Abstract
The current available literature evaluating feeding difficulties in children with esophageal atresia was reviewed. The published literature was searched through PubMed using a pre-defined search strategy. Feeding difficulties are commonly encountered in children and adults with repaired esophageal atresia [EA]. The mechanism for abnormal feeding includes both esophageal and oropharyngeal dysphagia. Esophageal dysphagia is commonly reported in patients with EA and causes include dysmotility, anatomic lesions, esophageal outlet obstruction and esophageal inflammation. Endoscopic evaluation, esophageal manometry and esophograms can be useful studies to evaluate for causes of esophageal dysphagia. Oropharyngeal dysfunction and aspiration are also important mechanisms for feeding difficulties in patients with EA. These patients often present with respiratory symptoms. Videofluoroscopic swallow study, salivagram, fiberoptic endoscopic evaluation of swallowing and high-resolution manometry can all be helpful tools to identify aspiration. Once diagnosed, management goals include reduction of aspiration during swallowing, reducing full column reflux into the oropharynx and continuation of oral feeding to maintain skills. We review specific strategies which can be used to reduce aspiration of gastric contents, including thickening feeds, changing feeding schedule, switching formula, trialing transpyloric feeds and fundoplication.
Keywords: Esophageal atresia, Tracheoesophageal fistula, Feeding difficulties, Oropharyngeal dysphagia, aspiration
INTRODUCTION
Esophageal atresia, with or without tracheoesophageal fistula (TEF), is a congenital anomaly of the esophagus affecting one in every 2500–3000 live births [1]. Infant survival of this condition is high, with reported survival rates of over 90% [2]. However, gastrointestinal and respiratory complications are well documented in children, adolescents and adults with repaired EA [3–9]. Feeding disorders in children with esophageal atresia are common in clinical practice but the literature supporting these observations is limited. Between 6% and 52% of patients have some abnormalities of feeding [7,10,11]. The majority of studies focus on esophageal abnormalities as source of feeding difficulties. There are no prospective studies on oropharyngeal dysfunction or aerodigestive abnormalities in patients with esophageal atresia.
OVERVIEW OF FEEDING DIFFICULTIES
Feeding difficulties have been described in patients with EA. Puntis et al., [11] first characterized feeding difficulties in 124 children with EA. Compared to healthy controls, children born with EA were significantly more likely to eat slowly, refuse meals, cough or choke during eating and vomit with meals. Chetcuti et al., [12] described similar feeding difficulties in childhood, but noted that overall, these difficulties lessen with age, with < 10% of patients age 15 or older reporting pervasive feeding difficulties. Patients who have undergone primary repair of long-gap esophageal atresia achieve major feeding milestones in a similar pattern to normal control infants, although do have much greater variability in achieving these milestones [13]. Baird et al., [10] administered a validated feeding questionnaire to 30 care-givers of children with EA. They found that, in comparison to controls, 17.5% of children with EA have feeding scores one standard deviation above the mean feeding difficulty score and 6.7% of cases are greater than two standard deviations above the mean. However, overall, feeding difficulties were classified as mild and in the subclinical range in the majority of patients. Schier et al., [14] administered questionnaires to 128 parents involved in an EA support group. 68% of respondents experienced feeding difficulties which included pain, regurgitation, vomiting and burping. Patients generally avoided meats and other tough or bulky foods and 69% of patients experienced at least 1 food impaction. Given the high prevalence of feeding difficulties in this population, Ramsey et al., [15] advocated for early involvement of a multidisciplinary team comprised of occupational therapy, nutrition and psychological support to assist families with feeding-related difficulties. However, despite the fact that feeding difficulties are common in patients with EA, only 11% of parents report discussing their concerns with medical staff [11].
MECHANISM OF ABNORMAL FEEDING
Esophageal dysphagia as a cause for feeding difficulty
Dysphagia is a common complaint in patients with EA and causes include dysmotility, anatomic lesions, esophageal outlet obstruction and esophageal inflammation. The reported prevalence of dysphagia in patients with EA ranges from 38% to 85% [3,8,9,12,16,17]. A recent systematic review and meta-analysis by Connor et al. found an overall pooled estimated prevalence of 50.3% (95% CI 35.7 – 64.8) [5]. The evaluation of dysphagia involves (1) an esophagram to assess for strictures or, when present, pooling above the fundoplication; (2) videofluroscopic imaging during swallowing to assess for aspiration; (3) upper endoscopy to assess for inflammation and; (4) esophageal motility study to assess for adequate contraction pressures, and when paired with impedance, to assess for bolus stasis.
Endoscopic evaluation
Esophageal inflammation is common in patients with EA and is often is often implicated as a cause of dysphagia. In a cross-sectional study by Castilloux et al. of 45 patients with esophageal atresia undergoing endoscopic assessment, 31% had histologic evidence of esophagitis and 36% had gastric metaplasia [16]. Interestingly, there was no association between symptoms of dysphagia and endoscopic findings, either grossly or histologically in this cohort. Similarly, Sistonen et al. reported histologic esophagitis in 25% of patients, however there were no significant differences in rates of dysphagia between patients with esophagitis and those with normal biopsies [17]. Deurloo et al. found that while patients who reported dysphagia more often had disturbed motility on esophageal manometry, there was no association between reported dysphagia and biopsy confirmed esophagitis [18]. Further supporting this observation is the finding that 38% of patients with food impactions have normal esophageal biopsies, suggesting that food impactions can occur even in the absence of inflammation and may be more related to abnormal esophageal motility [16].
Esophageal Manometry
Esophageal dysmotility is common in patients with EA. Sistonen et al. report normal propagating peristalsis in only 20% of the 101 adult patient studied [17]. Deurloo et al. described similar findings, with 70% of patients having low or moderate amplitude of esophageal body contractions and 35% of patients having retrograde contractions [18]. Furthermore, they found that patients reporting dysphagia more often had disturbed motility and significantly lower scores on a variety of health-related quality of life scales. Kawahara et al. also described a lack of distal esophageal contractions on esophageal manometry in patients with EA [19]. For centers where esophageal motility studies are not available, even radionucleotide esophagogastric studies reveal significantly longer esophageal transit time in patients with a history of long-gap EA, compared to those with non-long-gap EA suggesting that imaging may be helpful in identifying dysmotility. In these patients, the bolus accumulated mainly in the lower 2/3 of the esophagus below the anastomosis and persisted in this area for several minutes before being cleared into the stomach [20] (see Figures 1 and 2). This suggests that impaired clearing capacity may be playing a role in the dysphagia in these patients. There appears to be overall improvement in esophageal peristaltic function on manometric studies as patients age [21]. More recently, high-resolution manometry has been used in the EA population to better characterize both esophageal dysmotility and extraesophageal symptoms. Lemoine et al. used high-resolution manometry in 40 children with a history of EA repair [22]. All patients had abnormal manometry results: 38% of patients had aperistalsis, 15% had pressurization and 47% had abnormal distal contractions. They found both gastroesophageal reflux and pulmonary symptoms more commonly in the aperistalsis group. Kawahara et al. found the absence of significant contractions in the middle esophagus just below the anastomosis in 29 patients with repaired EA [19]. Lack of distal esophageal contractions with significantly correlated with development of gastroesophageal reflux in this population (P < 0.001). Patients with EA have also been shown to have significantly fewer complete bolus transits of both liquids and viscous materials, compared to controls [23]. While the majority of the literature describes dysmotility in EA patients after surgical repair, one recent case report found dysmotility on high-resolution manometry preoperatively in two children with isolated unrepaired tracheoesophagela fistula [24]. This suggests that dysmotility may stem from abnormal development of the innervation and smooth muscle of the esophagus, rather than from surgical intervention.
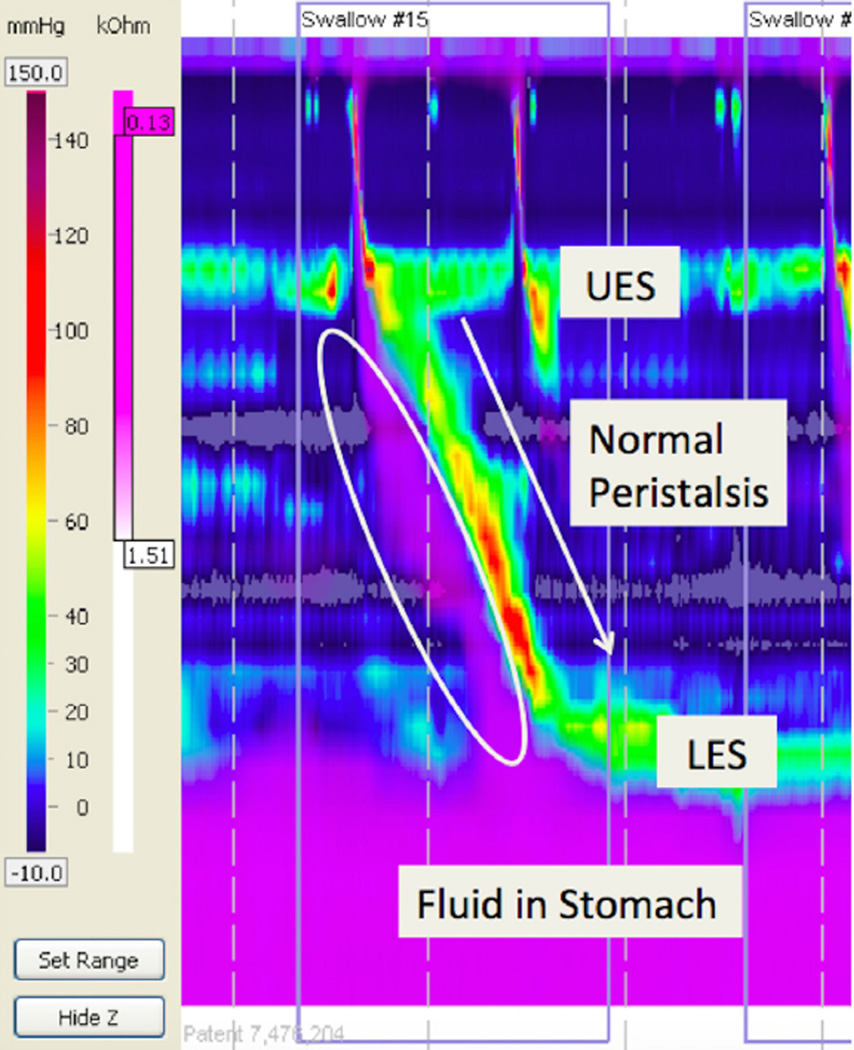
Figure 1.
Normal peristalsis with normal bolus clearance using high resolution esophageal manometry with impedance. Purple: Liquid. Note that with each peristaltic wave in yellow/red there is complete bolus clearance with no residual purple in the esophagus (white circle).
LES: Lower esophageal sphincter; UES: Upper esophageal sphincter.
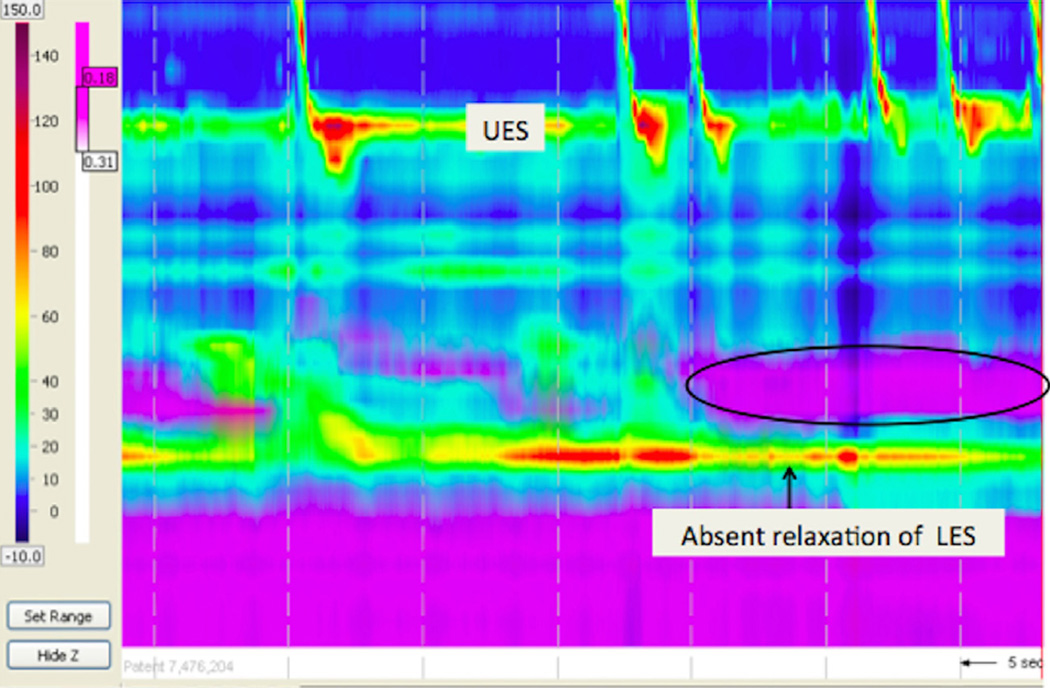
Figure 2.
Absent esophageal peristalsis in a patient with esophageal atresia and a fundoplication. Note the minimal to absent peristalsis, the stasis of liquid in the esophagus (circle), and the lack of LES relaxation with the fundoplication.
Esophogram
Barium imaging of the esophagus will delineate stricturing (congenital, GERD related, or anastomotic) and retention of food in the proximal esophageal pouch as a cause for dysphagia. Anastomotic strictures have been reported in 8%–59% of patients with EA, and may contribute to dysphagia and feeding difficulties in these patients [2,3,17,25]. Esophageal strictures may present with dysphagia, poor feeding, emesis or food impactions [26]. Esophagrams are also useful in EA patients who have undergone fundoplication, which can create an esophageal outlet obstruction in the setting of a dysmotile esophagus. Holschneider et al. found a higher rate of dysphagia in patients with EA undergoing fundoplication (17.2%) compared to children undergoing fundoplication for other indications (6.5%) [27]. There are no studies which address the impact of fundoplication on acquisition of feeding milestones or rates of significant food impactions.
Oropharyngeal dysphagia/aspiration as a cause for feeding difficulty
The most common symptoms of oropharyngeal dysphagia are respiratory symptoms. Respiratory problems in patients with esophageal atresia are common (See Table 1) and may be due in part to retained secretions in the proximal pouch above the anastomosis or in distal esophagus, aspiration during swallowing, recurrence of TEF or gastroesophageal reflux [4]. In the largest aerodigestive paper published, Checuti et al., [28] reported that 44% of patients were hospitalized with respiratory illnesses in the years following surgery. There does appear to be a decline in respiratory-related admissions with age, with 73% of admissions occurring in patients < 5 years of age but only 7% of admissions in patients 10–15 years of age. Symptoms range from cough and wheezing to recurrent respiratory infections. The frequency of respiratory symptoms is shown in Table 1. In these patients with respiratory symptoms, often, the extent of a GI evaluation consists of reflux testing. However, rarely is the answer as simple as reflux alone and the evaluation of children with recurrent respiratory symptoms should also include the same tests outlined above for dysphagia to determine, apart from reflux, if esophageal dysfunction (with resultant stasis) or oropharyngeal dysfunction (with aspiration) is occurring. In addition, the evaluation of children with respiratory symptoms must include an assessment of swallowing function. One of the most under-recognized causes of respiratory symptoms in EA patients is aspiration during swallowing because the symptoms of aspiration are identical to the symptoms of reflux: gagging, choking, head turning, arching, noisy breathing, poor growth and coughing. Hormann et al. studied videofluoroscopic findings (25 examinations) in 19 children with repaired EA [29]. They found that 16% of patients had nasopharyngeal regurgitation, 5% had had residue in the pharynx, 10% had laryngeal penetration and 37% had aspiration. Once aspiration or penetration on swallow study is diagnosed, the following differentials must be considered in order to predict prognosis:
Table 1.
Respiratory symptoms in patients with repaired esophageal atresia
| Symptom | Prevalence | Reference |
|---|---|---|
| Cough | 9–34% | Legrand [8] |
| Chetcuti [9] | ||
| Somppi [21] | ||
| Chetcuti [28] | ||
| Gatzinsky [65] | ||
| Wheezing | 14–40% | Malmstrom [4] |
| Chetcuti [9] | ||
| Chetcuti [28] | ||
| Gatzinsky [65] | ||
| Asthma | 22–30% | Connor [5] |
| Gatzinsky [65] | ||
| Beucher [66] | ||
| Pneumonia | 31–52% | Malmstrom [4] |
| Chetcuti [28] | ||
| Bronchitis | 24–42% | Legrand [8] |
| Chetcuti [9] | ||
| Abnormal pulmonary function tests | 68–88% | Legrand [8] |
| Obstructive lung disease | 19–50% | Banjar [59] |
| Restrictive lung disease | 11–23% | Beucher [66] |
| Mixed lung disease | 36% | Sistonen [67] |
| Chetcuti [68] |
Vocal Cord Paralysis/Paresis
Clinically significant vocal cord paralysis has been reported in 3%–17% of patients with EA [30–32] In a study of 174 patients with treated EA/TEF, 7 (4%) of patients had vocal cord paresis or paralysis. Longer length of intubation, long-gap EA, cervical esophagostomy and anastomotic leakage were all significantly more common in the patients with vocal cord paralysis [30]. There are high rates of recovery, however, reported in pediatric patients. In pediatric patients with vocal cord paralysis following cardiac surgery, 35% recovered vocal cord function with a median time to diagnosis of recovery of 6.6 months [33].
Laryngeal cleft
The differential diagnosis of aspiration also includes laryngeal clefts. In a case series of 183 pediatric patients diagnoses with laryngeal clefts, 22 (12%) had a tracheoesophageal fistula [32]. Half of these patients presented with aspiration and 18% had feeding difficulties. Seventeen of the 22 patients with laryngeal clefts and TEF required surgical repair. Postoperative modified barium swallow studies showed resolution of aspiration following cleft repair.
Neonatal swallowing dysfunction
Neonatal swallowing dysfunction causing aspiration is a common cause of morbidity in neonates. While there are no studies in EA patients, in a study of 148 former preterm neonates referred for a videofluoroscopic swallow study (VFSS), 68% of patients were found to aspirate thin liquids [34]. Those patients who failed the VFSS initially eventually passed after a median of 3.4 months from the first study. These results suggest that, whenever possible, patients should undergo a repeat MBS to assess for improvement in swallowing before consideration of surgical interventions such as gastrostomy tube placement.
DIAGNOSIS OF ASPIRATION
There are many different methods of diagnosing oropharyngeal dysphagia and all of them are complementary with no clear gold standard test. Studies have suggested that the agreement between studies is poor. Baikie et al. for example, studied the agreement between three tests for aspiration – barium videofluoroscopy, salivagram and milk scan - in 63 children with cerebral palsy [35]. The authors found that overall agreement between the tests was poor, with a maximum kappa of 0.20 suggesting that if aspiration is suspected, several different diagnostic modalities, including the following tests, should be considered.
Videofluoroscopic swallow study
The gold standard for diagnosing oropharyngeal aspiration is the VFSS, which allows for visualization of the oral and pharyngeal phases of swallowing. Oropharyngeal aspiration diagnosed on VFSS is common in children. One large study of 300 pediatric patients with feeding disorders found oropharyngeal aspiration in 34% of children [36]. Of these patients, 81% had silent aspiration. To determine if the results of VFSS predict clinical outcome, Weir et al. examined the risk of pneumonia in a cohort of 150 children with swallowing dysfunction diagnosed on videofluoroscopic swallow study [37]. They found that the odds ratio for pneumonia was significantly increased in patients with post-swallow residuals (OR 2.5) or aspiration of thin liquids (OR 2.4) on univariate analysis. There was no significant increase in risk of pneumonia with aspiration of other consistencies. However, on multivariate analysis, these factors were no longer significantly associated with pneumonia.
Salivogram
Radionucleotide scintigraphy can be used to detect aspiration of oral secretions and refluxed gastric contents. Unlike VFSS, salivagrams are used to detect aspiration of saliva rather than a bolus of food. Simons et al. studied salivagrams in 129 pediatric patients with suspected aspiration [38]. Aspiration was identified on 21% of studies. Positive salivagram results were significantly associated with developmental delay (OR 2.8), chronic respiratory infections or pneumonia (2.6) reactive airway disease exacerbations (2.8) and use of H2 blockers or proton pump inhibitors (OR 2.7). Drubach et al. found a similar rate of positive salivagrams (25%) in a study of 222 pediatric patients [39]. The authors compared agreement between salivagram and chest x-ray results and found high agreement (kappa = 0.891, P < 0.0001). 50 of the 55 patients with abnormal salivagrams also had positive chest radiography findings (consolidation, prominent peribronchial markings or bronchiectasis). Somasundaram et al. studied the utility of salivagrams in the routine evaluation of developmentally normal children who have recurrent lower respiratory tract infections [40]. The authors found a positive salivagram in 39% of infants and 16% of children ages 1–2 years. No aspiration was detected in children above the age of 2 years.
Fiberoptic endoscopic evaluation of swallowing
Fiberoptic Endoscopy Evaluation of Swallowing (FEES) uses a transnasal flexible fiberoptic laryngoscopy to visualize the pharynx and larynx while swallowing. It can be used to diagnose aspiration in both children and adults [41–45]. Da Silva et al. compared VFSS with FEES in 30 children to determine accuracy of FEES [43]. They found overall low diagnostic agreement between VFSS and FEES, however laryngeal penetration and aspiration on FEES showed the highest specificity and positive predictive value when compared to VFSS. Kelly et al. studied 15 dysphagic patients who underwent simultaneous VFSS and FEES. Fifteen independent reviewers from multiple sites reviewed the images and scored laryngeal penetration or aspiration. They found that agreement between experts was significantly higher on the FEES recordings compared to the VFSS. Aviv et al. randomized 126 adults with dysphagia to either FEES or MBS to guide management decisions [45]. They found no statistically significant differences between the two groups with respect to the incidence of pneumonia. There was also no difference in the pneumonia-free interval between the two groups.
High Resolution Manometry
Omari et al. reported on the use of pharyngeal manometry and impedance to assess swallow function [46]. 20 adult patients with suspected aspiration and 10 healthy controls were included in this study. The authors recorded manometry and impedance simultaneously with videofluoroscopy in these patients. They computed a Swallow Risk Index (SRI) from automated analysis of combined manometric and impedance variables. They found that SRI could be used to predict aspiration on fluoroscopy and offers the benefit of no radiation.
MANAGEMENT OF OROPHARYNGEAL DYSPHAGIA WITH ASPIRATION
Many causes of aspiration improve over time and thus management decisions regarding feeding must be made in the context of the likelihood of improvement in aspiration. Management goals include: reduction of aspiration during swallowing, reducing full column reflux into the oropharynx and continuation of oral feeding to maintain skills. While there are no studies on the management of aspiration in children with EA, conclusions can be drawn from the available literature in other populations.
Thickened oral or gastric feeds to reduce aspiration during swallowing and aspiration of gastric contents
Thickened feeds may improve swallowing, reduce reflux into the oropharynx and reduce the chance of retching. Wenzl et al. studied 14 otherwise healthy infants with reflux who were fed alternating thickened feeds vs. standard formula [47]. They measured GER episodes by simultaneous intraesophageal impedance measurement and pH monitoring. They found that regurgitation frequency and amount were significantly lower after feedings with thickened formula and, while the authors found no differences in mean maximal height reached by refluxate after thickened feeds, the benefit of less in the mouth (i.e. less vomited) may be of particular benefit in the aspirating child. Similarly, in a 2008 systematic review of 14 randomized controlled trials studying the efficacy and safety of thickened feeds for the treatment of GER in healthy infants, Horvath et al. [69] found significant improvement in regurgitation. However, again there was no effect on effect of thickening on the reflux index, number of acid GER episodes per hour or the number of reflux episodes lasting > 5 minutes. Given these results, there may be a role for thickened feeds in the aspirating child to try to prevent formula from entering the mouth.
Thickening may also have a beneficial effect on the stomach. For example, thickened feeds may also have a role in reducing gagging and retching in patients who have gastrostomy tubes and fundoplications. Pentiuk et al. reported that over half of the 33 children studied had > 75% reduction in gagging and retching when given a pureed diet via gastrostomy tube [48]. Nishiwaki et al. found similar results with thickened feeds in adults with percutaneous endoscopic gastrostomy (PEG) tubes [49]. The percentage of GER was significantly reduced in patients who received semi-solid compared to liquid nutrients, however this effect did not appear to be mediated by differences in gastric emptying time. Shizuku et al. similarly studied 42 adult patients with PEG tubes [50]. These patients received 8 weeks of feeding with a liquid diet and 8 weeks of feeding with a semi-solid diet. The percentage of observational days with fever during half-solid nutrient feeding was significantly lower than that during the liquid nutrient feeding (p =0.03).
Bolus vs. continuous feeds to reduce risk of aspirating gastric contents
The method of feeds to reduce aspiration is often debated. In a study by Lee et al., 178 adults were randomized to receive either continuous pump feedings or intermittent bolus feeds via nasogastric tube for 4 weeks [51]. They found no difference in the incidence of pneumonia between these groups (15% of patients in the bolus group and 14% of patients in the continuous group). Bowling et al. measured gastric emptying time and gastroesophageal reflux in 12 healthy volunteers who were given a liquid feed via an oral bolus, NG tube bolus and continuous NG tube infusion [52]. They found no significant differences in the median number or duration of reflux episodes between these three groups. However, there are no pediatric studies to determine the safest method of feeding from an airway perspective.
Changing feeding schedule or formula to reduce risk of aspirating gastric contents
Transient lower esophageal sphincter relaxation is the main mechanism of gastroesophageal reflux (GER) in infants. More frequent feedings are often recommended for management of gastroesophageal reflux. Indeed, feeding pattern has been shown to change the pattern of occurrence of transient lower esophageal sphincter relaxation, as well as the proportion of acid and nonacid GER [53,54]. However, only 51% of transient lower esophageal sphincter relaxations are associated with GER [53]. Non-acid reflux is the predominant type of reflux in children fed more frequently whereas acid reflux events are more common with longer time after initiation of a feed [54].
While there do not appear to be differences in GER characteristics between infants fed breastmilk and formula, the type of formula may be important [54,55]. Seventeen children with cow’s milk allergy and suspected GERD underwent intraluminal impedance monitoring while fed 24 hours of amino acid-based formula then 24 hours of cow’s milk challenge [55]. The authors found that the total reflux episodes and the number of weakly acid episodes were significantly higher during the cow’s milk challenge (P < 0.001 and P < 0.001 respectively).
Transpyloric feeding to reduce risk of aspirating gastric contents
In a study of 428 critically ill, mechanically ventilated adults, Metheny et al. found that transpyloric feeding was associated with significantly less aspiration compared to gastric feedings [56]. Pneumonia occurred less often when feedings were introduced at or beyond the second portion of the duodenum (P = 0.02). Reflux of gastric fluid can still occur during transplyoric feeding [57]. Despite this, there are no significant differences in the mean number of reflux-related hospitalizations in the year before or after the initiation of transpyloric feeds. Srivastava et al. compared fundoplication and gastrojejunal tube feedings in 366 children with neurologic impairment and GERD [58]. 43 children had a gastrojejunal feeding tube and 323 children underwent fundoplication. Children were followed for a median of 3.4 years. Pneumonia-free survival and overall survival were similar between the two groups, suggesting that neither option is superior in preventing subsequent aspiration pneumonia or improving overall survival.
Fundoplication to reduce risk of aspirating gastric contents
Fundoplications are commonly performed in children with repaired EA, with reported rates between 39% and 59% of all patients with EA [7,8,16,59]. Indications for anti-reflux surgery in children with repaired EA include refractory anastomotic stenosis, pure and long-gap EA and patients with EA and associated duodenal atresia [60]. While fundoplications are common in EA patients, there is no literature on objective outcomes in these patients, such as characterization of reflux events or hospitalizations. From published data in other patient populations, fundoplication does not consistently improve respiratory outcomes and in patients who aspirate and may even make symptoms worse. Barnhart et al. reported results of a large multicenter study of 4163 infants with neurological impairment who underwent gastrostomy tube placement with or without fundoplication [61]. The authors found that infants who underwent concomitant gastrostomy tube placement and fundoplication had more reflux-related hospitalizations during the first year post-procedure than those who underwent gastrostomy tube placement alone. Goldin et al. reported a nontrivial percentage of patients (22%) who had more reflux related hospitalizations following fundoplication compared to the preoperative period [62]. In a study of 342 pediatric patients with fundoplications, Lee et al. reported no significant difference in the rates of hospital admission for aspiration pneumonia, respiratory distress or failure to thrive before and after fundoplication [63]. However, following fundoplication, 20 patients with no history of previous hospitalization were admitted with aspiration pneumonia and 55 patients never previously hospitalized were admitted with respiratory distress. Preoperative reflux burden, including acid and non-acid reflux as measured by pH-multichannel intraluminal impedance, does not predict fundoplication outcome [64].
DISCUSSION
Feeding difficulties are common in patients with repaired EA. In this review, we investigate the mechanisms for abnormal feeding in these patients. Esophageal dysphagia is well-described in patients with EA and is often due to anatomic abnormalities such as strictures, esophageal dysmotility or mucosal inflammation. While respiratory symptoms are highly prevalent in patients with EA, there are very few studies evaluating oropharyngeal dysfunction in this population. Aspiration is an under-recognized cause of respiratory symptoms and feeding difficulties in EA patients. Symptoms of aspiration can be identical to the symptoms of reflux. Clinicians caring for patients with repaired EA should have a high index of suspicion for aspiration in their patients with feeding difficulties or persistent respiratory symptoms. Further studies are needed to identify the optimal method of managing aspiration in patients with EA.
EDUCATIONAL AIMS.
To describe the mechanism of esophageal dysphagia and oropharyngeal dysphagia/aspiration as mechanisms for feeding difficulties in patients with esophageal atresia
To highlight the prevalence of respiratory symptoms in patients with esophageal atresia
To review methods for diagnosing aspiration
To discuss treatment strategies for management of aspiration
DIRECTIONS FOR FUTURE RESEARCH.
To characterize oropharyngeal dysphagia and aspiration in patients with esophageal atresia.
To investigate the relationship between aspiration, respiratory symptoms and feeding difficulties in patients with esophageal atresia.
To determine the best method of diagnosing aspiration in patients with repaired esophageal atresia.
To optimize management of aspiration in patients with esophageal atresia.
Acknowledgments
FUNDING SOURCE
This work was supported by the Translational Research Program at Children’s Hospital Boston and NIH NIDDK R01 DK097112. It was funded by the NASPGHAN/ASTRA research award for disorders of the upper tract.
Abbreviations
- EA
Esophageal atresia
- TEF
Tracheoesophageal fistula
- VFSS
Videofluroscopic Swallow Study
Footnotes
FINANCIAL DISCLOSURES
None.
References
- 1.Spitz L. Oesophageal atresia. Orphanet J Rare Dis. 2007;2(1):24–13. doi: 10.1186/1750-1172-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lilja HE, Wester T. Outcome in neonates with esophageal atresia treated over the last 20 years. Pediatr Surg Int. 2008;24(5):531–536. doi: 10.1007/s00383-008-2122-z. [DOI] [PubMed] [Google Scholar]
- 3.Little DC, Rescorla FJ, Grosfeld JL, West KW, Scherer LR, Engum SA. Long-term analysis of children with esophageal atresia and tracheoesophageal fistula. Journal of Pediatric Surgery. 2003;38(6):852–856. doi: 10.1016/s0022-3468(03)00110-6. [DOI] [PubMed] [Google Scholar]
- 4.Malmström K, Lohi J, Lindahl H, et al. Longitudinal Follow-up of Bronchial Inflammation, Respiratory Symptoms, and Pulmonary Function in Adolescents after Repair of Esophageal Atresia with Tracheoesophageal Fistula. The Journal of Pediatrics. 2008;153(3):396.e1–401.e1. doi: 10.1016/j.jpeds.2008.03.034. [DOI] [PubMed] [Google Scholar]
- 5.Connor MJ, Springford LR, Kapetanakis VV, Giuliani S. Esophageal atresia and transitional care-step 1: a systematic review and meta-analysis of the literature to define the prevalence of chronic long-term problems. Am J Surg. 2015;209(4):747–759. doi: 10.1016/j.amjsurg.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 6.Kovesi T. Long-term respiratory complications of congenital esophageal atresia with or without tracheoesophageal fistula: an update. Dis Esophagus. 2013;26(4):413–416. doi: 10.1111/dote.12061. [DOI] [PubMed] [Google Scholar]
- 7.Koivusalo AI, Pakarinen MP, Rintala RJ. Modern outcomes of oesophageal atresia: Single centre experience over the last twenty years. Journal of Pediatric Surgery. 2013;48(2):297–303. doi: 10.1016/j.jpedsurg.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Legrand C, Michaud L, Salleron J, et al. Long-term outcome of children with oesophageal atresia type III. Archives of Disease in Childhood. 2012;97(9):808–811. doi: 10.1136/archdischild-2012-301730. [DOI] [PubMed] [Google Scholar]
- 9.Chetcuti P, Myers NA, Phelan PD, Beasley SW. Adults who survived repair of congenital oesophageal atresia and tracheo-oesophageal fistula. BMJ. 1988;297(6644):344–346. doi: 10.1136/bmj.297.6644.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baird R, Levesque D, Birnbaum R, Ramsay M. A pilot investigation of feeding problems in children with esophageal atresia. Dis Esophagus. 2015;28(3):224–228. doi: 10.1111/dote.12178. [DOI] [PubMed] [Google Scholar]
- 11.Puntis JW, Ritson DG, Holden CE, Buick RG. Growth and feeding problems after repair of oesophageal atresia. Archives of Disease in Childhood. 1990;65(1):84–88. doi: 10.1136/adc.65.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chetcuti P, Phelan PD. Gastrointestinal morbidity and growth after repair of oesophageal atresia and tracheo-oesophageal fistula. Archives of Disease in Childhood. 1993;68(2):163–166. doi: 10.1136/adc.68.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan KM, Krosch TC, Eickhoff JC, et al. Achievement of feeding milestones after primary repair of long-gap esophageal atresia. Early Human Development. 2009;85(6):387–392. doi: 10.1016/j.earlhumdev.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Schier F, Korn S, Michel E. Experiences of a parent support group with the long-term consequences of esophageal atresia. Journal of Pediatric Surgery. 2001;36(4):605–610. doi: 10.1053/jpsu.2001.22299. [DOI] [PubMed] [Google Scholar]
- 15.Ramsay M, Birnbaum R. Feeding difficulties in children with esophageal atresia: treatment by a multidisciplinary team. Dis Esophagus. 2013;26(4):410–412. doi: 10.1111/dote.12062. [DOI] [PubMed] [Google Scholar]
- 16.Castilloux J, Bouron-Dal Soglio D, Faure C. Endoscopic assessment of children with esophageal atresia: Lack of relationship of esophagitis and esophageal metaplasia to symptomatology. Can J Gastroenterol. 2010;24(5):312–316. doi: 10.1155/2010/902847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sistonen SJ, Koivusalo A, Nieminen U, et al. Esophageal Morbidity and Function in Adults With Repaired Esophageal Atresia With Tracheoesophageal Fistula. Annals of Surgery. 2010;251(6):1167–1173. doi: 10.1097/SLA.0b013e3181c9b613. [DOI] [PubMed] [Google Scholar]
- 18.Deurloo JA, Klinkenberg EC, Ekkelkamp S, Heij HA, Aronson DC. Adults with corrected oesophageal atresia: is oesophageal function associated with com-plaints and/or quality of life? Pediatr Surg Int. 2008;24(5):537–541. doi: 10.1007/s00383-008-2120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawahara H, Kubota A, Hasegawa T, et al. Lack of distal esophageal contractions is a key determinant of gastroesophageal reflux disease after repair of esophageal atresia. Journal of Pediatric Surgery. 2007;42(12):2017–2021. doi: 10.1016/j.jpedsurg.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 20.Lopes MF, Botelho MF. Midterm follow-up of esophageal anastomosis for esophageal atresia repair: long-gap versus non-long-gap. Dis Esophagus. 2007;20(5):428–435. doi: 10.1111/j.1442-2050.2007.00691.x. [DOI] [PubMed] [Google Scholar]
- 21.Somppi E, Tammela O, Ruuska T, et al. Outcome of patients operated on for esophageal atresia: 30 years’ experience. Journal of Pediatric Surgery. 1998;33(9):1341–1346. doi: 10.1016/s0022-3468(98)90003-3. [DOI] [PubMed] [Google Scholar]
- 22.Lemoine C, Aspirot A, Le Henaff G, Piloquet H, Lévesque D, Faure C. Characterization of esophageal motility following esophageal atresia repair using high-resolution esophageal manometry. Journal of Pediatric Gastroenterology and Nutrition. 2013;56(6):609–614. doi: 10.1097/MPG.0b013e3182868773. [DOI] [PubMed] [Google Scholar]
- 23.Fröhlich T, Otto S, Weber P, et al. Combined esophageal multichannel intra-luminal impedance and pH monitoring after repair of esophageal atresia. Journal of Pediatric Gastroenterology and Nutrition. 2008;47(4):443–449. doi: 10.1097/MPG.0b013e3181638ca2. [DOI] [PubMed] [Google Scholar]
- 24.Lemoine C, Aspirot A, Morris M, Faure C. Esophageal dysmotility is present before surgery in isolated tracheoesophageal fistula. Journal of Pediatric Gastro-enterology and Nutrition. 2015;60(5):642–644. doi: 10.1097/MPG.0000000000000667. [DOI] [PubMed] [Google Scholar]
- 25.Engum SA, Grosfeld JL, West KW, Rescorla FJ, Scherer LR. Analysis of morbidity and mortality in 227 cases of esophageal atresia and/or tracheoesophageal fistula over two decades. Arch Surg. 1995;130(5) doi: 10.1001/archsurg.1995.01430050052008. 502-8–discussion 508-9. [DOI] [PubMed] [Google Scholar]
- 26.Kovesi T, Rubin S. Long-term complications of congenital esophageal atresia and/or tracheoesophageal fistula. Chest. 2004;126(3):915–925. doi: 10.1378/chest.126.3.915. [DOI] [PubMed] [Google Scholar]
- 27.Holschneider P, Dübbers M, Engelskirchen R, Trompelt J, Holschneider AM. Results of the operative treatment of gastroesophageal reflux in childhood with particular focus on patients with esophageal atresia. Eur J Pediatr Surg. 2007;17(3):163–175. doi: 10.1055/s-2007-965087. [DOI] [PubMed] [Google Scholar]
- 28.Chetcuti P, Phelan PD. Respiratory morbidity after repair of oesophageal atresia and tracheo-oesophageal fistula. Archives of Disease in Childhood. 1993;68(2):167–170. doi: 10.1136/adc.68.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hörmann M, Pokieser P, Scharitzer M, et al. Videofluoroscopy of deglutition in children after repair of esophageal atresia. Acta Radiol. 2002;43(5):507–510. [PubMed] [Google Scholar]
- 30.Morini F, Iacobelli BD, Crocoli A, et al. Symptomatic vocal cord paresis/paralysis in infants operated on for esophageal atresia and/or tracheo-esophageal fistula. The Journal of Pediatrics. 2011;158(6):973–976. doi: 10.1016/j.jpeds.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 31.Mortellaro V, Pettiford J, St Peter S, Fraser J, Ho B, Wei J. Incidence, Diagnosis, and Outcomes of Vocal Fold Immobility After Esophageal Atresia (EA) and/or Tracheoesophageal Fistula (TEF) Repair. Eur J Pediatr Surg. 2011;21(06):386–388. doi: 10.1055/s-0031-1291269. [DOI] [PubMed] [Google Scholar]
- 32.Fraga JC, Adil EA, Kacprowicz A, et al. The association between laryngeal cleft and tracheoesophageal fistula: Myth or reality? The Laryngoscope. 2014;125(2):469–474. doi: 10.1002/lary.24804. [DOI] [PubMed] [Google Scholar]
- 33.Truong MT, Messner AH, Kerschner JE, et al. Pediatric vocal fold paralysis after cardiac surgery: rate of recovery and sequelae. Otolaryngol Head Neck Surg. 2007;137(5):780–784. doi: 10.1016/j.otohns.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 34.Davis NL, Liu A, Rhein L. Feeding Immaturity in Preterm Neonates. Journal of Pediatric Gastroenterology and Nutrition. 2013;57(6):735–740. doi: 10.1097/MPG.0b013e3182a9392d. [DOI] [PubMed] [Google Scholar]
- 35.Baikie G, South MJ, Reddihough DS, et al. Agreement of aspiration tests using barium videofluoroscopy, salivagram, and milk scan in children with cerebral palsy. Dev Med Child Neurol. 2005;47(2):86–93. doi: 10.1017/s0012162205000174. [DOI] [PubMed] [Google Scholar]
- 36.Weir KA, McMahon S, Taylor S, Chang AB. Oropharyngeal aspiration and silent aspiration in children. Chest. 2011;140(3):589–597. doi: 10.1378/chest.10-1618. [DOI] [PubMed] [Google Scholar]
- 37.Weir K, McMahon S, Barry L, Ware R, Masters IB, Chang AB. Oropharyngeal aspiration and pneumonia in children. Pediatr Pulmonol. 2007;42(11):1024–1031. doi: 10.1002/ppul.20687. [DOI] [PubMed] [Google Scholar]
- 38.Simons JP, Rubinstein EN, Mandell DL. Clinical predictors of aspiration on radionuclide salivagrams in children. Arch Otolaryngol Head Neck Surg. 2008;134(9):941–944. doi: 10.1001/archotol.134.9.941. [DOI] [PubMed] [Google Scholar]
- 39.Drubach LA, Zurakowski D, Palmer EL, Tracy DA, Lee EY. Utility of salivagram in pulmonary aspiration in pediatric patients: comparison of salivagram and chest radiography. AJR Am J Roentgenol. 2013;200(2):437–441. doi: 10.2214/AJR.12.8792. [DOI] [PubMed] [Google Scholar]
- 40.Somasundaram VH, Subramanyam P, Palaniswamy S. Salivagram revisited: justifying its routine use for the evaluation of persistent/recurrent lower respiratory tract infections in developmentally normal children. Ann Nucl Med. 2012;26(7):578–585. doi: 10.1007/s12149-012-0616-1. [DOI] [PubMed] [Google Scholar]
- 41.Baijens LWJ, Speyer R, Pilz W, Roodenburg N. FEES protocol derived estimates of sensitivity: aspiration in dysphagic patients. Dysphagia. 2014;29(5):583–590. doi: 10.1007/s00455-014-9549-2. [DOI] [PubMed] [Google Scholar]
- 42.Kelly AM, Drinnan MJ, Leslie P. Assessing penetration and aspiration: how do videofluoroscopy and fiberoptic endoscopic evaluation of swallowing compare? The Laryngoscope. 2007;117(10):1723–1727. doi: 10.1097/MLG.0b013e318123ee6a. [DOI] [PubMed] [Google Scholar]
- 43.da Silva AP, Lubianca Neto JF, Santoro PP. Comparison between videofluoroscopy and endoscopic evaluation of swallowing for the diagnosis of dysphagia in children. Otolaryngol Head Neck Surg. 2010;143(2):204–209. doi: 10.1016/j.otohns.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 44.Sitton M, Arvedson J, Visotcky A, et al. Fiberoptic Endoscopic Evaluation of Swallowing in children: feeding outcomes related to diagnostic groups and endoscopic findings. Int J Pediatr Otorhinolaryngol. 2011;75(8):1024–1031. doi: 10.1016/j.ijporl.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 45.Aviv JE. Prospective, randomized outcome study of endoscopy versus modified barium swallow in patients with dysphagia. The Laryngoscope. 2000;110(4):563–574. doi: 10.1097/00005537-200004000-00008. [DOI] [PubMed] [Google Scholar]
- 46.Omari TI, Dejaeger E, van Beckevoort D, et al. A method to objectively assess swallow function in adults with suspected aspiration. Gastroenterology. 2011;140(5):1454–1463. doi: 10.1053/j.gastro.2011.02.051. [DOI] [PubMed] [Google Scholar]
- 47.Wenzl TG, Schneider S, Scheele F, Silny J, Heimann G, Skopnik H. Effects of thickened feeding on gastroesophageal reflux in infants: a placebo-controlled crossover study using intraluminal impedance. PEDIATRICS. 2003;111(4 Pt 1):e355–e359. doi: 10.1542/peds.111.4.e355. [DOI] [PubMed] [Google Scholar]
- 48.Pentiuk S, O’Flaherty T, Santoro K, Willging P, Kaul A. Pureed by Gastrostomy Tube Diet Improves Gagging and Retching in Children With Fundoplication. Journal of Parenteral and Enteral Nutrition. 2011;35(3):375–379. doi: 10.1177/0148607110377797. [DOI] [PubMed] [Google Scholar]
- 49.Nishiwaki S, Araki H, Shirakami Y, et al. Inhibition of Gastroesophageal Reflux by Semi-solid Nutrients in Patients With Percutaneous Endoscopic Gastrostomy. Journal of Parenteral and Enteral Nutrition. 2009;33(5):513–519. doi: 10.1177/0148607108327045. [DOI] [PubMed] [Google Scholar]
- 50.Shizuku T, Adachi K, Furuta K, et al. Efficacy of half-solid nutrient for the elderly patients with percutaneous endoscopic gastrostomy. J Clin Biochem Nutr. 2011;48(3):226–229. doi: 10.3164/jcbn.10-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee JSW, Kwok T, Chui PY, et al. Can continuous pump feeding reduce the incidence of pneumonia in nasogastric tube-fed patients? A randomized controlled trial. Clin Nutr. 2010;29(4):453–458. doi: 10.1016/j.clnu.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 52.Bowling TE, Cliff B, Wright JW, Blackshaw PE, Perkins AC, Lobo DN. The effects of bolus and continuous nasogastric feeding on gastro-oesophageal reflux and gastric emptying in healthy volunteers: A randomised three-way crossover pilot study. Clinical Nutrition. 2008;27(4):608–613. doi: 10.1016/j.clnu.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 53.Omari TI, Barnett CP, Benninga MA, et al. Mechanisms of gastro-oesophageal reflux in preterm and term infants with reflux disease. Gut. 2002;51(4):475–479. doi: 10.1136/gut.51.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jadcherla SR, Chan CY, Moore R, Malkar M, Timan CJ, Valentine CJ. Impact of Feeding Strategies on the Frequency and Clearance of Acid and Nonacid Gastroesophageal Reflux Events in Dysphagic Neonates. Journal of Parenteral and Enteral Nutrition. 2012;36(4):449–455. doi: 10.1177/0148607111415980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Borrelli O, Mancini V, Thapar N, et al. Cow”s milk challenge increases weakly acidic reflux in children with cow”s milk allergy and gastroesophageal reflux disease. The Journal of Pediatrics. 2012;161(3):476.e1–481.e1. doi: 10.1016/j.jpeds.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 56.Metheny NA, Stewart BJ, McClave SA. Relationship Between Feeding Tube Site and Respiratory Outcomes. Journal of Parenteral and Enteral Nutrition. 2011;35(3):346–355. doi: 10.1177/0148607110377096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rosen R, Hart K, Warlaumont M. Incidence of Gastroesophageal Reflux During Transpyloric Feeds. Journal of Pediatric Gastroenterology and Nutrition. 2011;52(5):532–535. doi: 10.1097/MPG.0b013e31820596f8. [DOI] [PubMed] [Google Scholar]
- 58.Srivastava R, Downey EC, O’Gorman M, et al. Impact of fundoplication versus gastrojejunal feeding tubes on mortality and in preventing aspiration pneumonia in young children with neurologic impairment who have gastroesophageal reflux disease. PEDIATRICS. 2009;123(1):338–345. doi: 10.1542/peds.2007-1740. [DOI] [PubMed] [Google Scholar]
- 59.Banjar HH, Al-Nassar SI. Gastroesophageal reflux following repair of esophageal atresia and tracheoesophageal fistula. Saudi Med J. 2005;26(5):781–785. [PubMed] [Google Scholar]
- 60.Tovar JA, Fragoso AC. Anti-reflux surgery for patients with esophageal atresia. Dis Esophagus. 2013;26(4):401–404. doi: 10.1111/dote.12063. [DOI] [PubMed] [Google Scholar]
- 61.Barnhart DC, Hall M, Mahant S, et al. Effectiveness of Fundoplication at the Time of Gastrostomy in Infants With Neurological Impairment. JAMA Pediatr. 2013;167(10):911–918. doi: 10.1001/jamapediatrics.2013.334. [DOI] [PubMed] [Google Scholar]
- 62.Goldin AB, Sawin R, Seidel KD, Flum DR. Do antireflux operations decrease the rate of reflux-related hospitalizations in children? PEDIATRICS. 2006;118(6):2326–2333. doi: 10.1542/peds.2006-2212. [DOI] [PubMed] [Google Scholar]
- 63.Lee SL, Shabatian H, Hsu J-W, Applebaum H, Haigh PI. Hospital admissions for respiratory symptoms and failure to thrive before and after Nissen fundoplication. Journal of Pediatric Surgery. 2008;43(1):59–65. doi: 10.1016/j.jpedsurg.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 64.Rosen R, Levine P, Lewis J, Mitchell P, Nurko S. Reflux Events Detected by pH-MII Do Not Determine Fundoplication Outcome. Journal of Pediatric Gastroenterology and Nutrition. 2010;50(3):251–255. doi: 10.1097/MPG.0b013e3181b643db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gatzinsky V, Jönsson L, Ekerljung L, Friberg L-G, Wennergren G. Long-term respiratory symptoms following oesophageal atresia. Acta Paediatrica. 2011;100(9):1222–1225. doi: 10.1111/j.1651-2227.2011.02279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Beucher J, Wagnon J, Daniel V, et al. Long-term evaluation of respiratory status after esophageal atresia repair. Pediatr Pulmonol. 2013;48(2):188–194. doi: 10.1002/ppul.22582. [DOI] [PubMed] [Google Scholar]
- 67.Sistonen S, Malmberg P, Malmstrom K, et al. Repaired oesophageal atresia: respiratory morbidity and pulmonary function in adults. European Respiratory Journal. 2010;36(5):1106–1112. doi: 10.1183/09031936.00153209. [DOI] [PubMed] [Google Scholar]
- 68.Chetcuti P, Phelan PD, Greenwood R. Lung function abnormalities in repaired oesophageal atresia and tracheo-oesophageal fistula. Thorax. 1992;47(12):1030–1034. doi: 10.1136/thx.47.12.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Horvath A, Dziechciarz P, Szajewska H. The effect of thickened-feed interventions on gastroesophageal reflux in infants: systematic review and meta-analysis of randomized, controlled trials. Pediatrics. 2008;122(6):e1268–e1277. doi: 10.1542/peds.2008-1900. http://dx.doi.org/10.1542/peds.2008-1900. Epub 2008 Nov 10. Review. Erratum in: Pediatrics. 2009 Apr;123(4):1254. [DOI] [PubMed] [Google Scholar]