Abstract
Background & Aims
Little is known about the diagnostic utility of the eosinophilic esophagitis (EoE) endoscopic reference score (EREFS), and how scores change in response to treatment. We investigated the operating characteristics of the EREFS in diagnosis of EoE, how the score changes with treatment, and ways to optimize scoring system.
Methods
We performed a prospective study of adults undergoing outpatient upper endoscopy from August 2011 through December 2013 at the North Carolina School of Medicine. Incident cases of EoE were diagnosed per consensus guidelines and were treated with topical steroids or dietary elimination (n=67); 144 subjects without EoE were included as controls. EREFS scores were compared between cases and controls. For EoE cases, scores were compared before and after treatment. Area under the receiver operator characteristic curve (AUC) analysis was used to determine diagnostic utility of the EREFS system. An iterative analysis was performed to determine optimal EREFS scoring weights.
Results
The mean total EREFS score was 3.88 for EoE cases and 0.42 for controls (P>.001); the score identified subjects with EoE with an AUC of 0.934. After treatment of EoE cases, the mean score decreased from 3.88 to 2.01 (P>.001). This change was more prominent for patients with a histologic response (reduction to <15 eos/hpf), compared with non-responders; post-treatment scores were 0.45 for responders vs 3.24 for non-responders (P<.001). A weighted scoring system that doubled exudates, rings, and edema scores maximized the responsiveness of the total EREFS score.
Conclusions
The EREFS classification system identifies patients with EoE an AUC of 0.934; the score decreases with treatment, and histologic responders have significantly lower scores than non-responders. This system can therefore be used to identify individuals with EoE and used as an endoscopic outcome measure to follow their response to treatment.
Keywords: eosinophilic esophagitis, endoscopy, treatment response, outcomes, score, diagnosis
Introduction
Eosinophilic esophagitis (EoE) is a chronic esophageal inflammatory clinicopathologic disease defined by symptoms of esophageal dysfunction and esophageal eosinophilia.1, 2 EoE is frequently encountered in the endoscopy suite,3–5 but its multiple characteristic endoscopic signs including esophageal rings, strictures, narrowing, linear furrows, white plaques or exudates, decreased vascularity or edema, and fragile or “crêpe-paper” mucosa,6 are not currently part of the EoE diagnostic criteria.1, 2 This is largely because there has only been fair agreement between physicians in assessing the features,7 and individual features have not been shown to be either sensitive or specific enough to support diagnosis.8
The recent proposal of the EoE Endoscopic Reference Score (EREFS) by Hirano and colleagues allows for consistency in the description, recognition, and reporting of findings.9 After using a standard atlas illustrating this classification system, they showed good agreement between physicians in assessing exudates, rings, edema, furrows, and strictures. This agreement has now been shown in an independent population,10 and the ERFES has been recommended for use by clinicians to standardize reporting.11 Additionally, this system may be able to help identify inflammatory versus fibrostenotic features of EoE.12 However, it is still unknown whether EREFS has diagnostic utility for EoE, if the measure is responsive to treatment, and if so, what the best scoring approach would be.
The aims of this study were to 1) describe the range of EREFS findings in a large set of EoE cases and non-EoE controls; 2) determine diagnostic operating characteristics of EREFS for EoE; 3) determine whether the EREFS score was responsive to treatment in EoE cases; and 4) determine the optimal scoring system.
Methods
Study design, case definitions, and clinical data
We performed an analysis of a prospective cohort study of patients enrolled from August, 2011 (after implementation of the EREFS system after its first report13) through December, 2013. Details of this parent protocol have been reported previously.14–16 In brief, consecutive patients undergoing outpatient endoscopy were recruited if they were 18 years or older and had symptoms of esophageal dysfunction (ie dysphagia, food impaction, heartburn, reflux, or chest pain). There was a mix of open-access endoscopy and endoscopy performed by the patient’s physician. The study coordinator screened the schedule for every upper endoscopy and then approached patients to determine eligibility. Exclusion criteria were: known diagnosis of either EoE or of a non-EoE eosinophilic gastrointestinal disorder (EGID); known esophageal cancer; prior esophageal surgery; GI bleeding; anticoagulation; known esophageal varices; medical instability or multiple comorbidities precluding enrollment in the clinical opinion of the endoscopist; and inability to read or understand the consent form. This study was approved by the UNC Institutional Review Board. Subjects provided informed consent prior to enrollment and endoscopy.
Incident EoE cases were diagnosed per consensus guidelines.1, 2 They were required to have a typical symptom of esophageal dysfunction, an esophageal biopsy with at least 15 eosinophils per high-power field (eos/hpf) after an 8 week trial of a proton-pump inhibitor (PPI; 20–40 mg twice daily of any of the available agents, prescribed at the discretion of the clinician), and other causes of esophageal eosinophilia excluded. Non-EoE controls were subjects who, after endoscopy and biopsy, did not meet clinical and histologic criteria for EoE. Of note, patients with PPI-responsive esophageal eosinophilia were not included in this study.
Demographics, symptoms, and atopic conditions were recorded using standardized case report forms and a prospectively administered patient questionnaire. These data were recorded prior to the endoscopy and prior to knowing biopsy results and final case/control determination. Esophageal biopsies for research use were obtained (two from the proximal, one from the mid, and two from the distal esophagus) to maximize sensitivity for detecting an eosinophilic infiltrate.17, 18 To exclude concomitant eosinophilic gastroenteritis, gastric and duodenal biopsies were also collected. To quantify the maximum esophageal eosinophil density (eosinophils/mm2), slides were masked to case/control status, digitized, and eosinophil density was calculated using our previously validated methodology.19 This density was converted to an eosinophil count (eosinophils per high power field; eos/hpf) using a hpf size of 0.24 mm2, the most commonly reported field size in the literature,20 in order to compare results to prior studies.
Endoscopic data and the EREFS classification
During the baseline endoscopy, the study coordinator used a standardized case report form to prospectively record endoscopic findings and apply the EREFS classification system;9 the score was never calculated retrospectively based on captured images. As these were standard-of-care procedures, endoscopists were not blinded to the clinical details, including whether the exam was to assess treatment response. Exudates were recorded as absent (grade 0), mild (covering <10% of the esophageal mucosa; grade 1), or severe (involving ≥10% of the esophageal mucosa; grade 2). Rings were recorded as absent (grade 0), mild (circumferential ridges; grade 1), moderate (distinct rings that did not impair passage of a standard adult upper endoscope; grade 2), of severe (distinct rings that did not allow a standard adult upper endoscope to pass; grade 3). Edema was recorded as absent (grade 0) or present (loss of vascular markings; grade 1). Furrows were recorded as absent (grade 0), mild (present but without visible depth; grade 1), or severe (visible depth or mucosal indentation; grade 2). Strictures were recorded as absent (grade 0) or present (grade 1), and if present, the inner diameter of the stricture was estimated by the endoscopist prior to dilation. Fifteen endoscopists performed study procedures, but approximately three-quarters of exams were performed by the first author (ESD). An overview of the EREFS classification was provided, an image atlas was available, and the coordinator also provided the classification during the endoscopic examination to ensure that all endoscopists had access to the same knowledge base.
Treatment and follow-up
After identification, the EoE cases enrolled in this study were treated by their gastroenterologist as clinically indicated. Treatment options were either topical corticosteroids (either oral viscous budesonide 1 mg twice daily or fluticasone from a multi-dose inhaler, 880 mcg twice daily) for 8 weeks,21–23 or dietary therapy with the six-food elimination diet (avoidance of dairy, wheat, egg, soy, nuts, and seafood) for 6 weeks.24, 25 At the end of either treatment period, repeat upper endoscopy was performed, the full set of EREFS findings were again recorded, a follow-up set of esophageal biopsies were obtained, and histologic assessments were repeated. Response to treatment was defined histologically as a maximum eosinophil count of <15 eos/hpf.26
Statistical analysis
The features of the EoE cases and non-EoE controls were summarized using descriptive statistics. To assess the EREFS, we constructed several scores. First, individual features were scored by simply summing the severity grade; for example the score for exudates could range from 0 to 2, and the score for rings could range from 0 to 3. Second, a total EREFS score was creating by summing the grades for all five of the findings (this could range from 0 to 9). Third, inflammatory and fibrostenotic subscores were generated. The inflammatory score contained the sum of the exudate, edema, and furrows scores (this could range from 0 to 5), and the fibrostenotic score contained the sum of the rings and stricture scores (this could range from 0 to 4). For cases and controls, means were compared with a two-sample t-test, and proportions were compared with chi-square. To compare baseline and follow-up EREFS scores among EoE cases and assess the responsiveness of the system, paired t-tests were used for continuous variables and chi-square was used for categorical variables. This analysis was repeated after stratifying by baseline esophageal dilation status. We also compared the EREFS features of EoE cases who were histologic responders (<15 eos/hpf) to those who were not (≥15 eos/hpf).
In order to investigate the diagnostic utility of the EREFS to predict EoE case status (using the consensus diagnostic guidelines as the gold standard), we calculated the area under the receiver operator characteristic (ROC) curve (AUC). We then determined the EREFS score cut-point that would provide optimal sensitivity and specificity. We also evaluated the ability of both the baseline and follow-up EREFS scores to predict histologic treatment response, again utilizing ROC analysis. Finally, we examined various scoring paradigms to optimize the strength of association between change in EREFS score and either change in eosinophil count as a continuous variable or with a response threshold of 15 eosinophils per high power field. For each EREFS findings we assigned integer weights that varied from one to ten. For all possible combinations of these weights for all findings, we calculated weighted EREFS scores. The change before and after treatment in the weighted EREFS score was compared to the change in eosinophil counts on the basis of Pearson correlation coefficients for the continuous outcome and odds ratios for the threshold outcome. Strength of these associations was plotted as the mean of simulations for each finding. We then compared the best-performing weights, as measured by the change (the delta) between the pre- and post-treatment weights, to the unweighted EREFS score using ROC analysis. Analyses were performed with Stata (version 9.2; StataCorp; College Station, TX) and SAS (version 9.4; SAS Institute; Cary, NC).
Results
Range of EREFS findings and diagnostic utility in cases and controls
There were 67 EoE cases and 144 non-EoE controls included in this study (Table 1). When the EREFS classification was used to compare cases at baseline to controls, there were multiple highly significant differences between the groups (Table 2). Accordingly, the total EREFS score was 3.88 for EoE cases and 0.42 for controls (p < 0.001), with similar differences for the inflammatory and fibrostenotic sub-scores (Table 2).
Table 1.
Clinical, endoscopic, and histologic characteristics of the controls and EoE cases
| Non-EoE controls (n = 144) | EoE cases (n = 67) | p* | |
|---|---|---|---|
| Age at diagnosis (mean ± SD) | 51.6 ± 13.5 | 38.0 ± 13.0 | < 0.001 |
| Male (n, %) | 54 (38) | 39 (58) | 0.005 |
| White (n, %) | 118 (82) | 63 (94) | 0.02 |
| Symptoms/EGD indication (n, %) | |||
| Dysphagia | 108 (75) | 65 (97) | < 0.001 |
| Heartburn | 103 (72) | 12 (18) | < 0.001 |
| Abdominal pain | 9 (6) | 7 (10) | 0.28 |
| Nausea/vomiting | 12 (8) | 1 (1) | 0.05 |
| Atopic disorders (n, %) | |||
| Asthma | 33 (23) | 20 (30) | 0.28 |
| Atopic dermatitis | 10 (7) | 5 (7) | 0.89 |
| Allergic rhinitis/sinusitis | 69 (48) | 44 (66) | 0.02 |
| Food allergies | 21 (15) | 30 (45) | < 0.001 |
| Any atopic disease | 83 (58) | 50 (75) | 0.02 |
| EGD findings (n, %) | |||
| Normal | 23 (16) | 3 (4) | 0.02 |
| Rings | 14 (10) | 52 (78) | < 0.001 |
| Stricture | 26 (18) | 16 (24) | 0.32 |
| Narrowing | 5 (3) | 20 (30) | < 0.001 |
| Furrows | 9 (6) | 60 (90) | < 0.001 |
| Crêpe-paper | 3 (2) | 4 (6) | 0.14 |
| White plaques/exudates | 4 (3) | 33 (49) | < 0.001 |
| Decreased vascularity/edema | 5 (3) | 42 (63) | < 0.001 |
| Dilation performed | 44 (31) | 21 (31) | 0.91 |
| Baseline maximum eosinophil count (mean ± SD) | 2.5 ± 6.3 | 149.9 ± 124.2 | < 0.001 |
| Treatment (n, %) | |||
| Topical steroids | -- | 65 (96) | -- |
| Diet elimination | -- | 3 (4) | -- |
| Post-treatment eosinophil count (mean ± SD) | -- | 50.3 ± 84.2 | -- |
| Response with < 15 eos/hpf (n, %) | -- | 38 (57) | -- |
Proportions are compared with chi-square; means are compared with a two-sample t-test
Table 2.
EREFS features and scores for EoE cases before and after treatment, and for non-EoE controls
| EoE cases (n = 67)
|
Non-EoE controls (n = 144)
|
||||
|---|---|---|---|---|---|
| Baseline | Post-treatment | p* | p** | ||
|
|
|
||||
| EREFS findings | |||||
| Exudates (n, %) | |||||
| 0 | 34 (51) | 51 (76) | 0.005 | 140 (97) | < 0.001 |
| 1 | 19 (28) | 12 (18) | 4 (3) | ||
| 2 | 14 (21) | 4 (6) | 0 (0) | ||
| Score (mean ±SD) | 0.70 ± 0.80 | 0.30 ± 0.58 | < 0.001 | 0.03 ± 0.16 | < 0.001 |
| Rings (n, %) | |||||
| 0 | 15 (22) | 31 (46) | 0.001 | 130 (90) | < 0.001 |
| 1 | 27 (40) | 29 (43) | 12 (8) | ||
| 2 | 20 (30) | 7 (11) | 1 (1) | ||
| 3 | 5 (8) | 0 (0) | 1 (1) | ||
| Score (mean ±SD) | 1.22 ± 0.88 | 0.64 ± 0.67 | < 0.001 | 0.12 ± 0.40 | < 0.001 |
| Edema (n, %) | |||||
| 0 | 25 (37) | 48 (72) | < 0.001 | 139 (97) | < 0.001 |
| 1 | 42 (63) | 19 (28) | 5 (3) | ||
| Score (mean ±SD) | 0.62 ± 0.49 | 0.28 ± 0.45 | < 0.001 | 0.03 ± 0.18 | < 0.001 |
| Furrows (n, %) | |||||
| 0 | 7 (11) | 35 (52) | < 0.001 | 135 (94) | < 0.001 |
| 1 | 47 (70) | 28 (42) | 9 (6) | ||
| 2 | 13 (19) | 4 (6) | 0 (0) | ||
| Score (mean ±SD) | 1.09 ± 0.54 | 0.54 ± 0.61 | < 0.001 | 0.06 ± 0.24 | < 0.001 |
| Stricture (n, %) | |||||
| 0 | 51 (76) | 50 (75) | 0.84 | 118 (92) | 0.32 |
| 1 | 16 (24) | 17 (25) | 26 (18) | ||
| Score (mean ±SD) | 0.24 ± 0.43 | 0.25 ± 0.44 | 0.82 | 0.18 ± 0.39 | 0.33 |
| Stricture size (mean mm ± SD) † | 9.7 ± 4.0 | 13.2 ± 3.0 | 0.049 | 12.4 ± 3.4 | 0.03 |
| Inflammatory score (mean ± SD) | 2.41 ± 1.42 | 1.12 ± 1.25 | < 0.001 | 0.13 ± 0.39 | < 0.001 |
| Fibrostenotic score (mean ± SD) | 1.46 ± 1.11 | 0.89 ± 0.89 | < 0.001 | 0.30 ± 0.62 | < 0.001 |
| Total score (mean ± SD) | 3.88 ± 2.10 | 2.01 ± 1.75 | < 0.001 | 0.42 ± 0.87 | < 0.001 |
For comparison of EoE cases at baseline and post-treatment, chi-square or paired t-tests are used
For comparison of EoE cases at baseline and non-EoE controls, chi-square or two-sample t-tests are used.
n=7 for paired stricture diameter data for EoE cases
On ROC analysis, a model that contained all five components of the EREFS system as categorical variables had an AUC of 0.946, indicating an excellent ability to predict EoE case status based on endoscopic findings alone. A model using the total EREFS score had an AUC of 0.934. For this model, a score of 2.0 or greater optimized diagnostic utility, with a sensitivity of 88%, specificity of 92%, positive predictive value (PPV) of 84%, negative predictive value (NPV) of 94%, and accuracy of 91%. Interestingly, the majority of this predictive ability was in the inflammatory component. The inflammatory score had an AUC of 0.936, whereas the fibrostenotic score had an AUC of 0.825, primarily owing to the high prevalence of strictures in the control group.
Responsiveness of the EREFS classification system
Most of the EoE cases (96%) received treatment with topical steroids, and post-treatment data were available for all 67 cases. The mean of the maximum eosinophil counts decreased from 150 eos/hpf at baseline to 50 eos/hpf after treatment (p < 0.001), and 38 cases (57%) had a histologic response as defined by <15 eos/hpf.
The endoscopic findings significantly improved for EoE cases after treatment, as reflected in all individual and total EREFS scores, with the exception of strictures (Table 2). The total score decreased from 3.88 to 2.01, with the inflammatory score decreasing from 2.41 to 1.22 and the fibrostenotic score decreasing from 1.46 to 0.89 (p < 0.001 for all). While these trends persisted for subjects after stratification by baseline esophageal dilation status, the improvement in all endoscopic features was more notable in EoE cases that did not require baseline dilation (Supplemental Table). While strictures did not resolve in the group undergoing dilation, the esophageal caliber did improve with this treatment (9mm vs 12mm; p = 0.04).
The decreases in EREFS scores were even more prominent when comparing histologic responders and non-responders, with all scores significantly improving in the histologic responders (Table 3). Non-responders had total, inflammatory, and fibrostenotic scores of 3.24, 2.00, and 1.24, respectively, compared with 0.45, 0.63, and 1.08 for the responders (p < 0.001, p = 0.005, and p < 0.001, respectively). Mean post-treatment maximum eosinophil counts were 113 ± 98 eos/hpf in the non-responders compared with 3 ± 4 eos/hpf in the responders.
Table 3.
EREFS post-treatment features and scores stratified by histologic response
| EoE nonresponders (n =29) | EoE responders (<15 eos/hpf) (n = 38) | p* | |
|---|---|---|---|
| EREFS findings (n, %) and scores (mean ±SD) | |||
| Exudates | |||
| 0 | 17 (59) | 34 (89) | 0.013 |
| 1 | 9 (31) | 3 (8) | |
| 2 | 3 (10) | 1 (3) | |
| Score | 0.51 ± 0.69 | 0.13 ± 0.41 | 0.006 |
| Rings | |||
| 0 | 12 (41) | 19 (50) | 0.06 |
| 1 | 11 (38) | 18 (47) | |
| 2 | 6 (21) | 1 (3) | |
| 3 | 0 (0) | 0 (0) | |
| Score | 0.79 ± 0.77 | 0.53 ± 0.56 | 0.11 |
| Edema | |||
| 0 | 13 (45) | 35 (92) | < 0.001 |
| 1 | 16 (55) | 3 (8) | |
| Score | 0.55 ± 0.51 | 0.08 ± 0.27 | < 0.001 |
| Furrows | |||
| 0 | 6 (21) | 29 (76) | < 0.001 |
| 1 | 19 (65) | 9 (24) | |
| 2 | 4 (14) | 0 (0) | |
| Score | 0.93 ± 0.59 | 0.24 ± 0.43 | < 0.001 |
| Stricture | |||
| 0 | 16 (55) | 34 (89) | 0.001 |
| 1 | 13 (45) | 4 (11) | |
| Score | 0.45 ± 51 | 0.11 ± 0.31 | 0.001 |
| Stricture size (mean mm ± SD) | 13.1 ± 3.0 | 13.6 ± 3.5 | 0.75 |
| Inflammatory score (mean ± SD) | 2.00 ± 1.34 | 0.45 ± 0.60 | < 0.001 |
| Fibrostenotic score (mean ± SD) | 1.24 ± 0.99 | 0.63 ± 0.71 | 0.005 |
| Total score (mean ± SD) | 3.24 ± 1.79 | 1.08 ± 1.00 | < 0.001 |
Proportions are compared with chi-square; means are compared with a two sample t-test
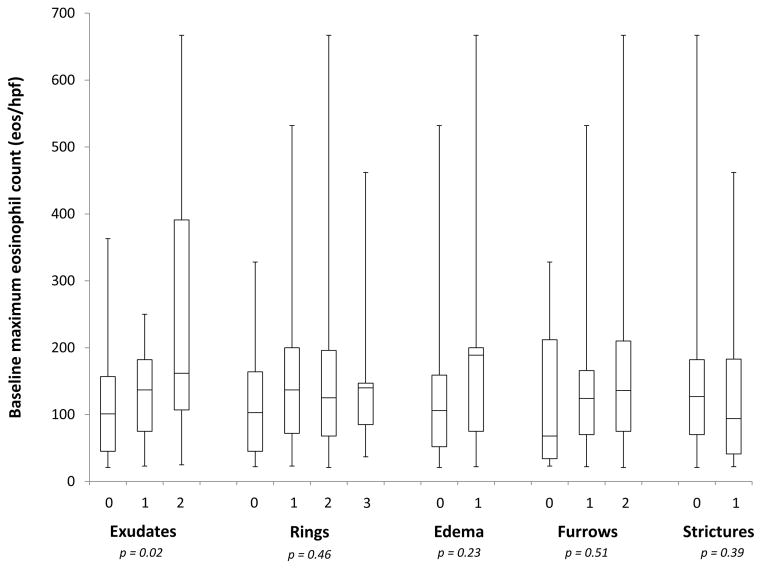
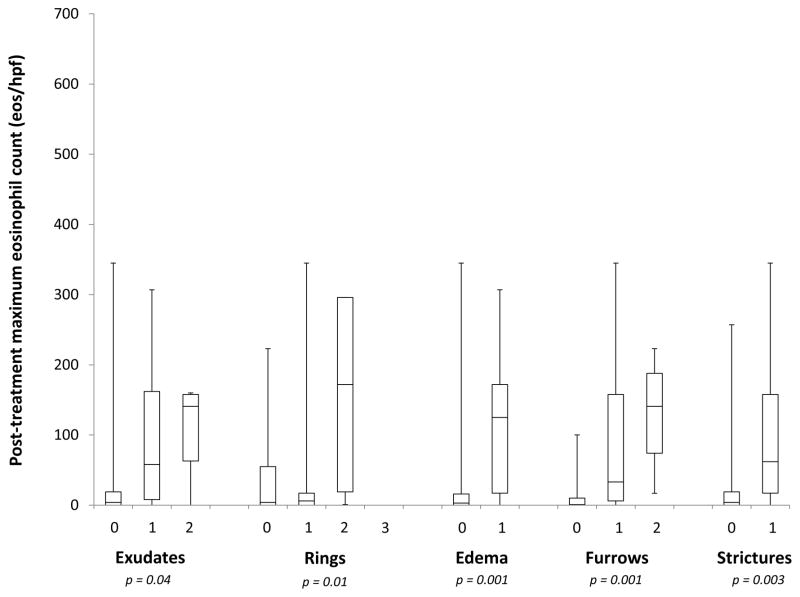
At baseline, the maximum eosinophil count among EoE cases was associated with the severity of the exudate score, but not with the other EREFS features (Figure 1A). For grade 0, 1, and 2 exudates, the mean eosinophil counts were 118, 135, and 248 eos/hpf, respectively (p = 0.02). At follow-up, however, all EREFS findings were associated the post-treatment eosinophil count, with more severe findings associated with higher counts (Figure 1B).
Figure 1.
Relationship between the maximum eosinophil count and grade of the EREFS features at baseline (A) and post-treatment (B). For the post-treatment cases, none had grade 3 rings.
Prediction of treatment response and optimization of EREFS scoring
On ROC analysis, baseline EREFS findings had a modest ability to predict histologic response to treatment. A model with all five components had an AUC of 0.730. The follow-up EREFS findings, however, were more predictive of post-treatment histologic response. The model with all five components had an AUC of 0.880.
The iterative analysis investigating weighing the EREFS features differently showed that increasing the weight of the exudate, rings, and edema score modestly increased the predictive power when the change in eosinophil counts was treated continuously (Supplemental Figure 1A), and that increasing the weight of exudates and rings was beneficial with a threshold eosinophil count (< 15 eos/hpf) for response (Supplemental Figure 1B). We created a set of EREFS scores based on these varying weights and found that doubling the exudates, rings, and edema scores maximized the responsiveness of the total EREFS score (delta of 3.19, compared with a delta of 1.87 for the standard score; Supplemental Figure 2) while minimizing the complexity of the weighting system. However, these more responsive scores did not change the overall predictive ability as measured by the AUC.
Discussion
It is only recently that the EREFS classification system has been proposed as a method for standardizing recognition and reporting of the main endoscopic features of EoE.9 While this system has been demonstrated to have good inter- and intra-observer agreement,9, 10 the diagnostic utility and responsiveness of the system are unknown. This prospective study, the first of its kind studying the EREFS system, aimed to address these knowledge gaps and there were several notable findings. First, the total EREFS score is highly predictive of EoE and potentially could have diagnostic utility. Second, the EREFS findings are highly responsive to treatment and therefore can be used as an outcome measure. Third, the initial scoring system is simple and appropriate for clinical use, though a weighed scoring system may be preferable for clinical trial outcomes to maximize responsiveness.
The characteristic endoscopic findings of EoE were noted in early reports of the disease,6 and confirmed as more experience accumulated over the past two decades.20 However, endoscopic features were not universal and they have not been included in diagnostic guidelines.1, 2 This decision was supported by data showing that inter- and intra-observer agreement on endoscopic findings was fair,7 as well as by a meta-analysis that showed individual endoscopic findings were not sensitive or specific enough to support a diagnosis of EoE.8 However, in that study, a sub-analysis of prospective studies found that 93% of patients had at least one endoscopic finding, suggesting that carefully performed examinations by endoscopists familiar with the manifestations of EoE would likely be positive. One goal of the EREFS classification was to increase awareness and recognition of EoE endoscopic finding by creating an atlas; a second goal was to create a standardized reporting system for accurately tracking findings over time.9 That this system is helpful for increasing agreement of reported endoscopic findings has already been documented.10 However, our study is the first to assess EREFS in cases and controls, the first to assess them prospectively, and the first to show that this system is responsive to treatment.
Our study confirms that the EREFS findings are highly predictive of EoE. With prospective upper endoscopic examination of patients prior to their diagnosis of EoE, we found that nearly all EoE patients (96%) had at least one endoscopic finding of the condition. Because these findings were much less common in non-EoE controls, the total EREFS score has diagnostic utility, with a score above 2 having good sensitivity and specificity. Moreover, the EREFS score was responsive to treatment; the scores decreased significantly in association with histologic improvement. This implies that the endoscopic features, as measured by the EREFS score, are a viable treatment outcome. It is notable that while exudates, rings, edema, and furrows improved with treatment, strictures did not. This is not unexpected as fibrostenotic changes of EoE12 have been shown to be treatment-resistant during an initial treatment period,22, 27, 28 though this is not universal.21 Two additional points are of interest in interpreting our findings. First, we found that with the exception of exudates, the baseline EREFS findings are not associated with pre-treatment eosinophil counts and have only a modest ability to predict histologic response. Second, the analysis of different weighting systems for creating an EREFS score showed that the initial grading classification proposed by Hirano et al9 is responsive and likely sufficient for routine use. However, if a greater change in score is desired in a clinical trial setting, doubling the exudates, rings, and edema scores maximized the responsiveness of the total EREFS score while minimizing the complexity of the weighting system.
There are several limitations of this study. First, because it was conducted at a tertiary care center with the majority of assessments performed by endoscopists experienced in EoE, it is not known if the results could be generalized to all gastroenterologists. Future studies should examine the performance of EREFS in a community setting to confirm the utility there. Second, it included only an adult population. Therefore, it is unknown if our results can be applied to children, particularly because they tend not to have fibrostenotic manifestations.8 It is, however, encouraging in this regard that the inflammatory component appeared to have the best discriminative capability between cases and controls. The majority of the subjects in our study were treated with topical steroids, with only a few treated with dietary elimination. However, there is little reason to think that the scoring system would be impacted by the type of treatment, as long as that treatment is successful. Additionally, because endoscopists were not blinded and were aware of the clinical history and cases status after treatment, we cannot eliminate the possibility that this introduced bias in their esophageal endoscopic examination. Finally, the score was optimized for histologic response, and this could have placed additional weight on the inflammatory features. While we did find that doubling the weight on the rings score increased the responsiveness, in the future differential weighting might be required if symptom outcomes are of primary importance.
This study also has a number of strengths. It was prospective and included a large number of both incident cases and controls. The baseline analysis of the cases and controls allowed us to study the diagnostic utility of EREFS, while the prospective cohort component allowed responsiveness assessment. Methodology was rigorous, with standardized data collection and treatment protocols, and careful patient retention, with no loss to follow-up. We were also able to stratify by baseline dilation status and perform an analysis to optimize scoring.
In conclusion, this prospective study found that the EREFS classification has diagnostic utility for EoE. Moreover, the score is responsive to treatment, decreasing significantly in histologic responders, and can be used as an outcome measure. In routine clinical practice, a score derived from simply summing the grades of endoscopic severity performs well. However, for research purposes or in clinical trials where a larger delta between pre- and post-treatment is desired, a scoring system with increased weights for exudates, rings, and edema may be preferable, though more prospective data would be needed to fully endorse this concept.
Supplementary Material
Supplemental Figure 1. Results of the iterative analysis for alternative weights for the EREFS score. Assessment of the association between a change in EREFS score and histologic response for all possible combinations of the EREFS score using (A) the eosinophil count as a continuous variable using Pearson correlation coefficients and (B) a threshold of 15 eos/hpf using odds ratio. Each line represents the association between change in EREFS score and change in eosinophil count at a given category weight. Categories that performed better with higher weights generally had an upward slope, while those that performed better with lower weights had a downward slope.
Supplemental Figure 2. Determination of change in the EREFS score from baseline (black bars) to post-treatment (gray bars) for a number of potential weighting schemes.
Supplemental Table: EREFS scores at baseline and post-treatment, as stratified by baseline dilation status
Acknowledgments
Grant support: This work was supported, in part, by NIH Awards K23DK090073 (ESD), K24DK100548 (NJS), and T32DK007634 (CCC), and uses resources from the UNC Center for GI Biology and Disease (P30DK34987).
Footnotes
Disclosures: There are no potential conflicts of interest for any of the authors pertaining to this study.
Author contributions:
Dellon: Project conception/design; obtained funding; data analysis/interpretation; drafting of the article; critical revision; approved final draft
Cotton: Data analysis/interpretation; drafting of the article; critical revision; approved final draft
Gebhart: Patient recruitment; data collection; critical revision; approved final draft
Higgins: Patient recruitment; data collection; critical revision; approved final draft
Beitia: Patient recruitment; data collection; critical revision; approved final draft
Woosley: Pathology supervision; critical revision; approved final draft
Shaheen: Project conception/design; data interpretation; critical revision; approved final draft
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Liacouras CA, Furuta GT, Hirano I, et al. Eosinophilic esophagitis: Updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128:3–20. e6. doi: 10.1016/j.jaci.2011.02.040. Epub 2011/04/12. [DOI] [PubMed] [Google Scholar]
- 2.Dellon ES, Gonsalves N, Hirano I, et al. ACG Clinical Guideline: Evidence based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis. Am J Gastroenterol. 2013;108(5):679–92. doi: 10.1038/ajg.2013.71. [DOI] [PubMed] [Google Scholar]
- 3.Veerappan GR, Perry JL, Duncan TJ, et al. Prevalence of Eosinophilic Esophagitis in an Adult Population Undergoing Upper Endoscopy: A Prospective Study. Clin Gastroenterol Hepatol. 2009;7:420–6. doi: 10.1016/j.cgh.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 4.Prasad GA, Alexander JA, Arora AS, et al. Eosinophilic esophagitis: Prevalence and predictive factors. Am J Gastroenterol. 2006;101(9 Suppl):S60–1. doi: 10.1111/j.1572-0241.2007.01512.x. A56. [DOI] [PubMed] [Google Scholar]
- 5.Dellon ES, Speck O, Woodward K, et al. Prospective determination of the prevalence of PPI-responsive esophageal eosinophilia in patients with dysphagia undergoing upper endoscopy. Am J Gastroenterol. 2012;107 (Suppl 1):S9. AB 20. [Google Scholar]
- 6.Dellon ES, Liacouras CA. Advances in Clinical Management of Eosinophilic Esophagitis. Gastroenterology. 2014;147(6):1238–54. doi: 10.1053/j.gastro.2014.07.055. Epub 2014/08/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peery AF, Cao H, Dominik R, et al. Variable reliability of endoscopic findings with white-light and narrow-band imaging for patients with suspected eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2011;9(6):475–80. doi: 10.1016/j.cgh.2011.02.026. Epub 2011/03/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim HP, Vance RB, Shaheen NJ, et al. The Prevalence and Diagnostic Utility of Endoscopic Features of Eosinophilic Esophagitis: A Meta-Analysis. Clin Gastroenterol Hepatol. 2012;10:988–96. e5. doi: 10.1016/j.cgh.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirano I, Moy N, Heckman MG, et al. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: validation of a novel classification and grading system. Gut. 2013;62:489–95. doi: 10.1136/gutjnl-2011-301817. Epub 2012/05/24. [DOI] [PubMed] [Google Scholar]
- 10.van Rhijn BD, Warners MJ, Curvers WL, et al. Evaluating the eosinophilic esophagitis Endoscopic Reference Score (EREFS): Moderate to substantial intra- and interobserver reliability. Endoscopy. 2014;46:1049–55. doi: 10.1055/s-0034-1377781. [DOI] [PubMed] [Google Scholar]
- 11.Dellon ES. Do you see what I see? Towards standardized reporting of endoscopic findings in eosinophilic esophagitis. Endoscopy. 2014;46(12):1043–5. doi: 10.1055/s-0034-1390706. Epub 2014/11/28. [DOI] [PubMed] [Google Scholar]
- 12.Dellon ES, Kim HP, Sperry SL, et al. A phenotypic analysis shows that eosinophilic esophagitis is a progressive fibrostenotic disease. Gastrointest Endosc. 2014;79:577–85. e4. doi: 10.1016/j.gie.2013.10.027. Epub 2013/11/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moy N, Heckman MG, Gonsalves N, et al. Inter-observer agreement on endoscopic esopahgeal findings in eosinophilic esophagitis. Gastroenterology. 2011;140 (Suppl 1):S236. Ab Sa1146. [Google Scholar]
- 14.Dellon ES, Rusin S, Gebhart JH, et al. Utility of a non-invasive serum biomarker panel for diagnosis and monitoring of EoE: A prospective study. Am J Gastroenterol. 2015;110:821–7. doi: 10.1038/ajg.2015.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dellon ES, Rusin S, Gebhart JH, et al. A clinical prediction tool identifies cases of eosinophilic esophagitis without endoscopic biopsy: A prospective study. Am J Gastroenterol. 2015 doi: 10.1038/ajg.2015.239. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dellon ES, Gebhart JH, Higgins LL, et al. The esophageal biopsy “pull” sign: a highly specific and treatment-responsive endoscopic finding in eosinophilic esophagitis (with video) Gastrointest Endosc. 2015 doi: 10.1016/j.gie.2015.05.046. In press. Epub 2015/07/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonsalves N, Policarpio-Nicolas M, Zhang Q, et al. Histopathologic variability and endoscopic correlates in adults with eosinophilic esophagitis. Gastrointest Endosc. 2006;64(3):313–9. doi: 10.1016/j.gie.2006.04.037. [DOI] [PubMed] [Google Scholar]
- 18.Dellon ES, Speck O, Woodward K, et al. Distribution and variability of esophageal eosinophilia in patients undergoing upper endoscopy. Mod Pathol. 2015;28:383–90. doi: 10.1038/modpathol.2014.110. Epub 2014/09/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dellon ES, Fritchie KJ, Rubinas TC, et al. Inter- and intraobserver reliability and validation of a new method for determination of eosinophil counts in patients with esophageal eosinophilia. Dig Dis Sci. 2010;55(7):1940–9. doi: 10.1007/s10620-009-1005-z. Epub 2009/10/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dellon ES, Aderoju A, Woosley JT, et al. Variability in diagnostic criteria for eosinophilic esophagitis: A systematic review. Am J Gastroenterol. 2007;102:2300–13. doi: 10.1111/j.1572-0241.2007.01396.x. [DOI] [PubMed] [Google Scholar]
- 21.Dellon ES, Sheikh A, Speck O, et al. Viscous Topical is More Effective than Nebulized Steroid Therapy for Patients with Eosinophilic Esophagitis. Gastroenterology. 2012;143:321–4. e1. doi: 10.1053/j.gastro.2012.04.049. Epub 2012/05/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alexander JA, Jung KW, Arora AS, et al. Swallowed Fluticasone Improves Histologic but Not Symptomatic Responses of Adults with Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2012;10:742–9. e1. doi: 10.1016/j.cgh.2012.03.018. Epub 2012/04/06. [DOI] [PubMed] [Google Scholar]
- 23.Butz BK, Wen T, Gleich GJ, et al. Efficacy, Dose Reduction, and Resistance to High-dose Fluticasone in Patients with Eosinophilic Esophagitis. Gastroenterology. 2014;147:324–33. e5. doi: 10.1053/j.gastro.2014.04.019. Epub 2014/04/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonsalves N, Yang GY, Doerfler B, et al. Elimination Diet Effectively Treats Eosinophilic Esophagitis in Adults; Food Reintroduction Identifies Causative Factors. Gastroenterology. 2012;142(7):1451–9. e1. doi: 10.1053/j.gastro.2012.03.001. Epub 2012/03/07. [DOI] [PubMed] [Google Scholar]
- 25.Wolf WA, Jerath MR, Sperry SL, et al. Dietary Elimination Therapy Is an Effective Option for Adults With Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2014;12(8):1272–9. doi: 10.1016/j.cgh.2013.12.034. Epub 2014/01/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolf WA, Green DJ, Hughes JT, et al. What cut-point should be used to define a histologic response to topical steroid use in eosinophilic esophagitis? A data-driven approach using symptoms and endoscopic findings. Gastroenterology. 2014;146 (Suppl 1):S665–6. Mo1832. [Google Scholar]
- 27.Straumann A, Conus S, Degen L, et al. Budesonide is effective in adolescent and adult patients with active eosinophilic esophagitis. Gastroenterology. 2010;139(5):1526–37. 37 e1. doi: 10.1053/j.gastro.2010.07.048. Epub 2010/08/05. [DOI] [PubMed] [Google Scholar]
- 28.Wolf WA, Cotton CC, Green DJ, et al. Predictors of response to steroid therapy for eosinophilic esophagitis and treatment of steroid-refractory patients. Clin Gastroenterol Hepatol. 2015;13:452–8. doi: 10.1016/j.cgh.2014.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Results of the iterative analysis for alternative weights for the EREFS score. Assessment of the association between a change in EREFS score and histologic response for all possible combinations of the EREFS score using (A) the eosinophil count as a continuous variable using Pearson correlation coefficients and (B) a threshold of 15 eos/hpf using odds ratio. Each line represents the association between change in EREFS score and change in eosinophil count at a given category weight. Categories that performed better with higher weights generally had an upward slope, while those that performed better with lower weights had a downward slope.
Supplemental Figure 2. Determination of change in the EREFS score from baseline (black bars) to post-treatment (gray bars) for a number of potential weighting schemes.
Supplemental Table: EREFS scores at baseline and post-treatment, as stratified by baseline dilation status