Abstract
Objective
To create isogenic human pluripotent stem cell-derived macrophages with and without ABCA1 expression as a model for reverse cholesterol transport.
Approach and Results
The clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated 9 (Cas9) genome-editing system was used to introduce frameshift mutations into the coding sequence of ABCA1. Individual human pluripotent stem cell clones with deleterious mutations were identified, expanded, and differentiated into mature macrophages with a cytokine-based, feeder-free differentiation protocol. Wild-type cells demonstrated effective cholesterol efflux to apolipoprotein A-I acceptor, while ABCA1−/− cells displayed significantly reduced efflux ability and increased expression of proinflammatory cytokines.
Conclusions
Human pluripotent stem cell-derived macrophages capable of reverse cholesterol transport can be rapidly generated and genetically edited with CRISPR/Cas9. Introduction of homozygous frameshift mutations results in loss of ABCA1 expression in differentiated macrophages and subsequent reduction of cholesterol efflux capability. This facile genome-editing approach and differentiation protocol pave the way for future studies of the molecular determinants of reverse cholesterol transport and other macrophage properties.
Keywords: cholesterol, differentiation, genetics, macrophage, stem cell
INTRODUCTION
The process by which peripheral cholesterol is returned to the liver for excretion in bile is termed reverse cholesterol transport (RCT). This multistep pathway is necessary for cells to balance cholesterol intake and de novo synthesis against the toxic effects of excess unesterified cholesterol accumulation. The efflux of cholesterol to extracellular acceptors occurs throughout the body; however, the link with atherosclerosis is hypothesized to be exclusively through macrophage RCT.1 In atherosclerotic lesions, the macrophage is the primary cell type overloaded with cholesterol, and recent epidemiological studies have linked in vitro macrophage cholesterol efflux capacity with risk of adverse cardiovascular events.2,3
The major cellular mediator of cholesterol efflux is ATP-binding cassette, sub-family A, member 1 (ABCA1), which functions as a cell surface transporter.4 It is required for normal lipidation of lipid-poor apolipoprotein A-I (apoA-I), which is rapidly catabolized in its absence. Lack of ABCA1 is the molecular basis for Tangier disease, an autosomal recessive disease characterized by undetectable HDL-C levels and cholesterol accumulation in peripheral macrophages.5 Other molecular mediators of RCT have been identified in mouse knockout models, including the ABCG16 and SR-BI7 pathways, but macrophage RCT requires further study for a full understanding of its determinants. A recent report made use of induced pluripotent stem cells (iPSCs) generated from individuals with Tangier disease and, after differentiation into macrophages, assessed them with respect to cholesterol efflux and inflammatory response to lipopolysaccharide (LPS).8 However, the ability to recruit patients with rare mutations for generation of these cells remains difficult. Here we demonstrate that genome editing and a simplified differentiation protocol can be employed in human pluripotent stem cells (hPSCs) to generate isogenic macrophage cell lines to test the molecular determinants of macrophage RCT.
MATERIALS AND METHODS
Materials and Methods are available in the online-only Data Supplement.
RESULTS
Human Pluripotent Stem Cell-Derived Macrophages have Cholesterol Efflux Capacity
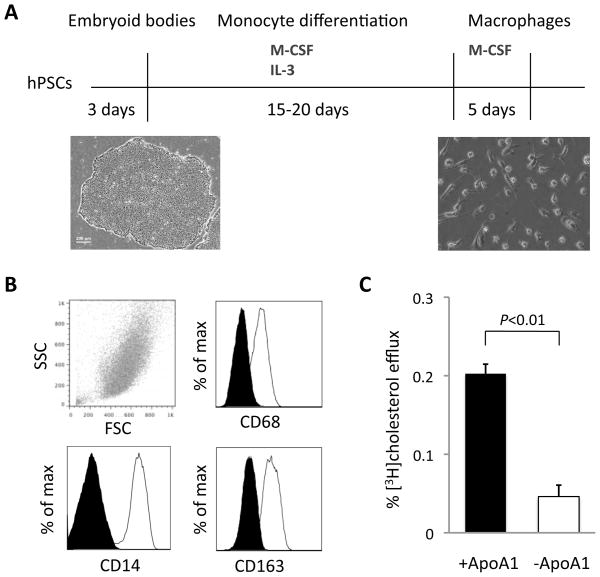
We validated the ability of hPSC-derived macrophages to efflux cholesterol after a 23 to 28 day differentiation protocol adapted from previously published protocols (Figure 1A).9,10 Monocytes floating in the culture medium were generated by day 15 and could be harvested every 4 to 5 days for approximately three months. After further maturation with macrophage colony-stimulating factor (M-CSF) for 5 days, the cells expressed the macrophage-specific cell surface markers CD14, CD68, and CD163 (Figure 1B and Supplemental Figure I). These differentiated macrophages retained phagocytic function in a fluorescence-labeled bioparticle assay (Supplemental Figure II) and effluxed cholesterol in an in vitro reverse cholesterol transport assay with apoA-I as the cholesterol acceptor (Figure 1C).
Figure 1.
Differentiation of cholesterol efflux-capable macrophages from human pluripotent stem cells. A, Adapted protocol for differentiation of stem cells into CD14+ monocytes and subsequent maturation into macrophages over approximately 23 days with a cytokine-based, feeder-free system. B, Stem cell-derived macrophages express macrophage-specific markers. Flow cytometry analysis of macrophages at day 23 of differentiation demonstrates expression of CD68, CD14, and CD163. C, Stem cell-derived macrophages have the ability to efflux cholesterol with apoA-I as an acceptor after 24-hour radiolabeling with [3H]cholesterol and 18-hour LXR agonist activation. Three biological replicates each of clones C10 and C11 were used; N = 6 for each experimental condition. Error bars indicate standard deviations.
Genome Editing of ABCA1 with CRISPR/Cas9
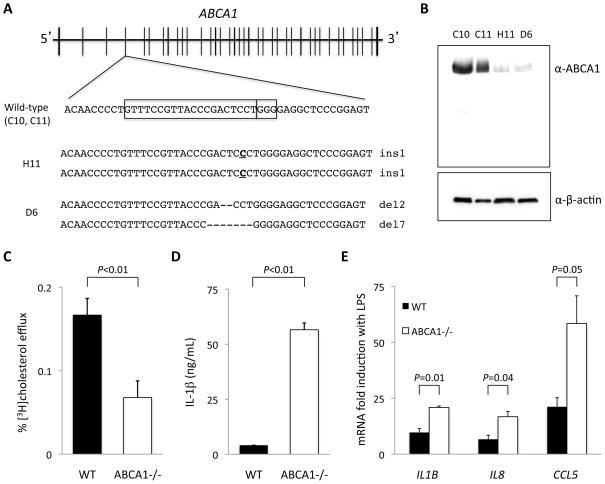
In order to employ the clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated 9 (Cas9) genome-editing system to knock out the ABCA1 gene, we designed a guide RNA to pair with Cas9 nuclease to target the fourth exon of ABCA1 (Figure 2A) in the hPSC line HUES 9. Cas9 nuclease was transiently expressed upon electroporation of dissociated single cells. The nuclease generated double-strand breaks at this specific site, and subclones were screened for evidence of frameshift insertions or deletions 10 days after transfection. There were 146 clones with at least one edited allele from a total of 192 screened clones (76% efficiency). Two unaltered, wild-type clones (C10 and C11) and 2 clones with indels at the predicted Cas9 cleavage site in both alleles (H11 and D6) were chosen for expansion and differentiation into macrophages. The frameshift indel mutations were a homozygous single base insertion in clone H11 and a pair of deletions (2-bp and 7-bp) in the compound heterozygous clone D6. Sites of possible off-target nuclease activity were identified using 2 independent prediction methods, and we tested a total of 14 sites deemed most likely to be off-target sites, based on DNA-RNA mismatches or bulges, in each of the hPSC lines (C10, C11, H11, and D6) (see Supplemental Methods for sites tested and primer sequences). No off-target mutations at these sites were observed. We additionally note that previous whole-genome sequencing studies of CRISPR/Cas9-targeted hPSC lines have documented that off-target mutations are exceedingly rare.11,12 To minimize the possibility of confounding by an off-target mutation in a single clone, two different hPSC clones were used for each condition in all experiments.
Figure 2.
CRISPR/Cas9 genome editing of ABCA1 results in loss of cholesterol efflux capacity and increased cytokine expression in macrophages. A, Region of ABCA1 exon 4 targeted by CRISPR/Cas9. The boxes indicate the protospacer sequence and the NGG motif. The mutant alleles in clones H11 and D6 are indicated along with the sizes of the indels. B, Western blot analysis demonstrates that two wild-type macrophage lines (C10, C11) robustly express ABCA1 while two mutant macrophage lines (H11 and D6) show loss of ABCA1 expression. C, ABCA1−/− macrophages display reduced cholesterol efflux to apoA-I acceptor (calculated as the difference in efflux in the presence and absence of apoA-I). Three biological replicates each of clones C10, C11, H11, and D6 were used; N = 6 for each experimental condition. Error bars indicate standard deviations. D, ABCA1−/− macrophages produce higher levels of IL-1β cytokine than wild-type macrophages after 18 hours of stimulation with LPS. E, ABCA1−/− macrophages display a larger degree of induction of proinflammatory cytokine mRNAs (relative to matched unstimulated macrophages) than wild-type macrophages after 18 hours of LPS stimulation.
Reduced Efflux Capacity of ABCA1−/− Macrophages
We confirmed the loss of ABCA1 protein expression in clones H11 and D6 by Western blot analysis (Figure 2B) in whole cell lysates from differentiated macrophages. The minimal residual ABCA1 expression observed in the knockout lysates may have resulted from cross-reaction with another protein or very-low-percentage admixture of the cell lines with wild-type cells. There were no smaller protein products detected by the anti-ABCA1 antibody to suggest expression from alternative ABCA1 transcripts. Confirming that loss of ABCA1 had no effect on the efficacy of the differentiation protocol, both wild-type and ABCA1−/− macrophages displayed similar levels of CD14, CD68, and CD163 expression (Supplemental Figure I) as well as phagocytic function (Supplemental Figure II). The ABCA1−/− macrophages had significantly reduced cholesterol efflux ability in comparison with isogenic wild-type macrophages that were differentiated in parallel (Figure 2C). ABCA1−/− macrophages also demonstrated higher production of IL-1β as well as higher expression of proinflammatory genes including IL1B, IL8, and CCL5 in response to LPS stimulation (Figure 2D and 2E).
DISCUSSION
In this report we outline a successful strategy for creation of a human cell-based assay for macrophage RCT. We found that hPSC-derived macrophages display effective cholesterol efflux, and we propose that these cells can be used to systematically interrogate the role of human genetic variation in this pathway. As a proof of principle, we used CRISPR/Cas9 genome editing to effect the loss of ABCA1 expression in differentiated macrophages. The macrophage RCT assay is designed to quantify the rate of cholesterol efflux from cultured cells to a plasma acceptor such as apoA-1.13 It has been used to measure cholesterol efflux in murine monocytes,14 primary human monocytes,13 and the acute monocytic leukemia cell line THP-1.15 With this assay, our genome-edited ABCA1−/− macrophages demonstrated reduced cholesterol efflux.
A role for ABCA1 in inflammation has been demonstrated in peritoneal macrophages isolated from macrophage-specific Abca1−/− mice16 but has not been confirmed with primary human macrophages due to difficulty in obtaining cells from Tangier disease patients. In a recent report, iPSC-derived macrophages from Tangier disease patients displayed a proinflammatory signature compared to iPSC-derived macrophages from control individuals.8 The use of isogenic cell lines to study genetic alterations eliminates the clone-to-clone variability and effects of differences in genetic background often observed when studying iPSC lines derived from patients and control individuals.17 We found with our genome-edited ABCA1−/− macrophages that loss of ABCA1 indeed results in increased expression of proinflammatory cytokines.
The ability to readily genome-edit wild-type hPSC lines allows for testing of mutations without the need for identifying patients with mutations, obtaining primary tissues from those patients, and generating iPSC lines. This may prove to be useful in characterizing novel ABCA1 variants of unknown functional significance that are uncovered in next-generation sequencing studies of large human cohorts. Furthermore, genome-edited, hPSC-derived macrophages could allow for the interrogation of candidate RCT genes via gene knockout or even for unbiased screens. Thus, the approach presented here provides a human-based model with which to dissect RCT and potentially other aspects of macrophage biology.
Supplementary Material
SIGNIFICANCE.
The use of induced pluripotent stem cells (iPSCs) for disease modeling presents an opportunity for genotype-to-phenotype characterization. The difficulty of generating patient-specific and well-matched control iPSC lines limits the generalizability of this approach. CRISPR/Cas9 genome editing enables the rapid and specific introduction of disease-causing mutations into wild-type iPSC lines, yielding isogenic cell lines with and without the mutations. Here we demonstrate the feasibility of CRISPR/Cas9 genome editing to generate a human cellular model with impaired reverse cholesterol transport. This is the first use of CRISPR/Cas9 to create a stem cell-based model of disease in macrophages, which will be a valuable resource for future experiments investigating the molecular determinants of reverse cholesterol transport.
Acknowledgments
SOURCES OF FUNDING
This work was supported by a LaDue Fellowship in Cardiovascular Medicine and Sarnoff Scholar Award to R.M.G. Additional support was provided by funding from Harvard University and the Harvard Stem Cell Institute.
ABBREVIATIONS
- RCT
reverse cholesterol transport
- ABCA1
ATP-binding cassette, sub-family A, member 1
- ABCG1
ATP-binding cassette, sub-family G, member 1
- SR-BI
scavenger receptor class B, type I
- apoA-I
apolipoprotein A-I
- HDL-C
high-density lipoprotein cholesterol
- iPSCs
induced pluripotent stem cells
- LPS
lipopolysaccharide
- hPSCs
human pluripotent stem cells
- M-CSF
macrophage colony-stimulating factor
Footnotes
DISCLOSURES
None.
References
- 1.Cuchel M, Rader DJ. Macrophage reverse cholesterol transport: key to the regression of atherosclerosis? Circulation. 2006;113:2548–2555. doi: 10.1161/CIRCULATIONAHA.104.475715. [DOI] [PubMed] [Google Scholar]
- 2.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE, Neeland IJ, Yuhanna IS, Rader DR, de Lemos JA, Shaul PW. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med. 2014;371:2383–2393. doi: 10.1056/NEJMoa1409065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oram JF, Lawn RM, Garvin MR, Wade DP. ABCA1 is the cAMP-inducible apolipoprotein receptor that mediates cholesterol secretion from macrophages. J Biol Chem. 2000;275:34508–34511. doi: 10.1074/jbc.M006738200. [DOI] [PubMed] [Google Scholar]
- 5.Bodzioch M1, Orsó E, Klucken J, et al. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat Genet. 1999;22:347–351. doi: 10.1038/11914. [DOI] [PubMed] [Google Scholar]
- 6.Kennedy MA, Barrera GC, Nakamura K, Baldan A, Tarr P, Fishbein MC, Frank J, Francone OL, Edwards PA. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab. 2005;1:121–131. doi: 10.1016/j.cmet.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Van Eck M, Twisk J, Hoekstra M, Van Rij BT, Van der Lans CA, Bos IS, Kruijt JK, Kuipers F, Van Berkel TJ. Differential effects of scavenger receptor BI deficiency on lipid metabolism in cells of the arterial wall and in the liver. J Biol Chem. 2003;278:23699–23705. doi: 10.1074/jbc.M211233200. [DOI] [PubMed] [Google Scholar]
- 8.Zhang H, Xue C, Shah R, et al. Functional analysis and transcriptomic profiling of iPSC-derived macrophages and their application in modeling Mendelian disease. Circ Res. 2015;117:17–28. doi: 10.1161/CIRCRESAHA.117.305860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karlsson KR, Cowley S, Martinez FO, Shaw M, Minger SL, James W. Homogeneous monocytes and macrophages from human embryonic stem cells following coculture-free differentiation in M-CSF and IL-3. Exp Hematol. 2008;36:1167–1175. doi: 10.1016/j.exphem.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panicker LM, Miller D, Park TS, et al. Induced pluripotent stem cell model recapitulates pathologic hallmarks of Gaucher disease. Proc Natl Acad Sci U S A. 2012;109:18054–18059. doi: 10.1073/pnas.1207889109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith C, Gore A, Yan W, Abalde-Atristain L, Li Z, He C, Wang Y, Brodsky RA, Zhang K, Cheng L, Ye Z. Whole-genome sequencing analysis reveals high specificity of CRISPR/Cas9 and TALEN-based genome editing in human iPSCs. Cell Stem Cell. 2014;15:12–13. doi: 10.1016/j.stem.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Veres A, Gosis BS, Ding Q, Collins R, Ragavendran A, Brand H, Erdin S, Cowan CA, Talkowski ME, Musunuru K. Low incidence of off-target mutations in individual CRISPR-Cas9 and TALEN targeted human stem cell clones detected by whole-genome sequencing. Cell Stem Cell. 2014;15:27–30. doi: 10.1016/j.stem.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Low H, Hoang A, Sviridov D. Cholesterol efflux assay. J Vis Exp. 2012;6:e3810. doi: 10.3791/3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ditiatkovski M, D’Souza W, Kesani R, Chin-Dusting J, de Haan JB, Remaley A, Sviridov D. An apolipoprotein A-I mimetic peptide designed with a reductionist approach stimulates reverse cholesterol transport and reduces atherosclerosis in mice. PLoS One. 2013;8:e68802. doi: 10.1371/journal.pone.0068802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang J, Cai S, Peterson BR, Kris-Etherton PM, Heuvel JP. Development of a cell-based, high-throughput screening assay for cholesterol efflux using a fluorescent mimic of cholesterol. Assay Drug Dev Technol. 2011;9:136–146. doi: 10.1089/adt.2010.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu X, Lee JY, Timmins JM, Brown JM, Boudyguina E, Mulya A, Gebre AK, Willingham MC, Hiltbold EM, Mishra N, Maeda N, Parks JS. Increased cellular free cholesterol in macrophage-specific Abca1 knock- out mice enhances pro-inflammatory response of macrophages. J Biol Chem. 2008;283:22930–22941. doi: 10.1074/jbc.M801408200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Musunuru K. Genome editing of human pluripotent stem cells to generate human cellular disease models. Dis Model Mech. 2013;6:896–904. doi: 10.1242/dmm.012054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.