Abstract
Tissue Factor Pathway Inhibitor (TFPI) is an anticoagulant protein that inhibits early phases of the procoagulant response. Alternatively spliced isoforms of TFPI are differentially expressed by endothelial cells and human platelets and plasma. The TFPIβ isoform localizes to the endothelium surface where it is a potent inhibitor of tissue factor-factor VIIa complexes that initiate blood coagulation. The TFPIα isoform is present in platelets. TFPIα contains a stretch of 9 amino acids nearly identical to those found in the B-domain of factor V (fV) that are well conserved in mammals. These amino acids provide exosite binding to activated fV, which allows for TFPIα to inhibit prothrombinase during the initiation phase of blood coagulation. Endogenous inhibition at this point in the coagulation cascade was only recently recognized and has provided a biochemical rationale to explain the pathophysiological mechanisms underlying several clinical disorders. These include the east Texas bleeding disorder that is caused by production of an altered form of factor V with high affinity for TFPI, and a paradoxical procoagulant effect of heparins. In addition, these findings have led to ideas for pharmacological targeting of TFPI that may reduce bleeding in hemophilia patients.
Keywords: TFPI, tissue factor, factor VII, factor V, prothrombinase, hemophilia
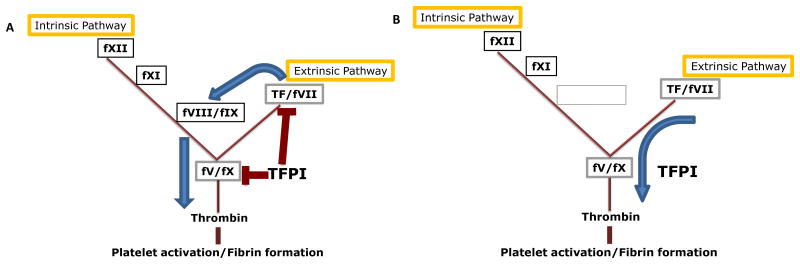
Blood coagulation proceeds as a series of proteolytic activation reactions of factors that sequentially amplify activities of each other and culminate with a final burst of thrombin.1, 2 Thrombin then activates platelets and generates fibrin to produce a blood clot (Figure 1A). The proteins of the blood clotting pathways include three major cofactors, tissue factor (TF), factor Va (fVa) and factor VIIIa (fVIIIa), that each combine with a target serine protease in a manner that greatly increases the speed and specificity of the protease for its biological substrate (Figure 1A). These cofactor proteins are essential for normal hemostasis and absence of any one causes severe bleeding. TF is expressed by cells within the tissues surrounding the vasculature (within the tissue), but is not typically present within the blood.3 It tightly binds to the plasma protease factor VIIa (fVIIa). The TF-fVIIa catalytic complex initiates blood coagulation by activating fX and factor IX (fIX).4 fVa is a cofactor protein for fXa.5 These two proteins combine in the presence of calcium ions on a phospholipid surface to form the prothrombinase complex, which rapidly converts prothrombin to thrombin.6 fVIIIa is a cofactor protein for fIXa. fVIIIa and fIXa combine form an enzymatic complex that rapidly converts fX to fXa.7 Deficiency of fVIII or fIX produces hemophilia A or hemophilia B, respectively. Since these two proteins act together in one enzymatic complex, deficiency of either one causes very similar symptoms such that laboratory testing is required to determine the type of hemophilia present in an individual patient.
Figure 1.
The blood coagulation pathway is a series of sequential proteolytic reactions that can be separated into intrinsic and extrinsic pathway. A) When TFPI is present, it dampens activation of clotting through the extrinsic pathways by serving as a fXa-dependent inhibitor of TF-fVIIa and of prothrombinase. Under these conditions, hemostasis occurs through activation of fIX by TF-VIIa, which amplifies fXa generation such that TFPI inhibition of clotting is overcome. B) When TFPI activity is blocked, sufficient thrombin can be generated for hemostasis without requiring amplification by fVIII/fIX, thereby providing the rationale for developing inhibitors of TFPI to treat hemophilia.
TFPI inhibits TF-fVIIa and prothrombinase
Tissue factor pathway inhibitor (TFPI) is a multivalent, Kunitz-type, serine protease inhibitor that was originally recognized as a plasma component that inhibited TF-fVIIa in a manner that required fX and calcium ions.8-10 Subsequent studies demonstrated its pathophysiological importance by showing that immunodepletion of TFPI in rabbit models increased susceptibility for development of disseminated intravascular coagulation following injection of TF or endotoxin.11, 12 It is now known that TFPI dampens the blood coagulation pathway by blocking the activity of two of the major protease-cofactor complexes, TF-fVIIa13 and prothrombinase (Figure1A).14 TFPI is the only endogenous protein that effectively inhibits these enzymatic complexes under physiological conditions. In doing so, it modulates the amount of procoagulant activity needed by the third major protease-cofactor complex, fVIIIa-fIXa, to produce a hemostatic blood clot (Figure 1B).15 This ability of TFPI to alter how the three major protease-cofactor complexes contribute to thrombin generation can be exploited to treat bleeding disorders, and pharmacological inhibitors of TFPI activity restore hemostasis in animal models of hemophilia.15-18 The biochemistry and physiology underlying TFPI inhibition of TF-fVIIa and prothrombinase, and the impact of TFPI on hemophilia bleeding, will be reviewed.
The structure and cellular expression of TFPI
TFPI is an alternatively spliced protein that is produced in two major isoforms, TFPIα and TFPIβ,19 which will be discussed here. TFPI is also produced in two minor isoforms, TFPIγ20 and TFPIδ.21 Diagrams of the structures of all the TFPI isoforms are presented in Maroney et al.21 TFPIα contains an acidic N-terminal region followed by three tandem Kunitz domains and a basic C-terminal region. The first Kunitz domain (K1) binds to the active site of fVIIa. The second Kunitz domain (K2) binds to the active site of fXa.13 The third Kunitz domain (K3) has not been found to inhibit any protease, but binds to protein S.22 The basic C-terminal region has a stretch of amino acids with sequence identity to a region within the FV B-domain.23 These amino acids in TFPIα tightly bind some forms of fVa allowing it to inhibit prothrombinase.14 TFPIα is secreted by human endothelial cells,24 is present in human plasma and binds human endothelium. Endothelium binding is likely mediated by electrostatic interactions with cell surface glycosaminoglycans, because the plasma TFPIα concentration promptly increases 2- to 4-fold upon heparin infusion.25, 26 TFPIα is also produced by megakaryocytes27 and is present within quiescent platelets, but not on their surface.28, 29 Interestingly, platelet TFPIα is not localized within the platelet α-granules or lysosomes and its intracellular location is not clear.28, 29 Following platelet activation, TFPI is externalized, with a portion released as a soluble protein and a portion associating with the surface of the activated platelet.28, 29 Murine TFPIα differs from its human counterpart in that it is not produced by endothelial cells and is not present in plasma.30 Murine platelet TFPIα is very similar to human platelet TFPIα in regards to the characteristics of its storage within the platelet and release following platelet activation.31
TFPIβ contains the identical first two Kunitz domains present in TFPIα, but has an alternatively spliced C-terminus that replaces K3 and basic C-terminal region present in TFPIα with a different amino acid sequence that encodes for attachment of a glycosylphosphatidyl inositol (GPI)-anchor.19, 32 This anchor binds TFPIβ to the surface of endothelial cells.30, 33 TFPIβ is not produced by megakaryocytes and is present neither within platelets nor on their surface.27 The molecular events that control the cellular and tissue expression of the TFPI isoforms are not well understood. It is known that exon 2 within the TFPI 5′ untranslated region undergoes alternative splicing and acts as a molecular switch that blocks TFPIβ translation.34 Further studies are needed to define how exon 2 expression contributes to temporal and tissue specific regulation of TFPI isoform production.
Inhibition of TF-fVIIa by TFPI
TFPI is a weak inhibitor of TF-fVIIa in the absence of fXa and, therefore, can be described as a fXa dependent inhibitor of TF-fVIIa. It is often stated that TFPI first binds fXa with K2, and then K1 within the TFPI-fXa complex, in a second inhibitory reaction, binds to TF-fVIIa. However, the rate limiting step in the reaction is the inhibition of fXa, not the inhibition of TF-fVIIa.35 Therefore, it appears that TFPI simultaneously inhibits fXa and TF-fVIIa immediately after fX is activated by TF-fVIIa.35 TFPIα and TFPIβ are both able to inhibit TF-fVIIa, since both have the first two Kunitz domains that mediate the inhibitory activity. However, the differences in the C-terminal structures of the two proteins modify their individual inhibitory activity in unique ways. The basic C-terminal region of TFPIα enhances direct inhibition of fXa in amidolytic assays, such that TFPIα inhibits fXa approximately 50-fold better than TFPI-160, a soluble, altered form of TFPI similar to TFPIβ in that contains only K1 and K2.36, 37 TFPIα inhibits TF-fVIIa approximately 3-fold better than TFPI-160 in fXa generation assays.37 These studies initially suggested that TFPIα is a more potent anticoagulant protein than TFPIβ. However, localization of TFPIβ to the cell surface via its GPI-anchor greatly increases its inhibitory activity, such that it is a slightly better inhibitor of fXa and TF-fVIIa than TFPIα in these assays. Further in vitro studies of TFPIβ have shown that it is a potent inhibitor of TF-mediated cellular migration through matrices that are not permeable to soluble forms of TFPI, while in vivo studies have shown that TFPIβ inhibits TF-mediated cellular migration into murine lung following tail vein injection and prevents development of a consumptive coagulopathy.37 Therefore, TFPIβ is a highly effective inhibitor of TF-mediated coagulation and cell signaling events that produce cellular migration.
Inhibition of prothrombinase by TFPI
TFPIα inhibits early forms of prothrombinase, the coagulation complex of fXa and fVa that rapidly converts prothrombin to thrombin.14 The inhibitory mechanism requires the basic C-terminal region of TFPIα and, therefore, prothrombinase inhibition is an isoform specific activity of TFPI that is not performed by TFPIβ. fV has a large B-domain of more than 800 amino acids. When the B-domain is intact, fV remains in an inactive conformation that does not promote blood clotting. This inactive conformation is maintained though interactions between a basic region near the N-terminus of the B-domain and an acidic region near its C-terminus.38 Proteolytic removal of either the basic or the acidic region of the B-domain produces a fully procoagulant form of fVa.38 Proteases that cleave within the fV B-domain include fXa, which rapidly removes the basic region, but only slowly removes the acidic region, and thrombin, which rapidly removes the entire B-domain.39 In addition, platelet α-granules contain forms of fVa that lack the basic region of the B-domain but retain the acidic region.40 The C-terminus of TFPIα and the basic region of the fV B-domain both contain the amino acid sequence LIKT followed by five basic amino acids (TFPI: LIKTKRKRK; fV: LIKTRKKKK).23 Early in the blood coagulation cascade, before thrombin is produced, prothrombinase assembles with forms of fVa that retain the acidic region of the B-domain. These early forms of prothrombinase tightly bind to the basic region of TFPIα allowing for rapid inhibition of prothrombinase that is directly mediated by binding of K2 to the active site of fXa.14 This inhibitory mechanism requires the presence of the acidic region of the fV B-domain that is rapidly removed by thrombin. Therefore, it is thought that TFPIα only inhibits prothrombinase during the initial stages of blood coagulation, perhaps preventing full procoagulant responses to sub-threshold stimuli that would otherwise occlude blood vessels.
Clinical relevance for TFPI inhibition of prothrombinase
TFPIα is the only endogenous protein recognized that inhibits prothrombinase at physiologically relevant rates and protein concentrations.14 The prothrombinase inhibitory activity of TFPIα is independent of its TF-fVIIa inhibitory activity.14 Identifying and characterizing the biochemical mechanism for inhibition of prothrombinase during the initiation of coagulation by TFPIα are directly relevant to understanding the pathogenesis of bleeding disorders and thrombotic disease. Three examples where the inhibition of prothrombinase by TFPIα may be relevant to patient care are as follows.
East Texas Bleeding Disorder
The east Texas bleeding disorder is an autosomal dominant condition associated with easy bruising, menorrhagia and life-threatening bleeding following trauma or surgery.41 Initial genetic studies identified an A2440G mutation encoding a glycine to serine amino acid substitution in the B-domain of fV that segregated with the disease phenotype. However, the presence of the serine residue did not alter clinical measurements of plasma fV antigen or activity. Thus, it was not initially thought to be related to the disease phenotype.41 Subsequent biochemical studies performed 10 years after identification of the genetic anomaly found that the A2440G mutation causes production of a fV splice variant, termed fV-short, that is missing 702 amino acids from the B-domain, including the basic region that is homologous with the basic region of TFPIα.42 The fV-short binds tightly to TFPIα. This stabilizes circulating TFPIα, such that patients with east Texas fV have an approximately 10-fold increase in plasma TFPIα.42 The TFPIα:fV-short complexes potently inhibit coagulation in in vitro assays, probably by rapid inhibition of prothrombinase, which is the likely cause of the bleeding disorder in these patients. Another mutation at a different site in the fV B-domain has subsequently been identified that produces a similar form of fV and a similar bleeding disorder, confirming this interaction between TFPI and fV-short in a separate patient population.43
A procoagulant property of heparin
Heparin is an anticoagulant drug that produces its activity by binding to antithrombin, and to a lesser extent, heparin cofactor II (HCII). Heparin binding greatly increases the inhibitory activity of these proteins towards several blood coagulation proteases, including thrombin and fXa.44 However, in plasma lacking antithrombin and HCII, heparin has paradoxical procoagulant activity.45 The biochemical mechanism underlying this procoagulant activity of heparin is important to understand because it may be clinically relevant in patients with conditions that cause simultaneous bleeding and clotting and are associated with low antithrombin, such as sepsis or disseminated intravascular coagulation. Heparin is a large, negatively charged polysaccharide. Its procoagulant effect is mediated, at least in part, by blocking the charge-dependent exosite interaction between the basic C-terminal region of TFPIα and the acidic region of the fVa B-domain. Consistent with this biochemical mechanism, the procoagulant activity of heparin is abolished by addition of thrombin-activated fVa to the plasma.14 Fucoidan is another large, negatively charged polysaccharide that also blocks the inhibition of prothrombinase by TFPIα, providing one plausible explanation for its therapeutic efficacy in a canine model of hemophilia A.16
New treatments for hemophilia
Blocking TFPI activity restores thrombin generation through the extrinsic blood coagulation pathway and restores hemostasis in several animal models of hemophilia.15-18 Pharmacological agents that block TFPI activity are currently under development for treatment of hemophilia.46 Blocking TFPI, or “inhibiting the inhibitor”, as a therapeutic strategy has several advantages over intravenous factor replacement therapy, which is currently used to treat patients with hemophilia. These include: the ability to treat hemophilia A and hemophilia B with the same drug; the ability to treat patients with antibody inhibitors directed at fVIII or fIX; the potential for subcutaneous, rather than intravenous, treatment options; and the potential for weekly or less frequent dosing. Characterization of the cellular expression patterns defining TFPIα as the TFPI isoform produced by platelets and definition of the biochemical mechanism for inhibition of prothrombinase by TFPIα has produced new insights into how therapeutic agents targeted against different structural regions of TFPI may produce hemostasis in patients with hemophilia. Studies performed in mice with hemophilia A found that total inhibition of plasma and endothelial pools of TFPI using an anti-TFPI polyclonal antibody dampened blood loss in a tail clip assay.47 While this was an expected finding, these experiments had another very interesting result. The amount of tail blood loss continued to progressively decrease even after all the plasma and endothelial TFPI was inhibited. This suggested that the TFPIα sequestered within platelets and released at the site of the tail wound had to be inhibited in order to obtain an optimal hemostatic response from the antibody. This idea was tested by producing mice with hemophilia A that lacked platelet TFPIα, but had normal plasma and endothelial TFPI, using fetal liver transplantation. When these mice were subjected to the tail clip assay, they had significantly less bleeding than mice containing normal amounts of platelet TFPIα, suggesting that platelet TFPIα modulates bleeding in hemophilia.47 These data also suggest that anti-TFPI agents designed to specifically block platelet TFPIα would improve hemostasis in patients with hemophilia without altering the anticoagulant properties of TFPIβ on endothelium.
Conclusions
TFPI is an anticoagulant protein that blocks the initiation of blood coagulation by inhibiting TF-fVIIa and early forms of prothrombinase. Alternative splicing of TFPI produces two primary isoforms, TFPIα and TFPIβ. TFPIα is a soluble protein secreted by endothelial cells and activated platelets. TFPIβ is a GPI-anchored protein expressed primarily on the surface of endothelium. Although TFPIα and TFPIβ are both capable of inhibiting TF-fVIIa activity, TFPIβ is a particularly effective inhibitor of TF-fVIIa present on cellular surfaces and may act in vivo to dampen intravascular procoagulant activity on the surface of inflamed vascular cells. TFPIα, but not TFPIβ, inhibits prothrombinase. The inhibitory mechanism for prothrombinase inhibition by TFPIα has identified fXa activated fV and forms of platelet fVa that retain the acidic portion of the B-domain, as key proteins for physiological regulation of blood coagulation. This has produced new understanding of the pathophysiology of clinical disorders including the east Texas bleeding disorder, a potential procoagulant activity of heparin, and new ideas for how to best target inhibitors of TFPI activity for treatment of hemophilia.
Supplementary Material
Significance.
TFPI dampens very early intravascular procoagulant activity thereby minimizing the development of occlusive thrombi and consumptive coagulopathy. TFPI is an alternatively spliced protein with two primary isoforms in humans, TFPIα and TFPIβ. The TFPI isoforms block early events in blood coagulation through two separate mechanisms: 1) inhibition of TF-FVIIa by K1 and K2, which is mediated by TFPIβ on endothelium; and 2) the inhibition of early forms of prothrombinase by K2 and the C-terminal region of TFPIα released from within platelets. The inhibition of prothrombinase by TFPIα is recently recognized. Further characterization of prothrombinase inhibition by TFPIα will generate important new knowledge of human bleeding and thrombotic disorders and potentially lead to targeted new therapies.
Acknowledgments
Sources of Funding: This work was supported by grant R01 HL068835 from the National Heart, Lung, and Blood Institute of the National Institutes of Health.
Disclosures: The author receives research grant funding from Novo Nordisk.
Abbreviations
- fV
factor V
- fVII
factor VII
- fX
factor X
- fIX
factor IX
- TF
tissue factor
- TFPI
tissue factor pathway inhibitor
- GPI
glycosylphosphatidyl inositol
- K1
Kunitz domain 1
- K2
Kunitz domain 2
- K3
Kunitz domain 3
Reference List
- 1.Davie EW, Ratnoff OD. Waterfall sequence for intrinsic blood clotting. Science. 1964;145:1310–1312. doi: 10.1126/science.145.3638.1310. [DOI] [PubMed] [Google Scholar]
- 2.Macfarlane RG. An enzyme cascade in the blood clotting mechanism, and its function as a biochemical amplifier. Nature. 1964;202:498–499. doi: 10.1038/202498a0. [DOI] [PubMed] [Google Scholar]
- 3.Drake TA, Morrissey JH, Edgington TS. Selective cellular expression of tissue factor in human tissues. Implications for disorders of hemostasis and thrombosis. Am J Pathol. 1989;134:1087–1097. [PMC free article] [PubMed] [Google Scholar]
- 4.Osterud B, Rapaport SI. Activation of factor IX by the reaction product of tissue factor and factor VII: additional pathway for initiating blood coagulation. Proc Natl Acad Sci U S A. 1977;74:5260–5264. doi: 10.1073/pnas.74.12.5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barton PG, Jackson CM, Hanahan DJ. Relationship between factor V and activated factor X in the generation of prothrombinase. Nature. 1967;214:923–924. doi: 10.1038/214923a0. [DOI] [PubMed] [Google Scholar]
- 6.Nesheim ME, Taswell JB, Mann KG. The contribution of bovine Factor V and Factor Va to the activity of prothrombinase. J Biol Chem. 1979;254:10952–10962. [PubMed] [Google Scholar]
- 7.van DG, Tans G, Rosing J, Hemker HC. The role of phospholipid and factor VIIIa in the activation of bovine factor X. J Biol Chem. 1981;256:3433–3442. [PubMed] [Google Scholar]
- 8.Sanders NL, Bajaj SP, Zivelin A, Rapaport SI. Inhibition of tissue factor/factor VIIa activity in plasma requires factor X and an additional plasma component. Blood. 1985;66:204–212. [PubMed] [Google Scholar]
- 9.Rao LV, Rapaport SI. Studies of a mechanism inhibiting the initiation of the extrinsic pathway of coagulation. Blood. 1987;69:645–651. [PubMed] [Google Scholar]
- 10.Broze GJ, Jr, Miletich JP. Characterization of the inhibition of tissue factor in serum. Blood. 1987;69:150–155. [PubMed] [Google Scholar]
- 11.Warr TA, Rao LV, Rapaport SI. Disseminated intravascular coagulation in rabbits induced by administration of endotoxin or tissue factor: effect of anti-tissue factor antibodies and measurement of plasma extrinsic pathway inhibitor activity. Blood. 1990;75:1481–1489. [PubMed] [Google Scholar]
- 12.Sandset PM, Warn-Cramer BJ, Maki SL, Rapaport SI. Immunodepletion of extrinsic pathway inhibitor sensitizes rabbits to endotoxin-induced intravascular coagulation and the generalized Shwartzman reaction. Blood. 1991;78:1496–1502. [PubMed] [Google Scholar]
- 13.Girard TJ, Warren LA, Novotny WF, Likert KM, Brown SG, Miletich JP, Broze GJ., Jr Functional significance of the Kunitz-type inhibitory domains of lipoprotein-associated coagulation inhibitor. Nature. 1989;338:518–520. doi: 10.1038/338518a0. [DOI] [PubMed] [Google Scholar]
- 14.Wood JP, Bunce MW, Maroney SA, Tracy PB, Camire RM, Mast AE. Tissue factor pathway inhibitor-alpha inhibits prothrombinase during the initiation of blood coagulation. Proc Natl Acad Sci U S A. 2013;110:17838–17843. doi: 10.1073/pnas.1310444110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erhardtsen E, Ezban M, Madsen MT, Diness V, Glazer S, Hedner U, Nordfang O. Blocking of tissue factor pathway inhibitor (TFPI) shortens the bleeding time in rabbits with antibody induced haemophilia A. Blood Coagul Fibrinolysis. 1995;6:388–394. doi: 10.1097/00001721-199507000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Prasad S, Lillicrap D, Labelle A, Knappe S, Keller T, Burnett E, Powell S, Johnson KW. Efficacy and safety of a new-class hemostatic drug candidate, AV513, in dogs with hemophilia A. Blood. 2008;111:672–679. doi: 10.1182/blood-2007-07-098913. [DOI] [PubMed] [Google Scholar]
- 17.Waters EK, Genga RM, Schwartz MC, Nelson JA, Schaub RG, Olson KA, Kurz JC, McGinness KE. Aptamer ARC19499 mediates a procoagulant hemostatic effect by inhibiting tissue factor pathway inhibitor. Blood. 2011;117:5514–5522. doi: 10.1182/blood-2010-10-311936. [DOI] [PubMed] [Google Scholar]
- 18.Hilden I, Lauritzen B, Sorensen BB, et al. Hemostatic effect of a monoclonal antibody mAb 2021 blocking the interaction between FXa and TFPI in a rabbit hemophilia model. Blood. 2012;119:5871–5878. doi: 10.1182/blood-2012-01-401620. [DOI] [PubMed] [Google Scholar]
- 19.Chang JY, Monroe DM, Oliver JA, Roberts HR. TFPIbeta, a second product from the mouse tissue factor pathway inhibitor (TFPI) gene. Thromb Haemost. 1999;81:45–49. [PubMed] [Google Scholar]
- 20.Maroney SA, Ferrel JP, Collins ML, Mast AE. Tissue factor pathway inhibitor-gamma is an active alternatively spliced form of tissue factor pathway inhibitor present in mice but not in humans. J Thromb Haemost. 2008;6:1344–1351. doi: 10.1111/j.1538-7836.2008.03033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maroney SA, Ellery PE, Mast AE. Alternatively spliced isoforms of tissue factor pathway inhibitor. Thromb Res. 2010;125(Suppl 1):S52–S56. doi: 10.1016/j.thromres.2010.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hackeng TM, Sere KM, Tans G, Rosing J. Protein S stimulates inhibition of the tissue factor pathway by tissue factor pathway inhibitor. Proc Natl Acad Sci U S A. 2006;103:3106–3111. doi: 10.1073/pnas.0504240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wood JP, Ellery PE, Maroney SA, Mast AE. Biology of tissue factor pathway inhibitor. Blood. 2014;123:2934–2943. doi: 10.1182/blood-2013-11-512764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bajaj MS, Kuppuswamy MN, Saito H, Spitzer SG, Bajaj SP. Cultured Normal Human Hepatocytes do not Synthesize Lipoprotein-Associated Coagulation Inhibitor: Evidence that Endothelium is the Principal Site of Its Synthesis. PNAS. 1990;87:8869–8873. doi: 10.1073/pnas.87.22.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Novotny WF, Brown SG, Miletich JP, Rader DJ, Broze GJ., Jr Plasma antigen levels of the lipoprotein-associated coagulation inhibitor in patient samples. Blood. 1991;78:387–393. [PubMed] [Google Scholar]
- 26.Sandset PM, Abildgaard U, Larsen ML. Heparin induces release of extrinsic coagulation pathway inhibitor (EPI) Thromb Res. 1988;50:803–813. doi: 10.1016/0049-3848(88)90340-4. [DOI] [PubMed] [Google Scholar]
- 27.Maroney SA, Haberichter SL, Friese P, Collins ML, Ferrel JP, Dale GL, Mast AE. Active tissue factor pathway inhibitor is expressed on the surface of coated platelets. Blood. 2007;109:1931–1937. doi: 10.1182/blood-2006-07-037283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maroney SA, Mast AE. Expression of tissue factor pathway inhibitor by endothelial cells and platelets. Transfus Apher Sci. 2008;38:9–14. doi: 10.1016/j.transci.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Novotny WF, Girard TJ, Miletich JP, Broze GJ., Jr Platelets secrete a coagulation inhibitor functionally and antigenically similar to the lipoprotein associated coagulation inhibitor. Blood. 1988;72:2020–2025. [PubMed] [Google Scholar]
- 30.Maroney SA, Ferrel JP, Pan S, White TA, Simari RD, McVey JH, Mast AE. Temporal expression of alternatively spliced forms of tissue factor pathway inhibitor in mice. J Thromb Haemost. 2009;7:1106–1113. doi: 10.1111/j.1538-7836.2009.03454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maroney SA, Cooley BC, Ferrel JP, Bonesho CE, Mast AE. Murine hematopoietic cell tissue factor pathway inhibitor limits thrombus growth. Arterioscler Thromb Vasc Biol. 2011;31:821–826. doi: 10.1161/ATVBAHA.110.220293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang J, Piro O, Lu L, Broze GJ., Jr Glycosyl phosphatidylinositol anchorage of tissue factor pathway inhibitor. Circulation. 2003;108:623–627. doi: 10.1161/01.CIR.0000078642.45127.7B. [DOI] [PubMed] [Google Scholar]
- 33.Girard TJ, Tuley E, Broze GJ., Jr TFPIbeta is the GPI-anchored TFPI isoform on human endothelial cells and placental microsomes. Blood. 2012;119:1256–1262. doi: 10.1182/blood-2011-10-388512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ellery PE, Maroney SA, Martinez ND, Wickens MP, Mast AE. Translation of Human Tissue Factor Pathway Inhibitor-beta mRNA Is Controlled by Alternative Splicing Within the 5′ Untranslated Region. Arterioscler Thromb Vasc Biol. 2014;34:187–195. doi: 10.1161/ATVBAHA.113.302660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baugh RJ, Broze GJ, Jr, Krishnaswamy S. Regulation of extrinsic pathway factor Xa formation by tissue factor pathway inhibitor. J Biol Chem. 1998;273:4378–4386. doi: 10.1074/jbc.273.8.4378. [DOI] [PubMed] [Google Scholar]
- 36.Lockett JM, Mast AE. Contribution of regions distal to glycine-160 to the anticoagulant activity of tissue factor pathway inhibitor. Biochemistry. 2002;41:4989–4997. doi: 10.1021/bi016058n. [DOI] [PubMed] [Google Scholar]
- 37.Maroney SA, Ellery PE, Wood JP, Ferrel JP, Martinez ND, Mast AE. Comparison of the inhibitory activities of human tissue factor pathway inhibitor (TFPI)alpha and TFPIbeta. J Thromb Haemost. 2013;11:911–918. doi: 10.1111/jth.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bos MH, Camire RM. A bipartite autoinhibitory region within the B-domain suppresses function in factor V. J Biol Chem. 2012;287:26342–26351. doi: 10.1074/jbc.M112.377168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Monkovic DD, Tracy PB. Activation of human factor V by factor Xa and thrombin. Biochemistry. 1990;29:1118–1128. doi: 10.1021/bi00457a004. [DOI] [PubMed] [Google Scholar]
- 40.Monkovic DD, Tracy PB. Functional characterization of human platelet-released factor V and its activation by factor Xa and thrombin. J Biol Chem. 1990;265:17132–17140. [PubMed] [Google Scholar]
- 41.Kuang SQ, Hasham S, Phillips MD, Wolf D, Wan Y, Thiagarajan P, Milewicz DM. Characterization of a novel autosomal dominant bleeding disorder in a large kindred from east Texas. Blood. 2001;97:1549–1554. doi: 10.1182/blood.v97.6.1549. [DOI] [PubMed] [Google Scholar]
- 42.Vincent LM, Tran S, Livaja R, Bensend TA, Milewicz DM, Dahlback B. Coagulation factor V(A2440G) causes east Texas bleeding disorder via TFPIalpha. J Clin Invest. 2013;123:3777–3787. doi: 10.1172/JCI69091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cunha ML, Bakhtiari K, Peter J, Marquart JA, Meijers JC, Middeldorp S. A novel mutation in the F5 gene (factor V Amsterdam) associated with bleeding independent of factor V procoagulant function. Blood. 2015;125:1822–1825. doi: 10.1182/blood-2014-08-592733. [DOI] [PubMed] [Google Scholar]
- 44.Damus PS, Hicks M, Rosenberg RD. Anticoagulant action of heparin. Nature. 1973;246:355–357. doi: 10.1038/246355a0. [DOI] [PubMed] [Google Scholar]
- 45.Smith SA, Morrissey JH. Heparin is procoagulant in the absence of antithrombin. Thromb Haemost. 2008;100:160–162. doi: 10.1160/TH08-05-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chowdary P, Lethagen S, Friedrich U, Brand B, Hay C, Karim FA, Klamroth R, Knoebl P, Laffan M, Mahlangu J, Miesbach W, Nielsen JD, Martin-Salces M, Angchaisuksiri P. Safety and pharmacokinetics of anti-TFPI antibody (concizumab) in healthy volunteers and patients with hemophilia: a randomized first human dose trial. J Thromb Haemost. 2015;13:743–754. doi: 10.1111/jth.12864. [DOI] [PubMed] [Google Scholar]
- 47.Maroney SA, Cooley BC, Ferrel JP, Bonesho CE, Nielsen LV, Johansen PB, Hermit MB, Petersen LC, Mast AE. Absence of hematopoietic tissue factor pathway inhibitor mitigates bleeding in mice with hemophilia. Proc Natl Acad Sci U S A. 2012;109:3927–3931. doi: 10.1073/pnas.1119858109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.