Abstract
PURPOSE
Patients with systemic lupus erythematosus (SLE) frequently develop lupus nephritis (LN), a complication frequently leading to end stage kidney disease. Immune complex deposition in the glomerulus is central to the development of LN. Using a targeted proteomic approach, we tested the hypothesis that autoantibodies targeting glomerular antigens contribute to the development of LN.
EXPERIMENTAL DESIGN
Human podocyte and glomerular proteins were separated by SDS-PAGE and immunoblotted with sera from SLE patients with and without LN. The regions of those gels corresponding to reactive bands observed with sera from LN patients were analyzed using LC-MS/MS.
RESULTS
LN reactive bands were seen at approximately 50 kDa in podocyte extracts and between 36-50 kDa in glomerular extracts. Those bands were analyzed by LC-MS/MS and 102 overlapping proteins were identified. Bioinformatic analysis determined that 36 of those proteins were membrane associated, including a protein previously suggested to contribute to glomerulonephritis and LN, annexin A2. By ELISA, patients with proliferative LN demonstrated significantly increased antibodies against annexin A2.
CONCLUSION AND CLINICAL RELEVANCE
Proteomic approaches identified multiple candidate antigens for autoantibodies in patients with LN. Serum antibodies against annexin A2 were significantly elevated in subjects with proliferative LN, validating those antibodies as potential biomarkers.
Keywords: autoantibodies, glomerulonephritis, lupus nephritis, systemic lupus erythematosus, target antigens
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by loss of self-tolerance and the development of autoantibodies. Abnormal clearance of apoptotic bodies results in the production of antibodies against components of nucleosomes, including anti-nuclear antibodies [1]. Anti-nuclear antibodies that react to double stranded DNA (anti-dsDNA) are highly specific for SLE, and serum titers often correlate with disease activity [2]. SLE can affect multiple organ systems including musculoskeletal, skin, cardiovascular, hematologic, and kidneys. Up to two-thirds of patients with SLE develop glomerular injury termed lupus nephritis (LN) due to deposition of immune complexes. Approximately 60% of those patients develop a proliferative form of LN (PLN) (termed class III or IV), while 20% to 40% develop membranous LN (MLN) (class V) [3]. The location of immune complex deposits determines the histologic pattern of injury. Proliferative LN is associated with deposits in the mesangium and subendothelial space, while deposits in the subepithelial space occur in membranous LN [4].
Three hypotheses have been advanced to explain glomerular immune complex deposition in LN. First, circulating pre-formed immune complexes directly deposit in the glomerular vasculature. Second, autoantibodies recognize an endogenous glomerular antigen leading to in situ immune complex formation. Third, antibodies bind to an antigen that is normally not a glomerular component, but is planted in the glomerulus. The targets of antibodies that form glomerular immune complexes in lupus nephritis and produce disease remain to be identified. Anti-dsDNA antibodies were proposed to play a major role through binding to nucleosomes deposited in the glomerulus or through their cross-reactivity with a number of cellular or extracellular matrix proteins [5-7]. Data supporting a role of anti-dsDNA include a correlation of serum levels with development of renal disease and the presence of anti-dsDNA in glomerular immune complexes [5, 8, 9]. However, Mannik et al. [5] reported that antibody eluted from glomeruli of patients with LN reacted to histones or chromatin in only 50% of patients, and reacted to dsDNA in only 25%. Additionally, anti-dsDNA accounted for less than 1% of eluted IgG in those 25% of patients. Identification of the target antigens for nephritogenic autoantibodies is critical to understanding the pathophysiology of LN. The present study was designed to determine if endogenous glomerular proteins serve as targets for autoantibodies in different histologic classes of LN. We combined patient serum-reactive immunoblotting of endogenous glomerular proteins with LC-MS/MS analysis of corresponding gel bands. A number of membrane-associated candidate proteins were identified, and annexin A2 was validated as a target for autoantibodies in patients with proliferative LN.
Materials and Methods
Study Subjects
Sera were obtained from a total of 45 research subjects. 10 subjects were lupus controls (LC), 10 had PLN, 15 had MLN, and 10 were normal controls (NC). All lupus controls met ACR criteria for the diagnosis of SLE, never had a diagnosis of lupus nephritis, and had no clinical evidence of kidney disease. All PLN subjects had biopsy proven class III or IV lesions. All MLN subjects had biopsy proven class V lesions. SLE samples were obtained from the Ohio SLE Study cohort [10] and the Lupus Family Registry and Repository [11]. Normal controls were obtained from healthy adult volunteers at the University of Louisville. Sample donation and sharing were approved by all human studies committees at all institutions.
Glomerular and Podocyte Protein Extracts
Glomerular protein extracts were prepared from human kidneys obtained from deceased donors that were unsuitable for transplantation (courtesy of Kentucky Organ Donor Affiliates). Glomeruli were isolated from kidney cortical slices using a set of three different stainless steel mesh sieves placed in a series as previously described [12]. The purity of glomerular fractions was greater than 90%, as determined by light microscopy. Proteins were extracted from isolated glomeruli by sonication in lysis buffer containing 50 mM Tris, 150 mM NaCl, 1 mM EDTA, 7.5% glycerol, 0.2% NP-40 with protease inhibitor cocktail (Santa Cruz Biotech, Dallas, TX) and phosphatase inhibitor cocktail (Sigma-Aldrich, St. Louis, MO) combined according to the package insert. Glomerular extracts were incubated overnight with Protein G agarose beads (Pierce, Rockford, IL) to remove contaminating IgG and depletion was validated by anti-human IgG immunoblot.
To obtain proteins from cultured podocytes, conditionally immortalized human podocytes (obtained from Dr. Moin Saleem [13]) were cultured in RPMI-1640 containing 10% FBS, penicillin/streptomycin, and insulin/transferrin/selenium X at 33°C prior to differentiation by cultivation on collagen at 37°C. To enrich for membrane and cytosolic proteins, cells were lysed by homogenization in 50 mM mannitol, 5 mM Tris-HCl buffer with the previously described protease and phosphatase inhibitors. The homogenate was cleared of cells and debris by sequential centrifugation at 2500 × g and 10,000 × g. That supernatant was then centrifuged at 100,000 × g for 30 min to separate cytosol from plasma membrane. The supernatant was collected as the cytosolic fraction. The pellet was re-suspended in the lysis buffer and stored as the membrane fraction. Enrichment of podocyte cytosol and membrane proteins was confirmed by immunoblot for WT-1, PLA2R, and NAK-α1.
Immunoblot Analysis with Sera
Podocyte membrane and human glomerular extracts were separated by 5-15% gradient SDS/PAGE and transferred to nitrocellulose or PVDF membranes. For the podocyte proteins, gel electrophoresis was conducted under non-reducing conditions. For the human glomerular extracts, gel electrophoresis was conducted under reducing conditions. Following overnight incubation with pooled or individual subject sera (1:200-1:10,000), membranes were washed and incubated with 0.01% HRP conjugated rabbit anti-human IgG secondary antibody (Santa Cruz 2769, Santa Cruz, CA) for 1 h. Membranes were then washed and incubated for 5 min with chemoluminescent substrate (SuperSignal West Pico, Thermo Scientific or Clarity Western ECL, Biorad, Hercules, CA). Images were captured using film or Biorad chemi-doc. The molecular size and density of each band on individual blots was determined using the Biorad chemiDoc MP imager.
Mass Spectrometry Analysis
Proteomic Data Collection
SDS-PAGE gel bands containing candidate target antigens were processed and analyzed by mass spectrometry analysis with modifications of previously described methods [14-17]. Briefly, supernatants from in-gel trypsin digestion of gel slices were lyophilized, then re-dissolved in 2% acetonitrile / 0.1% formic acid for separation using an EASY n-LC (ThermoElectron, Waltham, MA) UHPLC system. The peptides were loaded onto a Dionex Acclaim PepMap 100 trap column and separated with a 90 min 2% to 40% acetonitrile gradient on a RSLC Pepmap 100 C18 reversed phase resolving column prior to introduction by nanoelectrospray using a Nanospray Flex source (ThermoElectron) into a LTQ-Velos-Orbitrap ELITE (ThermoElectron) mass spectrometer. Data dependent tandem mass spectra (top 10 with dynamic exclusion) were collected using CID and/or ETD fragmentation using an Nth Order Double Play with ETD Decision Tree method created in Xcalibur v2.2.
Database Searching
RAW files were converted to DTA files using msconvert.exe from Trans-Proteomic Pipeline (ver4.6.3) without charge state calculation or de-isotoping. The data were analyzed using Sequest Sorcerer2 (SageN) database search strategy against the Uniprot KB human reference proteome (canonical and isoform sequences, 4/8/2014 version, 88703 entries). Data dependent spectra were acquired and then searched using Sorcerer v5.1 (Sage-N Research, Inc., Milpitas, CA) using UniprotKB Homo sapiens reference proteome with canonical and isoform sequences (version 4/8/2014). Search parameters included: variable methionine oxidation (+16 on M), correction of search strategy for ETD fragmentation spectra with addition of +17 to the N-termini and −16 on C-termini, fixed cysteine carbamidomethylation (+57 on C), up to 2 missed tryptic cleavages, 50ppm precursor error for MS1 Orbitrap FTMS data and 1 Da error for MS2 LTQ data. Sage-N Sorcerer decoy peptide generation was enabled to allow for false discovery rate (FDR) calculations. The resulting files were loaded into Scaffold Q+S (ver4.3.4, Proteome Software, Portland, OR) to validate MS2 based peptide and protein identifications using the Peptide and Protein Prophet algorithms [18, 19]. Protein probabilities were accepted for assignments with >95% peptide and protein confidence intervals with a minimum of 2 unique peptides for protein assignment. Proteins that contained similar peptides and could not be differentiated based on MS/MS analysis alone were grouped to a single best explained protein to satisfy the principles of parsimony. The results were annotated with human gene ontology information (NCBI download, Jul 31, 2014) from the Gene Ontology Annotations Database (ftp.ebi.ac.uk) [20]. Proteins in the various groups were analyzed using Ingenuity Pathway Analysis (IPA®, QIAGEN Redwood City, www.qiagen.com/ingenuity).
Annexin A2 ELISA
Recombinant annexin A2 was expressed and purified using the expression plasmid, pMCSG7, obtained from the Biodesign Institute at Arizona State University, a non-profit plasmid depository [21]. Ninety-six well plates were coated with mouse monoclonal annexin A2 antibody (Santa Cruz 47696) at a concentration of 1 μg/ml in coating buffer (Biolegend, San Diego, CA). The plate was incubated overnight at 4°C. The coating solution was removed and the plate was washed 5X with 300 μl/well of wash solution (Biolegend) by automated plate washer. Sample dilution buffer (Biolegend 1% BSA in PBS) was added and incubated for 1 h at room temperature to block non-specific binding. Recombinant annexin A2 was added at a concentration of 0.2 μg per well and incubated for 2 h at room temperature with shaking. The plate was washed 5X as described above. Sera were diluted 1:200 in sample dilution buffer, and duplicate samples from each subject incubated at room temperature for 1 h. Dilution buffer without sera was incubated for 1 h in duplicate as a control. Following a 5X wash step, HRP conjugated rabbit anti-human IgG secondary antibody (Santa Cruz 2769) at 1:1000 was added to each well for 30 min at room temperature with shaking. The plate was washed again 5X, and TMB substrate (Biolegend) was added for 15 min in the dark and then the reaction was stopped with addition of 1 M sulfuric acid. Immediately following, the optical density (OD) was measured at 450 nM and normalized at 570 nM. The OD of the sera free control was subtracted from the measured OD of all samples to eliminate non-specific reactivity.
Results and Discussion
Analysis of autoantibody reactivity against glomerular proteins
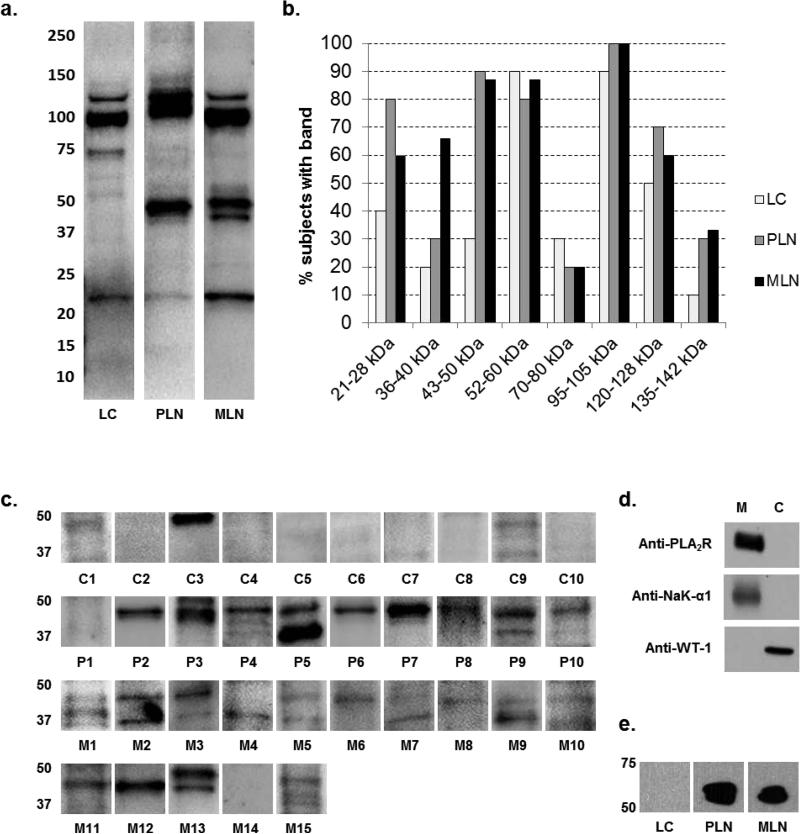
Identifying target antigens for nephritogenic autoantibodies would significantly advance the understanding of the pathogenesis and diagnosis of LN. The rationale for the current study was based on reports that autoantibodies to glomerular proteins play a role in idiopathic membranous nephropathy and focal glomerulosclerosis [14, 22] and autoantibodies directed against nerve cell proteins are present in patients with lupus cerebritis [23]. To examine the hypothesis that patients with LN generate autoantibodies against glomerular proteins, the present study used an approach similar to that which identified PLA2R on podocytes as a target antigen in idiopathic membranous nephropathy [14]. Sera from 10 patients with class III/IV lupus nephritis (PLN), 15 patients with class V lupus nephritis (MLN), and 10 SLE patients without renal disease (LC) were immunoblotted against proteins extracted from glomeruli isolated from normal human kidneys retrieved for transplantation. Figure 1a shows representative blots using sera from an individual patient in each group. As expected, a number of bands were observed in all SLE patients, suggesting the presence of circulating autoantibodies against many proteins. Analysis of the molecular size of bands for each individual patient was performed to identify bands most likely to contain nephritogenic autoantibodies. Figure 1b shows the percent of patients in each group with reactivity to proteins observed at various molecular sizes. Bands between 43-50 kDa were present in about 90% of patients with PLN and MLN, but only 30% of LC patients. Bands between 36-40 kDa were present in nearly 70% of patients with MLN, while present in only 20-30% of patients in the other two groups. Bands between 21-28 kDa were present in 80% of PLN patients, 60% of MLN, and 40% of SLE patients without LN. Reactive bands between 52-60 kDa, 95-105 kDa, and 120-128 kDa were present in a majority of patients with SLE, whether or not they had renal disease. Figure 1c shows immunoblots from each subject, illustrating the reactivity of sera against proteins in the 36 kDa to 50 kDa range. The findings suggest that SLE patients contain a number of autoantibodies, some of which are unique to those patients with PLN and MLN.
Figure 1. Sera from LN subjects demonstrate selective reactivity to glomerular and podocyte proteins.
a. Representative immunoblots of individual lupus subjects with human glomerular proteins. Sera at a dilution of 1:200 from individual lupus subjects were immunoblotted against 30 μg of human glomerular proteins. Immunoblots from a lupus subject without nephritis (LC), a lupus subject with proliferative nephritis (PLN), and a lupus subject with membranous nephritis (MLN) are shown.
b. Patterns of sera reactivity to human glomerular proteins among subjects with SLE. Graph demonstrating patterns sera reactivity of individual subjects against human glomerular extracts. 10 subjects with SLE without nephritis (LC), 10 subjects with proliferative lupus nephritis (PLN), and 15 subjects with membranous lupus nephritis (MLN) were compared. The percentage of subjects with reactive bands in each region is displayed.
c. Immunoblots of individual lupus subjects with human glomerular proteins highlighting the region of interest from 36-50 kDa. Sera from individual lupus subjects were immunoblotted against 30 μg of human glomerular proteins at 1:200 dilution. Immunoblots from 10 lupus subjects without nephritis (C1-C10), 10 subjects with proliferative lupus nephritis (P1-P10), and 15 subjects with membranous lupus nephritis (M1-M15) are shown.
d. Human podocyte immunoblot demonstrating separation of membrane and cytosolic fractions. Podocyte plasma membrane (M) and cytosol (C) fractions were probed with antibodies against plasma membrane proteins PLA2R and NAK-α1, and cytosolic WT-1.
e. Immunoblot of pooled sera with human podocyte membrane proteins. Pooled sera from 10 lupus subjects without nephritis (LC), 5 subjects with MLN, and 10 subjects with PLN were immunoblotted against 20 μg of podocyte membrane proteins at 1:10,000 dilution. A reactive band at approximately 50 kDa was seen in the MLN and PLN subjects, but not in the LC subjects.
Podocytes are highly specialized glomerular epithelial cells characterized by foot processes which wrap around glomerular capillaries and maintain the glomerular filtration barrier. Slit diaphragms connect adjacent foot processes and act as the final barrier to protein filtration [24]. Disruption of podocytes or their slit diaphragms results in proteinuria, and reduced expression of podocyte slit diaphragm proteins in lupus nephritis correlates with disease severity [25]. Autoantibodies directed against PLA2R on podocytes are postulated to cause in situ immune complex formation in idiopathic membranous nephropathy, and autoantibodies to CD40 on podocytes may contribute to development of focal glomerulosclerosis [14, 22]. To determine if patients with LN develop autoantibodies against podocyte proteins, proteins extracted from cultured human podocyte membranes were used for immunoblot analysis with patient sera. Expression of membrane (PLA2R and NAK-α1) and cytosolic (WT-1) markers of podocytes confirmed enrichment of proteins from podocyte membranes (fig 1d). Pooled sera from 10 subjects with PLN, 5 subjects with MLN, and 10 LC subjects were used as primary antibody. Figure 1e shows that sera from PLN and MLN subjects (dilution 1:10,000) reacted to proteins at a molecular size of about 50 kDa, while sera from LC patients failed to react. Thus, patients with LN contain antibodies that recognize podocyte proteins at the upper molecular size limit of bands recognized from proteins extracted for intact glomeruli.
Identification of Candidate Proteins
We interpreted the data shown in figure 1 to suggest that glomerular and podocyte targets of autoantibodies in patients with LN were present in the 36-50 kDa range. To identify candidates, proteins were extracted from podocyte membranes and human glomerular extracts, separated by SDS-PAGE, and gel lanes containing the 36-50 kDa range were excised. Peptides generated by in-gel trypsin digestion were identified by tandem mass spectrometry. A total of 294 proteins were identified from podocyte membrane extracts and 310 proteins from human glomerular extracts (Supplementary Tables 1 and 2). To identify high probability candidates, the two groups of proteins were analyzed for common proteins and cellular location. A total of 102 proteins were common to podocyte and human glomerular extracts (Supplementary Table 3). As targets of nephritogenic autoantibodies in idiopathic MN and focal segmental glomerulosclerosis were present in the podocyte membrane [14, 22], the list of common proteins was analyzed for membrane associated proteins by the Gene Ontology database (ftp.ebi.ac.uk) [20]. This resulted in a final candidate list of 36 proteins.
Ingenuity Pathway Analysis (IPA®, QIAGEN Redwood City, www.qiagen.com/ingenuity) is a curated database platform that is a widely used bioinformatics tool for high-throughput functional characterization of large list proteins resulting from LC-MS/MS experiments. This approach aids in identification of proteins with known functions relevant to target molecular or disease process, as high-priority candidates for follow-up validations. The actin cytoskeleton is necessary for maintaining foot process integrity of podocytes [26]. The slit diaphragm is a modified adherens junction that is linked to, and regulates, the actin cytoskeleton in foot processes [24]. Rho GTPase signaling pathways play a critical role in linking slit diaphragm proteins to actin cytoskeleton remodeling [26, 27]. Disruption of Rho signaling pathways was shown to be involved in the pathogenesis of the nephrotic syndrome [28, 29]. Thus, IPA analysis was used to determine if any of the 36 candidate proteins are associated with these processes.
To more completely characterize the final candidates, each protein was also evaluated by PubMed search for involvement in actin regulation, for participation in glomerular diseases, and for association with the slit diaphragm. Table 1 shows the results of these analyses. Seventeen proteins were associated with actin regulation, 6 proteins involved in canonical Rho signaling, 10 proteins associated with glomerulonephritis, and 8 proteins associated with the slit diaphragm.
Table 1.
Membrane-associated Proteins Common to Podocytes and Glomeruli.
| Protein | Gene Name | Actin Reg. | Canonical Rho Signaling* | GN | SD Protein** |
|---|---|---|---|---|---|
| Annexin A2 | ANXA2 | [30] | [31] | + | |
| ATP synthase subunit alpha, mitochondrial | ATP5A1 | ||||
| ATP synthase subunit beta, mitochondrial | ATP5B | ||||
| Alpha-enolase | ENO1 | [38] | [39, 40] | ||
| Moesin | MSN | [41] | + | [41-43] | + |
| Glyceraldehyde-3-phosphate dehydrogenase | GAPDH | ||||
| Elongation factor 1-alpha 1 | EEF1A1 | [44] | |||
| Guanine nucleotide-binding protein G(i) subunit alpha-2 | GNAI2 | [45] | + | + | |
| Isoform 2 of AP-2 complex subunit mu | AP2M1 | [46] | + | ||
| Isoform 2 of Protein disulfide-isomerase A6 | PDIA6 | ||||
| Pyruvate kinase PKM | PKM | ||||
| 60 kDa heat shock protein, mitochondrial | HSPD1 | [47] | [48] | ||
| Actin-related protein 3 | ACTR3 | [49] | + | [50] | |
| V-type proton ATPase subunit B, brain isoform | ATP6V1B2 | [51] | |||
| Myosin-9 | MYH9 | [52] | + | ||
| Isoform 3 of Heterogeneous nuclear ribonucleoprotein Q | SYNCRIP | [53] | |||
| Septin-7 | SEPT7 | [54] | + | ||
| Isoform 2 of Coronin-1C | CORO1C | [55] | |||
| Tubulin beta-4A chain | TUBB4A | ||||
| T-complex protein 1 subunit gamma | CCT3 | [56] | |||
| Isoform 2 of ATP-dependent RNA helicase | DDX3X | ||||
| Isoform 2 of Basigin | BSG | [57] | [58] | ||
| Dolichyl-diphosphooligosaccharide--protein glycosyltransferase 48 kDa subunit | DDOST | ||||
| Heat shock protein HSP 90-beta | HSP90AB1 | ||||
| Ezrin | EZR | [59] | + | [59, 60] | + |
| Isoform 2 of Neutral cholesterol ester hydrolase 1 | NCEH1 | ||||
| Isoform 2 of Heat shock cognate 71 kDa protein | HSPA8 | + | |||
| Heterogeneous nuclear ribonucleoprotein U | HNRNPU | ||||
| Rho GTPase-activating protein 1 | ARHGAP1 | [61] | + | ||
| Isoform 2 of Fatty aldehyde dehydrogenase | ALDH3A2 | ||||
| Serine palmitoyltransferase 1 | SPTLC1 | ||||
| Isoform 2 of ATP-citrate synthase | ACLY | ||||
| Phosphatidylinositol 4-kinase type 2-alpha | PI4K2A | ||||
| Na(+)/H(+) exchange regulatory cofactor NHERF1 | SLC9A3R1 | [62] | |||
| Alpha-parvin | PARVA | [63] | [63] | + | |
| Isoform 2 of Sorting nexin-17 | SNX17 |
Canonical Rho Pathway determined by Ingenuity Pathway Analysis
Slit diaphragm proteins identified by Pierchala et al [32]
The identification of annexin A2 was of particular interest, as table 1 shows that annexin A2 has been reported to regulate the actin cytoskeleton, to contribute to glomerulonephritis and LN, and to associate with the slit diaphragm [30-32]. The annexin family contains 12 proteins that are Ca2+ regulated phospholipid binding proteins [33]. Annexin A2 is expressed in many human tissues, including glomerular mesangial cells, endothelial cells, and epithelial cells [30, 31]. The cellular location of annexin A2 is variable, and includes cytoplasm, intracellular membranes, and the external surface of the plasma membrane [30]. Multiple functions have been described and vary with cell type and cellular location. In epithelial cells, such as podocytes, annexin A2 plays a critical role in dynamic remodeling of the actin cytoskeleton, effecting cell adhesion and motility through interactions with Rho GTPases [30, 34]. Cross-reactivity of anti-dsDNA with mesangial annexin A2 was previously reported, and annexin A2 co-localizes with glomerular IgG and C3 deposition in patients with LN [31]. Annexin A2 on the cell surface interacts with beta 2-glycoprotein I and toll like receptor 4, leading to proinflammatory and prothrombotic effects, which may be enhanced by autoantibody binding [35]. Annexin A2 has also been described as a ligand for C1q to bind to apoptotic cells [36]. C1q opsonization of apoptotic cells is critical to phagocytosis and in cases where this function is impaired, such as hereditary C1q deficiency, SLE ensues [37].
Patients with PLN demonstrate elevated Anti-Annexin A2
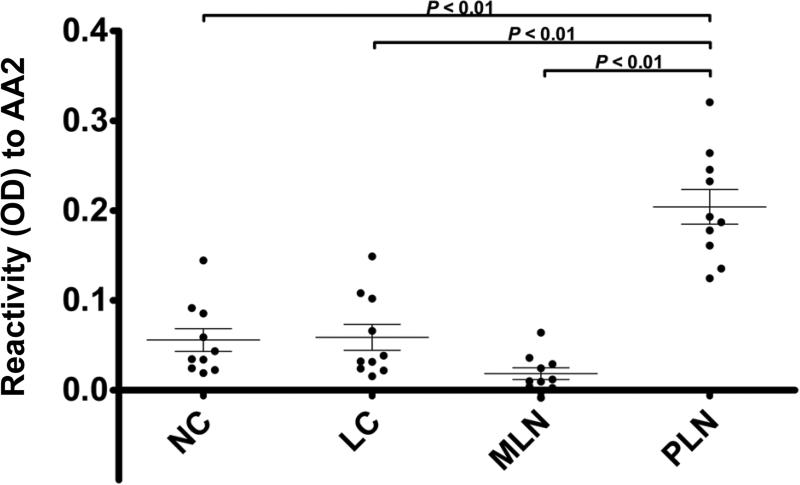
Based on our finding of annexin A2 in the final list of candidates, and its prior identification as a target antigen in LN, we chose to validate it as a target antigen in our cohort. We developed a sandwich ELISA protocol to reduce non-specific binding of sera to contaminants present in recombinant protein and were able to achieve very low background reactivity in both NC and LC samples. Using this approach, we confirmed the presence of antibodies reactive to annexin A2 in our PLN subjects. Sera at 1:200 from 10 PLN, 10 MLN, 10 LC, and 10 normal (NC) subjects were analyzed in duplicate. Fig. 2 shows that sera from PLN subjects demonstrated a significantly higher reactivity to annexin A2 than those from MLN, LC, and NC subjects. One way analysis of variance (ANOVA) comparing individual reactivity to anti-annexin A2 demonstrated a p-value of 1.25 X 10−10 and Tukey analysis verified statistical differences among groups comparing PLN to MLN, LC, and NC subjects (P < 0.01). Thus, sera from patients with class III/IV LN contain autoantibodies that recognize annexin A2, while sera from patients with MLN or SLE without LN do not differ from normal subjects. While our studies did not distinguish whether reactivity to annexin A2 was the result of cross-reacting anti-dsDNA or separate tissue-specific autoantibodies, the results suggest that anti-annexin A2 may serve as a biomarker for the proliferative forms of LN.
Figure 2. Annexin A2 ELISA.
Sera from 10 proliferative lupus nephritis (PLN), 10 membranous lupus nephritis (MLN), 10 lupus without nephritis-lupus control (LC), and 10 normal control (NC) subjects were analyzed in duplicate at a 1:200 dilution. PLN subjects demonstrated a mean OD value of 0.204, MLN subjects demonstrated a mean OD value of 0.018, LC subjects demonstrated a mean OD value of 0.059, and NC subjects demonstrated a mean OD value of 0.056. One way analysis of variance (ANOVA) comparing individual anti-annexin A2 levels demonstrated a p-value of 1.25 X 10−10 and Tukey analysis verified statistical difference among groups comparing PLN to MLN, LC, and NC subjects (P < 0.01). Error bars demonstrate standard error of the mean.
Concluding Remarks
A targeted, translational proteomic approach led to the identification of glomerular proteins as candidate targets of autoantibodies in lupus nephritis. A majority of the plasma membrane-associated candidates are involved in actin function and regulation. This provides predictive information that actin regulation may play a role in the pathogenesis of LN, but experimental confirmation will be needed. Annexin A2 is a multifunctional protein that belongs to a large family of Ca2+ regulated phospholipid binding proteins that was reported to participate in LN. Our study is unique because it compares anti-annexin A2 activity in patients with proliferative LN (PLN), membranous LN (MLN), lupus controls (LC), and normal controls (NC). Current lupus biomarkers, such as anti-dsDNA, do not always correlate with nephritis and are usually present in both MLN and PLN. We demonstrate that patients with active PLN have significantly increased reactivity to annexin A2 when compared with MLN, LC, and NC. Quantitative serum levels of IgG to annexin A2 may be a useful biomarker that specifically identifies patients with proliferative lupus nephritis, the most clinically significant form of lupus nephritis.
Supplementary Material
Statement of Clinical Relevance.
Over one-half of patients with systemic lupus erythematosus (SLE) develop clinical renal disease termed lupus nephritis (LN), a complication that carries significant morbidity and can lead to end stage kidney disease. Glomerular immune complex deposition is central to the development of LN. The targets of nephritogenic autoantibodies and the mechanism by which they deposit in the glomerulus are not fully understood. Identification of target antigens for the autoantibodies that induce LN will enhance understanding of the pathogenesis of LN, provide novel biomarkers, and lead to more targeted treatment strategies.
Acknowledgements
The authors were supported by grants from the National Institutes of Health AR063124 (to D.W.P.), AI103980 (to D.W.P. and K.R.M.), AI24717, TR000077, HG006828 (to J.B.H), and the Juvenile Diabetes Research Foundation international grant 1-2011-588 (to D.W.P.).
J.B.H. and K.R.M were supported by the US Department of Veterans Affairs.
The authors wish to thank Vilius Stribinskis, Ph.D., for the production of recombinant annexin A2.
Abbreviations
- SLE
systemic lupus erythematosus
- LN
lupus nephritis
- ANA
anti-nuclear antibodies
- Anti-dsDNA
anti-double stranded DNA antibodies
- MLN
membranous lupus nephritis
- PLN
proliferative lupus nephritis
- SLE
without nephritis
- LC
lupus control
- NC
normal control
Footnotes
The authors declare no conflicts of interest.
References
- 1.Tsokos GC. Systemic lupus erythematosus. The New England journal of medicine. 2011;365:2110–2121. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- 2.Cozzani E, Drosera M, Gasparini G, Parodi A. Serology of Lupus Erythematosus: Correlation between Immunopathological Features and Clinical Aspects. Autoimmune diseases. 2014;2014:321359. doi: 10.1155/2014/321359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhinder S, Singh A, Majithia V. Membranous (class V) renal disease in systemic lupus erythematosus may be more common than previously reported: results of a 6-year retrospective analysis. Am J Med Sci. 2010;339:230–232. doi: 10.1097/MAJ.0b013e3181c9529c. [DOI] [PubMed] [Google Scholar]
- 4.Weening JJ, D'Agati VD, Schwartz MM, Seshan SV, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney international. 2004;65:521–530. doi: 10.1111/j.1523-1755.2004.00443.x. [DOI] [PubMed] [Google Scholar]
- 5.Mannik M, Merrill CE, Stamps LD, Wener MH. Multiple autoantibodies form the glomerular immune deposits in patients with systemic lupus erythematosus. The Journal of rheumatology. 2003;30:1495–1504. [PubMed] [Google Scholar]
- 6.Seret G, Le Meur Y, Renaudineau Y, Youinou P. Mesangial cell-specific antibodies are central to the pathogenesis of lupus nephritis. Clinical & developmental immunology. 2012;2012:579670. doi: 10.1155/2012/579670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanrotel-Saliou C, Segalen I, Le Meur Y, Youinou P, Renaudineau Y. Glomerular antibodies in lupus nephritis. Clinical reviews in allergy & immunology. 2011;40:151–158. doi: 10.1007/s12016-010-8204-4. [DOI] [PubMed] [Google Scholar]
- 8.Olson SW, Lee JJ, Prince LK, Baker TP, et al. Elevated subclinical double-stranded DNA antibodies and future proliferative lupus nephritis. Clin J Am Soc Nephrol. 2013;8:1702–1708. doi: 10.2215/CJN.01910213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalaaji M, Mortensen E, Jorgensen L, Olsen R, Rekvig OP. Nephritogenic lupus antibodies recognize glomerular basement membrane-associated chromatin fragments released from apoptotic intraglomerular cells. Am J Pathol. 2006;168:1779–1792. doi: 10.2353/ajpath.2006.051329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birmingham DJ, Hebert LA, Song H, Noonan WT, et al. Evidence that abnormally large seasonal declines in vitamin D status may trigger SLE flare in non-African Americans. Lupus. 2012;21:855–864. doi: 10.1177/0961203312439640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rasmussen A, Sevier S, Kelly JA, Glenn SB, et al. The lupus family registry and repository. Rheumatology (Oxford) 2011;50:47–59. doi: 10.1093/rheumatology/keq302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamamoto T. Renal and Urinary Proteomics. Wiley-VCH Verlag GmbH & Co. KGaA; 2009. pp. 1–7. [Google Scholar]
- 13.Saleem MA, O'Hare MJ, Reiser J, Coward RJ, et al. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. Journal of the American Society of Nephrology : JASN. 2002;13:630–638. doi: 10.1681/ASN.V133630. [DOI] [PubMed] [Google Scholar]
- 14.Beck LH, Jr., Bonegio RG, Lambeau G, Beck DM, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. The New England journal of medicine. 2009;361:11–21. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Powell DW, Rane MJ, Joughin BA, Kalmukova R, et al. Proteomic identification of 14-3-3zeta as a mitogen-activated protein kinase-activated protein kinase 2 substrate: role in dimer formation and ligand binding. Molecular and Cellular Biology. 2003;23:5376–5387. doi: 10.1128/MCB.23.15.5376-5387.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cummins TD, Barati MT, Coventry SC, Salyer SA, et al. Quantitative mass spectrometry of diabetic kidney tubules identifies GRAP as a novel regulator of TGF-beta signaling. Biochim Biophys Acta. 2010;1804:653–661. doi: 10.1016/j.bbapap.2009.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baba SP, Hoetker JD, Merchant M, Klein JB, et al. Role of aldose reductase in the metabolism and detoxification of carnosine-acrolein conjugates. The Journal of biological chemistry. 2013;288:28163–28179. doi: 10.1074/jbc.M113.504753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keller A, Nesvizhskii AI, Kolker E, Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Analytical chemistry. 2002;74:5383–5392. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- 19.Nesvizhskii AI, Keller A, Kolker E, Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Analytical chemistry. 2003;75:4646–4658. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- 20.Ashburner M, Ball CA, Blake JA, Botstein D, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nature genetics. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seiler CY, Park JG, Sharma A, Hunter P, et al. DNASU plasmid and PSI:Biology-Materials repositories: resources to accelerate biological research. Nucleic acids research. 2014;42:D1253–1260. doi: 10.1093/nar/gkt1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delville M, Sigdel TK, Wei C, Li J, et al. A circulating antibody panel for pretransplant prediction of FSGS recurrence after kidney transplantation. Sci Transl Med. 2014;6:256ra136. doi: 10.1126/scitranslmed.3008538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lefranc D, Launay D, Dubucquoi S, de Seze J, et al. Characterization of discriminant human brain antigenic targets in neuropsychiatric systemic lupus erythematosus using an immunoproteomic approach. Arthritis Rheum. 2007;56:3420–3432. doi: 10.1002/art.22863. [DOI] [PubMed] [Google Scholar]
- 24.Reiser J, Kriz W, Kretzler M, Mundel P. The glomerular slit diaphragm is a modified adherens junction. Journal of the American Society of Nephrology : JASN. 2000;11:1–8. doi: 10.1681/ASN.V1111. [DOI] [PubMed] [Google Scholar]
- 25.Perysinaki GS, Moysiadis DK, Bertsias G, Giannopoulou I, et al. Podocyte main slit diaphragm proteins, nephrin and podocin, are affected at early stages of lupus nephritis and correlate with disease histology. Lupus. 2011;20:781–791. doi: 10.1177/0961203310397412. [DOI] [PubMed] [Google Scholar]
- 26.Welsh GI, Saleem MA. The podocyte cytoskeleton--key to a functioning glomerulus in health and disease. Nature reviews. Nephrology. 2012;8:14–21. doi: 10.1038/nrneph.2011.151. [DOI] [PubMed] [Google Scholar]
- 27.Greka A, Mundel P. Calcium regulates podocyte actin dynamics. Seminars in nephrology. 2012;32:319–326. doi: 10.1016/j.semnephrol.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gee HY, Saisawat P, Ashraf S, Hurd TW, et al. ARHGDIA mutations cause nephrotic syndrome via defective RHO GTPase signaling. The Journal of clinical investigation. 2013;123:3243–3253. doi: 10.1172/JCI69134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meyer-Schwesinger C, Dehde S, Sachs M, Mathey S, et al. Rho-kinase inhibition prevents proteinuria in immune-complex-mediated antipodocyte nephritis. Am J Physiol Renal Physiol. 2012;303:F1015–1025. doi: 10.1152/ajprenal.00380.2011. [DOI] [PubMed] [Google Scholar]
- 30.Bharadwaj A, Bydoun M, Holloway R, Waisman D. Annexin A2 heterotetramer: structure and function. International journal of molecular sciences. 2013;14:6259–6305. doi: 10.3390/ijms14036259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yung S, Cheung KF, Zhang Q, Chan TM. Anti-dsDNA antibodies bind to mesangial annexin II in lupus nephritis. Journal of the American Society of Nephrology : JASN. 2010;21:1912–1927. doi: 10.1681/ASN.2009080805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pierchala BA, Munoz MR, Tsui CC. Proteomic analysis of the slit diaphragm complex: CLIC5 is a protein critical for podocyte morphology and function. Kidney international. 2010;78:868–882. doi: 10.1038/ki.2010.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Markoff A, Gerke V. Expression and functions of annexins in the kidney. Am J Physiol Renal Physiol. 2005;289:F949–956. doi: 10.1152/ajprenal.00089.2005. [DOI] [PubMed] [Google Scholar]
- 34.Rescher U, Ludwig C, Konietzko V, Kharitonenkov A, Gerke V. Tyrosine phosphorylation of annexin A2 regulates Rho-mediated actin rearrangement and cell adhesion. Journal of cell science. 2008;121:2177–2185. doi: 10.1242/jcs.028415. [DOI] [PubMed] [Google Scholar]
- 35.Canas F, Simonin L, Couturaud F, Renaudineau Y. Annexin A2 autoantibodies in thrombosis and autoimmune diseases. Thrombosis research. 2015;135:226–230. doi: 10.1016/j.thromres.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 36.Martin M, Leffler J, Blom AM. Annexin A2 and A5 serve as new ligands for C1q on apoptotic cells. The Journal of biological chemistry. 2012;287:33733–33744. doi: 10.1074/jbc.M112.341339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leffler J, Bengtsson AA, Blom AM. The complement system in systemic lupus erythematosus: an update. Ann Rheum Dis. 2014;73:1601–1606. doi: 10.1136/annrheumdis-2014-205287. [DOI] [PubMed] [Google Scholar]
- 38.Diaz-Ramos A, Roig-Borrellas A, Garcia-Melero A, Lopez-Alemany R. alpha-Enolase, a multifunctional protein: its role on pathophysiological situations. Journal of biomedicine & biotechnology. 2012;2012:156795. doi: 10.1155/2012/156795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bruschi M, Sinico RA, Moroni G, Pratesi F, et al. Glomerular Autoimmune Multicomponents of Human Lupus Nephritis In Vivo: alpha-Enolase and Annexin AI. Journal of the American Society of Nephrology : JASN. 2014 doi: 10.1681/ASN.2013090987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bruschi M, Carnevali ML, Murtas C, Candiano G, et al. Direct characterization of target podocyte antigens and auto-antibodies in human membranous glomerulonephritis: Alfa-enolase and borderline antigens. Journal of proteomics. 2011;74:2008–2017. doi: 10.1016/j.jprot.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 41.Hsu HH, Hoffmann S, Endlich N, Velic A, et al. Mechanisms of angiotensin II signaling on cytoskeleton of podocytes. Journal of molecular medicine. 2008;86:1379–1394. doi: 10.1007/s00109-008-0399-y. [DOI] [PubMed] [Google Scholar]
- 42.Hugo C, Hugo C, Pichler R, Gordon K, et al. The cytoskeletal linking proteins, moesin and radixin, are upregulated by platelet-derived growth factor, but not basic fibroblast growth factor in experimental mesangial proliferative glomerulonephritis. The Journal of clinical investigation. 1996;97:2499–2508. doi: 10.1172/JCI118697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suzuki K, Nagao T, Itabashi M, Hamano Y, et al. A novel autoantibody against moesin in the serum of patients with MPO-ANCA-associated vasculitis. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2013 doi: 10.1093/ndt/gft469. [DOI] [PubMed] [Google Scholar]
- 44.Doyle A, Crosby SR, Burton DR, Lilley F, Murphy MF. Actin bundling and polymerisation properties of eukaryotic elongation factor 1 alpha (eEF1A), histone H2A-H2B and lysozyme in vitro. Journal of structural biology. 2011;176:370–378. doi: 10.1016/j.jsb.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 45.Wiege K, Le DD, Syed SN, Ali SR, et al. Defective macrophage migration in Galphai2- but not Galphai3-deficient mice. J Immunol. 2012;189:980–987. doi: 10.4049/jimmunol.1200891. [DOI] [PubMed] [Google Scholar]
- 46.Zhou CJ, Lo WK. Association of clathrin, AP-2 adaptor and actin cytoskeleton with developing interlocking membrane domains of lens fibre cells. Experimental eye research. 2003;77:423–432. doi: 10.1016/s0014-4835(03)00171-4. [DOI] [PubMed] [Google Scholar]
- 47.Zanin-Zhorov A, Nussbaum G, Franitza S, Cohen IR, Lider O. T cells respond to heat shock protein 60 via TLR2: activation of adhesion and inhibition of chemokine receptors. FASEB J. 2003;17:1567–1569. doi: 10.1096/fj.02-1139fje. [DOI] [PubMed] [Google Scholar]
- 48.Lang A, Benke D, Eitner F, Engel D, et al. Heat shock protein 60 is released in immune-mediated glomerulonephritis and aggravates disease: in vivo evidence for an immunologic danger signal. Journal of the American Society of Nephrology : JASN. 2005;16:383–391. doi: 10.1681/ASN.2004040276. [DOI] [PubMed] [Google Scholar]
- 49.Hurst IR, Zuo J, Jiang J, Holliday LS. Actin-related protein 2/3 complex is required for actin ring formation. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2004;19:499–506. doi: 10.1359/JBMR.0301238. [DOI] [PubMed] [Google Scholar]
- 50.Akiyama K, Morita H, Suetsugu S, Kuraba S, et al. Actin -related protein 3 (Arp3) is mutated in proteinuric BUF/Mna rats. Mammalian genome : official journal of the International Mammalian Genome Society. 2008;19:41–50. doi: 10.1007/s00335-007-9078-5. [DOI] [PubMed] [Google Scholar]
- 51.Lu Y, Liu X, Shi S, Su H, et al. Bioinformatics analysis of proteomic profiles during the process of anti-Thy1 nephritis. Molecular & cellular proteomics : MCP. 2012;11:M111 008755. doi: 10.1074/mcp.M111.008755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin CP, Adrianto I, Lessard CJ, Kelly JA, et al. Role of MYH9 and APOL1 in African and non-African populations with lupus nephritis. Genes and immunity. 2012;13:232–238. doi: 10.1038/gene.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen HH, Yu HI, Chiang WC, Lin YD, et al. hnRNP Q regulates Cdc42-mediated neuronal morphogenesis. Molecular and cellular biology. 2012;32:2224–2238. doi: 10.1128/MCB.06550-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kremer BE, Adang LA, Macara IG. Septins regulate actin organization and cell-cycle arrest through nuclear accumulation of NCK mediated by SOCS7. Cell. 2007;130:837–850. doi: 10.1016/j.cell.2007.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rosentreter A, Hofmann A, Xavier CP, Stumpf M, et al. Coronin 3 involvement in F-actin-dependent processes at the cell cortex. Experimental cell research. 2007;313:878–895. doi: 10.1016/j.yexcr.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 56.Liang P, MacRae TH. Molecular chaperones and the cytoskeleton. Journal of cell science. 1997;110(Pt 13):1431–1440. doi: 10.1242/jcs.110.13.1431. [DOI] [PubMed] [Google Scholar]
- 57.Khunkeawla P, Moonsom S, Staffler G, Kongtawelert P, Kasinrerk W. Engagement of CD147 molecule-induced cell aggregation through the activation of protein kinases and reorganization of the cytoskeleton. Immunobiology. 2001;203:659–669. doi: 10.1016/S0171-2985(01)80015-2. [DOI] [PubMed] [Google Scholar]
- 58.Kosugi T, Maeda K, Sato W, Maruyama S, Kadomatsu K. CD147 (EMMPRIN/Basigin) in kidney diseases: from an inflammation and immune system viewpoint. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2014 doi: 10.1093/ndt/gfu302. [DOI] [PubMed] [Google Scholar]
- 59.Wasik AA, Koskelainen S, Hyvonen ME, Musante L, et al. Ezrin is down-regulated in diabetic kidney glomeruli and regulates actin reorganization and glucose uptake via GLUT1 in cultured podocytes. Am J Pathol. 2014;184:1727–1739. doi: 10.1016/j.ajpath.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 60.Ostalska-Nowicka D, Zachwieja J, Nowicki M, Kaczmarek E, et al. Ezrin--a useful factor in the prognosis of nephrotic syndrome in children: an immunohistochemical approach. Journal of clinical pathology. 2006;59:916–920. doi: 10.1136/jcp.2005.031732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ahn YH, Gibbons DL, Chakravarti D, Creighton CJ, et al. ZEB1 drives prometastatic actin cytoskeletal remodeling by downregulating miR-34a expression. The Journal of clinical investigation. 2012;122:3170–3183. doi: 10.1172/JCI63608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schmieder S, Nagai M, Orlando RA, Takeda T, Farquhar MG. Podocalyxin activates RhoA and induces actin reorganization through NHERF1 and Ezrin in MDCK cells. Journal of the American Society of Nephrology : JASN. 2004;15:2289–2298. doi: 10.1097/01.ASN.0000135968.49899.E8. [DOI] [PubMed] [Google Scholar]
- 63.Zha D, Chen C, Liang W, Chen X, et al. Nephrin phosphorylation regulates podocyte adhesion through the PINCH-1-ILK-alpha-parvin complex. BMB reports. 2013;46:230–235. doi: 10.5483/BMBRep.2013.46.4.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.