Abstract
Background & Aims
Hepatocellular carcinoma (HCC) can develop in individuals without cirrhosis. We investigated risk factors for development of HCC in the absence of cirrhosis in a US population.
Methods
We identified a national cohort of 1500 patients with verified HCC during 2005–2010 in the US Veterans Administration (VA), and reviewed their full VA medical records for evidence of cirrhosis and risk factors for HCC. Patients without cirrhosis were assigned to categories of level 1 evidence for no cirrhosis (very high probability) or level 2 evidence for no cirrhosis (high probability), based on findings from histologic analyses, laboratory test results, markers of fibrosis from non-invasive tests, and imaging features.
Results
A total of 43 (2.9%) of the 1500 patients with HCC had level 1 evidence for no cirrhosis and 151 (10.1%) had level 2 evidence for no cirrhosis; the remaining 1203 patients (80.1%) had confirmed cirrhosis. Compared to patients with HCC in presence of cirrhosis, greater proportions of patients with HCC without evidence of cirrhosis had metabolic syndrome, non-alcoholic fatty liver disease (NAFLD), or no identifiable risk factors. Patients with HCC without evidence of cirrhosis were less likely to have abused alcohol or have HCV infection than patients with cirrhosis. Patients with HCC and NAFLD (unadjusted odds ratio, 5.4; 95% confidence interval, 3.4–8.5) or metabolic syndrome (unadjusted odds ratio, 5.0; 95% confidence interval, 3.1–7.8) had more than a 5-fold risk of having HCC in the absence of cirrhosis, compared to patients with HCV-related HCC.
Conclusions
Approximately 13% of patients with HCC in the VA system do not appear to have cirrhosis. NAFLD and metabolic syndrome are the main risk factors HCC in the absence of cirrhosis.
Keywords: Liver cancer, Fatty liver, NASH, steatosis
INTRODUCTION
Hepatocellular carcinoma (HCC) is the most common primary malignancy of liver.1 Cirrhosis is the precursor lesion for most HCC cases. Nevertheless HCC is known to occur in the absence of cirrhosis. Among the major etiological risk factors for HCC, hepatitis B virus (HBV) is known to be involved directly in liver mutagenesis. A recent study of patients treated with entecavir reported that up to 44.6% of HBV-related HCC developed in the absence of cirrhosis.2 Outside the US, a multicenter study conducted in Italy among all HCC patients diagnosed during a 1-year period reported that only 6.9% of patients developed HCC in the absence of cirrhosis.3 Although some studies have reported that hepatitis C virus (HCV) and alcohol can be associated with development of HCC in the absence of cirrhosis, the causal relationship between HCV, alcohol, and development of HCC in the absence of cirrhosis remains controversial.4–6 Recent literature has documented non-alcoholic fatty liver disease (NAFLD) associated HCC in the absence of cirrhosis or advanced hepatic fibrosis.7
There is a lack of systematic studies examining the epidemiologic and etiologic risk profile of HCC in the absence of cirrhosis. The majority of published studies on HCC in the absence of cirrhosis included patients undergoing either resection or liver transplantation.5, 8–10 Furthermore, most studies reported data from tertiary referral centers11–12 and may not be representative of the actual prevalence and risk factors of HCC in the absence of cirrhosis in the general population. Given that most strategies for preventing HCC, including surveillance and chemoprevention, have targeted individuals with cirrhosis, it is important to obtain a better understanding about the development of HCC in the absence of cirrhosis because of its potential implications on current clinical paradigms.
Using automated as well as medical record data obtained from the national Veterans Health Administration (VA) system, we conducted a retrospective cohort study among HCC patients diagnosed during 2005–2011. The aims of our study were to 1) to estimate the frequency of HCC that developed in the absence of cirrhosis, and 2) to examine and compare the etiological risk factor distribution between HCC patients without cirrhosis to those with cirrhosis.
METHODS
Study population
Data were obtained from VA administrative data files combined with review of patient electronic medical records (EMR) and relevant data abstracted using a structured data abstraction tools by trained medical record abstractors (ST and SM) (details in supplement 1). We identified a cohort of 10,695 patients who had a HCC diagnosis across VA hospitals between October 1, 2004 and September 30, 2011 (fiscal years 2005–2010). Patients with possible HCC were initially identified based on the presence of ICD-9 CM code 155.0 (malignant neoplasm of liver) in the absence of code 155.1 (intrahepatic cholangiocarcinoma).14 Based on a desired sample size of 1500, we selected a random computer generated sample of patients for chart review to determine the study eligibility criteria. We included patients in the study if they had a diagnosis of HCC confirmed either by histopathology or imaging criteria according to the 2005 American Association for the Study of Liver Disease or European Association for the Study of Liver Disease guidelines.15 We reviewed charts 2,719 patients with possible HCC to arrive at 1500 study subjects. We excluded 830 patients due to insufficient evidence for HCC diagnosis. We further excluded 389 patients without recent VA healthcare utilization (defined as at least one inpatient or outpatient encounter at any VA facility within the 1-year prior to the date of HCC diagnosis); cases with HCC recurrence and first diagnosis of HCC prior to study period, or those who recieved treatment before establishing guideline based diagnosis. Thus our final study cohort included 1,500 patients with verified HCC.
Categorization of cirrhosis status
Patients with HCC were categorized into 3 mutually exclusive groups as either 1) level 1 evidence of no cirrhosis (very high probability) or 2) level 2 evidence of no cirrhosis (high probability) or 3) confirmed cirrhosis (Table 1).
Table 1.
Study definition for classification of cirrhosis categories
| Cirrhosis Category | Definition |
|---|---|
| Level 1 evidence of no cirrhosis (very high probability), n= 43 | No evidence of cirrhosis on a resection specimen or liver biopsy performed within 1 year prior to or at time of HCC diagnosis AND No features suggestive of cirrhosis on abdominal imaging available nearest to HCC diagnosis within 3 years prior to HCC diagnosis |
| Level 2 evidence of no cirrhosis (high probability), n=151 | Aspartate aminotransferase to platelet ratio index (APRI) <1 based on laboratory results available nearest to HCC diagnosis within 6 months prior and 4 weeks after HCC diagnosis AND No features suggestive of cirrhosis on abdominal imaging performed nearest to HCC diagnosis within 3 years prior to HCC diagnosis AND Two of three tests values in normal range based on laboratory results available nearest to HCC diagnosis within 6 months prior and 4 weeks after HCC diagnosis (albumin > 3.5 g/l, platelets >200,000/microliter or INR <1.1) |
| Confirmed cirrhosis, n=1201 | Documented cirrhosis on a resection specimen or liver biopsy performed any time prior to or at time of HCC diagnosis OR Features suggestive of cirrhosis on abdominal imaging performed nearest to HCC diagnosis within 3 years prior to HCC diagnosis OR Documented presence of ascites, varices, or hepatic encephalopathy OR Abnormal values on two of three laboratory tests available nearest to HCC diagnosis within 6 months prior and 4 weeks after HCC diagnosis (albumin <3.0g/l, platelets <200,000 microliter, INR >1.1) |
| Unclassified, n=105 | Insufficient information to classify in any cirrhosis category |
Risk factors for HCC
HCV status was determined by the presence of positive anti-HCV or HCV ribonucleic acid (RNA) tests detected any time before or after HCC diagnosis. HBV was defined by a positive surface antigen (HBsAg) detected any time before or after HCC diagnosis. Alcohol abuse was defined as history of more than 3 drinks a day, documentation of alcoholism/alcohol abuse in a physician progress notes, enrollment in a substance abuse treatment program, or history of alcoholic hepatitis. NAFLD was determined based on documented evidence of hepatic steatosis on liver biopsy, or in the absence of liver biopsy, by the presence of metabolic syndrome in the absence of other causes of chronic liver disease (HCV, HBV, alcohol abuse, and no documentation of primary biliary cirrhosis, primary sclerosing cholangitis, autoimmune hepatitis, hemochromatosis or Wilson disease) prior to HCC diagnosis. Metabolic syndrome was defined using U.S. National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATP III) guidelines16, except for replacing elevated waist circumference with body mass index (BMI) > 28.8 kg/m2 in both men and women.17 Less common causes of HCC such as hemochromatosis, Wilson’s disease, alpha-1 anti-trypsin deficiency or autoimmune hepatitis were captured when diagnostic laboratory tests results were positive (e.g., homozygosity for C282Y) or diagnoses were listed in the problem list or progress notes. Patients having none of the above risk factors were classified as idiopathic HCC.
Patient characteristics
Patient characteristics such as demographics, Model for End Stag Liver Disease (MELD) score liver disease complications (ascites, encephalopathy, varices), performance status (Eastern Cooperative Oncology Group performance status 0–5), Barcelona Clinic Liver Cancer (BCLC) HCC stage at diagnosis (A–D), presence of portal vein thrombosis, medical comorbidities and mental health disorders were manually extracted from EMR.
Statistical analysis
Demographic features, HCC risk factors, clinical factors and tumor characteristics were compared among the three groups of HCC patients: Level 1 evidence of no cirrhosis (very high probability), Level 2 evidence of no cirrhosis (high probability), and confirmed cirrhosis using chi-square test for discrete variables and t-test for continuous variables. Logistic regression models were used to identify variables independently associated with HCC in the absence of cirrhosis. Patient with level 1 and level 2 evidence of no cirrhosis (very high probability or high probability) were combined for these analyses. Patient and clinical factors that were significant at a p-value < 0.10 in the univariate analysis were retained in the final multivariable model, except for co-morbidities which were excluded to avoid multicollinearity as they are part of the definition of NAFLD and metabolic syndrome. Odds ratios (OR) and their accompanying 95% CI were calculated. Analyses were performed using SAS version 9.1 (SAS Institute, Cary, NC).
RESULTS
The mean age of the study cohort at the time of HCC diagnosis was 63.7 years (standard deviation=9.5) and the vast majority were men (99.8%). The greatest proportions of patients were white non-Hispanic (59.8%), followed by blacks (26.1%) and Hispanics (11.9%). As compared to race distribution in the whole VA population, a higher proportion of HCC patients in our cohort were either blacks or Hispanics and lesser proportion were white. HCV testing was performed in 96.1%, HBV testing in 89.5% and liver biopsy in 52.4% of the study cohort. Among patients in the cohort, 67.5% had HCV, 4.6% HBV, 80.6% alcoholic liver disease, 8% NAFLD, 1.7% had other risk factors (hemochromatosis, alpha-1 anti-trypsin deficiency or autoimmune hepatitis) and 2.6% were idiopathic with no identifiable risk factor.
A total of 43 (2.9%) had level 1 evidence of no cirrhosis (very high probability), 151 (10.1%) had level 2 evidence of no cirrhosis (high probability), 1203 (80.1%) had confirmed cirrhosis, and in 105 (6.7%) there was insufficient information to classify these patients into any of the above categories. Among HCC patients with level 1 evidence of no cirrhosis, the hepatic fibrosis stage was 0 in 4 (9.3%), 1 in 13 (30.2%), 2 in 21 (48.9 %) and 3 in 5 patients (11.6%) according to the Metavir scoring system.
HCC risk factor distribution stratified by cirrhosis status is presented in Table 2. Among patients with NAFLD related HCC, 34.6% had level 1 or level 2 evidence of no cirrhosis and only 65.4% patients had confirmed cirrhosis at time of HCC diagnosis. HCC patients having metabolic syndrome irrespective of presence of other risk factors, 88.9% of them had confirmed cirrhosis and only 19% had no evidence of cirrhosis. However among patients with HCC and metabolic syndrome as the only risk factor (excluding HCV, HBV and alcohol abuse) 32.7% had level 1 or level 2 evidence of no cirrhosis and only 67.3% had confirmed cirrhosis at time of HCC diagnosis. On the other hand, among HCC patients with HCV, only 8.9% had level 1 or level 2 evidence of no cirrhosis and most (91.1%) had confirmed cirrhosis at time of HCC diagnosis. Similarly among HCC patients with HBV or alcohol abuse, 92.3% and 88.9% had confirmed cirrhosis respectively at time of HCC diagnosis.
Table 2.
Risk factor distribution in the HCC cohort by cirrhosis category (N=1395)*. Risk factor distribution is not mutually exclusive as many patients had more than 1 risk factor.
| Risk factor n (%) |
Level 1 evidence of no cirrhosis n= 43 |
Level 2 evidence of no cirrhosis n= 151 |
Confirmed Cirrhosis n=1201 | P value |
|---|---|---|---|---|
| NAFLD | 6 (5.6) | 31 (28.9) | 70 (65.4) | <0.01 |
| HCV | 18 (1.9) | 67 (7.0) | 867 (91.1) | <0.01 |
| HBV | 2 (3.1) | 3 (4.6) | 60 (92.3) | 0.25 |
| Alcohol abuse | 29 (2.6) | 96 (8.5) | 1008 (88.9) | <0.01 |
| Metabolic syndrome | 14 (3.9) | 54 (15.1) | 289 (80.9) | <0.01 |
| Others** | 2 (8.0) | 3 (12.0) | 20 (80.0) | 0.45 |
| Idiopathic*** | 2 (5.9) | 11 (32.4) | 21 (61.8) | <0.01 |
HCV-hepatitis C virus; HBV-hepatitis B virus; NAFLD-nonalcoholic fatty liver disease
Of 1500, 105 had insufficient information to classify in any cirrhosis category
Others – Hemochromatosis, autoimmune hepatitis, alpha-1 antitrypsin deficiency
None of the above
HCC patients with level 1 or level 2 evidence of no cirrhosis were more likely to be older as compared to those with confirmed cirrhosis (Table 3). The mean MELD scores were significantly lower in HCC patients with level 1 (mean score 8.8, SD 3.2) and level 2 evidence of no cirrhosis (mean score 9.0, SD 3.6) compared to HCC patients with confirmed cirrhosis (mean score 12.2, SD 4.8) (P<0.01). No significant differences were observed by race. Patients with HCC in the presence of level 1 evidence of no cirrhosis were more likely to have AFP < 20 ng/ml compared to HCC in presence of confirmed cirrhosis (Table 3), while the prevalence of portal vein thrombosis was significantly lower in HCC patient with very high (2.3%) or high (11.9%) probability of no cirrhosis as compared to HCC in presence of cirrhosis (18.5%) (P<0.01). At the time of diagnosis, HCC patients with level 1 evidence of no cirrhosis were more likely to have BCLC stage B tumor (vs. C or D) as compared to HCC with cirrhosis. Tumor differentiation was not significantly different by cirrhosis status.
Table 3.
Comparison of demographics, co-morbidities and tumor characteristics among HCC patients according to cirrhosis status
| Variables | Level 1 evidence of no cirrhosis n= 43 | Level 2 evidence of no cirrhosis n= 151 | Confirmed Cirrhosis n=1201 | P-value |
|---|---|---|---|---|
| Age Mean (S.D.) | 65.5 (8.5) | 69.7 (10.7) | 62.6 (9.0) | <0.01 |
| Sex (%) | ||||
| Male | 43 (100) | 150 (99.3) | 1199 (99.8) | 0.44 |
| Race (%) | 0.05 | |||
| White | 23 (53.5) | 95 (62.9) | 711 (59.2) | |
| Black | 17 (39.5) | 44 (29.1) | 301 (25.1) | |
| Hispanic | 3 (6.9) | 9 (5.9) | 160 (13.3) | |
| Other | 0 | 3 (1.9) | 29 (2.4) | |
| BMI (%) | 0.74 | |||
| <25 | 10 (23.3) | 39 (25.8) | 261 (21.7) | |
| 25–29.9 | 14 (32.6) | 56 (37.1) | 443 (36.9) | |
| 30+ | 19 (44.2) | 56 (37.1) | 497 (41.4) | |
| Ascites | 0 (0) | 0 (0) | 585 (48.7) | <0.01 |
| Encephalopathy | 0 (0) | 0 (0) | 211 (17.8) | <0.01 |
| Varices | 0 (0) | 0 (0) | 513 (42.7) | <0.01 |
| Medical Comorbidities (%) | ||||
| Diabetes | 23 (53.5) | 68 (45.0) | 477 (39.7) | 0.10 |
| Hypertension | 38 (88.4) | 131 (86.8) | 872 (72.6) | <0.01 |
| HIV | 1 (2.3) | 7 (4.6) | 37 (3.1) | 0.56 |
| Myocardial infarction | 5 (11.6) | 28 (18.5) | 90 (7.5) | <0.01 |
| Peripheral Vascular Disease | 5 (11.6) | 30 (19.9) | 114 (9.5) | <0.01 |
| AFP <20ng/ml (%) | 26 (60.5) | 53 (35.1) | 437 (36.4) | <0.01 |
| Portal vein thrombosis (%) | 1 (2.3) | 18 (11.9) | 222 (18.5) | <0.01 |
| BCLC Stage (%) | <0.01 | |||
| Stage A | 5 (11.6) | 6 (3.9) | 177 (14.7) | |
| Stage B | 21 (48.8) | 42 (27.8) | 265 (22.1) | |
| Stage C | 11 (25.6) | 72 (47.7) | 444 (36.9) | |
| Stage D | 0 | 15 (9.9) | 231 (19.2) | |
| Missing | 6 (13.9) | 16 (10.6) | 84 (6.9) | |
| Tumor differentiation (%) | 0.63 | |||
| Well | 15 (37.5) | 27 (25) | 148 (26) | |
| Moderate | 9 (22.5) | 24 (22) | 122 (21) | |
| Poorly | 4 (10) | 8 (7) | 55 (10) | |
| Missing | 12 (30) | 50 (46) | 244 (43) |
Association between etiologic risk factors and risk of HCC in the absence of cirrhosis
We performed multiple logistic regression to examine the association of etiologic risk factors and risk of HCC in absence of cirrhosis. For this analysis we combined HCC with very high or high probability of no cirrhosis into single group in one level of the outcome variable (vs. cirrhosis). Model 1 examined NAFLD as the main exposure variable while Model 2 examined metabolic syndrome (Table 4); we did not combine these two variables in one model because of overlap (metabolic syndrome was used to define NAFLD in the absence of liver biopsy). In the unadjusted analysis, HCC patients with NAFLD had more than a five-fold risk (OR 5.4, 95% CI 3.4–8.5) of having HCC in the absence of cirrhosis compared to patients with HCV related HCC. Patients with HCC and underlying metabolic syndrome as the only risk factor for liver disease were also five-fold (OR 5.0, 95% CI 3.1–7.8) more likely to develop HCC in absence of cirrhosis as compared to patients with HCV related HCC. Patients with HCC with alcohol abuse as the only risk factor had greater than 2-fold risk of having HCC in absence of cirrhosis as compared to HCC with underlying HCV. HCC patients with HBV infection did not have a higher risk of HCC in absence of cirrhosis as compared to HCV related HCC. Adjusting for age, race and BCLC stage, MELD score, AFP and portal vein thrombus did not change the magnitude or direction of the association between etiologic risk factors and risk of HCC in absence of cirrhosis.
Table 4.
Association between etiologic risk factors and risk of HCC in absence of cirrhosis – Results of logistic regression analysis examining two models (model 1 contains NAFLD, model 2 contains metabolic syndrome)
| Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|
| Variable | n | Unadjusted OR (95% CI) | * Adjusted OR (95% CI) | Variable | Unadjusted OR (95% CI) | * Adjusted OR (95% CI) |
| Etiology | Etiology | |||||
| HCV | 952 | 1.0 | 1.0 | HCV | 1.0 | 1.0 |
| NAFLD | 107 | 5.4 (3.4–8.5) | 3.9 (2.1–7.3) | Metabolic syndrome | 5.0 (3.1–7.8) | 3.4 (1.9–6.4) |
| Alcohol abuse | 280 | 2.6 (1.8–3.8) | 2.5 (1.6–3.9) | Alcohol abuse | 2.6 (1.8–3.8) | 2.5 (1.5–3.9) |
| HBV | 15 | 1.6 (0.4–7.1) | 1.3 (0.2–7.2) | HBV | 1.6 (0.4–7.1) | 1.3 (0.2–7.2) |
| Idiopathic | 41 | 4.7 (2.4–9.5) | 3.6 (1.6–8.3) | Idiopathic | 6.0 (3.0–11.9) | 5.1 (2.2–11.7) |
| Age | 139 5 | 1.0 (1.0–1.1) | 1.0 (1.0–1.1) | |||
| Race | ||||||
| White | 829 | 1.0 | 1.0 | |||
| Black | 362 | 2.2 (1.5–3.4) | 2.2 (1.5–3.4) | |||
| Hispanic | 172 | 0.4 (0.2–0.7) | 0.4 (0.2–0.7) | |||
| Others | 32 | 0.9 (0.2–3.4) | 0.9 (0.2–3.4) | |||
| BCLC Stage | ||||||
| A | 188 | 1.0 | 1.0 | |||
| B | 328 | 2.9 (1.4–5.8) | 2.9 (1.4–5.9) | |||
| C | 527 | 2.5 (1.3–5.2) | 2.6 (1.3–5.2) | |||
| D | 246 | 1.2 (0.5–2.9) | 1.2 (0.5–2.9) | |||
| Unknown | 106 | 2.5 (1.0–6.0) | 2.5 (1.0–2.9) | |||
| MELD score | ||||||
| < 10 | 551 | 1.0 | 1.0 | |||
| 10–19.9 | 641 | 0.2 (0.1–0.3) | 0.2 (0.1–0.3) | |||
| 20+ | 73 | 0.1 (0.0–0.5) | 0.1 (0.0–0.5) | |||
| Unknown | 130 | 0.7 (0.4–1.2) | 0.7 (0.4–1.3) | |||
| AFP | ||||||
| <20 | 516 | 1.0 | 1.0 | |||
| 20+ | 780 | 0.8 (0.5–1.2) | 0.8 (0.5–1.2) | |||
| Unknown | 99 | 1.6 (0.9–2.8) | 1.6 (0.9–2.8) | |||
| PVT | ||||||
| Absent | 111 3 | 1.0 | 1.0 | |||
| Present | 241 | 0.6 (0.3–1.1) | 0.6 (0.3–1.1) | |||
| Unknown | 41 | 2.3 (1.1–4.9) | ||||
Adjusted for age, race, BCLC stage, MELD score, AFP and portal vein thrombus
DISCUSSION
In a large national cohort of randomly identified US veterans with HCC, we conducted a comprehensive medical record review to confirm diagnosis of cirrhosis and underlying risk factors for HCC, and found that approximately 13% of HCC cases developed in patients without any evidence of cirrhosis. Compared with HCC in the presence of cirrhosis, these patients were more likely to have metabolic syndrome or NAFLD or no identifiable risk factor and less likely to have alcohol abuse or HCV infection. Amongst etiologic risk factors for HCC, patients with metabolic syndrome or NAFLD related HCC had the highest risk, followed by alcohol related HCC of developing HCC in absence of cirrhosis compared to HCV related HCC. HCC patients with HBV infection did not have a higher risk of HCC in absence of cirrhosis as compared to HCV related HCC.
The risk of HCC developing in the absence of cirrhosis varies according to etiology of the liver disease. A systemic review of studies published between 1998 and 2009 reported that among patients with HBV-related HCC who received anti-viral treatment, 9.5% of patients had no evidence of cirrhosis.18 However, a study that examined at long-term outcomes of chronic hepatitis B among Caucasian patients reported that HCC risk was mostly limited to patients with cirrhosis, findings similar to our study results.19 In the Hepatitis C Antiviral Long Term Treatment against Cirrhosis (HALT-C) study conducted in the United States, the annual risk of HCV related HCC among non-cirrhotics was 0.8% compared to 2–8% per year in cirrhotics.15, 20 Among patients with alcohol abuse related HCC, estimates of HCC without evidence of cirrhosis varies widely from 11.5 to 49% depending upon geographical location of study and definition of alcohol abuse.21 In our study, approximately 10% of those with alcohol abuse or HCV related HCC and 7% of those with HBV related HCC did not have evidence of cirrhosis at time of HCC diagnosis. In contrast, 35% of NAFLD related HCC and 33.8% of idiopathic HCC did not have evidence of cirrhosis at the time of HCC diagnosis (Figure 1). It is however possible that we have missed diagnosis of NAFLD among some patients with idiopathic HCC due to absence of liver biopsy or undiagnosed components of metabolic syndrome.
Figure 1.
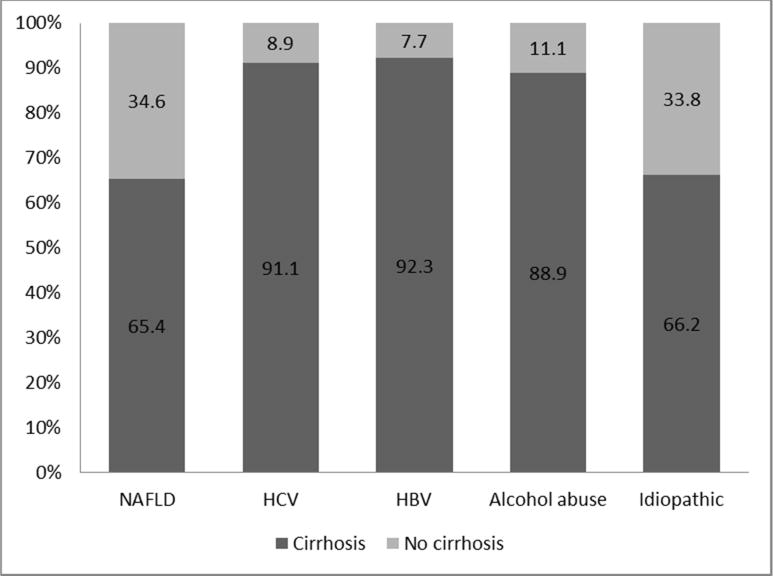
Proportion of HCC patients with or without evidence of cirrhosis by risk factor
Studies from other parts of the world have also reported high proportion of NAFLD related HCC occurring in absence of cirrhosis. In a study from Japan, only 51% of 87 cases of NAFLD related HCC had cirrhosis at time of HCC diagnosis.22 Similarly, among 31 HCC patients with metabolic syndrome as the only risk factor undergoing liver resection in a French hospital only 35% had advanced hepatic fibrosis.9 In a study from Germany, only 58% of 36 NAFLD related HCC had cirrhosis at time of HCC diagnosis.12 Another study from US on HCC patients undergoing curative treatment found that only about 73% of 52 cases NAFLD-HCC patients had underlying cirrhosis.23 A study using health system database found only 46% of patients with NAFLD/NASH and HCC had underlying cirrhosis.24 However in this study authors relied only on non validated ICD codes to capture diagnosis of NAFLD. In our study we performed manual chart review to confirm diagnosis of HCC, presence or absence of underlying cirrhosis and risk factor. Our study findings extend the findings of these previous and highlight that the sequence of events from steatohepatitis to cirrhosis and finally HCC may not be linear in a substantial proportion of patients with NAFLD or metabolic syndrome related HCC. It seems that the increased risk of HCC in these patients is due to both progression to cirrhosis and oncogenic potential of NAFLD or metabolic syndrome per se.
We used findings on liver biopsy performed within 1 year of HCC diagnosis to evaluate for diagnosis of cirrhosis. Liver biopsy is prone to sampling error and can miss cirrhosis.25 This is especially true for cases with stage 3 hepatic fibrosis.26 However among HCC patients with no evidence of cirrhosis on liver biopsy in our study, only a small fraction (< 12%) had stage 3 fibrosis. Moreover, we labelled HCC patients having no evidence of cirrhosis only when biopsy evidence was supplemented by absence of features of cirrhosis on abdominal imaging. Further we used biopsy findings within 1 year of HCC diagnosis thus minimizing the possibility of fibrosis progression in the time interval between biopsy and HCC diagnosis. In absence of liver biopsy we relied upon non-invasive marker AST to platelet ratio (APRI) to rule out cirrhosis. A recent meta-analysis on APRI with cut off 1.0 had summary receiver operating characteristic curve (AUROC) of 0.83 with negative predictive value of 69% in excluding cirrhosis.27 A diagnostic tool with AUROC of 100% is considered perfect and anything above 80% is considered good. To further reduce the chances of misclassification of cirrhosis status in the absence of liver biopsy, we supplemented APRI score with no evidence of cirrhosis on imaging and absence of any laboratory parameters suggestive of cirrhosis. Thus a patient had to fulfill all three criteria in order to qualify for level 2 evidence of no cirrhosis. HCC patients with no evidence of cirrhosis had significantly lower MELD score and lower prevalence of portal vein thrombosis thus providing internal validity of our findings. We observed that HCC patients without evidence of cirrhosis were older as compared to HCC in presence of cirrhosis. This may be due to the higher prevalence of NAFLD among non-cirrhotic HCC while cirrhotic HC had a higher proportion of HCV or alcohol abuse related HCC.
Our findings have important implications for HCC surveillance practices and paradigms in patients with NAFLD. Current guidelines do not recommend surveillance in patients with NAFLD who do not have evidence of cirrhosis.15 Studies have shown that HCC screening is under-utilized among cirrhotics who are at the highest risk of developing HCC and most likely to benefit from HCC surveillance.28 It will be logistically impractical to expand the risk pool by including the large NAFLD population. Although evidence suggests that a substantial proportion of NAFLD patients can develop HCC in absence of cirrhosis the absolute risk of HCC in non-cirrhotics is not known and the strategies and benefits of screening for HCC in NAFLD patients without cirrhosis has not been examined. Chemoprevention may also be a feasible strategy if an intervention has low toxicity and high efficacy. Given the epidemiologic association of diabetes mellitus and obesity with HCC it seems reasonable to seek and treat concomitant metabolic conditions in patients with NASH to reduce the risk of HCC. There is evidence to suggest that metformin reduces the risk of HCC among diabetics.29,30 Studies of these and other risk factors of HCC among NAFLD with and without cirrhosis are needed.
Our study has several limitations. The study population was predominantly male and may limit generalizability of results. Large liver specimen obtained during liver transplantation or hepatic resection for HCC treatment provides for more accurate staging of fibrosis and severity of underlying liver disease. However limiting the study to such patients would have introduced considerable selection bias by overestimating the number of patients with preserved liver function and consequently less advanced liver disease and defeated our primary objective to study the prevalence of HCC in absence of cirrhosis and its risk factors among the general population. We used all available VA electronic medical records to identify risk factors including a search of all progress notes for any evidence of alcohol abuse prior to HCC diagnosis. However there is still chance of occult alcohol use and therefore misclassification of risk category between alcohol use and NAFLD. It is also plausible that HCC that we attributed to NAFLD due to the presence of metabolic syndrome in the absence of other risk factors is due to other currently unknown genetic or metabolic causes. However this definition of NAFLD has been successfully employed in several NAFLD studies.17, 31
In conclusion, we found that up to 13.0 % of HCC patients had no evidence of cirrhosis at the time of HCC diagnosis. The main risk factors for this entity were NAFLD or metabolic syndrome (vs. HCV, HBV or alcohol abuse). Future research is needed to identify actionable risk factors and/or biomarkers to predict NAFLD patients at higher risk of developing HCC.
Supplementary Material
Acknowledgments
GRANT SUPPORT: This project was supported in part by the National Cancer Institute (R01 CA160738, PI: J. Davila) the facilities and resources of the Houston Veterans Affairs Health Services Research and Development Center of Excellence (HFP90-020), Michael E. DeBakey Veterans Affairs Medical Center, and the Dan Duncan Cancer Center, Houston, Texas, United States of America. The views expressed in this article are those of the authors and do not necessarily represent the views of the funding institutions.
Abbreviations
- NAFLD
Non-alcoholic fatty liver disease
- HCC
Hepatocellular carcinoma
- VA
Veterans Administration
- AFP
Alpha-fetoprotein
- HCV
Hepatitis C
- EMR
Electronic medical records
- ICD-9-CM
International Classification of Diseases, 9th Revision, Clinical Modification
- CPT
Common Procedural Terminology
- CAPRI
Compensation and Pension Records Interchange
- CPRS
Computerized Patient Record System
- HBV
Hepatitis B
- OPC
Outpatient Care File
- PTF
Patient Treatment File
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: No conflicts of interest exist for Drs. Mittal, Sada, El-Serag, Kanwal, Ms. Temple, Ms. May, Duan, Kramer, Richardson, and Davila.
AUTHOR CONTRIBUTIONS: Study concept and design: Davila, El-Serag, Kanwal, Mittal and Sada. Acquisition of data: May, Richardson, Sada and Temple. Analysis and interpretation of data: Duan, Davila, El-Serag and Mittal. Drafting of manuscript: Davila, El-Serag and Mittal. Critical revision of the manuscript for important intellectual content: Davila, El-Serag, Kanwal, Kramer, and Mittal. Statistical Analysis: Duan and Richardson. Study supervision: Davila.
Data Sources
Administrative data included the Medical SAS (MedSAS) Outpatient and Inpatient files, and the VA Vital Status File. The MedSAS files contain patient demographic data as well as diagnoses according to International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) and procedures according to Common Procedural Terminology (CPT) codes. We determined date of death, if any, in the Vital Status File that uses an algorithm to select the most accurate date of death using the VA MedSAS Inpatient file, Beneficiary Identification & Records Locator System Death File, Medicare Vital Status file, and Social Security Administration death file.16 Patient EMR information were obtained by accessing the Compensation and Pension Records Interchange (CAPRI), which is a VA application that provides access to the EMR found in the Computerized Patient Record System (CPRS) at any VA facility nationwide.
References
- 1.Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: Consider the population. J Clin Gastroenterol. 2013;47(Suppl):S2–6. doi: 10.1097/MCG.0b013e3182872f29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong GL, Chan HL, Chan HY, et al. Accuracy of risk scores for patients with chronic hepatitis B receiving entecavir treatment. Gastroenterology. 2013;144:933–944. doi: 10.1053/j.gastro.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Stroffolini T, Andreone P, Andriulli A, et al. Characteristics of hepatocellular carcinoma in Italy. J Hepatol. 1998;29:944–952. doi: 10.1016/s0168-8278(98)80122-0. [DOI] [PubMed] [Google Scholar]
- 4.Albeldawi M, Soliman M, Lopez R, Zein NN. Hepatitis C virus-associated primary hepatocellular carcinoma in non-cirrhotic patients. Dig Dis Sci. 2012;57:3265–3270. doi: 10.1007/s10620-012-2260-y. [DOI] [PubMed] [Google Scholar]
- 5.Bralet MP, Regimbeau JM, Pineau P, et al. Hepatocellular carcinoma occurring in nonfibrotic liver:Epidemiologic and histopathologic analysis of 80 French cases. Hepatology. 2000;32:200–204. doi: 10.1053/jhep.2000.9033. [DOI] [PubMed] [Google Scholar]
- 6.Grazi GL, Cescon M, Ravaioli M, et al. Liver resection for hepatocellular carcinoma in cirrhotics and noncirrhotics. Evaluation of clinicopathologic features and comparison of risk factors for long-term survival and tumour recurrence in a single centre. Aliment Pharmacol Ther. 2003;17(Suppl 2):119–129. doi: 10.1046/j.1365-2036.17.s2.9.x. [DOI] [PubMed] [Google Scholar]
- 7.Guzman G, Brunt EM, Petrovic LM, et al. Does nonalcoholic fatty liver disease predispose patients to hepatocellular carcinoma in the absence of cirrhosis? Arch Pathol Lab Med. 2008;132:1761–1766. doi: 10.5858/132.11.1761. [DOI] [PubMed] [Google Scholar]
- 8.Lerut J, Mergental H, Kahn D, et al. Place of liver transplantation in the treatment of hepatocellular carcinoma in the normal liver. Liver Transpl. 2011;17(Suppl 2):S90–7. doi: 10.1002/lt.22393. [DOI] [PubMed] [Google Scholar]
- 9.Paradis V, Zalinski S, Chelbi E, et al. Hepatocellular carcinomas in patients with metabolic syndrome often develop without significant liver fibrosis: A pathological analysis. Hepatology. 2009;49:851–859. doi: 10.1002/hep.22734. [DOI] [PubMed] [Google Scholar]
- 10.Smoot RL, Nagorney DM, Chandan VS, et al. Resection of hepatocellular carcinoma in patients without cirrhosis. Br J Surg. 2011;98:697–703. doi: 10.1002/bjs.7401. [DOI] [PubMed] [Google Scholar]
- 11.Yeh MM, Daniel HD, Torbenson M. Hepatitis C-associated hepatocellular carcinomas in non-cirrhotic livers. Mod Pathol. 2010;23:276–283. doi: 10.1038/modpathol.2009.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ertle J, Dechene A, Sowa JP, et al. Non-alcoholic fatty liver disease progresses to hepatocellular carcinoma in the absence of apparent cirrhosis. Int J Cancer. 2011;128:2436–2443. doi: 10.1002/ijc.25797. [DOI] [PubMed] [Google Scholar]
- 13.VA information resource center. accessed at http://Www.virec.reserach.va.gov/VSF/overview.htm.
- 14.Davila JA, Weston A, Smalley W, El-Serag HB. Utilization of screening for hepatocellular carcinoma in the United States. J Clin Gastroenterol. 2007;41:777–782. doi: 10.1097/MCG.0b013e3180381560. [DOI] [PubMed] [Google Scholar]
- 15.Bruix J, Sherman M, American Association for the Study of Liver Diseases Management of hepatocellular carcinoma: An update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 17.Ascha MS, Hanouneh IA, Lopez R, et al. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51:1972–1978. doi: 10.1002/hep.23527. [DOI] [PubMed] [Google Scholar]
- 18.Papatheodoridis GV, Lampertico P, Manolakopoulos S, Lok A. Incidence of hepatocellular carcinoma in chronic hepatitis B patients receiving nucleos(t)ide therapy: A systematic review. J Hepatol. 2010;53:348–356. doi: 10.1016/j.jhep.2010.02.035. [DOI] [PubMed] [Google Scholar]
- 19.Fattovich G1, Olivari N, Pasino M, D’Onofrio M, Martone E, Donato F. Long-term outcome of chronic hepatitis B in Caucasian patients: mortality after 25 years. Gut. 2008;57:84–90. doi: 10.1136/gut.2007.128496. [DOI] [PubMed] [Google Scholar]
- 20.Lok AS, Seeff LB, Morgan TR, et al. Incidence of hepatocellular carcinoma and associated risk factors in hepatitis C-related advanced liver disease. Gastroenterology. 2009;136:138–148. doi: 10.1053/j.gastro.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trevisani F, Frigerio M, Santi V, Grignaschi A, Bernardi M. Hepatocellular carcinoma in non-cirrhotic liver: A reappraisal. Dig Liver Dis. 2010;42:341–347. doi: 10.1016/j.dld.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Yasui K, Hashimoto E, Komorizono Y, Koike K, Arii S, Imai Y, Shima T, et al. Characteristics of patients with nonalcoholic steatohepatitis who develop hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2011;9:428–433. doi: 10.1016/j.cgh.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 23.Reddy SK, Steel JL, Chen HW, et al. Outcomes of curative treatment for hepatocellular cancer in nonalcoholic steatohepatitis versus hepatitis C and alcoholic liver disease. Hepatology. 2012;55:1809–1819. doi: 10.1002/hep.25536. [DOI] [PubMed] [Google Scholar]
- 24.Sanyal A, Poklepovic A, Moyneur E, Barghout V. Population-based risk factors and resource utilization for HCC: US perspective. Curr Med Res Opin. 2010;26:2183–2191. doi: 10.1185/03007995.2010.506375. [DOI] [PubMed] [Google Scholar]
- 25.Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD, American Association for the Study of Liver Diseases Liver biopsy. Hepatology. 2009;49:1017–1044. doi: 10.1002/hep.22742. [DOI] [PubMed] [Google Scholar]
- 26.Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, Grimaldi A, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898–1906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 27.Lin ZH1, Xin YN, Dong QJ, Wang Q, Jiang XJ, Zhan SH, Sun Y, Xuan SY. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology. 2011 Mar;53(3):726–36. doi: 10.1002/hep.24105. [DOI] [PubMed] [Google Scholar]
- 28.Davila JA, Henderson L, Kramer JR, et al. Utilization of surveillance for hepatocellular carcinoma among hepatitis C virus-infected veterans in the United States. Ann Intern Med. 2011;154:85–93. doi: 10.7326/0003-4819-154-2-201101180-00006. [DOI] [PubMed] [Google Scholar]
- 29.Lai SW, Chen PC, Liao KF, Muo CH, Lin CC, Sung FC. Risk of hepatocellular carcinoma in diabetic patients and risk reduction associated with anti-diabetic therapy: A population-based cohort study. Am J Gastroenterol. 2012;107:46–52. doi: 10.1038/ajg.2011.384. [DOI] [PubMed] [Google Scholar]
- 30.Hassan MM, Curley SA, Li D, Kaseb A, et al. Association of diabetes duration and diabetes treatment with the risk of hepatocellular carcinoma. Cancer. 2010;116:1938–1946. doi: 10.1002/cncr.24982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marrero JA, Fontana RJ, Su GL, Conjeevaram HS, Emick DM, Lok AS. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology. 2002;36:1349–1354. doi: 10.1053/jhep.2002.36939. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


