available information is unfortunately too limited to allow further informed speculation on the properties of these nerve type.
4. Afferent nerves mediating sensitization of cough reflex from the esophagus
The identity of a nerve type mediating cough sensitization from the esophagus has not been established. The esophageal sensory innervation have been reviewed in details elsewhere [56, 57]. Briefly, based on data from guinea pig and other species, the esophagus receives dual extrinsic afferent innervation from vagal and spinal afferent neurons. Vagal afferent neurons are located in the vagal nodose and jugular sensory ganglia, while spinal afferent neurons are located in the spinal dorsal root ganglia (DRG). Approximately half of nodose neurons project low threshold mechnanosensitive A-fibers tension mechanosensors. These non-nociceptive nerve fibers are unlikely critical for sensitization of cough reflex from the esophagus inasmuch as their terminals are unresponsive to acid and located deep in the muscle. In contrast, all three nodose, jugular and DRG ganglia project capsaicin-sensitive C-fibers into the esophagus. Neurophysiological and pharmacological properties of esophageal C-fibers depend on their embryonic origin in that the neural crest-derived jugular and DRG C-fibers have certain similar properties that are distinct from nodose C-fibers [57–62].
The esophageal vagal C-fibers are the candidates for mediating cough sensitization because of their sensitivity to esophageal noxious stimuli including acid. We hypothesize that cough sensitization is mediated by vagal nociceptive C-fibers innervating the esophagus, most probably the jugular C-fibers. This conjecture is based on their sensory properties including vagal origin, responsiveness to acid, putative location of their nerve terminals in esophageal mucosa, and a precedent for cough reflex sensitization by jugular C-fibers in the respiratory system [63]. Nonetheless, the role of esophageal nodose and DRG C-fibers in cough sensitization from the esophagus has not been ruled out.
In humans, the instillation of capsaicin into the esophagus induces pain (heartburn, retrosternal burning and epigastric burning) that is consistent with activation of esophageal spinal DRG C-fibers [64–66]. Intraesophageal capsaicin also induced modulation of esophageal motor reflexes (e.g. swallowing, secondary peristalsis, LES pressure) that are regulated by vagal afferent nerves [65–67]. The latter observation strongly indicates that vagal capsaicin-sensitive afferent nerves (vagal C-fibers) innervate esophageal mucosa in humans consistent with the conclusions from animal studies.
The acid is considered to be the key mediator that induces sensitization of cough reflex by activation of esophageal sensory nerves [25, 29–32, 48]. Indeed, as predicted from the expression of the capsaicin receptor TRPV1 in the esophageal C-fibers, their nerve terminals are robustly activated by acid in high concentration (pH<5) [61, 68–70]. However, it is often overlooked that acid in high concentration is not the only C-fiber activator in reflux.
Components of gastroesophageal reflux other than acid may stimulate esophageal sensory nerves to increase cough reflex sensitivity. This issue has been extensively studied in patients with GERD in who the symptoms of reflux persisted despite effective suppression of gastric acid secretion by proton pumps inhibitors (PPI). These studies revealed that in most patients on PPI treatment the reflux is still present (the number and duration of reflux episodes do not change) and that the symptoms are still associated with reflux that becomes only weakly acidic due to PPI therapy). These observations are consistent with the hypothesis that factors in gastroesophageal other than acid in high concentration activate esophageal nerves. While the nature of these factors is unknown at present, clinical and laboratory studies suggest that mild acid, bile acid and possibly the mechanical effectx of the reflux volume are involved.
When compared to gastric acidity (pH=1.0–2.0), it is easy to overlook that there is still considerable amount of acid in weakly acidic reflux on PPI therapy relative to physiological pH in the tissue (pH=7.4). If the pH of weakly acidic reflux is pH=5.0–6.0, the concentration of acid (H+ hydrogen ions) is 30–300-fold higher than in the tissue. Increased mucosal permeability in GERD facilitates acid diffusion. We have reported that acid in concentration similar to the concentration of acid in weakly acidic reflux (mild acid, pH=6.0) robustly stimulates esophageal C-fibers [70]. The receptor mechanism(s) of this activation are unknown, but our expression analysis indicate that certain members of two-pore domain (K2P) potassium channel family (e.g. TASK1) [71], acid sensing ion channels (ASICs) [72] family and proton-sensing G-protein coupled receptors family (OGR1) may be involved because of their high acid sensitivity (threshold close to pH=6.0–7.0) [73] and abundant expression in afferent neurons innervating the esophagus [61, 70].
Bile acids are often present in gastroesophageal reflux and can reach relatively high concentrations in some patients [74, 75]. Acid suppression treatment can even create conditions that facilitate bile acid reflux, solubility and diffusion due to an increase in pH, reduced gastric secretion and bacterial deconjugation [76, 77]. Infusion of bile acids into the esophagus can induce heartburn and pain at neutral pH [78] and bile acid-containing reflux associates with reflux symptoms in refractory GERD [79, 80]. These clinical observations suggest that bile acids can activate C-fibers. Our unpublished data indicate that bile acids can stimulate vagal esophageal C-fibers and that vagal C-fiber neurons innervating the esophagus express the receptors for bile acids TGR5 [72]. Intriguingly, many patients with chronic cough have impaired motility and experience weak peristalsis with large breaks [81]. These mechanisms may aid mucosal exposure to acid and non-acidic components.
The mechanical effect of the volume of gastroesophageal reflux is the least understood presumably because of the difficulty to measure the volume with currently available 24h esophageal monitoring techniques. Nonetheless, a proportion of vagal C-fibers are responsive to esophageal distention [58]. While large, often noxious levels of esophageal distention are required to meaningfully activate esophageal C-fibers, these fibers can be strongly sensitized by various mediators to respond to smaller levels of distention that may be perhaps occur during reflux [82–84].
Because the components of non-acidic reflux have high potential to stimulate esophageal C-fibers, the lack of response of chronic cough to acid suppression with PPI may not be sufficient to exclude gastroesophageal reflux as a contributing factor to chronic cough. Further basic and clinical studies are needed to elucidate the mechanisms of esophageal sensory nerve activation by non-acidic reflux and their role in chronic cough due to GERD, and possibly to identify novel drug targets for treatment of chronic cough.
Acknowledgements
This work was supported by BioMed Martin (ITMS: 26220220187) co-funded by EU (Slovakia). M.K. received support from NIH DK074480 (US).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chung KF, Pavord ID. Prevalence, pathogenesis, and causes of chronic cough. Lancet. 2008;371:1364–1374. doi: 10.1016/S0140-6736(08)60595-4. [DOI] [PubMed] [Google Scholar]
- 2.Irwin RS, French CL, Curley FJ, Zawacki JK, Bennett FM. Chronic cough due to gastroesophageal reflux. Clinical, diagnostic, and pathogenetic aspects. Chest. 1993;104:1511–1517. doi: 10.1378/chest.104.5.1511. [DOI] [PubMed] [Google Scholar]
- 3.Kahrilas PJ, Howden CW, Hughes N, Molloy-Bland M. Response of chronic cough to acid-suppressive therapy in patients with gastroesophageal reflux disease. Chest. 2013;143:605–612. doi: 10.1378/chest.12-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ing AJ, Ngu MC, Breslin AB. Pathogenesis of chronic persistent cough associated with gastroesophageal reflux. Am J Respir Crit Care Med. 1994;149:160–167. doi: 10.1164/ajrccm.149.1.8111576. [DOI] [PubMed] [Google Scholar]
- 5.Morice AH, Fontana GA, Belvisi MG, Birring SS, Chung KF, Dicpinigaitis PV, et al. ERS guidelines on the assessment of cough. Eur Respir J. 2007;29:1256–1276. doi: 10.1183/09031936.00101006. [DOI] [PubMed] [Google Scholar]
- 6.Choudry NB, Fuller RW. Sensitivity of the cough reflex in patients with chronic cough. Eur Respir J. 1992;5:296–300. [PubMed] [Google Scholar]
- 7.Dicpinigaitis PV. Experimentally induced cough. Pulm Pharmacol Ther. 2007;20:319–324. doi: 10.1016/j.pupt.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 8.McGarvey LP, Heaney LG, Lawson JT, Johnston BT, Scally CM, Ennis M, et al. Evaluation and outcome of patients with chronic non-productive cough using a comprehensive diagnostic protocol. Thorax. 1998;53:738–743. doi: 10.1136/thx.53.9.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higenbottam T. Chronic cough and the cough reflex in common lung diseases. Pulm Pharmacol Ther. 2002;15:241–247. doi: 10.1006/pupt.2002.0341. [DOI] [PubMed] [Google Scholar]
- 10.Chung KF, McGarvey L, Mazzone SB. Chronic cough as a neuropathic disorder. The Lancet Respiratory medicine. 2013;1:414–422. doi: 10.1016/S2213-2600(13)70043-2. [DOI] [PubMed] [Google Scholar]
- 11.Morice AH, Millqvist E, Belvisi MG, Bieksiene K, Birring SS, Chung KF, et al. Expert opinion on the cough hypersensitivity syndrome in respiratory medicine. Eur Respir J. 2014;44:1132–1148. doi: 10.1183/09031936.00218613. [DOI] [PubMed] [Google Scholar]
- 12.Pecova R, Zucha J, Pec M, Neuschlova M, Hanzel P, Tatar M. Cough reflex sensitivity testing in in seasonal allergic rhinitis patients and healthy volunteers. J Physiol Pharmacol. 2008;59(Suppl 6):557–564. [PubMed] [Google Scholar]
- 13.Pecova R, Vrlik M, Tatar M. Cough sensitivity in allergic rhinitis. J Physiol Pharmacol. 2005;56(Suppl 4):171–178. [PubMed] [Google Scholar]
- 14.Khosravi M, Collins PB, Lin RL, Hayes D, Jr, Smith JA, Lee LY. Breathing hot humid air induces airway irritation and cough in patients with allergic rhinitis. Respir Physiol Neurobiol. 2014;198:13–19. doi: 10.1016/j.resp.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu L, Xu X, Wang L, Yang Z, Lu H, Qiu Z. Capsaicin-sensitive cough receptors in lower airway are responsible for cough hypersensitivity in patients with upper airway cough syndrome. Medical science monitor : international medical journal of experimental and clinical research. 2013;19:1095–1101. doi: 10.12659/MSM.889118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrari M, Olivieri M, Sembenini C, Benini L, Zuccali V, Bardelli E, et al. Tussive effect of capsaicin in patients with gastroesophageal reflux without cough. Am J Respir Crit Care Med. 1995;151:557–561. doi: 10.1164/ajrccm.151.2.7842220. [DOI] [PubMed] [Google Scholar]
- 17.O'Connell F, Thomas VE, Pride NB, Fuller RW. Capsaicin cough sensitivity decreases with successful treatment of chronic cough. Am J Respir Crit Care Med. 1994;150:374–380. doi: 10.1164/ajrccm.150.2.8049818. [DOI] [PubMed] [Google Scholar]
- 18.Benini L, Ferrari M, Sembenini C, Olivieri M, Micciolo R, Zuccali V, et al. Cough threshold in reflux oesophagitis: influence of acid and of laryngeal and oesophageal damage. Gut. 2000;46:762–767. doi: 10.1136/gut.46.6.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ziora D, Jarosz W, Dzielicki J, Ciekalski J, Krzywiecki A, Dworniczak S, et al. Citric acid cough threshold in patients with gastroesophageal reflux disease rises after laparoscopic fundoplication. Chest. 2005;128:2458–2464. doi: 10.1378/chest.128.4.2458. [DOI] [PubMed] [Google Scholar]
- 20.Braunstahl GJ, Fokkens WJ, Overbeek SE, KleinJan A, Hoogsteden HC, Prins JB. Mucosal and systemic inflammatory changes in allergic rhinitis and asthma: a comparison between upper and lower airways. Clin Exp Allergy. 2003;33:579–587. doi: 10.1046/j.1365-2222.2003.01652.x. [DOI] [PubMed] [Google Scholar]
- 21.Tatar M, Plevkova J, Brozmanova M, Pecova R, Kollarik M. Mechanisms of the cough associated with rhinosinusitis. Pulm Pharmacol Ther. 2009;22:121–126. doi: 10.1016/j.pupt.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 22.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152:S2–S15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Athanasiadis T, Allen JE. Chronic cough: an otorhinolaryngology perspective. Current opinion in otolaryngology & head and neck surgery. 2013;21:517–522. doi: 10.1097/MOO.0b013e3283658eca. [DOI] [PubMed] [Google Scholar]
- 24.Tsoukali E, Sifrim D. Investigation of extraesophageal gastroesophageal reflux disease. Annals of gastroenterology : quarterly publication of the Hellenic Society of Gastroenterology. 2013;26:290–295. [PMC free article] [PubMed] [Google Scholar]
- 25.Javorkova N, Varechova S, Pecova R, Tatar M, Balaz D, Demeter M, et al. Acidification of the oesophagus acutely increases the cough sensitivity in patients with gastro-oesophageal reflux and chronic cough. Neurogastroenterol Motil. 2008;20:119–124. doi: 10.1111/j.1365-2982.2007.01020.x. [DOI] [PubMed] [Google Scholar]
- 26.Ing AJ, Ngu MC, Breslin AB. Chronic persistent cough and gastro-oesophageal reflux. Thorax. 1991;46:479–483. doi: 10.1136/thx.46.7.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ours TM, Kavuru MS, Schilz RJ, Richter JE. A prospective evaluation of esophageal testing and a double-blind, randomized study of omeprazole in a diagnostic and therapeutic algorithm for chronic cough. Am J Gastroenterol. 1999;94:3131–3138. doi: 10.1111/j.1572-0241.1999.01504.x. [DOI] [PubMed] [Google Scholar]
- 28.Paterson WG, Murat BW. Combined ambulatory esophageal manometry and dual-probe pH-metry in evaluation of patients with chronic unexplained cough. Dig Dis Sci. 1994;39:1117–1125. doi: 10.1007/BF02087567. [DOI] [PubMed] [Google Scholar]
- 29.Blondeau K, Dupont LJ, Mertens V, Tack J, Sifrim D. Improved diagnosis of gastro-oesophageal reflux in patients with unexplained chronic cough. Aliment Pharmacol Ther. 2007;25:723–732. doi: 10.1111/j.1365-2036.2007.03255.x. [DOI] [PubMed] [Google Scholar]
- 30.Sifrim D, Dupont L, Blondeau K, Zhang X, Tack J, Janssens J. Weakly acidic reflux in patients with chronic unexplained cough during 24 hour pressure, pH, and impedance monitoring. Gut. 2005;54:449–454. doi: 10.1136/gut.2004.055418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith JA, Decalmer S, Kelsall A, McGuinness K, Jones H, Galloway S, et al. Acoustic cough-reflux associations in chronic cough: potential triggers and mechanisms. Gastroenterology. 2010;139:754–762. doi: 10.1053/j.gastro.2010.06.050. [DOI] [PubMed] [Google Scholar]
- 32.Blondeau K, Mertens V, Dupont L, Pauwels A, Farre R, Malfroot A, et al. The relationship between gastroesophageal reflux and cough in children with chronic unexplained cough using combined impedance-pH-manometry recordings. Pediatric pulmonology. 2011;46:286–294. doi: 10.1002/ppul.21365. [DOI] [PubMed] [Google Scholar]
- 33.Abdulqawi R, Houghton LA, Smith JA. Gastro-oesophageal reflux and cough. The Journal of the Association of Physicians of India. 2013;61:17–19. [PubMed] [Google Scholar]
- 34.Philip G, Baroody FM, Proud D, Naclerio RM, Togias AG. The human nasal response to capsaicin. J Allergy Clin Immunol. 1994;94:1035–1045. doi: 10.1016/0091-6749(94)90122-8. [DOI] [PubMed] [Google Scholar]
- 35.Secher C, Kirkegaard J, Borum P, Maansson A, Osterhammel P, Mygind N. Significance of H1 and H2 receptors in the human nose: rationale for topical use of combined antihistamine preparations. J Allergy Clin Immunol. 1982;70:211–218. doi: 10.1016/0091-6749(82)90044-6. [DOI] [PubMed] [Google Scholar]
- 36.Plevkova J, Brozmanova M, Pecova R, Tatar M. Effects of intranasal histamine on the cough reflex in subjects with allergic rhinitis. J Physiol Pharmacol. 2005;56(Suppl 4):185–195. [PubMed] [Google Scholar]
- 37.Plevkova J, Brozmanova M, Pecova R, Tatar M. Effects of intranasal capsaicin challenge on cough reflex in healthy human volunteers. J Physiol Pharmacol. 2004;55(Suppl 3):101–106. [PubMed] [Google Scholar]
- 38.Plevkova J, Brozmanova M, Pecova R, Tatar M. The effects of nasal histamine challenge on cough reflex in healthy volunteers. Pulm Pharmacol Ther. 2006;19:120–127. doi: 10.1016/j.pupt.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 39.Taylor-Clark TE, Kollarik M, MacGlashan DW, Jr, Undem BJ. Nasal sensory nerve populations responding to histamine and capsaicin. J Allergy Clin Immunol. 2005;116:1282–1288. doi: 10.1016/j.jaci.2005.08.043. [DOI] [PubMed] [Google Scholar]
- 40.Plevkova J, Kollarik M, Brozmanova M, Revallo M, Varechova S, Tatar M. Modulation of experimentally-induced cough by stimulation of nasal mucosa in cats and guinea pigs. Respir Physiol Neurobiol. 2004;142:225–235. doi: 10.1016/j.resp.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 41.Brozmanova M, Plevkova J, Tatar M, Kollarik M. Cough reflex sensitivity is increased in the guinea pig model of allergic rhinitis. J Physiol Pharmacol. 2008;59 in press. [PubMed] [Google Scholar]
- 42.Poussel M, Varechova S, Demoulin B, Chalon B, Schweitzer C, Marchal F, et al. Nasal stimulation by water down-regulates cough in anesthetized rabbits. Respir Physiol Neurobiol. 2012;183:20–25. doi: 10.1016/j.resp.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 43.Plevkova J, Kollarik M, Poliacek I, Brozmanova M, Surdenikova L, Tatar M, et al. The role of trigeminal nasal TRPM8-expressing afferent neurons in the antitussive effects of menthol. J Appl Physiol (1985) 2013;115:268–274. doi: 10.1152/japplphysiol.01144.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Driessen AK, Farrell MJ, Mazzone SB, McGovern AE. Multiple neural circuits mediating airway sensations: recent advances in the neurobiology of the urge-to-cough. Respir Physiol Neurobiol. 2015 doi: 10.1016/j.resp.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 45.Hilton E, Marsden P, Thurston A, Kennedy S, Decalmer S, Smith JA. Clinical features of the urge-to-cough in patients with chronic cough. Respiratory medicine. 2015;109:701–707. doi: 10.1016/j.rmed.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 46.Mazzone SB, McLennan L, McGovern AE, Egan GF, Farrell MJ. Representation of capsaicin-evoked urge-to-cough in the human brain using functional magnetic resonance imaging. Am J Respir Crit Care Med. 2007;176:327–332. doi: 10.1164/rccm.200612-1856OC. [DOI] [PubMed] [Google Scholar]
- 47.Buday T, Brozmanova M, Biringerova Z, Gavliakova S, Poliacek I, Calkovsky V, et al. Modulation of cough response by sensory inputs from the nose - role of trigeminal TRPA1 versus TRPM8 channels. Cough. 2012;8:11. doi: 10.1186/1745-9974-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu DN, Yamauchi K, Kobayashi H, Tanifuji Y, Kato C, Suzuki K, et al. Effects of esophageal acid perfusion on cough responsiveness in patients with bronchial asthma. Chest. 2002;122:505–509. doi: 10.1378/chest.122.2.505. [DOI] [PubMed] [Google Scholar]
- 49.Jordan D, Wood LM. A convergent input from nasal receptors and the larynx to the rostral sensory trigeminal nuclei of the cat. J Physiol. 1987;393:147–155. doi: 10.1113/jphysiol.1987.sp016816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McGovern AE, Davis-Poynter N, Yang SK, Simmons DG, Farrell MJ, Mazzone SB. Evidence for multiple sensory circuits in the brain arising from the respiratory system: an anterograde viral tract tracing study in rodents. Brain structure & function. 2015;220:3683–3699. doi: 10.1007/s00429-014-0883-9. [DOI] [PubMed] [Google Scholar]
- 51.McGovern AE, Driessen AK, Simmons DG, Powell J, Davis-Poynter N, Farrell MJ, et al. Distinct brainstem and forebrain circuits receiving tracheal sensory neuron inputs revealed using a novel conditional anterograde transsynaptic viral tracing system. J Neurosci. 2015;35:7041–7055. doi: 10.1523/JNEUROSCI.5128-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Silver WL, Finger TE. The anatomical and electrophysiological basis of peripheral nasal trigeminal chemoreception. Ann N Y Acad Sci. 2009;1170:202–205. doi: 10.1111/j.1749-6632.2009.03894.x. [DOI] [PubMed] [Google Scholar]
- 53.Lucier GE, Egizii R. Central projections of the ethmoidal nerve of the cat as determined by the horseradish peroxidase tracer technique. J Comp Neurol. 1986;247:123–132. doi: 10.1002/cne.902470108. [DOI] [PubMed] [Google Scholar]
- 54.Hunter DD, Dey RD. Identification and neuropeptide content of trigeminal neurons innervating the rat nasal epithelium. Neuroscience. 1998;83:591–599. doi: 10.1016/s0306-4522(97)00324-2. [DOI] [PubMed] [Google Scholar]
- 55.Grunditz T, Uddman R, Sundler F. Origin and peptide content of nerve fibers in the nasal mucosa of rats. Anat Embryol (Berl) 1994;189:327–337. doi: 10.1007/BF00190589. [DOI] [PubMed] [Google Scholar]
- 56.Page AJ, Blackshaw LA. Roles of gastro-oesophageal afferents in the mechanisms and symptoms of reflux disease. Handb Exp Pharmacol. 2009;194:227–257. doi: 10.1007/978-3-540-79090-7_7. [DOI] [PubMed] [Google Scholar]
- 57.Kollarik M, Ru F, Brozmanova M. Vagal afferent nerves with the properties of nociceptors. Auton Neurosci. 2010;153:12–20. doi: 10.1016/j.autneu.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu S, Undem BJ, Kollarik M. Vagal afferent nerves with nociceptive properties in guinea-pig oesophagus. J Physiol. 2005;563:831–842. doi: 10.1113/jphysiol.2004.079574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu S, Ru F, Ouyang A, Kollarik M. 5-Hydroxytryptamine selectively activates the vagal nodose C-fibre subtype in the guinea-pig oesophagus. Neurogastroenterol Motil. 2008;20:1042–1050. doi: 10.1111/j.1365-2982.2008.01136.x. [DOI] [PubMed] [Google Scholar]
- 60.Ru F, Surdenikova L, Brozmanova M, Kollarik M. Adenosine-induced activation of esophageal nociceptors. Am J Physiol Gastrointest Liver Physiol. 2011;300:G485–G493. doi: 10.1152/ajpgi.00361.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dusenkova S, Ru F, Surdenikova L, Nassenstein C, Hatok J, Dusenka R, et al. The expression profile of acid-sensing ion channel (ASIC) subunits ASIC1a, ASIC1b, ASIC2a, ASIC2b, and ASIC3 in the esophageal vagal afferent nerve subtypes. Am J Physiol Gastrointest Liver Physiol. 2014;307:G922–G930. doi: 10.1152/ajpgi.00129.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yu X, Hu Y, Ru F, Kollarik M, Undem BJ, Yu S. TRPM8 function and expression in vagal sensory neurons and afferent nerves innervating guinea pig esophagus. Am J Physiol Gastrointest Liver Physiol. 2015;308:G489–G496. doi: 10.1152/ajpgi.00336.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mazzone SB, Mori N, Canning BJ. Synergistic interactions between airway afferent nerve subtypes regulating the cough reflex in guinea-pigs. J Physiol. 2005;569:559–573. doi: 10.1113/jphysiol.2005.093153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kindt S, Vos R, Blondeau K, Tack J. Influence of intra-oesophageal capsaicin instillation on heartburn induction and oesophageal sensitivity in man. Neurogastroenterol Motil. 2009;21 doi: 10.1111/j.1365-2982.2009.01332.x. 1032-e82. [DOI] [PubMed] [Google Scholar]
- 65.Chen CL, Liu TT, Yi CH, Orr WC. Effects of capsaicin-containing red pepper sauce suspension on esophageal secondary peristalsis in humans. Neurogastroenterol Motil. 2010;22:1177–1182. e312–e313. doi: 10.1111/j.1365-2982.2010.01561.x. [DOI] [PubMed] [Google Scholar]
- 66.Liu TT, Yi CH, Lei WY, Hung XS, Yu HC, Chen CL. Influence of repeated infusion of capsaicin-contained red pepper sauce on esophageal secondary peristalsis in humans. Neurogastroenterol Motil. 2014;26:1487–1493. doi: 10.1111/nmo.12414. [DOI] [PubMed] [Google Scholar]
- 67.Gonzalez R, Dunkel R, Koletzko B, Schusdziarra V, Allescher HD. Effect of capsaicin-containing red pepper sauce suspension on upper gastrointestinal motility in healthy volunteers. Dig Dis Sci. 1998;43:1165–1171. doi: 10.1023/a:1018831018566. [DOI] [PubMed] [Google Scholar]
- 68.Zhang S, Liu Z, Heldsinger A, Owyang C, Yu S. Intraluminal acid activates esophageal nodose C fibers after mast cell activation. Am J Physiol Gastrointest Liver Physiol. 2014;306:G200–G207. doi: 10.1152/ajpgi.00142.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu X, Hu Y, Yu S. Effects of acid on vagal nociceptive afferent subtypes in guinea pig esophagus. Am J Physiol Gastrointest Liver Physiol. 2014;307:G471–G478. doi: 10.1152/ajpgi.00156.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ru F, Banovcin P, Jr, Kollarik M. Acid sensitivity of the spinal dorsal root ganglia C-fiber nociceptors innervating the guinea pig esophagus. Neurogastroenterol Motil. 2015;27:865–874. doi: 10.1111/nmo.12561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Enyedi P, Czirjak G. Molecular background of leak K+ currents: two-pore domain potassium channels. Physiol Rev. 2010;90:559–605. doi: 10.1152/physrev.00029.2009. [DOI] [PubMed] [Google Scholar]
- 72.Deval E, Lingueglia E. Acid-Sensing Ion Channels and nociception in the peripheral and central nervous systems. Neuropharmacology. 2015;94:49–57. doi: 10.1016/j.neuropharm.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 73.Holzer P. Acid sensing by visceral afferent neurones. Acta Physiol (Oxf) 2011;201:63–75. doi: 10.1111/j.1748-1716.2010.02143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kauer WK, Peters JH, DeMeester TR, Feussner H, Ireland AP, Stein HJ, et al. Composition and concentration of bile acid reflux into the esophagus of patients with gastroesophageal reflux disease. Surgery. 1997;122:874–881. doi: 10.1016/s0039-6060(97)90327-5. [DOI] [PubMed] [Google Scholar]
- 75.Nehra D, Howell P, Williams CP, Pye JK, Beynon J. Toxic bile acids in gastro-oesophageal reflux disease: influence of gastric acidity. Gut. 1999;44:598–602. doi: 10.1136/gut.44.5.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shindo K, Machida M, Fukumura M, Koide K, Yamazaki R. Omeprazole induces altered bile acid metabolism. Gut. 1998;42:266–271. doi: 10.1136/gut.42.2.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Theisen J, Nehra D, Citron D, Johansson J, Hagen JA, Crookes PF, et al. Suppression of gastric acid secretion in patients with gastroesophageal reflux disease results in gastric bacterial overgrowth and deconjugation of bile acids. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2000;4:50–54. doi: 10.1016/s1091-255x(00)80032-3. [DOI] [PubMed] [Google Scholar]
- 78.Siddiqui A, Rodriguez-Stanley S, Zubaidi S, Miner PB., Jr Esophageal visceral sensitivity to bile salts in patients with functional heartburn and in healthy control subjects. Dig Dis Sci. 2005;50:81–85. doi: 10.1007/s10620-005-1282-0. [DOI] [PubMed] [Google Scholar]
- 79.Gasiorowska A, Navarro-Rodriguez T, Wendel C, Krupinski E, Perry ZH, Koenig K, et al. Comparison of the degree of duodenogastroesophageal reflux and acid reflux between patients who failed to respond and those who were successfully treated with a proton pump inhibitor once daily. Am J Gastroenterol. 2009;104:2005–2013. doi: 10.1038/ajg.2009.240. [DOI] [PubMed] [Google Scholar]
- 80.Tack J, Koek G, Demedts I, Sifrim D, Janssens J. Gastroesophageal reflux disease poorly responsive to single-dose proton pump inhibitors in patients without Barrett's esophagus: acid reflux, bile reflux, or both? Am J Gastroenterol. 2004;99:981–988. doi: 10.1111/j.1572-0241.2004.04171.x. [DOI] [PubMed] [Google Scholar]
- 81.Almansa C, Smith JA, Morris J, Crowell MD, Valdramidou D, Lee AS, et al. Weak peristalsis with large breaks in chronic cough: association with poor esophageal clearance. Neurogastroenterol Motil. 2015;27:431–442. doi: 10.1111/nmo.12513. [DOI] [PubMed] [Google Scholar]
- 82.Yu S, Gao G, Peterson BZ, Ouyang A. TRPA1 in mast cell activation-induced long-lasting mechanical hypersensitivity of vagal afferent C-fibers in guinea pig esophagus. Am J Physiol Gastrointest Liver Physiol. 2009;297:G34–G42. doi: 10.1152/ajpgi.00068.2009. [DOI] [PubMed] [Google Scholar]
- 83.Yu S, Stahl E, Li Q, Ouyang A. Antigen inhalation induces mast cells and eosinophils infiltration in the guinea pig esophageal epithelium involving histamine-mediated pathway. Life Sci. 2008;82:324–330. doi: 10.1016/j.lfs.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 84.Yu S, Kollarik M, Ouyang A, Myers AC, Undem BJ. Mast cell-Mediated Long- Lasting Increases in Excitability of Vagal C-Fibers in Guinea Pig Esophagus. Am J Physiol Gastrointest Liver Physiol. 2007 doi: 10.1152/ajpgi.00277.2007. [DOI] [PubMed] [Google Scholar]