Abstract
Itch and pain are closely related but also clearly distinct sensations. Pain is known to suppress itch, while analgesics such as morphine can provoke itch. However, in pathological and chronic conditions, pain and itch also have similarities. Dysfunction of the nervous system, as manifested by neural plastic changes in primary sensory neurons of the peripheral nervous system (peripheral sensitization) and spinal cord and brain stem neurons in the central nervous system (central sensitization) will result in chronic pain and itch. Importantly, these diseases also result from immune dysfunction, since inflammatory mediators can directly activate or sensitize nociceptive and pruriceptive neurons in the peripheral and central nervous system, leading to pain and itch hypersensitivity. In this mini-review, I discuss the roles of Toll-like receptors (TLRs), transient receptor potential ankyrin 1 (TRPA1) ion channel, and Nav1.7 sodium channel in regulating itch and inflammation, with special emphasis of neuronal TLR signaling and the interaction of TLR7 and TRPA1. Chronic pain and chronic itch are debilitating diseases and dramatically impact the life quality of patients. Targeting TLRs for the control of inflammation, neuroinflammation (inflammation restricted in the nervous system), and hyperexcitability of nociceptors and pruriceptors will lead to new therapeutics for the relief of chronic pain and chronic itch. Finally, given the shared mechanisms among chronic cough, chronic pain, and chronic itch and the demonstrated efficacy of the neuropathic pain drug gabapentin in treating chronic cough, novel therapeutics targeting TRPA1, Nav1.7, and TLRs may also help to alleviate refractory cough via modulating neuron-immune interaction.
Keywords: Central sensitization, chronic cough, chronic itch, chronic pain, neuroinflammation, peripheral sensitization
1. Introduction
Itch and pain are closely related but distinct sensations. Itch (pruritus) elicits scratching response, whereas pain causes withdrawal responses. In physiological conditions, acute itch can be inhibited by scratching and painful stimuli. The antagonistic interaction between itch and pain is further revealed by the fact that analgesic compounds such as morphine and bile acid can evoke itch (1;2). Itch and pain also share similarities, especially in pathological and chronic conditions (3). Both itch and pain are detected by primary sensory neurons in dorsal root ganglion (DRG) and trigeminal ganglion. Indeed, pruriceptors and nociceptors are overlapped in DRGs, and the pruriceptors are a subset of small population of C-fiber nociceptors. Pain and itch also employ largely overlapping transduction machinery, such as transient receptor potential ion channel subtype V1 (TRPV1) and A1 (TRPA1), Toll-like receptors (TLRs), and proteinase activated receptors (PARs), although the G-protein coupled receptor (GPCR) MrgprA3 and thymic stromal lymphopoietin (TSLP) receptor are identified as itch-specific receptors (4;5). Under pathological and chronic conditions, dysfunction of the nervous system, as manifested by neural plastic changes in primary sensory neurons of the peripheral nervous system (peripheral sensitization) and spinal cord, brain stem, and cortical neurons in the central nervous system (central sensitization) will not only result in chronic pain but also lead to chronic itch (3;6). For example, central sensitization underlies both touch-evoked pain (allodynia) and touch-evoked itch (alloknesis) (7;8). Loss of inhibitory synaptic transmission (disinhibition) in the spinal cord has also been attributed to both chronic pain and chronic itch; both can be suppressed by spinal implantation of forebrain GABAergic neurons (9;10). Importantly, both diseases are a direct consequence of immune dysfunction, since inflammatory mediators, produced by immune cells and epithelial cells after tissue injury, can directly activate or sensitize nociceptive and pruriceptive neurons, leading to pain and itch hypersensitivity (3;11). I focus this review on emerging roles of TLRs, TRPA1, and Nav1.7 in the regulation of pain, itch, and neuroinflammation, an inflammation that is restricted in the nervous system but is particularly important for the pathogenesis of neurological diseases (12). Finally, I also discuss the shared mechanisms and treatments among chronic pain, chronic itch, and chronic cough.
2. Itch mediators and transduction mechanisms
As one of the best studied itch mediators, histamine is released from mast cells and binds to histamin H1 receptor (H1R), which is coupled with Gαq, phospholipase Cβ3, and TRPV1 in DRG neurons to evoke itch (13;14). Although antihistamines are widely-used as anti-itch drugs, chronic itch is often resistant to anti-histamine treatments (15). It is generally believed that unmyelinated C-fibers especially TRPV1-expressing C-fibers are responsible for generating both histamine dependent and independent itch, although some myelinated fibers were also implicated in cowhage-induced itch (16). Nonhistaminergic itch (e.g., cowhage and chloroquine) activates distinct neural signaling pathways via PAR2, MrgprA3, and TRPA1 (17–19). Thymic stromal lymphopoietin (TSLP) is an epithelial cell produced cytokine, which can directly act on primary sensory neurons to elicit itch via activation of TRPA1 (5). At spinal cord level, the neuropeptides gastrin releasing peptide (GRP), substance P, and neuropeptide natriuretic polypeptide b (Nppb) have been implicated in itch sensation by activating respective GRPR, NK-1, and Npra in spinal cord neurons (20–22). These peptides may also modulate itch via peripheral mechanisms. Some of these itch mediators and transducers are summarized in Table-1.
Table 1.
Peripheral itch mediators and transducers in pruriceptive sensory neurons. The mediators can be produced by immune cells, epithelial cells, keratinocytes, and even neurons after tissue injury and infection. They act on their respective receptors in pruriceptive neurons to elicit itch via transducers (e.g., TRPA1 and TRPV1).
| Challenges | Cell types | Mediators | Receptors | Effectors |
|---|---|---|---|---|
| Pruriceptive sensory neurons | ||||
| Tissue injury | Keratinocytes | NGF | TrkA | TRPV1? |
| ET-1 | ETA | TRPA1 | ||
| TSLP1 | TSLPR | TRPA1 | ||
| Mast cells | Serotonin | 5HTR | TRPA1 | |
| Tryptase | PAR2 | TRPV1 | ||
| Histamine | H1 | TRPV1/Nav1.7 | ||
| Macrophages | TNF | TNFR1 | TRPV1? | |
| T cells | IL-31 | IL-31R | TRPV1 / TRPA1 | |
| Neurons | SP | NK1 | TRPA1/TRPV1? | |
| GRP | GRPR | Unknown | ||
| miRNAs (ss) | TLR7 | TRPA1 | ||
| Bacteria and virus infection | LPS | TLR4 | TRPV1/TRPA1? | |
| RNAs (ds) | TLR3 | TRPA1? | ||
| RNAs (ss) | TLR7 | TRPA1 | ||
| Drugs | Chloroquine | MagprA3 | TRPA1/Nav1.7 | |
| Oxidative stress | ROS (H2O2) | Unknown | TRPA1 | |
Oxidative stress has been strongly implicated in the pathogenesis of chronic pain (23). Interestingly, oxidative stress also induces histamine-independent itch via activation of TRPA1 (24) (Table-1). Intradermal injection of the oxidants hydrogen peroxide and tert-butylhydroperoxide into the nape of mouse was shown to evoke robust scratching behavior. Although TRPV1 as a transduction molecule is dispensable for oxidants-induced itch, TRPV1-expressing C-fibers are indispensable for oxidant-induced itch. Furthermore, hydrogen peroxide-induced itch was suppressed by TRPA1 antagonist and antioxidants such as vitamin E (24;25).
Sodium channel subunit Nav1.7 has been strongly implicated in human pain sensation, based on gain-of-function and loss-of function mutations (26;27). It was also reported that paroxysmal itch results from gain-of-function mutation of Nav1.7 (28). In particular, a monoclonal antibody targeting the voltage sensor paddle of Nav1.7 can suppress both histaminergic itch (induced by compound 48/80) and non-histaminergic itch (induced by chloroquine) by inhibiting TTX-sensitive sodium currents and action potentials in C-fiber DRG neurons (29) (Table-1). This monoclonal antibody can also suppress acute inflammatory pain and inhibit synaptic transmission in spinal nociceptive circuit (29). Given the efficacy of this antibody in inhibiting both pain and itch, it should act on the common neural circuit of pain and itch.
Chronic itch models are critical to study the molecular and cellular mechanisms of refractory itch and test new anti-itch therapies. These models include dry skin model induced by a mixture of acetone/ether (1:1) and water (AEW), contact dermatitis model induced by diphenylcyclopropenone (DCP), and allergic contact dermatitis model induced by 2,4-Dinitro-1-fluorobenzene (DNFB). Remarkably, TRPA1 is not only necessary for dry skin-induced chronic itch but also essential for dry skin-induced pathological changes triggered by dry skin-evoked itch and scratching (30). bRAF and ERK (extracelluar signal-regulated kinase) mediated intracellular signaling in primary sensory neurons is also sufficient to drive chronic itch (31). Following allergic contact dermatitis or dry skin elicited chronic itch, mice with constitutive activation of bRAF not only had enhanced and spontaneous pruritus but also exhibited enhanced GRP expression and sustained ERK phosphorylation in sensory neurons (31). Notably, genetic ablation or pharmacological inhibition of TRPA1, but not TRPV1, was shown to inhibit skin edema, keratinocyte hyperplasia, nerve growth, leukocyte infiltration, and antihistamine-resistant scratching behavior in mice following allergic contact dermatitis induced by oxazolone and urushiol, a contact allergen of poison ivy (32).
3. Emerging roles of TLRs in pain, itch, and neuroinflammation
TLRs are typically expressed in immune cells to mediate innate immunity by recognition of pathogen-associated molecular patterns (PAMPs) (33). Most TLRs (except TLR3) requires the intracellular adaptor protein MyD88 (myeloid differentiation primary response gene 88) to mediate down-stream signaling via activation of NF-κB and MAP kinase pathways (34;35), leading to the synthesis of a wide range of inflammatory mediators, including cytokines, chemokines, and reactive oxygen/nitrogen intermediates (36). Apart from peripheral and systemic inflammation, TLRs also play an important role in neuroinflammation, an inflammation restricted in the peripheral nervous system (PNS) and central nervous system (CNS). Neuroinflammation is characterized by infiltration of leukocytes into the PNS and CNS, activation of glial cells such as microglia, astrocytes in the CNS and satellite glial cells in PNS, and production of proinflammatory cytokines and chemokines (12). TLR2 and TLR4 have been shown to modulate spinal cord glial activation and contribute to the development of neuropathic pain after nerve injury and chemotherapy (37–40). TLR4 is also important to maintain chronic arthritis pain (41) and promote chronic opioid-induced glial activation (42). We found that TLRs also have an active role in chronic itch. For example, TLR3 deficiency resulted in a substantial reduction in dry skin-induced chronic itch (43), whereas loss of TLR4 lead to remarkable reduction of chronic itch following dry skin, contact dermatitis, and allergic contact dermatitis. TLR4 is also required for dry skin-induced astrocyte activation in the spinal cord (Liu et al., 2015, unpublished data).
It is of particular interest that TLRs, such as TLR3, TLR4, TLR7 and TLR9 were also found in primary sensory neurons and play a role in detecting exogenous pathogens or endogenous danger signals that can induce pain and itch (44–48). We identified TLR7 in a subset of DRG neurons that co-express TRPV1, GRP, TRPA1, and MrgprA3 (46;49). TLR7 is known to recognize synthetic ligands imiquimod and loxoribine (50). Intradermal injection of TLR7 ligands induced remarkable scratching behavior in mice. Of note, TLR7 ligands imiquimod and loxoribine also elicit rapid inward currents and action potentials in dissociated DRG neurons, and these actions required TLR7 (46). TLR7 deficiency caused a reduction in non-histaminergic itch (46). TLR3 is also expressed by small-diameter TRPV1-positive DRG neurons, and activation of TLR3 with synthetic ligand polyinosine-polycytidylic acid (poly(I:C)) evoked inward currents and action potentials in DRG neurons and also elicited scratching in WT mice, but these actions were reduced in Tlr3−/− mice (47). Both histamine-dependent and independent scratching responses were compromised after Tlr3 deletion (47). In contrast to TLR3 and TLR7 ligands, activation of TLR4 by LPS in DRG neurons does not trigger inward currents and action potentials, although TLR4 modulates histamine-induced itch by transcriptional control of TRPV1 expression (51). Primary sensory neurons also express MyD88, an intracellular scaffold protein for canonical TLR signaling. Selective deletion of Myd88 in Nav1.8-expressing primary sensory neurons lead to reduced expression of CCL2 in DRG neurons and decreased neuropathic pain following the treatment of paclitaxel, a chemotherapy agent (52). Interestingly, paclitaxel-induced innate immunity and adaptive immunity in DRGs was abrogated after sensory neuron deletion of MyD88, suggesting that MyD88 in sensory neurons can control neuroinflammation (52). It remains to be tested whether MyD88 signaling in sensory neurons also regulates chronic itch.
What are the endogenous ligands of TLRs? While TLR3 detects double-strand RNAs, TLR7 is known to sense single-strand RNAs (ssRNAs) including microRNAs (miRNAs) (34). We found total RNAs collected from brain (dsRNAs) are sufficient to induce inward currents in DRG neurons via activation of TLR3 (43). Intracellular microRNAs (miRNAs) are key regulators of gene expression, yet the role of extracellular miRNAs in neuronal activation and sensory behaviors have not been investigated. We recently revealed an unconventional role of extracellular miRNAs for rapid excitation of nociceptor neurons via TLR7 (49) (Figure 1). In particular, miRNA let-7b binds to TLR7 in HEK293 cells, and application of let-7b to DRG neurons is able to induce rapid inward currents and action potentials in DRG neurons. These electrophysiological responses require the GUUGUGU motif of Let-7b and TLR7. Intriguingly, TLR7 is coupled to TRPA1, and let-7b-induced inward currents and action potentials are abolished after deletion of Trpa1 or pharmacological blockade of TRPA1. Remarkably, intraplantar injection of let-7b elicits rapid spontaneous pain, which depends on TLR7 and TRPA1. Finally, let-7b can be released from DRG neurons by neuronal activation, and also let-7b inhibitor reduces formalin-induced TRPA1 currents and spontaneous pain (49). Thus, miRNAs are both sufficient and required to elicit pain via TLR7/TRPA1. Increasing evidence suggests that secreted miRNAs can be detected in body fluids such as serum, CSF, and urine and serve as biomarkers of diseases such as cancer (53). Future studies are needed to investigate whether the similar signaling pathway also controls pruritus.
Figure 1.
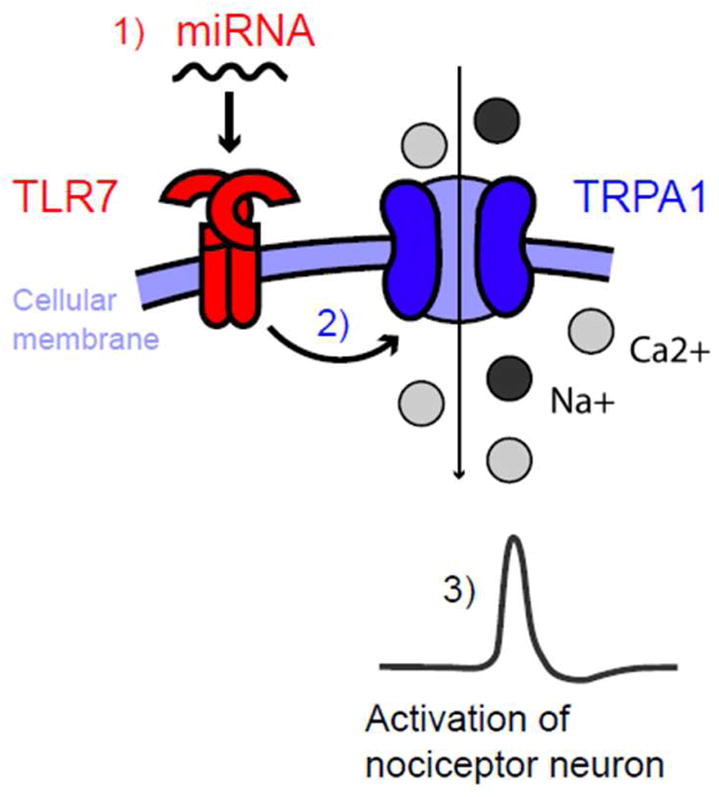
Interaction of TLR7 and TRPA1 in nociceptive primary sensory neurons (including pruriceptors). Tissue injury results in the release of miRNAs such as let-7b from damaged and inflamed tissue. Let-7b binds to TLR7 (step-1), which is coupled with TRPA1 (step-2) on the surface of nociceptive neurons. Activation of TLR7 and TRPA1 by let-7b increases the excitability of sensory neurons (step-3), leading to pain and peripheral sensitization.
4. Do chronic itch, chronic pain, and chronic cough share similar mechanisms?
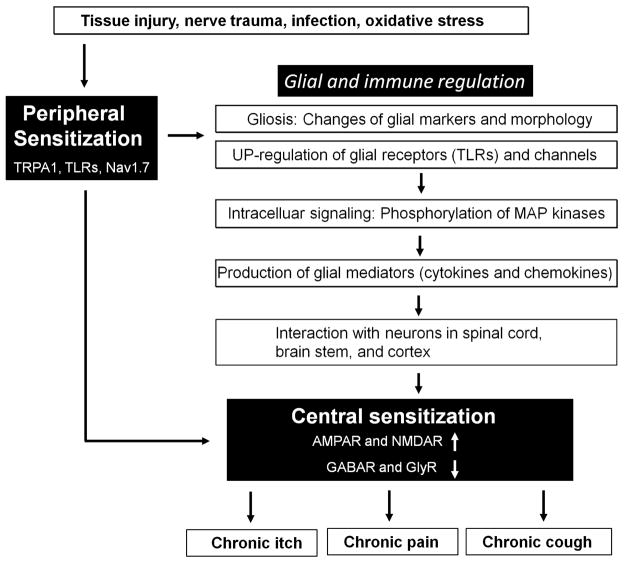
Despite clear differences between acute pain and acute itch, chronic pain and chronic itch share similar mechanisms, including peripheral sensitization, central sensitization, and glial and immune regulation (3;12), as illustrated in Figure 2. Peripheral sensitization can be induced by inflammatory mediators produced after tissue injury and requires the activation of TRPA1 and Nav1.7, as well as TRPV1 and Nav1.8 (54). While activation of TLRs in non-neuronal cells produces inflammatory mediators, activation of TLRs in sensory neurons will lead to TRPA1 activation via TLR7 (Figure 1), increased TRPV1 expression (51) and TRPV1 sensitization (55) via TLR4, and increased CCL2 production via MyD88 (52). Peripheral sensitization will result in increased release of neurotransmitters such as glutamate and brain-derived neurotrophic factor (BDNF) in central terminals of primary afferents, leading to hyperactivity of postsynaptic neurons in the spinal cord and brain stem (central sensitization)(56), which can be induced not only by enhanced excitatory synaptic transmission via glutamate NMDA and AMPA receptors but also by decreased inhibitory synaptic transmission via GABA and glycine receptors (57;58) (Figure 2). Peripheral sensitization also triggers the activation of glial cells such as microglia and astrocytes in the spinal cord, by releasing ATP, chemokines, and proteases (59). Upregulation of TLRs (e.g. TLR2 and TLR4) and activation of MAP kinases (ERK, p38, JNK) in activated microglia and astrocytes produce glial mediators such as TNF, IL-1β, IL-18, and BDNF from spinal microglia and CCL2, CXCL1, and IL-1β from spinal astrocytes (60;61). These glial mediators (cytokines, chemokines, and growth factor) can directly modulate excitatory and inhibitory synaptic transmission to drive central sensitization (57;62). On the other hand, central sensitization can also further potentiate glial activation and neuroinflammation in the CNS as part of “neurogenic inflammation” in the CNS (63).
Figure 2.
Shared mechanisms of peripheral sensitization, central sensitization, and neuro-immune interaction underlying chronic itch, chronic pain, and chronic cough. Chronic itch, pain and cough often occur in different pathophysiological condition. They are also induced in different tissues, cell types, and neural pathways. Nevertheless, these different pathological conditions activate very similar molecular machineries to trigger chronic itch, pain and cough. Under these different disease conditions, inflammatory mediators induce peripheral sensitization, which is partly mediated by ion channels TRPA1 and Nav1.8 and TLRs. Peripheral sensitization further triggers central sensitization, which is mediated by increased excitatory synaptic transmission and also by decreased inhibitory synaptic transmission. Peripheral sensitization as well as central sensitization will also cause glial activation and neuroinflammation in the CNS (in part mediated by TLRs), which can produce cytokines (TNF, IL-1β, IL-6), chemokines (CCL2, CXCL1) and growth factor (BDNF) to maintain central sensitization, leading to chronic pain, itch, and cough.
Several recent reviews compared the molecular and cellular mechanisms underlying chronic pain, chronic itch, and chronic cough. We recently discussed shared similarities in chronic pain and itch (3). O’Nell, McMahon, and Undem compared the basic pain and cough pathways and pointed out similar mechanisms of peripheral and central sensitization which may underlie symptoms such as hyperalgesia and allodynia in chronic pain and hypertussivity and allotussivity in chronic cough (64). Lavinka and Dong compared molecular signaling and targets of itch and cough and highlighted common aspects of itch and cough: both cough and itch result from irritation and involve mast cells, small-diameter sensory fibers, and neuroinflammation (65). Importantly, gabapentin, a common treatment for neuropathic pain has shown clear efficacy in alleviating chronic itch (66–68). A randomized, double-blind, placebo-controlled trial reported that gabapentin significantly inhibited refractory chronic cough and improved cough-specific quality of life compared with placebo (69). Recent guidelines for adult and pediatric cough emphasize cough reflex hypersensitivity as a key feature for patients with refractory or idiopathic chronic cough and highlight gabapentin as a new option (70).
5. Concluding remarks and future directions
It is well established that chronic pain is a hypersensitivity state that results from peripheral and central sensitization (6;54). It is increasingly appreciated that chronic itch and chronic cough are also hypersensitivity syndromes (3;71). While the role of glial cells in the pathogenesis of chronic pain is well recognized (59), their involvement in chronic itch and chronic cough needs further investigation. However, inflammation and neuroinflammation have been implicated in the development and maintenance of chronic pain, itch, and cough, involving the activation of TLRs in immune cells, glial cells and sensory neurons. Nav1.7 is highly expressed in DRG sensory neurons and plays an important role in pain and itch conduction and transmission, as well as in peripheral and central sensitization (26;28;29;72). Nav1.7 is also highly expressed in nodose ganglia and Jugular ganglia (64), and strikingly, Nav1.7 silencing in the nodose ganglia suppressed excitability and conduction in vagal sensory neurons and nearly abolished cough in guinea pigs (73;74). In addition to a prominent role of TRPA1 in pain and itch sensitization (30;75), TRPA1 has also been proposed for anti-tussive treatment (76). Notably, TRPA1 and Nav1.7 not only modulate neuronal activities but also play an important role in neurogenic inflammation. Taken together, targeting neuro-immune interaction via TLR modulators and Nav1.7 and TRPA1 inhibitors will lead to the development of novel therapies for chronic pain, itch, and cough syndromes that share similar mechanisms.
Acknowledgments
This work is supported by NIH RO1 grants DE17794, DE22743, NS87988, and NS89479 to R.R.J.
Footnotes
The author has no financial interest in this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Liu XY, Liu ZC, Sun YG, Ross M, Kim S, Tsai FF, Li QF, Jeffry J, Kim JY, Loh HH, et al. Unidirectional cross-activation of GRPR by MOR1D uncouples itch and analgesia induced by opioids. Cell. 2011;147:447–458. doi: 10.1016/j.cell.2011.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alemi F, Kwon E, Poole DP, Lieu T, Lyo V, Cattaruzza F, Cevikbas F, Steinhoff M, Nassini R, Materazzi S, et al. The TGR5 receptor mediates bile acid-induced itch and analgesia. J Clin Invest. 2013;123:1513–1530. doi: 10.1172/JCI64551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu T, Ji RR. New insights into the mechanisms of itch: are pain and itch controlled by distinct mechanisms? Pflugers Arch. 2013;465:1671–85. doi: 10.1007/s00424-013-1284-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han L, Ma C, Liu Q, Weng HJ, Cui Y, Tang Z, Kim Y, Nie H, Qu L, Patel KN, et al. A subpopulation of nociceptors specifically linked to itch. Nat Neurosci. 2013;16:174–182. doi: 10.1038/nn.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson SR, The L, Batia LM, Beattie K, Katibah GE, McClain SP, Pellegrino M, Estandian DM, Bautista DM. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell. 2013;155:285–295. doi: 10.1016/j.cell.2013.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152:S2–15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simone DA, Alreja M, LaMotte RH. Psychophysical studies of the itch sensation and itchy skin (“alloknesis”) produced by intracutaneous injection of histamine. Somatosens Mot Res. 1991;8:271–279. doi: 10.3109/08990229109144750. [DOI] [PubMed] [Google Scholar]
- 8.Akiyama T, Carstens MI, Ikoma A, Cevikbas F, Steinhoff M, Carstens E. Mouse model of touch-evoked itch (alloknesis) J Invest Dermatol. 2012;132:1886–1891. doi: 10.1038/jid.2012.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braz JM, Sharif-Naeini R, Vogt D, Kriegstein A, Alvarez-Buylla A, Rubenstein JL, Basbaum AI. Forebrain GABAergic neuron precursors integrate into adult spinal cord and reduce injury-induced neuropathic pain. Neuron. 2012;74:663–675. doi: 10.1016/j.neuron.2012.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braz JM, Juarez-Salinas D, Ross SE, Basbaum AI. Transplant restoration of spinal cord inhibitory controls ameliorates neuropathic itch. J Clin Invest. 2014;124:3612–3616. doi: 10.1172/JCI75214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akiyama T, Carstens E. Neural processing of itch. Neuroscience. 2013;250:697–714. doi: 10.1016/j.neuroscience.2013.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ji RR, Xu ZZ, Gao YJ. Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov. 2014;13:533–548. doi: 10.1038/nrd4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han SK, Mancino V, Simon MI. Phospholipase Cbeta 3 mediates the scratching response activated by the histamine H1 receptor on C-fiber nociceptive neurons. Neuron. 2006;52:691–703. doi: 10.1016/j.neuron.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 14.Imamachi N, Park GH, Lee H, Anderson DJ, Simon MI, Basbaum AI, Han SK. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc Natl Acad Sci U S A. 2009;106:11330–11335. doi: 10.1073/pnas.0905605106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeffry J, Kim S, Chen ZF. Itch signaling in the nervous system. Physiology (Bethesda) 2011;26:286–292. doi: 10.1152/physiol.00007.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ringkamp M, Schepers RJ, Shimada SG, Johanek LM, Hartke TV, Borzan J, Shim B, LaMotte RH, Meyer RA. A role for nociceptive, myelinated nerve fibers in itch sensation. J Neurosci. 2011;31:14841–14849. doi: 10.1523/JNEUROSCI.3005-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.SHELLEY WB, ARTHUR RP. Mucunain, the active pruritogenic proteinase of cowhage. Science. 1955;122:469–470. doi: 10.1126/science.122.3167.469. [DOI] [PubMed] [Google Scholar]
- 18.Reddy VB, Iuga AO, Shimada SG, LaMotte RH, Lerner EA. Cowhage-evoked itch is mediated by a novel cysteine protease: a ligand of protease-activated receptors. J Neurosci. 2008;28:4331–4335. doi: 10.1523/JNEUROSCI.0716-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, Ru F, Guan Y, Weng HJ, Geng Y, et al. Sensory Neuron-Specific GPCR Mrgprs Are Itch Receptors Mediating Chloroquine-Induced Pruritus. Cell. 2009;139:1353–1365. doi: 10.1016/j.cell.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun YG, Chen ZF. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature. 2007;448:700–703. doi: 10.1038/nature06029. [DOI] [PubMed] [Google Scholar]
- 21.Akiyama T, Nguyen T, Curtis E, Nishida K, Devireddy J, Delahanty J, Carstens MI, Carstens E. A central role for spinal dorsal horn neurons that express neurokinin-1 receptors in chronic itch. Pain. 2015 Mar 31; doi: 10.1097/j.pain.0000000000000172. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mishra SK, Hoon MA. The cells and circuitry for itch responses in mice. Science. 2013;340:968–971. doi: 10.1126/science.1233765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwartz ES, Kim HY, Wang J, Lee I, Klann E, Chung JM, Chung K. Persistent pain is dependent on spinal mitochondrial antioxidant levels. J Neurosci. 2009;29:159–168. doi: 10.1523/JNEUROSCI.3792-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu T, Ji RR. Oxidative stress induces itch via activation of transient receptor potential subtype ankyrin 1 in mice. Neurosci Bull. 2012;28:145–154. doi: 10.1007/s12264-012-1207-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nilius B, Appendino G, Owsianik G. The transient receptor potential channel TRPA1: from gene to pathophysiology. Pflugers Arch. 2012;464:425–458. doi: 10.1007/s00424-012-1158-z. [DOI] [PubMed] [Google Scholar]
- 26.Raouf R, Quick K, Wood JN. Pain as a channelopathy. J Clin Invest. 2010;120:3745–3752. doi: 10.1172/JCI43158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waxman SG. Painful Na-channelopathies: an expanding universe. Trends Mol Med. 2013;19:406–409. doi: 10.1016/j.molmed.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 28.Devigili G, Eleopra R, Pierro T, Lombardi R, Rinaldo S, Lettieri C, Faber CG, Merkies IS, Waxman SG, Lauria G. Paroxysmal itch caused by gain-of-function Nav1.7 mutation. Pain. 2014;155:1702–1707. doi: 10.1016/j.pain.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 29.Lee JH, Park CK, Chen G, Han Q, Xie RG, Liu T, Ji RR, Lee SY. A monoclonal antibody that targets a NaV1.7 channel voltage sensor for pain and itch relief. Cell. 2014;157:1393–1404. doi: 10.1016/j.cell.2014.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Wilson SR, Nelson AM, Batia L, Morita T, Estandian D, Owens DM, Lumpkin EA, Bautista DM. The ion channel TRPA1 is required for chronic itch. J Neurosci. 2013;33:9283–9294. doi: 10.1523/JNEUROSCI.5318-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao ZQ, Huo FQ, Jeffry J, Hampton L, Demehri S, Kim S, Liu XY, Barry DM, Wan L, Liu ZC, et al. Chronic itch development in sensory neurons requires BRAF signaling pathways. J Clin Invest. 2013;123:4769–4780. doi: 10.1172/JCI70528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu B, Escalera J, Balakrishna S, Fan L, Caceres AI, Robinson E, Sui A, McKay MC, McAlexander MA, Herrick CA, et al. TRPA1 controls inflammation and pruritogen responses in allergic contact dermatitis. FASEB J. 2013;27:3549–3563. doi: 10.1096/fj.13-229948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 34.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 35.Barton GM, Medzhitov R. Toll-like receptor signaling pathways. Science. 2003;300:1524–1525. doi: 10.1126/science.1085536. [DOI] [PubMed] [Google Scholar]
- 36.Liu T, Gao YJ, Ji RR. Emerging role of Toll-like receptors in the control of pain and itch. Neurosci Bull. 2012;28:131–144. doi: 10.1007/s12264-012-1219-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanga FY, Nutile-McMenemy N, DeLeo JA. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc Natl Acad Sci U S A. 2005;102:5856–5861. doi: 10.1073/pnas.0501634102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nicotra L, Loram LC, Watkins LR, Hutchinson MR. Toll-like receptors in chronic pain. Exp Neurol. 2011;234:316–29. doi: 10.1016/j.expneurol.2011.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim D, Kim MA, Cho IH, Kim MS, Lee S, Jo EK, Choi SY, Park K, Kim JS, Akira S, et al. A critical role of toll-like receptor 2 in nerve injury-induced spinal cord glial cell activation and pain hypersensitivity. J Biol Chem. 2007;282:14975–14983. doi: 10.1074/jbc.M607277200. [DOI] [PubMed] [Google Scholar]
- 40.Li Y, Zhang H, Zhang H, Kosturakis AK, Jawad AB, Dougherty PM. Toll-like receptor 4 signaling contributes to Paclitaxel-induced peripheral neuropathy. J Pain. 2014;15:712–725. doi: 10.1016/j.jpain.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Christianson CA, Dumlao DS, Stokes JA, Dennis EA, Svensson CI, Corr M, Yaksh TL. Spinal TLR4 mediates the transition to a persistent mechanical hypersensitivity after the resolution of inflammation in serum-transferred arthritis. Pain. 2011;152:2881–2891. doi: 10.1016/j.pain.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hutchinson MR, Zhang Y, Shridhar M, Evans JH, Buchanan MM, Zhao TX, Slivka PF, Coats BD, Rezvani N, Wieseler J, et al. Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain Behav Immun. 2010;24:83–95. doi: 10.1016/j.bbi.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu T, Berta T, Xu ZZ, Park CK, Zhang L, Lu N, Liu Q, Liu Y, Gao YJ, Liu YC, et al. TLR3 deficiency impairs spinal cord synaptic transmission, central sensitization, and pruritus in mice. J Clin Invest. 2012;122:2195–2207. doi: 10.1172/JCI45414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Due MR, Piekarz AD, Wilson N, Feldman P, Ripsch MS, Chavez S, Yin H, Khanna R, White FA. Neuroexcitatory effects of morphine-3-glucuronide are dependent on Toll-like receptor 4 signaling. J Neuroinflammation. 2012;9:200. doi: 10.1186/1742-2094-9-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qi J, Buzas K, Fan H, Cohen JI, Wang K, Mont E, Klinman D, Oppenheim JJ, Howard OM. Painful pathways induced by TLR stimulation of dorsal root ganglion neurons. J Immunol. 2011;186:6417–6426. doi: 10.4049/jimmunol.1001241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu T, Xu ZZ, Park CK, Berta T, Ji RR. Toll-like receptor 7 mediates pruritus. Nat Neurosci. 2010;13:1460–1462. doi: 10.1038/nn.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu T, Berta T, Xu ZZ, Park CK, Zhang L, Lu N, Liu Q, Liu Y, Gao YJ, Liu YC, et al. TLR3 deficiency impairs spinal cord synaptic transmission, central sensitization, and pruritus in mice. J Clin Invest. 2012;122:2195–2207. doi: 10.1172/JCI45414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chiu IM, von Hehn CA, Woolf CJ. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat Neurosci. 2012;15:1063–1067. doi: 10.1038/nn.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park CK, Xu ZZ, Berta T, Han Q, Chen G, Liu XJ, Ji RR. Extracellular MicroRNAs Activate Nociceptor Neurons to Elicit Pain via TLR7 and TRPA1. Neuron. 2014;82:47–54. doi: 10.1016/j.neuron.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, Horiuchi T, Tomizawa H, Takeda K, Akira S. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- 51.Min H, Lee H, Lim H, Jang YH, Chung SJ, Lee CJ, Lee SJ. TLR4 enhances histamine-mediated pruritus by potentiating TRPV1 activity. Mol Brain. 2014;7:59. doi: 10.1186/s13041-014-0059-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu XJ, Zhang Y, Liu T, Xu ZZ, Park CK, Berta T, Jiang D, Ji RR. Nociceptive neurons regulate innate and adaptive immunity and neuropathic pain through MyD88 adapter. Cell Res. 2014;24:1374–1377. doi: 10.1038/cr.2014.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, Guo J, Zhang Y, Chen J, Guo X, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997–1006. doi: 10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]
- 54.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Diogenes A, Ferraz CC, Akopian AN, Henry MA, Hargreaves KM. LPS Sensitizes TRPV1 via Activation of TLR4 in Trigeminal Sensory Neurons. J Dent Res. 2011 doi: 10.1177/0022034511400225. [DOI] [PubMed] [Google Scholar]
- 56.Ji RR, Kohno T, Moore KA, Woolf CJ. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci. 2003;26:696–705. doi: 10.1016/j.tins.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 57.Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- 58.Lu Y, Dong H, Gao Y, Gong Y, Ren Y, Gu N, Zhou S, Xia N, Sun YY, Ji RR, et al. A feed-forward spinal cord glycinergic neural circuit gates mechanical allodynia. J Clin Invest. 2013;123:4050–4062. doi: 10.1172/JCI70026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ji RR, Berta T, Nedergaard M. Glia and pain: Is chronic pain a gliopathy? Pain. 2013;154(Suppl 1):S10–28. doi: 10.1016/j.pain.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ren K, Dubner R. Central nervous system plasticity and persistent pain. J Orofac Pain. 1999;13:155–163. [PubMed] [Google Scholar]
- 61.Grace PM, Hutchinson MR, Maier SF, Watkins LR. Pathological pain and the neuroimmune interface. Nat Rev Immunol. 2014;14:217–231. doi: 10.1038/nri3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kawasaki Y, Zhang L, Cheng JK, Ji RR. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci. 2008;28:5189–5194. doi: 10.1523/JNEUROSCI.3338-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xanthos DN, Sandkuhler J. Neurogenic neuroinflammation: inflammatory CNS reactions in response to neuronal activity. Nat Rev Neurosci. 2014;15:43–53. doi: 10.1038/nrn3617. [DOI] [PubMed] [Google Scholar]
- 64.O’Neill J, McMahon SB, Undem BJ. Chronic cough and pain: Janus faces in sensory neurobiology? Pulm Pharmacol Ther. 2013;26:476–485. doi: 10.1016/j.pupt.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 65.Lavinka PC, Dong X. Molecular signaling and targets from itch: lessons for cough. Cough. 2013;9:8. doi: 10.1186/1745-9974-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gunal AI, Ozalp G, Yoldas TK, Gunal SY, Kirciman E, Celiker H. Gabapentin therapy for pruritus in haemodialysis patients: a randomized, placebo-controlled, double-blind trial. Nephrol Dial Transplant. 2004;19:3137–3139. doi: 10.1093/ndt/gfh496. [DOI] [PubMed] [Google Scholar]
- 67.Yosipovitch G, Bernhard JD. Clinical practice. Chronic pruritus. N Engl J Med. 2013;368:1625–1634. doi: 10.1056/NEJMcp1208814. [DOI] [PubMed] [Google Scholar]
- 68.Manenti L, Vaglio A, Costantino E, Danisi D, Oliva B, Pini S, Prati E, Testori A. Gabapentin in the treatment of uremic itch: an index case and a pilot evaluation. J Nephrol. 2005;18:86–91. [PubMed] [Google Scholar]
- 69.Ryan NM, Birring SS, Gibson PG. Gabapentin for refractory chronic cough: a randomised, double-blind, placebo-controlled trial. Lancet. 2012;380:1583–1589. doi: 10.1016/S0140-6736(12)60776-4. [DOI] [PubMed] [Google Scholar]
- 70.Birring SS, Kavanagh J, Lai K, Chang AB. Adult and paediatric cough guidelines: Ready for an overhaul? Pulm Pharmacol Ther. 2015 doi: 10.1016/j.pupt.2015.01.007. S1094–5539(15)00019-X. [DOI] [PubMed] [Google Scholar]
- 71.Chung KF. Chronic ‘cough hypersensitivity syndrome’: a more precise label for chronic cough. Pulm Pharmacol Ther. 2011;24:267–271. doi: 10.1016/j.pupt.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 72.Black JA, Frezel N, Dib-Hajj SD, Waxman SG. Expression of Nav1.7 in DRG neurons extends from peripheral terminals in the skin to central preterminal branches and terminals in the dorsal horn. Mol Pain. 2012;8:82. doi: 10.1186/1744-8069-8-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Muroi Y, Ru F, Kollarik M, Canning BJ, Hughes SA, Walsh S, Sigg M, Carr MJ, Undem BJ. Selective silencing of Na(V)1.7 decreases excitability and conduction in vagal sensory neurons. J Physiol. 2011;589:5663–5676. doi: 10.1113/jphysiol.2011.215384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Muroi Y, Ru F, Chou YL, Carr MJ, Undem BJ, Canning BJ. Selective inhibition of vagal afferent nerve pathways regulating cough using Nav 1.7 shRNA silencing in guinea pig nodose ganglia. Am J Physiol Regul Integr Comp Physiol. 2013;304:R1017–R1023. doi: 10.1152/ajpregu.00028.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 76.Grace MS, Belvisi MG. TRPA1 receptors in cough. Pulm Pharmacol Ther. 2011;24:286–288. doi: 10.1016/j.pupt.2010.11.002. [DOI] [PubMed] [Google Scholar]