Abstract
Background
Calpain over-expression is implicated in aberrant angiogenesis. We hypothesized that calpain inhibition (CI, MDL28170) would improve collateral perfusion in a swine model with hypercholesterolemia and chronic myocardial ischemia.
Methods and Results
Yorkshire swine fed a high cholesterol diet for 4 weeks underwent surgical placement of an ameroid constrictor to their left circumflex coronary artery. Three weeks later, animals received either: no drug, high cholesterol control group (HCC; n= 8); low dose CI (0.12 mg/kg; LCI, n= 9); or high dose CI (0.25 mg/kg; HCI, n= 8). The heart was harvested after 5 weeks. There was a trend toward increased right to left collateral vessels on angiography with HCI. Myocardial perfusion in ischemic myocardium significantly improved with HCI at rest and with demand pacing (p = 0.016 and 0.011). Endothelium-dependent microvessel relaxation was significantly improved with LCI (p = 0.001). There was a significant increase in capillary density, with LCI and HCI (p= 0.01 and 0.01), and arteriolar density with LCI (p= 0.001). CI significantly increased several proangiogenic proteins including VEGF (p= 0.02), VEGFR1 (p= 0.003), VEGFR2 (p= 0.003) and talin, a microvascular structural protein (p= 0.0002). There was a slight increase in proteins implicated in endothelial-dependent (NO Mediated) relaxation including ERK, p-ERK and iNOS with CI.
Conclusions
In the setting of hypercholesterolemia, CI improved perfusion, with a trend toward increased collateralization on angiography and increased capillary and arteriolar densities in ischemic myocardium. CI also improved endothelium-dependent microvessel relaxation and increased expression of proteins implicated in angiogenesis and vasodilatation.
Keywords: Calpain inhibition; Ischemic heart disease; Collateral circulation; Angiogenesis, Perfusion; Myocardial revascularization; Animal model surgery
Introduction
Despite advances in percutaneous and surgical interventions in the treatment of coronary artery disease (CAD), up to one-third of patients are either not candidates for or receive suboptimal revascularization with these therapies 1. The incidence of incomplete revascularization in patients with severe CAD who undergo surgical intervention is an independent predictor for operative and peri-operative morbidity and mortality2, 3. With an increased prevalence of obesity and metabolic syndrome (MS), the incidence of severe CAD not amenable to surgical treatment is likely to increase 1,4,5. Inducing angiogenesis through medical therapies remains a promising therapeutic option for these patients. However, a deeper understanding of the pro-angiogenic and anti-angiogenic pathways in the setting of hypercholesterolemia and chronic ischemic disease is necessary to treat this complicated and growing population of patients. Our lab has created a pig model for chronic myocardial ischemia in the setting of metabolic syndrome (weight gain, glucose intolerance, dyslipidemia and hypertension). 4
Calpains, calcium-dependent thiol proteases expressed ubiquitously in mammals, are an important potential mediator of these angiogenic pathways. When activated, calpains regulate a broad spectrum of functionally important protein targets that involve cytoskeletal organization, cell adhesion and cell migration. Hypoxia is known to induce calpain activity resulting in disruption of cardiac endothelial cell cytoskeletal structure and function6-10. Modest suppression of calpain activity has been shown to improve functional neovasculature. 7, 8 Though the mechanism for this improvement remains largely unknown, there is evidence in small animal (rodent) models that calpain inhibition allows for upregulation of pro-angiogenic proteins and scaffolding proteins that are essential for new vessel growth and maturation 7, 8. Although these studies are promising, they have only been performed in small, otherwise healthy animal models. Given the considerable potential for the proangiogenic effects of calpain inhibition, we sought to investigate their effects in a clinically relevant porcine model of metabolic syndrome. We hypothesized that in the setting of chronic myocardial ischemia, CI would result in improved collateral dependent myocardial perfusion and vascular function.
Materials and Methods
Animal Model and Surgical Interventions
Juvenile male Yorkshire swine (Parsons Research, Amherst, MA) were divided into 3 groups, fed a high cholesterol diet for 4 weeks, then underwent surgical placement of a titanium ameroid constrictor (Research Instruments SW, Escondito, CA) on the proximal left circumflex coronary artery (LCx). Males were used in an effort to limit variables (male vs. female) between pigs. Three weeks later animals received either: no drug, high cholesterol control group (HCC; n= 8); an oral form of a low dose CI (0.12 mg/kg; LCI, n= 9); or an oral form of a high dose CI (0.25 mg/kg; HCI, n= 8) (CI MDL28170; EMD Millipore, Danvers, MA). The diets and oral form of the CI were continued for 5 weeks until completion of the study, then the animals were anesthesized and underwent x-ray coronary angiography. The heart was then exposed through a midline sternotomy and microspheres were injected at rest and with ventricular pacing (160 beats per minute). The animals were euthanized and their hearts were harvested. Tissue samples from chronically ischemic myocardium (IM - LCx territory) and non-ischemic myocardium (NIM) were rapidly frozen in liquid nitrogen. Tissue samples for microvessel reactivity studies were placed in Krebs solution. Detailed methods on surgical procedures, anesthesia, and tissue harvesting can be found in prior studies 11. The Institutional Animal Care and Use Committee of the Rhode Island Hospital approved all experiments. Animals were cared for in compliance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals.
Microvessel Studies, Coronary Angiography
Our group has previously described detailed methods for microvessel studies and coronary angiography11. Coronary arterioles taken from ischemic myocardium were isolated and microvascular relaxation responses were measured after being exposed to endothelium-dependent and endothelium-independent agents. Relaxation responses were defined as percent relaxation of the preconstricted (thromboxane analog U46619) diameter. Coronary angiography was performed during the final procedure to demonstrate occlusion of the LCx. A cardiologist interpreted recorded images in a blinded fashion to assess collateral formation. Collaterals were graded according to the well validated Rentrop system of 0 to 3 5,12. Rentrop Score is a scoring system to grade collateral filling vessels: Grade 0 (no visible filling of any collateral channels), Grade 1 (collateral filling branches of vessel without any dye reaching the epicardial segment of that vessel), Grade 2 (partial collateral filling of the epiardial segment of the vessel being dilated) Grade 3 (complete collateral filling of the vessel being dilated).
Myocardial Perfusion
Methods for myocardial perfusion have been previously described as rest and during rapid cardiac pacing 11. Briefly, gold isotope-labeled microspheres (Biophysics Assay Laboratory) were injected into the left atrium during transient Lcx occlusion at the time of initial ameroid placement to determine the territory at risk. Lutetium and Europium isotope-labeled microspheres were injected at rest and with demand pacing (160 beats per minute) respectively (all microspheres obtained from (Biophysics Assay Laboratory, Worcester, MA). Tissue samples obtained after euthanasia were divided into 10 ventricular sections for isotope-labeled microsphere assays, which were exposed to neutron beams and measured with a gamma counter.
Protein Expression
Preparation of protein lysates and technique for western blotting and band quantification has been previously described 13. Primary antibodies used were vascular endothelial growth factor (VEGF), receptor 1 (VEGF R1), receptor 2 (VEGF R2), vascular endothelial cadherin (VE cadherin), extracellular signal-regulated kinase (ERK), phosphorylated ERK (pERK), inducible nitric oxide synthase (iNOS), endostatin, angiopoietin 1, ras homolog gene family member A (RhoA), tau, talin, paxillin, and vinculin (all from Cell Signaling, Beverly, MA). All membranes were probed with glyceraldehyde-3-phosphate (Cell Signaling, Beverly, MA) to correct for loading error.
Immunohistochemical Staining for Angiogenesis
Detailed description for tissue preparation and immunohistochemical staining for angiogenesis has been previously described14. Formalin fixed tissue sections were incubated with antibodies against porcine endothelial marker CD-31 (R&D Systems, Minneapolis, MN) and smooth muscle actin (Sigma-Aldrich) followed by appropriate Alexa-Flour conjugated antibody (Jackson ImmunoResearch, West Grove, PA). Images were captured at 20X magnification with a Nikon E800 Eclipse microscope (Nikon, Tokyo, Japan) at the same exposure in 3 random fields. Capillaries were defined as structures 5-25 μm2 in a cross-sectional area. Arterioles were defined by localization of smooth muscle actin and CD31 staining. Arteriolar and capillary density was measured using Image J software (National Institutes of Health, Bathesda, MD) in a blinded fashion. Representative images have been included in this manuscript.
Data Analysis
ANOVA was performed with Bonferroni corrections for multiple comparisons. We used Graphpad to obtain P-values and make all graphs. Reported probability values (P-values) were considered significant if less than 0.05. All results are expressed as fold change ± standard error of the mean as compared to the HCC group. Results in Tables are expressed as fold change +/− standard deviation as compared to the HCC group.
Results
Animal Model and Coronary Angiography
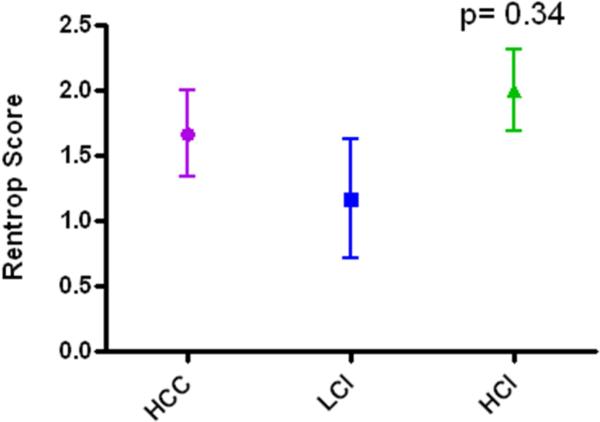
All animals included for analysis survived to completion of the study. Prior to sacrifice, coronary catheterization was performed and demonstrated occlusion of the left circumflex artery secondary to ameroid placement in all animals. There was a trend toward increased collateral vessel formation in the HCI group vs. either HCC or LCI groups, but this difference did not reach statistical significance. Rentrop scores: HCC, 1.67+/− 0.33 LCI, 1.17 +/− 0.45 HCI, 2.0 +/− 0.32 (p= 0.34) (Figure 1).
Figure 1.
Rentrop scores interpreted from x-ray coronary angiography. HCC: High Cholesterol Control; LCI: Low Dose Calpain Inhibitor; HCI: High Dose Calpain Inhibitor
Myocardial Perfusion
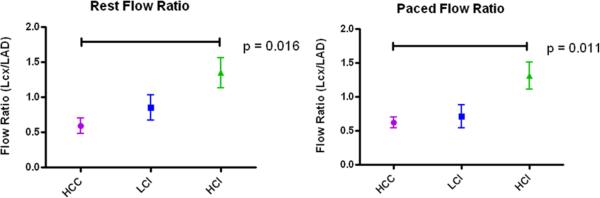
Myocardial perfusion ratios (Lcx/LAD) were calculated at rest (HCC, 0.59 +/− 0.11; LCI, 0.85 +/− 0/18; HCI, 1.34 +/− 0.21) and with demand pacing (HCC, 0.62 +/− 0.08; LCI, 0.71 +/− 0.16; HCI, 1.31 +/− 0.20). When compared with the HCC group, myocardial perfusion ratios were significantly improved in the HCI group (p= 0.016). Similarly, when the heart was stressed with ventricular demand pacing to 160 bpm there was a significant improvement in flow ratio in the HCI group when compared with the HCC group (p= 0.011) (Figure 2).
Figure 2.
Myocardial perfusion. Myocardial perfusin at rest and with demand pacing. There was a significant improvement in the HCI group compared with the HCC group at rest and with demand pacing at 160 beats/minute. HCC: High Cholesterol Control; LCI: Low Dose Calpain Inhibitor; HCI: High Dose Calpain Inhibitor
Microvessel Analysis
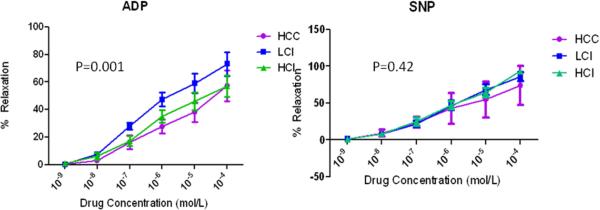
Microvessels (100-160 μm diameter) were precontracted with the thromboxane analog U46619 by 20-50% of the baseline diameter. There were no significant differences in size or precontraction between groups (Table 1). When compared with the control group, microvascular endothelium-dependent relaxation was greater in the HCI group and significantly improved in the LCI group as demonstrated by the relaxation response to ADP (p= 0.001). Endothelium–independent microvessel relaxation, as demonstrated by microvascular response to SNP, was similar among the groups (p= 0.42). There were no differences in baseline microvessel diameter or percentage of preconstruction in the ADP- or SNP- treated microvessels (Table 1, Figure 3).
Table 1.
Baseline Microvessel Diameter and Percentage Preconstriction
| Targets | HCC | LCI | HCI | p value |
|---|---|---|---|---|
| ADP Baseline Diameter μL | 132.5 ± 41.5 | 114.3 ± 15.0 | 117.6 ± 35.8 | 0.62 |
| ADP % Preconstriction | 32.0 ± 10.4 | 35.1 ± 7.3 | 37.1 ± 6.8 | 0.56 |
| SNP Baseline Diameter μL | 151.3 ± 11.1 | 114.4 ± 28.5 | 116.4 ± 29.4 | 0.08 |
| SNP % Preconstriction | 34.5 ± 7.3 | 32.2 ± 8.8 | 35.7 ± 10.1 | 0.75 |
Values reported ± SD compared to HCC. One way analysis of variance performed to determine p value. Bonferroni multiple comparison tests were performed. HCC: High Cholesterol Control; LCI: Low Dose Calpain Inhibitor; HCI: High Dose Calpain Inhibitor
Figure 3.
Microvessel reactivity. There was an improvement in endothelium-dependent (adenosine diphosphate [ADP] microvessel reactivity in the CI groups compared with the control group, with a significant improvement in the LCI group. No differences were seen in microvessel reactivity endothelium-independent (sodium nitroprusside [SNP]) vasodilators in the groups. There were no differences in baseline microvessel diameter and percentage of preconstriction. No difference was seen in baseline diameter or percentage of preconstriction in ADP- or SNP-treated microvessels. Control: High Cholesterol Control; LCI: Low Dose Calpain Inhibitor; HCI: High Dose Calpain Inhibitor
Angiogenesis and Protein Expression
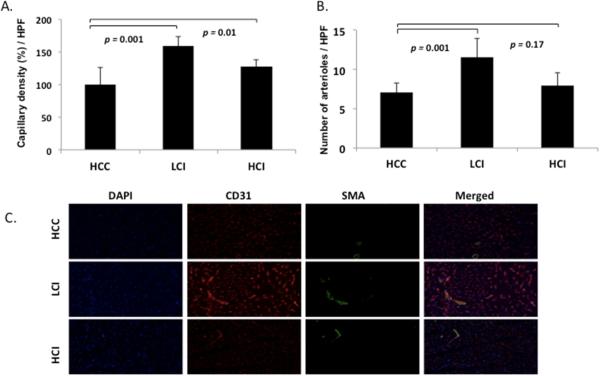
Compared to the control group, capillary density (percentage of capillary density/high power field) was significantly increased in the LCI group (p= 0.001) and the HCI group (p= 0.001) (Figure 4). Arteriolar counts (number of arterioles/high power field) were also significantly increased in the LCI group (p= 0.001) (Figure 4).
Figure 4.
A. Endothelial cell density staining for CD31. Significant increase seen in capillary density in LCI and HCI groups compared with HCC group. B. Arteriolar cell density staining for smooth muscle actin (SMA). Significant increase in arteriolar counts in LCI group compared with control. C. Representative images in HPF. CD31 is red, SMA is green. HCC: High Cholesterol Control; LCI: Low Dose Calpain Inhibitor; HCI: High Dose Calpain Inhibitor
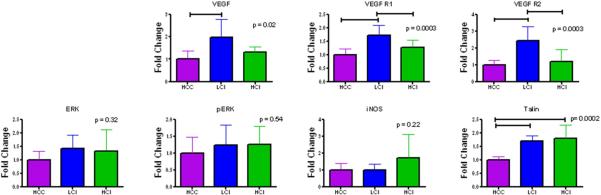
In the ischemic myocardium there was also a significant increase in several proangiogenic proteins including VEGF (p= 0.02), VEGFR1 (p= 0.003), VEGFR2 (p= 0.003) in the LCI group compared with the HCC group. When compared with the control group, there was a slight increase in proteins implicated in endothelial relaxation including ERK and pERK in the LCI and HCI groups, and iNOS in the HCI group (Figure 5, Table 2). There were no significant differences seen in the expression of VE cadherin, endostatin, or angiopoietin-1 among the groups (Table 2). Proteins integral for focal adhesion of cellular structures were also evaluated. There was a significant increase in talin in the LCI and HCI groups compared with the HCC group (p= 0.0002) (Figure 5, Table 1). There were no significant differences detected in other structural proteins including RhoA, tau, paxillin, and vinculin (Table 2).
Figure 5.
Protein expression in collateral dependent myocardium. HCC: High Cholesterol Control; LCI: Low Dose Calpain Inhibitor; HCI: High Dose Calpain Inhibitor
Table 2.
Protein Expression in Collateral Dependent Myocardium
| Targets | HCC | LCI | HCI | p value |
|---|---|---|---|---|
| ANGIOGENESIS PROTEINS | ||||
| VEGF | 1 ± 0.366 | 1.96 ± 0.819 | 1.30 ± 0.245 | 0.02* |
| VEGF R1 | 1 ± 0..208 | 1.71 ± 0.380 | 1.26 ± 0.289 | 0.0003*‡ |
| VEGF R2 | 1 ± 0.249 | 2.42 ± 0.839 | 1.19 ± 0.244 | 0.0003*‡ |
| VE Cadherin | 1 ± 0.258 | 1.23 ± 0.172 | 1.77 ± 0.576 | 0.58 |
| iNOS | 1 ± 0.379 | 1.01 ± 0.344 | 1.71 ± 1.390 | 0.21 |
| ERK | 1 ± 0.322 | 1.41 ± 0.508 | 1.33 ± 0.794 | 0.32 |
| pERK | 1 ± 0.469 | 1.23 ± 0.594 | 1.25 ± 0.538 | 0.54 |
| Endostatin | 1 ± 0.401 | 1.02 ± 0.278 | 0.97 ± 0.484 | 0.99 |
| Angiopoietin 1 | 1 ± 0.710 | 1.16 ± 0.599 | 0.90 ± 0.479 | 0.67 |
| STRUCTURAL PROTEINS | ||||
| RhoA | 1 ± 0.198 | 1.08 ± 0.305 | 1.03 ± 0.226 | 0.21 |
| Tau | 1 ± 0.342 | 1.08 ± 0.373 | 1.06 ± 0.372 | 0.91 |
| Talin | 1 ± 0.115 | 1.71 ± 0.179 | 1.79 ± 0.496 | 0.0002*† |
| Paxillin | 1 ± 0.386 | 0.91 ± 0.309 | 1.47 ± 0.296 | 0.11 |
| Vinculin | 1 ± 1.179 | 0.65 ± 0.424 | 0.71 ± 0.342 | 0.60 |
Protein expression listed as fold change ± SD compared to HCC. One way analysis of variance performed to determine p value. Bonferroni multiple comparison tests were performed:
indicates significant difference between HCC and LCI group.
indicates significant difference between HCC and HCI.
indicates significant difference between LCI and HCI group. HCC: High Cholesterol Control; LCI: Low Dose Calpain Inhibitor; HCI: High Dose Calpain Inhibitor
Discussion
In a hypercholesterolemic swine model of chronic myocardial ischemia, we demonstrate that CI improved proangiogenic protein expression, capillary density and arteriolar density, increased endothelial-dependent microvascular relaxation and myocardial blood flow.
Prior studies have demonstrated that moderate inhibition of calpain activity promotes neovascularization in the setting of hypoxia, though the exact mechanism for this remains under investigation 7. These prior studies are supported by our current study as CI resulted in an increase in capillary and arteriolar density, along with an improvement in myocardial perfusion. Data for myocardial blood flow is shown in Figure 2. There was a significant improvement in the HCI group compared to HCC group at rest and with demand pacing at 160 beats/minute demonstrating that myocardial perfusion was increased by high dose calpain inhibition. Figure 1 displays that more HCI animals demonstrated collaterals than HCC or LCI, but the increase in Rentrop score was not statistically significant. This suggests that calpain inhibition may work to increase blood flow to the myocardial tissue by increasing collateral vessels to that area. The lack of significance in collateral vessel formation improvement could be the time course of the calpain inhibitor drug. It is possible that 5 weeks is not long enough to see these changes. This would be interesting to investigate further with more pigs in each group. Figure 4 shows that both the LCI and HCI groups had a significant increase in capillary density compared to the control HCC group. And the LCI had a significant increase in arteriole counts compared to the control. Figure 3/Table 1 show there was an improvement in endothelium-dependent microvessel reactivity in the LCI compared to the control. There was no difference in endothelium-independent microvessel activity amongst the three groups. This information together suggests that calpain is working to increase blood flow to the ischemic myocardial tissue by increasing vessel density in that tissue via an endothelium dependent manner. Future work will need to evaluate the effect of calpain inhibition in the non-ischemic cardiac tissue.
Angiogenesis is an intricate process involving endothelial cell migration, proliferation, and differentiation. Figure 5 and Table 2 show that LCI and HCI showed significant increase in proteins VEGFR2, VEGFR1, and talin, while only LCI showed a significant increase in VEGF compared to the HCC group. VEGF is known to stimulate and regulate these processes. VEGF binds VEGF R1 and VEGF R2, which promote cell proliferation and survival by activating prosurvival proteins, like ERK, and inhibiting apoptosis 14, 15. Our current results demonstrate that CI results in an upregulation in VEGF. These results suggest that calpain inhibition may play a role in the regulation of VEGF mediated angiogenesis.
VEGF has been shown to induce calpain activity in endothelial cells and to paradoxically induce abnormalities in vasculature in the setting of hypoxia 7, 8. Typically these abnormalities result in architectural defects leading to poor blood vessel function and resulting poor blood flow. VEGF-induction of calpain activity results in impairments in cytoskeletal architecture and moderate inhibition of calpain improves these structures 7, 8. Calpains are known to mediate cytoskeletal and cell adhesion through the regulation of focal adhesion proteins. Our study demonstrated that CI results in a significant upregulation in one such protein, talin. Calpain dependent regulation of talin has been well demonstrated in other studies 16, and CI resulting in increased talin has been shown to improve microvascular contractility17. Another study utilizing a porcine model of right heart failure demonstrated that right ventricular (RV) pressure overload resulted in disrupted talin organization, while CI resulted in preservation in abundance and organization of talin, with an associated improvement in contractility18.
Interestingly, there was no significant difference among the three groups for VE-Cadherin, iNOS, ERK, pERK, Endostatin, Angiopoietin 1, RhoA, Tau, Paxillin, or Vinculin. These findings suggest that calpain may not have a significant effect on the ERK/iNOS pathways and may not modulate cytoskeletal structure through upregulation of RhoA, Tau, PAxillin or Vinculin.
Other possible mechanisms for the beneficial effects of CI are through the prevention of calpain induced endothelial dysfunction. A number of groups have demonstrated that hypoxia induces upregulation of calpain in endothelial cells 19, 20. In a rodent model of ischemic retinopathy, Hoang et al. found that hypoxia activates calpain in endothelial cells and resulted in disruption of the actin cytoskeleton, while moderate inhibition of calpain resulted in improved vascular perfusion and improved capillary morphogenesis and organization of actin cytoskeleton 8. Another group reported that calpain inhibitors have anti-inflammatory effects in the setting of hyperglycemia 20. Other studies have demonstrated beneficial effects of CI with respect to circulatory failure 21, 22. In a rodent model of hemorrhagic shock Mcdonald et al. demonstrated that calpain inhibition reduced circulatory failure 21. Reutten et al. demonstrated that CI attenuated circulatory failure and multi-organ dysfunction through prevention of vascular hypereactivity in a rat model of endotoxic shock 22. In our current study utilizing a large animal model of chronic ischemia we similarly demonstrate improved microvascular response in the setting of hypercholesterolemia with CI. These results taken together suggest that calpains likely play an important role in hyperglycemia induced inflammation and microvascular dysfunction in the cardiovascular system.
Interestingly, we demonstrated that collateral dependent blood flow was most improved in the HCI group, whereas microvessel relaxation, angiogenic protein expression, arteriolar density, capillary density was most improved in the LCI group. However, our do demonstrate that some amount of moderate calpain inhibition (either the high/HCI or low dose/LCI) in the setting of hypercholesterolemia and chronic myocardial ischemia improves myocardial perfusion (as indicated by the increase in blood flow), microvascular relaxation (as indicated by the endothelium dependent microsvessel reactivity study), and proangiogenic protein expression (VEGFR1, VEGFR2, and talin), however the data presented in this study cannot be used to determine the optimal dose of calpain inhibition required to improve the cardiovascular parameters in our metabolic syndrome model. It appears that the optimal dose of calpain inhibition to bring about these positive effects may lie in between the doses used in our LCI and HCI protocol. In other words, it is plausible that LCI and HCI are somewhere on the dose response curve of Calpain Inhibition where changes in dose do not have a significant change in response. Future dose-response studies are required to obtain precise level of calpain inhibition necessary for positive cardiovascular effects. We believe that these remarks are in line with our hypothesis that a certain range/levels of calpain inhibition (somewhere between LCI and HCI doses) improves myocardial blood flow.
Our study indicates that calpain inhibition is working to increase blood flow to ischemic myocardial tissue in an endothelial dependent manner. However, future work will be needed to identify exactly how calpain inhibition is having an effect. Our lab has since found that moderate calpain inhibition decreases tissue oxidative stress in the ischemic myocardial tissue of the same pig model23. Our lab is currently studying this hypothesis, which is beyond the scope of the current study. Functional data such as regional oxygen consumption will be addressed in future studies.
Limitations
There are limitations to this study. Given that this is a large animal model we have kept the number of animals to a minimum while still allowing appropriate statistical power. As mentioned earlier, sampling error at the time of harvest can certainly affect the interpretation of the data. Certainly further studies with larger animal groups and sample distributions would help diminish these types of errors. Although we provide important functional and molecular data as to the possible benefits of CI, the mechanisms by which these drugs exert their beneficial effects remains unclear. Furthermore, though we used two different doses of CI, the drug was given over a fixed period of time (for 5 weeks) and the tissue analyzed only represents one time-point at the completion of our study. As calpain regulates several different and dynamic pathways the ideal dose and time for treatment requires further investigation. The pigs were started on calpain inhibition at 3 weeks because this is the amount of time it takes for the ameroid to fully occlude the artery. Given that this is a chronic ischemia model, we wanted to mimic a completely occluded vessel in the setting of a high fat diet before beginning therapy. It would have been interesting to give the drug at different intervals, and doses, but for the scope of this study we utilized the 3 week time-point to avoid any of the acute disturbances/ischemia that may have resulted post-ameroid and prior to having had an established high fat diet. Moreover, the side effects of CI have not been described. Though our study was not specifically designed to evaluate the adverse effects of the drug, there were none clearly observed during the course of treatment.
Conclusions
In summary, moderate calpain inhibition in the setting of hypercholesterolemia and chronic myocardial ischemia improves proangiogenic protein expression, microvascular relaxation and myocardial perfusion. These findings may have important clinical implications for the treatment of patients with severe CAD and microvascular dysfunction resulting from hypercholesterolemia. Perhaps the greatest limitation for studying and developing regenerative myocardial and collateral therapies in this patient population is the lack of validation in small and large animal models. Several studies have demonstrated favorable outcomes in small animal models, or otherwise healthy large models. These studies have often not been clinically applicable, or relevant, due to the known microvascular, functional and molecular differences resulting from hyperglycemia and hypercholesterolemia 1, 4,24. These differences are also been well demonstrated in clinical investigations 1,4,5. We feel the use of a diseased large animal model provides a better indication of the utility of regenerative therapies, whether growth factor, gene- or cell-based therapies. In this study we demonstrate that calpain inhibition helps overcome some of the negative effects of hypercholesterolemia on the regenerative process and collateral vessel formation. Currently, calpain inhibitors are experimental drugs and have not been developed as therapeutic agents in patients. The results of this study are encouraging and further studies are merited to help elucidate the mechanism and extent by which calpain inhibitors exert their beneficial effects.
Perspective Statement.
Moderate calpain inhibition in the setting of hypercholesterolemia and chronic myocardial ischemia improves proangiogenic protein expression, microvascular relaxation and myocardial perfusion. These findings may have important clinical implications for the treatment of patients with severe CAD and microvascular dysfunction resulting from hypercholesterolemia.
Acknowledgments
Funding: Funding for this research was provided by the National Heart, Lung, and Blood Institute (R01HL46716, R01HL69024 Dr. Sellke), NIH Training grant 5T32-HL094300-03, (Dr. Sabe and Dr. Elmadhun), NIH Centers of Biomedical Research Excellence grant 5P20 GM1P20GM103652 (Project-3, Dr. Abid; and Dr. Feng), American Heart Association Grant-in-Aid 14GRNT20460291 (Dr. Abid), Rhode Island Foundation-RIF-20123834 (Dr. Feng), and NIH/NIGMS Training Grant 2T32 GM065085-11A1 (Dr. Potz)
Glossary
- CI
Calpain Inhibition, Drug MDL28170
- LCI
Low Dose Calpain Inhibitor High Cholesterol Pig Group
- HCI
High Dose Calpain Inhibition High Cholesterol Pig Group
- HCC
High Cholesterol Control Pig Group
- LCx
Left Circumflex Artery
- VEGF
vascular endothelial growth factor (VEGF), receptor 1 (VEGF R1), receptor 2 (VEGF R2)
- VE Cadherin
Vascular endothelial cadherin
- ERK
extracellular signal-regulated kinase
- pERK
phosphorylated ERK
- iNOS
inducible nitric oxide synthase
- RhoA
ras homolog gene family member A
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Central Message: Calpain inhibition in the setting chronic myocardial ischemia improves proangiogenic protein expression and myocardial perfusion.
Conflict of Interest: No Conficts of Interest Exist for Any of the Authors
Disclosures: None
References
- 1.Lassaletta AD, Chu LM, Sellke FW. Therapeutic neovascularization for coronary disease: Current state and future prospects. Basic research in cardiology. 2011;106:897–909. doi: 10.1007/s00395-011-0200-1. [DOI] [PubMed] [Google Scholar]
- 2.Bell MR, Gersh BJ, Schaff HV, Holmes DR, Jr., Fisher LD, Alderman EL, Myers WO, Parsons LS, Reeder GS. Effect of completeness of revascularization on long-term outcome of patients with three-vessel disease undergoing coronary artery bypass surgery. A report from the coronary artery surgery study (cass) registry. Circulation. 1992;86:446–457. doi: 10.1161/01.cir.86.2.446. [DOI] [PubMed] [Google Scholar]
- 3.Lawrie GM, Morris GC, Jr., Silvers A, Wagner WF, Baron AE, Beltangady SS, Glaeser DH, Chapman DW. The influence of residual disease after coronary bypass on the 5-year survival rate of 1274 men with coronary artery disease. Circulation. 1982;66:717–723. doi: 10.1161/01.cir.66.4.717. [DOI] [PubMed] [Google Scholar]
- 4.Boodhwani M, Sodha NR, Mieno S, Xu SH, Feng J, Ramlawi B, Clements RT, Sellke FW. Functional, cellular, and molecular characterization of the angiogenic response to chronic myocardial ischemia in diabetes. Circulation. 2007;116:I31–37. doi: 10.1161/CIRCULATIONAHA.106.680157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abaci A, Oguzhan A, Kahraman S, Eryol NK, Unal S, Arinc H, Ergin A. Effect of diabetes mellitus on formation of coronary collateral vessels. Circulation. 1999;99:2239–2242. doi: 10.1161/01.cir.99.17.2239. [DOI] [PubMed] [Google Scholar]
- 6.Franco SJ, Huttenlocher A. Regulating cell migration: Calpains make the cut. Journal of cell science. 2005;118:3829–3838. doi: 10.1242/jcs.02562. [DOI] [PubMed] [Google Scholar]
- 7.Hoang MV, Nagy JA, Fox JE, Senger DR. Moderation of calpain activity promotes neovascular integration and lumen formation during vegf-induced pathological angiogenesis. PloS one. 2010;5:e13612. doi: 10.1371/journal.pone.0013612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoang MV, Smith LE, Senger DR. Calpain inhibitors reduce retinal hypoxia in ischemic retinopathy by improving neovascular architecture and functional perfusion. Biochimica et biophysica acta. 2011;1812:549–557. doi: 10.1016/j.bbadis.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakajima E, David LL, Bystrom C, Shearer TR, Azuma M. Calpain-specific proteolysis in primate retina: Contribution of calpains in cell death. Investigative ophthalmology & visual science. 2006;47:5469–5475. doi: 10.1167/iovs.06-0567. [DOI] [PubMed] [Google Scholar]
- 10.Potter DA, Tirnauer JS, Janssen R, Croall DE, Hughes CN, Fiacco KA, Mier JW, Maki M, Herman IM. Calpain regulates actin remodeling during cell spreading. The Journal of cell biology. 1998;141:647–662. doi: 10.1083/jcb.141.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robich MP, Osipov RM, Nezafat R, Feng J, Clements RT, Bianchi C, Boodhwani M, Coady MA, Laham RJ, Sellke FW. Resveratrol improves myocardial perfusion in a swine model of hypercholesterolemia and chronic myocardial ischemia. Circulation. 2010;122:S142–149. doi: 10.1161/CIRCULATIONAHA.109.920132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rentrop KP, Cohen M, Blanke H, Phillips RA. Changes in collateral channel filling immediately after controlled coronary artery occlusion by an angioplasty balloon in human subjects. Journal of the American College of Cardiology. 1985;5:587–592. doi: 10.1016/s0735-1097(85)80380-6. [DOI] [PubMed] [Google Scholar]
- 13.Sabe AA, Elmadhun NY, Sadek AA, Chu LM, Bianchi C, Sellke FW. Differential effects of atorvastatin on autophagy in ischemic and nonischemic myocardium in ossabaw swine with metabolic syndrome. The Journal of thoracic and cardiovascular surgery. 2014;148:3172–3178. doi: 10.1016/j.jtcvs.2014.07.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elmadhun NY, Lassaletta AD, Chu LM, Liu Y, Feng J, Sellke FW. Atorvastatin increases oxidative stress and modulates angiogenesis in ossabaw swine with the metabolic syndrome. The Journal of thoracic and cardiovascular surgery. 2012;144:1486–1493. doi: 10.1016/j.jtcvs.2012.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrara N, Gerber HP, LeCouter J. The biology of vegf and its receptors. Nature medicine. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 16.Franco SJ, Rodgers MA, Perrin BJ, Han J, Bennin DA, Critchley DR, Huttenlocher A. Calpain-mediated proteolysis of talin regulates adhesion dynamics. Nature cell biology. 2004;6:977–983. doi: 10.1038/ncb1175. [DOI] [PubMed] [Google Scholar]
- 17.Kotecki M, Zeiger AS, Van Vliet KJ, Herman IM. Calpain- and talin-dependent control of microvascular pericyte contractility and cellular stiffness. Microvascular research. 2010;80:339–348. doi: 10.1016/j.mvr.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmad HA, Lu L, Ye S, Schwartz GG, Greyson CR. Calpain inhibition preserves talin and attenuates right heart failure in acute pulmonary hypertension. American journal of respiratory cell and molecular biology. 2012;47:379–386. doi: 10.1165/rcmb.2011-0286OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Su Y, Block ER. Role of calpain in hypoxic inhibition of nitric oxide synthase activity in pulmonary endothelial cells. American journal of physiology. Lung cellular and molecular physiology. 2000;278:L1204–1212. doi: 10.1152/ajplung.2000.278.6.L1204. [DOI] [PubMed] [Google Scholar]
- 20.Stalker TJ, Skvarka CB, Scalia R. A novel role for calpains in the endothelial dysfunction of hyperglycemia. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2003;17:1511–1513. doi: 10.1096/fj.02-1213fje. [DOI] [PubMed] [Google Scholar]
- 21.McDonald MC, Mota-Filipe H, Paul A, Cuzzocrea S, Abdelrahman M, Harwood S, Plevin R, Chatterjee PK, Yaqoob MM, Thiemermann C. Calpain inhibitor i reduces the activation of nuclear factor-kappab and organ injury/dysfunction in hemorrhagic shock. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2001;15:171–186. doi: 10.1096/fj.99-0645com. [DOI] [PubMed] [Google Scholar]
- 22.Ruetten H, Thiemermann C. Effect of calpain inhibitor i, an inhibitor of the proteolysis of i kappa b, on the circulatory failure and multiple organ dysfunction caused by endotoxin in the rat. British journal of pharmacology. 1997;121:695–704. doi: 10.1038/sj.bjp.0701180. [DOI] [PMC free article] [PubMed] [Google Scholar]