Abstract
The dorsal medulla encompassing the nucleus of the tractus solitarius (NTS) and surrounding reticular formation (RF) has an important role in processing sensory information from the upper and lower airways for the generation and control of airway protective behaviors. These behaviors, such as cough and swallow, historically have been studied in isolation. However, recent information indicates that these and other airway protective behaviors are coordinated to minimize risk of aspiration. The dorsal medullary neural circuits that include the NTS are responsible for rhythmogenesis for repetitive swallowing, but previous models have assigned a role for this portion of the network for coughing that is restricted to monosynaptic sensory processing. We propose a more complex NTS/RF circuit that controls expression of swallowing and coughing and the coordination of these behaviors. The proposed circuit is supported by recordings of activity patterns of selected neural elements in vivo and simulations of a computational model of the brainstem circuit for breathing, coughing, and swallowing. This circuit includes separate rhythmic sub-circuits for all three behaviors. The revised NTS/RF circuit can account for the mode of action of antitussive drugs on the cough motor pattern, as well as the unique coordination of cough and swallow by a meta-behavioral control system for airway protection.
Keywords: cough, swallow, breathing, respiratory reflex, central pattern generator, medullary neuron, brainstem, airway protection
INTRODUCTION
Airway protection is the prevention and/or correction of aspiration. Aspiration is prevented by the pharyngeal phase of swallow, which closes the vocal folds, changes the breathing pattern, and protects the laryngeal orifice by appropriate movement of the epiglottis. Additional behaviors, such as laryngeal adduction and apnea, also participate in the prevention of aspiration. If aspiration occurs, cough corrects this problem by the production of high velocity airflows that create shear forces to dislodge and eject material from the airway [1, 2].
In contrast to the high importance of airway protective behaviors in preventing or correcting aspiration, relatively little is known about how they are regulated. Until recently, the various behaviors that contribute to protecting the airway were studied primarily in isolation. Impetus for a more unified approach has come from clinical studies that have established a strong relationship between objective metrics of cough and swallow impairment in patients at high risk of aspiration [3–5]. Further, objective metrics of cough can predict risk of aspiration with high values of sensitivity and specificity [4, 6]. More recently, specific spatiotemporal coordinating processes between cough and swallow have been discovered that indicate the response to aspiration actually represents a meta-behavior [7, 8]. In this context, the term meta-behavior represents multiple different behaviors that are actuated and controlled by the nervous system as a functional unit to prevent and limit the consequences of aspiration. Impairment of both cough and swallow across multiple neurological disorders in patients at risk for aspiration provides further support for the existence of a control system in which the normal execution of these behaviors is linked by common neurological mechanisms.
The nature of this control system has been probed with pharmaceutical approaches, employing intra-arterial and direct microinjection routes of administration. These studies have focused on either cough or swallow as endpoints of the airway protection control system. Progress in understanding the central mechanisms of airway protective behaviors has included identification of a functional gating mechanism for coughing that is separate from the brainstem circuit for breathing. Further, the caudal medulla has cough suppressant circuits that can be actuated by microinjection of excitatory amino acid agonists, GABA receptor agonists, or several cough suppressants drugs including codeine and nicotine [9–13]. Work in the rabbit has indicated that a site of action of cough suppressants is in the nucleus of the tractus solitarius (NTS) [14–18]. Collectively, these studies support an anatomically disseminated circuit that is sensitive to the effects of cough suppressant drugs. However, they also highlight an important role for the NTS in the mechanism of action of these drugs.
Current understanding of the role of the NTS in mediating cough and swallow
Airway sensory feedback related to coughing is processed by second order interneurons located near to and in various subnuclei of the NTS [19, 20]. The NTS cough-related circuit has been proposed to have primarily a relay function for transmission of information downstream including the ventrolateral (VL) medulla, midline raphe nuclei, and pons [21–24]. The core of the cough network is proposed to be located in the VL medulla and is hypothesized to participate in breathing and rhythm regulation for cough [21, 23–26]. In the rabbit, microinjection of cough suppressants into the NTS modifies cough cycle durations [16, 17]. This pattern of perturbation is typically associated with the function of a rhythm generator. These observations suggest that the role of the NTS in the neurogenesis of coughing is more extensive than “sensory pass through”. Further, these studies emphasize the incomplete knowledge base that exists regarding the NTS circuits that represent potential antitussive-sensitive elements.
Like cough, the minimal neural circuitry for generation of a swallow is restricted to the brainstem, although suprapontine mechanisms can modify swallowing[27]. Swallow is also mediated by the NTS and surrounding circuits as well as by the ventrolateral (VL) medulla [27]. Jean proposed that the core of the swallow circuit is in and around the NTS and termed this area the dorsal swallow group (DSG) [27]. He also identified the “ventral swallow group“ (VSG) in the VL medulla tasked primarily with motor and premotor control of the upper airway[27]. This hypothesis is based on activity patterns of neurons in NTS and medial reticular formation as well as in the VL medulla during swallow as well as results following perturbations of the DSG including microinjection of neurotransmitter agonists and antagonists [27–30]. This region, along with the surrounding medial reticular formation, has been proposed to process and shape vagal sensory information that induces swallowing [27, 31]. Jean and co-workers [27, 31, 32] concluded that the dorsal swallow group had a sensory processing function because short latency neuronal responses to electrical stimulation of the superior laryngeal nerve were frequently observed in the NTS region relative to longer latency responses of neurons to this stimulus that were seen in the ventrolateral medulla. However, microinjection of NMDA and glutamate into the NTS induces rhythmic swallowing[28, 33] and lesion of this area eliminates swallowing induced by electrical stimulation of supra-pontine structures [34], which supports a role for this medullary region in rhythmogenesis of this behavior. The roles of other brainstem regions known to contain swallow-responsive neurons and/or receive anatomical projections from the DSG/VSG, such as the pons and rostromedial reticular formation, are not fully understood [27]. The conclusions of Jean and co-workers regarding the role of the NTS and surrounding reticular formation in sensory processing and swallow rhythmogenesis highlight the critical importance of this area in airway protection.
Proposed coupled oscillator model of the medullary circuit controlling cough, swallow, and breathing
Our recent computational modeling efforts have been focused on NTS circuits the support the production of cough and swallowing. This computational model has been informed by a variety of different studies, including the results of multi-electrode recordings from brainstem neurons that participate in cough, swallow, and breathing [19, 21, 22, 24, 35–49]. These recordings of neuronal spike trains have been analyzed with a variety of methods intended to provide information regarding functional connectivity between neurons. Spike train cross correlation is the most common method that has been employed and is an average of the firing rate of a target neuron triggered by spikes in a reference cell. Significant differences from background firing rates allow interpretations regarding functional connectivity between the neurons. Several classes of connectivity can be inferred from features in the cross correlation histograms [50, 51]. A central peak suggests inputs shared by the two neurons and/or a one-way excitatory interaction. A peak offset in time relative to the trigger event suggests excitation of the target neuron or an unobserved shared input that influences both cells with different delays. An offset trough suggests an inhibitory process, defined as any mono-or pauci-synaptic relationship that reduces target cell firing probability following trigger neuron spikes. Cross-correlation and related approaches do not allow a unique determination of the underlying neural circuit. Their goal is to define a restricted set of possible connections [52]. As such, computational models constructed based on metrics of functional connectivity between neurons represent plausible networks based on the data. For example, functional connectivity between neuronal populations in Figure 1 is shown as “synapses” that are coded as excitatory or inhibitory and that coding is based on accumulated experimental data from cross-correlation (and other related methods) between members of the designated populations.
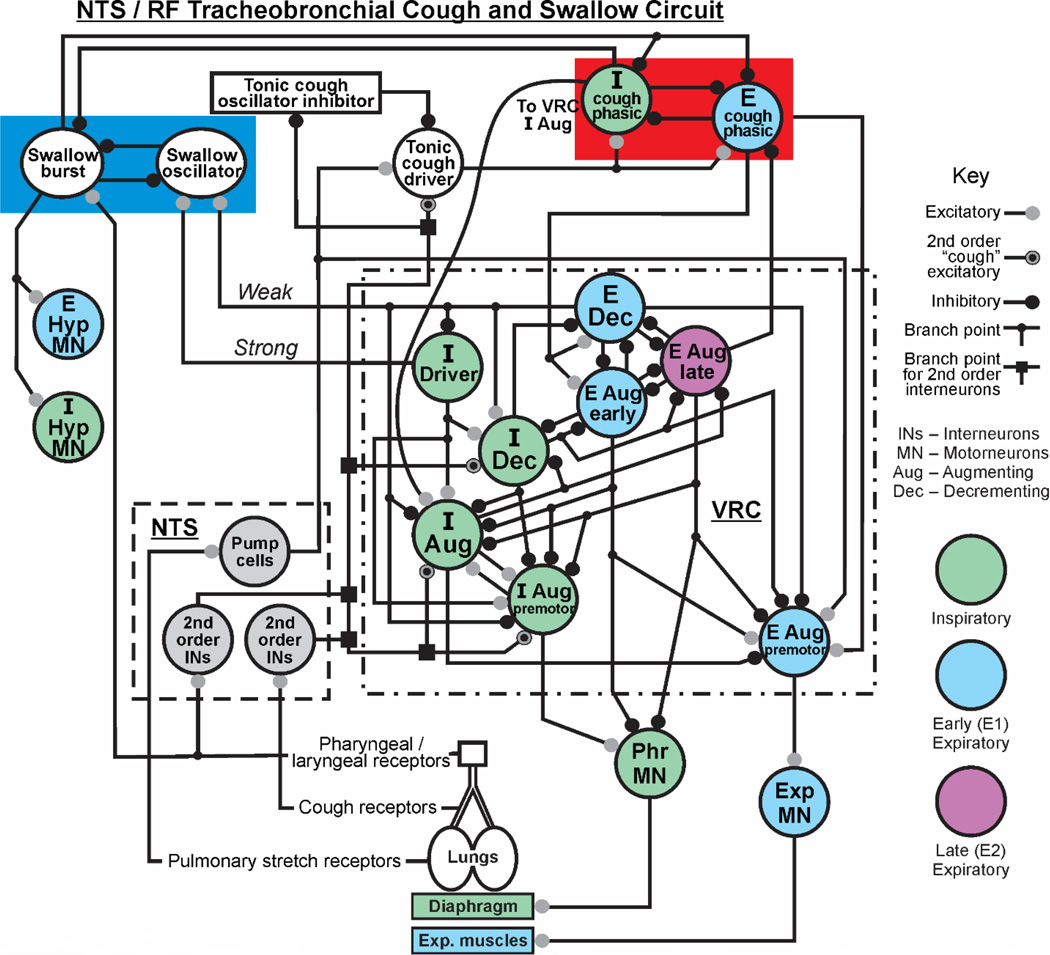
Figure 1. Proposed model of NTS/RF circuit for airway protection.
This circuit incorporates hypotheses on the coordination of cough and swallow, and preliminary results from recordings of NTS neurons. In the left section of the upper red box, a swallow oscillator circuit consisting of swallow burst and oscillator neurons is represented [1]. This circuit inhibits phasic cough inspiratory and expiratory neurons proposed to form a cough oscillatory network in the NTS/RF. The left side of the figure also shows excitatory drive to hypoglossal motoneuron pools from the swallow burst population. Preclinical experiments have identified NTS/RF neurons that are candidates for each population in the red box. The terms weak and strong represent synaptic excitation from the Exp Dec and I Driver populations, respectively, to the swallow oscillator population. These processes have been modeled [1]. Abbreviations: I-inspiratory, E-expiratory, Dec-decrementing, Aug-augmenting, VRC ventral respiratory column, Hyp-hypoglossal.
Simulations of the computational model were implemented through a C language network simulation program written for the UNIX environment. The program includes the functionality of the program SYSTM11 [53] used in previous simulations of the respiratory network [37]. The simulated networks include both discrete “integrate and fire” (IF) populations with cell parameters similar to those in SYSTEM 11 and a “hybrid” IF conditional burster” population with parameters based on the model of Breen et al. [54]. The latter population uses Hodgkin-Huxley style equations for subthreshold currents. The program allows neuron excitability to be modulated by injected current and added noise. The program also incorporates features found in NSM 2.0 [55] that aid modelling of brainstem respiratory networks, including a population type that mimics pulmonary slowly adapting stretch receptors. IF models are generally stochastic in nature, but they can be integrated with deterministic models [48]. The brainstem circuit shown in Fig. 1 is proposed to control breathing, coughing, and swallowing. This model depicts a circuit map that has been simulated as an IF (stochastic) model, but a similar network arrangement has been simulated as part of a hybrid stochastic/deterministic model that includes detailed equations that describe pulmonary and respiratory mechanics [48] during cough and breathing. The circuit does not include the ponsSimulations with the model produce cough airflows similar to those observed in humans, and has, as noted, generated predictions on circuit mechanisms for gain control of inspiratory motor drive during cough that were subsequently supported by coordinated in vivo experiments[56, 57]. The NTS circuit has been informed by the activity patterns of neurons recorded in the region of the NTS during cough and swallow and simulations of the proposed swallow oscillator (Fig. 2)[27, 58]. This proposed circuit represents the simplest modeling that is consistent with the data but not been informed by specific knowledge of functional relationships with other elements of the network. A recent study using herpes simplex virus by McGovern et al[59] mapped central pathways from the extrathoracic trachea and identified a large neuronal network in the NTS and surrounding areas. These transneuronal tracing results indicate that the size and complexity of the dorsal brainstem network for processing tracheal afferent information have been significantly underestimated. There is a major gap in our understanding of the role of the NTS and surrounding reticular formation in the neurogenesis of airway reflexes. This gap in understanding has motivated our efforts to model and simulate a circuit that will enable predictions regarding the specific functional interactions between dorsal medullary neurons and the rest of the medullary network that participates in airway defense.
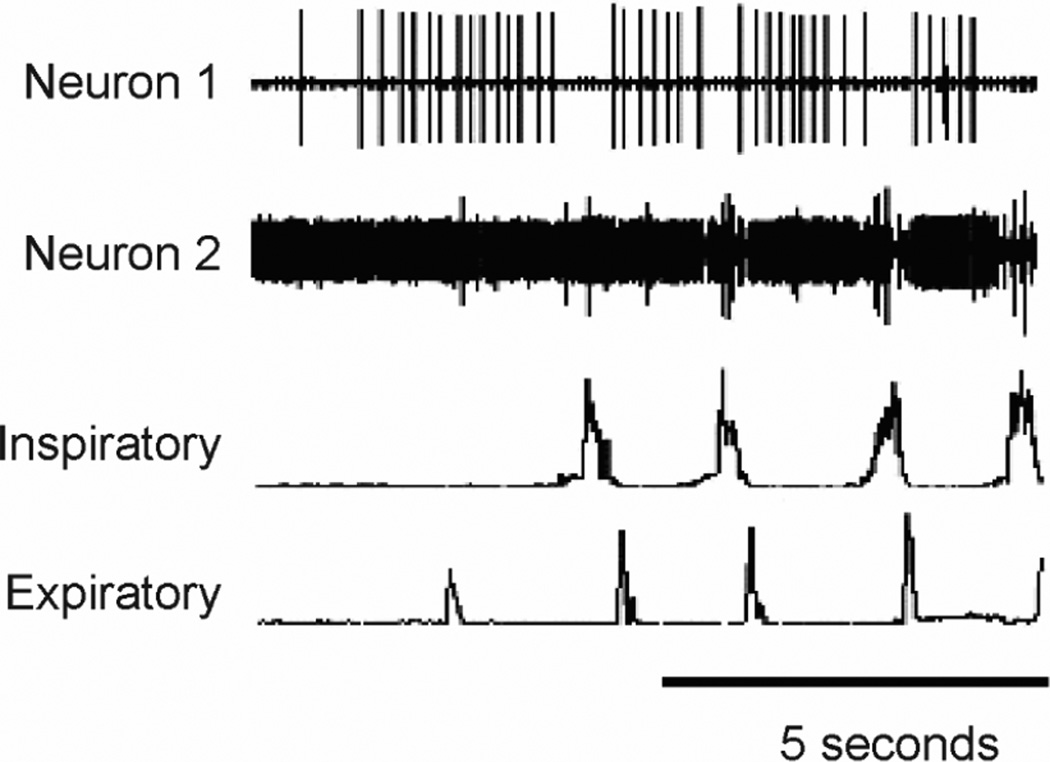
Figure 2. Activity patterns of putative E and I cough phasic neurons in the NTS.
Neuron 1 (a cough expiratory neuron) was silent during breathing and recruited before coughing began and neuron 2 is the large action potential that is recruited during the cough I phase. The recording for neuron 2 also has a smaller amplitude neuron that is tonically active but is transiently depressed during cough. Inspiratory-parasternal muscle EMG, Expiratory-transversus abdominis muscle EMG. Cough was induced by mechanical stimulation of the intrathoracic trachea in an anesthetized cat. Extracellular action potentials of NTS neurons were recorded with tungsten microelectrodes.
We have proposed that a rhythmic circuit exists within the NTS for coughing and swallowing [58] (Fig. 1). This hypothesis is supported by work in the rabbit [16, 17] as well as our preliminary results in the anesthetized cat with microinjection of the excitatory amino acid receptor antagonist, kynurenic acid. We bilaterally microinjected kynurenic acid into the rostral NTS of anesthetized cats and observed apneusis and co-activation of expiratory abdominal electromyograms during mechanical stimulation of the intrathoracic trachea, consistent with disruption of cough rhythmogenesis (data not shown). Previous versions of our model [21, 48, 56, 58, 60] that include NTS interneurons outlined in Fig. 1 by the dashed box as well as the ventral respiratory column (dotted-dashed box) produce coughing motor patterns that conform to the classical definition of this behavior: inspiration, compression, expulsion. However, sequential coughing in the human represents a hybrid motor pattern that differs from animals and is composed of an initial inspiration, compression, expulsion sequence but then converts to a repeating sequence of compression-expulsion without intervening inspiratory phases[61]. The repetitive compression-expulsions termed “re-accelerations” describes the mechanics of the airflow patterns [61, 62]. This may represent an evolutionary advantage to move mucus and aspirated material against gravity from smaller to larger airways in an upright lung [63–65]. This unique human cough pattern [61, 63] is disordered in patients with Parkinson’s disease, even if the single voluntary coughs remain relatively normal [3, 4, 66]. This compression-expulsion oscillatory behavior in humans cannot be explained by the current unified model [48] of the brainstem respiratory control network which requires inspiratory/expiratory oscillation to produce repetitive coughing.
Our preliminary simulations motivated us to add an additional “half-center” rhythmic circuit to the model specific for coughing (Fig. 1, red box right) that would allow for suppression of this behavior by antitussive drugs without affecting the core circuit for breathing (Fig. 1, dotted-dashed lines). This half-center consists of I and E cough phasic neurons that inhibit one another (Fig. 1). Candidate NTS neurons with activity patterns consistent with these populations are shown in Figure 2. Neuron 2 in this figure is a candidate I cough phasic neuron (Fig. 1) while neuron 1 is a candidate tonic cough oscillator inhibitor. Shown also is a circuit that processes afferent input from second order cough interneurons, composed of tonic cough oscillator inhibitor and tonic cough driver populations. These populations control the excitability of coughing by regulating excitatory afferent input to the cough half-center circuit. The populations are termed “tonic” because they are proposed to be active without phasic modulation during the phases of coughing. The tonic cough oscillator inhibitor population is proposed to be suppressed by inhibitory synaptic input from second-order cough receptor interneurons. This suppression would, in turn, disinhibit tonic cough driver neurons that excite the half-center circuit, enabling cough I and E oscillation to occur. The tonic cough driver population is excited by second-order cough receptor interneurons and pump cells, which are second-order interneurons that mediate pulmonary slowly adapting stretch receptor feedback (Fig. 1). A key element of this circuit arrangement is the excitation of I augmenting neurons in the ventral respiratory column in the core circuit by the I cough phasic population. This synaptic relationship would allow suppression of cough expiratory motor drive by an action of antitussive drugs on the E cough phasic population while allowing large inspiratory cough-like bursts during mechanical stimulation of the intrathoracic trachea [56, 67].
As such, this proposed circuit represents a model of multiple coupled oscillators, each of which drives a different behavior (breathing, coughing, and swallowing). Simulations of the proposed swallow oscillator circuit [58] resulted in the occurrence of this behavior primarily during expiration phase of breathing, which supports experimentally observed relationships between swallowing and breathing [68, 69]. This swallow circuit also is an example of a half-center oscillator. Linkage of the swallow half-center with the core respiratory rhythm generator occurs through strong and weak excitatory synaptic effects on I Driver and E Dec populations, respectively, which result in stronger inhibition of the swallow burst population (and disfacilitation of swallow motoneurons) during the inspiratory phase of breathing. We propose that this mechanism accounts for the expiratory phase preference of swallow during breathing.
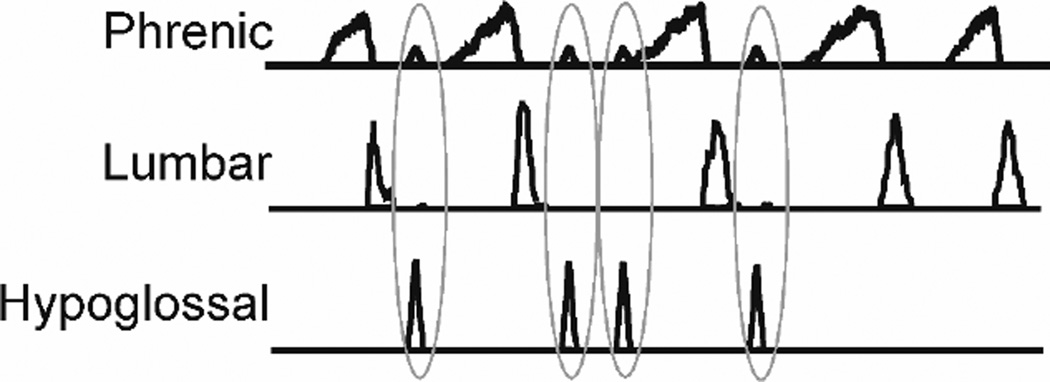
During repetitive coughing, swallow rarely, if ever, occurs during the cough inspiratory phase [8], consistent with phase restriction. The simulated coughing and swallowing shown in Fig. 3 illustrates phase restriction. Swallows occur only during the E2 phase, the period in the cough cycle in which active expulsion has ended but before the next inspiratory phase begins (Fig. 3). This phenomenon is accounted for in Fig. 1 by strong inhibition of the swallow burst population by I cough phasic neurons. This action of the cough I phasic neurons would prevent initiation of swallowing during the inspiratory phase of cough. Swallow burst neurons inhibit both I and E cough phasic populations Fig. 1, red box). This mechanism accounts for our observations that swallows only occur during the cough E phase, and suppress the onset of the subsequent cough I phase. The reciprocal inhibitory mechanism between the swallow and cough oscillators ensures that a) swallows can occur during repetitive coughing, and b) swallows can be initiated and completed before the next cough I phase begins. This coordinating mechanism supports transition of mucus that may be deposited in the pharynx by coughing into the esophagus before a subsequent cough inspiratory effort aspirates this material.
Figure 3. Simulation of repetitive coughing and swallowing.
Moving averages of phrenic (inspiratory), lumbar (expiratory), and hypoglossal (upper airway) motor outputs are shown in response to activation of both pharyngeal/laryngeal and tracheal sensory afferents. Repetitive cough is indicated by phrenic then lumbar bursts. Simulated swallows (hypoglossal bursts) are outlined by the grey ovoids.
The network model in Fig. 1 does not account for the compression-expulsion oscillation pattern exhibited by humans during coughing. However, we propose that this modeling solution represents a template that supports hypothesis development regarding cough oscillation that does not require the inspiratory phase. Our preliminary simulations support the conceptual feasibility for expulsive-adductor oscillator circuit that could account for the unique human repetitive coughing pattern. As such, our modeling efforts provide a path to predicting the minimal neural substrate that is necessary for producing the unique human coughing pattern.
Our proposed circuit, while considerably more complex than current models that address the role of NTS neurons in coughing, probably under-represents the first few synapses in the reflex pathway. Mifflin [45] identified neurons responding with monosynaptic and polysynaptic responses to electrical stimulation of the superior laryngeal nerve (SLN). Fifty percent of the monosynaptic and 100% of the polysynaptic neurons exhibited significant reductions in EPSP amplitude in response to increasing stimulus frequency applied to the SLN from 1–10 Hz [45] with virtually complete inhibition of EPSP responses at 25 Hz. One-half of the monosynaptic neurons showed little depression of SLN-induced EPSPs at 25 Hz [45]. Neurons with polysynaptic responses to electrical stimulation of the SLN often exhibited EPSP/IPSP patterns [45]. Mifflin also observed time-dependent disfacilitation of many neurons, which was consistent with primary afferent depolarization first reported by Rudomin [70]. These findings support the presence of feedforward, and possibly feedback, inhibitory mechanisms among the NTS interneuronal population that processes SLN afferent feedback.
Although Mifflin’s work [45] suggests the presence of filtering circuits based on rate coding, other neural strategies controlling information transmission have been identified, such as detailed balance [71], and gating controlled by oscillating circuits [72]. In the brainstem respiratory control system, complex processing circuits have been identified including rate-independent patterning that may regulate gain in baroreceptor pathways [73], logical gates [73], phase-dependent correlation [43] (a form of oscillatory gating), and multi-layer expiratory neuron circuits [74] that may control inspiratory drive through a mechanism analogous to detailed balance. The extent to which these core sophisticated sensory processing mechanisms participate in the regulation of afferent feedback from the airways is unknown.
Acknowledgements
Supported by NIH R01 HL103415 to DCB, R00 HL 111215 to TEP, R01 HL 109025 to PWD.
Abbreviations
- Aug
augmenting
- Dec
decrementing
- DSG
dorsal swallow group
- E
expiratory
- hyp
hypoglossal
- I
inspiratory
- NTS
nucleus of the tractus solitarius
- RF
reticular formation
- SLN
superior laryngeal nerve
- VL
ventrolateral
- VRC
ventral respiratory column
- VSG
ventral swallow group
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Bolser DC, Gestreau C, Morris KF, Davenport PW, Pitts TE. Central neural circuits for coordination of swallowing, breathing, and coughing: predictions from computational modeling and simulation. Otolaryngologic Clinics of North America. 2013;46:957–964. doi: 10.1016/j.otc.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leith DE, Butler JP, Sneddon SL, Brain JD. Cough. Handbook of Physiology The Respiratory System,V III Mechanics of Breathing, Part I. Bethesda: American Physiological Society; 1986. pp. 315–336. [Google Scholar]
- 3.Pitts T, Bolser D, Rosenbek J, Troche M, Sapienza C. Voluntary cough production and swallow dysfunction in Parkinson's disease. Dysphagia. 2008;23:297–301. doi: 10.1007/s00455-007-9144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pitts T, Troche M, Mann G, Rosenbek J, Okun MS, Sapienza C. Using voluntary cough to detect penetration and aspiration during oropharyngeal swallowing in patients with Parkinson disease. Chest. 2010;138:1426–1431. doi: 10.1378/chest.10-0342. [DOI] [PubMed] [Google Scholar]
- 5.Smith Hammond CA, Goldstein LB, Zajac DJ, Gray L, Davenport PW, Bolser DC. Assessment of aspiration risk in stroke patients with quantification of voluntary cough. Neurology. 2001;56:502–506. doi: 10.1212/wnl.56.4.502. [DOI] [PubMed] [Google Scholar]
- 6.Smith Hammond CA, Goldstein LB, Horner RD, Ying J, Gray L, Gonzalez-Rothi L, et al. Predicting aspiration in patients with ischemic stroke: comparison of clinical signs and aerodynamic measures of voluntary cough. Chest. 2009;135:769–777. doi: 10.1378/chest.08-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pitts T, Morris KF, Lindsey BG, Davenport PW, Poliacek I, Bolser DC. Co-ordination of cough and swallow in vivo and in silico. Experimental Physiology. 2012;97:469–473. doi: 10.1113/expphysiol.2011.063362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pitts T, Rose MJ, Mortensen AN, Poliacek I, Sapienza CM, Lindsey BG, Morris KF, Davenport PW, Bolser DC. Coordination of cough and swallow: a meta-behavioral response to aspiration. Respir Physiol Neurobiol. 2013;189:543–551. doi: 10.1016/j.resp.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poliacek I, Corrie LW, Wang C, Rose MJ, Bolser DC. Microinjection of DLH into the region of the caudal ventral respiratory column in the cat: evidence for an endogenous coughsuppressant mechanism. J Appl Physiol. 2007;102:1014–1021. doi: 10.1152/japplphysiol.00616.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poliacek I, Wang C, Corrie LW, Rose MJ, Bolser DC. Microinjection of codeine into the region of the caudal ventral respiratory column suppresses cough in anesthetized cats. J Appl Physiol. 2010;108:858–865. doi: 10.1152/japplphysiol.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poliacek I, Rose MJ, Pitts TE, Mortensen A, Corrie LW, Davenport PW, Bolser DC. Central administration of nicotine suppresses tracheobronchial cough in anesthetized cats. J Appl Physiol (1985) 2015;118:265–272. doi: 10.1152/japplphysiol.00075.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cinelli E, Bongianni F, Pantaleo T, Mutolo D. Modulation of the cough reflex by GABA(A) receptors in the caudal ventral respiratory group of the rabbit. Front Physiol. 2012;3:403. doi: 10.3389/fphys.2012.00403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mutolo D, Bongianni F, Cinelli E, Pantaleo T. Depression of cough reflex by microinjections of antitussive agents into caudal ventral respiratory group of the rabbit. J Appl Physiol (1985) 2010;109:1002–1010. doi: 10.1152/japplphysiol.00406.2010. [DOI] [PubMed] [Google Scholar]
- 14.Mutolo D, Bongianni F, Cinelli E, Fontana GA, Pantaleo T. Modulation of the cough reflex by antitussive agents within the caudal aspect of the nucleus tractus solitarii in the rabbit. Am J Physiol Regul Integr Comp Physiol. 2008;295:R243–R251. doi: 10.1152/ajpregu.00184.2008. [DOI] [PubMed] [Google Scholar]
- 15.Mutolo D, Bongianni F, Fontana GA, Pantaleo T. The role of excitatory amino acids and substance P in the mediation of the cough reflex within the nucleus tractus solitarii of the rabbit. Brain Res Bull. 2007;74:284–293. doi: 10.1016/j.brainresbull.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Mutolo D, Cinelli E, Bongianni F, Pantaleo T. Inhibitory control of the cough reflex by galanin receptors in the caudal nucleus tractus solitarii of the rabbit. Am J Physiol Regul Integr Comp Physiol. 2014;307:R1358–R1367. doi: 10.1152/ajpregu.00237.2014. [DOI] [PubMed] [Google Scholar]
- 17.Cinelli E, Bongianni F, Pantaleo T, Mutolo D. Suppression of the cough reflex by alpha 2-adrenergic receptor agonists in the rabbit. Physiol Rep. 2013;1:e00122. doi: 10.1002/phy2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cinelli E, Bongianni F, Pantaleo T, Mutolo D. The cough reflex is upregulated by lisinopril microinjected into the caudal nucleus tractus solitarii of the rabbit. Respir Physiol Neurobiol. 2015;219:9–17. doi: 10.1016/j.resp.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 19.Lipski J, Ezure K, Wong She RB. Identification of neurons receiving input from pulmonary rapidly adapting receptors in the cat. The Journal of physiology. 1991;443:55–77. doi: 10.1113/jphysiol.1991.sp018822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ezure K, Otake K, Lipski J, Wong She RB. Efferent projections of pulmonary rapidly adapting receptor relay neurons in the cat. Brain Research. 1991;564:268–278. doi: 10.1016/0006-8993(91)91463-b. [DOI] [PubMed] [Google Scholar]
- 21.Shannon R, Baekey DM, Morris KF, Lindsey BG. Ventrolateral medullary respiratory network and a model of cough motor pattern generation. J Appl Physiol. 1998;84:2020–2035. doi: 10.1152/jappl.1998.84.6.2020. [DOI] [PubMed] [Google Scholar]
- 22.Baekey DM, Morris KF, Gestreau C, Li Z, Lindsey BG, Shannon R. Medullary respiratory neurones and control of laryngeal motoneurones during fictive eupnoea and cough in the cat. The Journal of physiology. 2001;534:565–581. doi: 10.1111/j.1469-7793.2001.t01-1-00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shannon R, Baekey D, Morris K, Lindsey B. Brainstem respiratory networks and cough. Pulmonary Pharmacology. 1996;9:343–347. doi: 10.1006/pulp.1996.0045. [DOI] [PubMed] [Google Scholar]
- 24.Shannon R, Baekey DM, Morris KF, Li Z, Lindsey BG. Functional connectivity among ventrolateral medullary respiratory neurones and responses during fictive cough in the cat. The Journal of physiology. 2000;525(Pt 1):207–224. doi: 10.1111/j.1469-7793.2000.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shannon R, Baekey D, Morris K, Nuding S, Segers L, Lindsey BG. Production of reflex cough by brainstem respiratory networks. Pulmonary Pharmacology & Therapeutics. 2004;17:369–376. doi: 10.1016/j.pupt.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 26.Shannon R, Baekey DM, Morris KF, Lindsey BG. Brainstem respiratory networks and cough. Pulm Pharmacol. 1996;9:343–347. doi: 10.1006/pulp.1996.0045. [DOI] [PubMed] [Google Scholar]
- 27.Jean A. Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiological Review. 2001;81:929–969. doi: 10.1152/physrev.2001.81.2.929. [DOI] [PubMed] [Google Scholar]
- 28.Kessler JP, Jean A. Evidence that activation of N-methyl-D-aspartate (NMDA) and non-NMDA receptors within the nucleus tractus solitarii triggers swallowing. Eur J Pharmacol. 1991;201:59–67. doi: 10.1016/0014-2999(91)90323-i. [DOI] [PubMed] [Google Scholar]
- 29.Kessler JP, Jean A. Inhibition of the swallowing reflex by local application of serotonergic agents into the nucleus of the solitary tract. Eur J Pharmacol. 1985;118:77–85. doi: 10.1016/0014-2999(85)90665-x. [DOI] [PubMed] [Google Scholar]
- 30.Kessler JP, Jean A. Effect of catecholamines on the swallowing reflex after pressure microinjections into the lateral solitary complex of the medulla oblongata. Brain Res. 1986;386:69–77. doi: 10.1016/0006-8993(86)90142-3. [DOI] [PubMed] [Google Scholar]
- 31.Jean A. Control of the central swallowing program by inputs from the peripheral receptors. A review. Journal of the autonomic nervous system. 1984;10:225–233. doi: 10.1016/0165-1838(84)90017-1. [DOI] [PubMed] [Google Scholar]
- 32.Kessler JP, Jean A. Identification of the medullary swallowing regions in the rat. Exp Brain Res. 1985;57:256–263. doi: 10.1007/BF00236530. [DOI] [PubMed] [Google Scholar]
- 33.Kessler JP, Cherkaoui N, Catalin D, Jean A. Swallowing responses induced by microinjection of glutamate and glutamate agonists into the nucleus tractus solitarius of ketamine-anesthetized rats. Exp Brain Res. 1990;83:151–158. doi: 10.1007/BF00232203. [DOI] [PubMed] [Google Scholar]
- 34.Jean A, Car A. Inputs to the swallowing medullary neurons from the peripheral afferent fibers and the swallowing cortical area. Brain Res. 1979;178:567–572. doi: 10.1016/0006-8993(79)90715-7. [DOI] [PubMed] [Google Scholar]
- 35.Baekey DM, Morris KF, Nuding SC, Segers LS, Lindsey BG, Shannon R. Medullary raphe neuron activity is altered during fictive cough in the decerebrate cat. J Appl Physiol. 2003;94:93–100. doi: 10.1152/japplphysiol.00341.2002. [DOI] [PubMed] [Google Scholar]
- 36.Baekey DM, Morris KF, Nuding SC, Segers LS, Lindsey BG, Shannon R. Ventrolateral medullary respiratory network participation in the expiration reflex in the cat. J Appl Physiol. 2004;96:2057–2072. doi: 10.1152/japplphysiol.00778.2003. [DOI] [PubMed] [Google Scholar]
- 37.Balis UJ, Morris KF, Koleski J, Lindsey BG. Simulations of a ventrolateral medullary neural network for respiratory rhythmogenesis inferred from spike train cross-correlation. Biol Cybern. 1994;70:311–327. doi: 10.1007/BF00200329. [DOI] [PubMed] [Google Scholar]
- 38.Gestreau C, Milano S, Bianchi AL, Grelot L. Activity of dorsal respiratory group inspiratory neurons during laryngeal-induced fictive coughing and swallowing in decerebrate cats. Experimental brain research Experimentelle Hirnforschung Experimentation cerebrale. 1996;108:247–256. doi: 10.1007/BF00228098. [DOI] [PubMed] [Google Scholar]
- 39.Lindsey B, Hernandez Y, Morris K, Shannon R. Functional connectivity between brain stem midline neurons with respiratory-modulated firing rates. Journal of neurophysiology. 1992;67:890–904. doi: 10.1152/jn.1992.67.4.890. [DOI] [PubMed] [Google Scholar]
- 40.Lindsey B, Hernandez Y, Morris K, Shannon R, Gerstein G. Dynamic reconfiguration of brain stem neural assemblies: respiratory phase-dependent synchrony versus modulation of firing rates. J Neurophysiol. 1992;67:923–930. doi: 10.1152/jn.1992.67.4.923. [DOI] [PubMed] [Google Scholar]
- 41.Lindsey B, Morris K, Shannon R, Gerstein G. Repeated patterns of distributed synchrony in neuronal assemblies. J Neurophysiol. 1997;78:1714–1719. doi: 10.1152/jn.1997.78.3.1714. [DOI] [PubMed] [Google Scholar]
- 42.Lindsey B, Ott M, Nuding S, Segers L, O'Connor R, Morris KF. Central chemoreceptors modulate breathing via multipath tuning in ventrolateral respiratory column (VRC) circuits. FASEB J. 2011;25:847.27. doi: 10.1152/jn.00808.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lindsey BG, Hernandez YM, Morris KF, Shannon R, Gerstein GL. Dynamic reconfiguration of brain stem neural assemblies: respiratory phase-dependent synchrony versus modulation of firing rates. J Neurophysiol. 1992;67:923–930. doi: 10.1152/jn.1992.67.4.923. [DOI] [PubMed] [Google Scholar]
- 44.Lindsey BG, Hernandez YM, Morris KF, Shannon R, Gerstein GL. Respiratory-related neural assemblies in the brain stem midline. J Neurophysiol. 1992;67:905–922. doi: 10.1152/jn.1992.67.4.905. [DOI] [PubMed] [Google Scholar]
- 45.Mifflin SW. Laryngeal afferent inputs to the nucleus of the solitary tract. The American journal of physiology. 1993;265:R269–R276. doi: 10.1152/ajpregu.1993.265.2.R269. [DOI] [PubMed] [Google Scholar]
- 46.Morris KF, Arata A, Shannon R, Lindsey BG. Long-term facilitation of phrenic nerve activity in cats: responses and short time scale correlations of medullary neurones. J Physiol. 1996;490(Pt 2):463–480. doi: 10.1113/jphysiol.1996.sp021158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morris KF, Shannon R, Lindsey BG. Changes in cat medullary neurone firing rates and synchrony following induction of respiratory long-term facilitation. J Physiol. 2001;532:483–497. doi: 10.1111/j.1469-7793.2001.0483f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O'Connor R, Segers LS, Morris KF, Nuding SC, Pitts T, Bolser DC, et al. A joint computational respiratory neural network-biomechanical model for breathing and airway defensive behaviors. Front Physiol. 2012;3:264. doi: 10.3389/fphys.2012.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ott MM, Nuding SC, Segers LS, Lindsey BG, Morris KF. Ventrolateral medullary functional connectivity and the respiratory and central chemoreceptor-evoked modulation of retrotrapezoid-parafacial neurons. J Neurophysiol. 2011;105:2960–2975. doi: 10.1152/jn.00262.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aertsen AM, Gerstein GL. Evaluation of neuronal connectivity: sensitivity of crosscorrelation. Brain Res. 1985;340:341–354. doi: 10.1016/0006-8993(85)90931-x. [DOI] [PubMed] [Google Scholar]
- 51.Moore GP, Segundo JP, Perkel DH, Levitan H. Statistical signs of synaptic interaction in neurons. Biophys J. 1970;10:876–900. doi: 10.1016/S0006-3495(70)86341-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aertsen AMHJ, Gerstein GL, Habib MK, Palm G. Dynamics of Neuronal Firing Correlation - Modulation of Effective Connectivity. Journal of Neurophysiology. 1989;61:900–917. doi: 10.1152/jn.1989.61.5.900. [DOI] [PubMed] [Google Scholar]
- 53.MacGregor RJ. Neural and brain modeling. San Diego, Calif.: Academic Press; 1987. [Google Scholar]
- 54.Breen BJ, Gerken WC, Butera RJ., Jr Hybrid integrate-and-fire model of a bursting neuron. Neural Comput. 2003;15:2843–2862. doi: 10.1162/089976603322518768. [DOI] [PubMed] [Google Scholar]
- 55.Rybak IA, Shevtsova NA, St-John WM, Paton JF, Pierrefiche O. Endogenous rhythm generation in the pre-Botzinger complex and ionic currents: modelling and in vitro studies. Eur J Neurosci. 2003;18:239–257. doi: 10.1046/j.1460-9568.2003.02739.x. [DOI] [PubMed] [Google Scholar]
- 56.Poliacek I, Morris KF, Lindsey BG, Segers LS, Rose MJ, Corrie LW, Wang C, Pitts TE, Davenport PW, Bolser DC. Blood pressure changes alter tracheobronchial cough: computational model of the respiratory-cough network and in vivo experiments in anesthetized cats. J Appl Physiol. 2011;111:861–873. doi: 10.1152/japplphysiol.00458.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Segers LS, Nuding SC, Vovk A, Pitts T, Baekey DM, O’Connor R, Morris KF, Lindsey BG, Shannon R, Bolser DC. Discharge identity of medullary inspiratory neurons is altered during repetitive fictive cough. Frontiers in physiology. 2012:3. doi: 10.3389/fphys.2012.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bolser DC, Gestreau C, Morris KF, Davenport PW, Pitts TE. Central neural circuits for coordination of swallowing, breathing, and coughing: predictions from computational modeling and simulation. Otolaryngologic clinics of North America. 2013;46:957–964. doi: 10.1016/j.otc.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McGovern AE, Davis-Poynter N, Farrell MJ, Mazzone SB. Transneuronal tracing of airways-related sensory circuitry using herpes simplex virus 1, strain H129. Neuroscience. 2012;207:148–166. doi: 10.1016/j.neuroscience.2012.01.029. [DOI] [PubMed] [Google Scholar]
- 60.Bolser DC, Pitts TE, Morris KF. The use of multiscale systems biology approaches to facilitate understanding of complex control systems for airway protection. Current Opinion in Pharmacology. 2011 doi: 10.1016/j.coph.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vovk A, Bolser DC, Hey JA, Danzig M, Vickroy T, Berry R, Martin AV, Davenport PW. Capsaicin exposure elicits complex airway defensive motor patterns in normal humans in a concentration-dependent manner. Pulm Pharmacol Ther. 2007;20:423–432. doi: 10.1016/j.pupt.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chung KF, Bolser D, Davenport P, Fontana G, Morice A, Widdicombe J. Semantics and types of cough. Pulm Pharmacol Ther. 2009;22:139–142. doi: 10.1016/j.pupt.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Loudon RG, Shaw GB. Mechanics of cough in normal subjects and in patients with obstructive respiratory disease. Am Rev Respir Dis. 1967;96:666–677. doi: 10.1164/arrd.1967.96.4.666. [DOI] [PubMed] [Google Scholar]
- 64.Mahajan RP, Singh P, Murty GE, Aitkenhead AR. Relationship between expired lung volume, peak flow rate and peak velocity time during a voluntary cough manoeuvre. Br J Anaesth. 1994;72:298–301. doi: 10.1093/bja/72.3.298. [DOI] [PubMed] [Google Scholar]
- 65.Ross BB, Gramiak R, Rahn H. Physical dynamics of the cough mechanism. J Appl Physiol. 1955;8:264–268. doi: 10.1152/jappl.1955.8.3.264. [DOI] [PubMed] [Google Scholar]
- 66.Pitts T, Bolser D, Rosenbek J, Troche M, Okun MS, Sapienza C. Impact of expiratory muscle strength training on voluntary cough and swallow function in Parkinson disease. Chest. 2009;135:1301–1308. doi: 10.1378/chest.08-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bolser DC, Hey JA, Chapman RW. Influence of central antitussive drugs on the cough motor pattern. J Appl Physiol. 1999;86:1017–1024. doi: 10.1152/jappl.1999.86.3.1017. [DOI] [PubMed] [Google Scholar]
- 68.Bautista TG, Sun QJ, Pilowsky PM. The generation of pharyngeal phase of swallow and its coordination with breathing: interaction between the swallow and respiratory central pattern generators. Progress in brain research. 2014;212:253–275. doi: 10.1016/B978-0-444-63488-7.00013-6. [DOI] [PubMed] [Google Scholar]
- 69.Dick TE, Oku Y, Romaniuk JR, Cherniack NS. Interaction between central pattern generators for breathing and swallowing in the cat. The Journal of physiology. 1993;465:715–730. doi: 10.1113/jphysiol.1993.sp019702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rudomin P. Excitability changes of superior laryngeal, vagal and depressor afferent terminals produced by stimulation of the solitary tract nucleus. Exp Brain Res. 1968;6:156–170. doi: 10.1007/BF00239169. [DOI] [PubMed] [Google Scholar]
- 71.Vogels TP, Abbott LF. Gating multiple signals through detailed balance of excitation and inhibition in spiking networks. Nature neuroscience. 2009;12:483–491. doi: 10.1038/nn.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Akam T, Kullmann DM. Oscillations and filtering networks support flexible routing of information. Neuron. 2010;67:308–320. doi: 10.1016/j.neuron.2010.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arata A, Hernandez YM, Lindsey BG, Morris KF, Shannon R. Transient configurations of baroresponsive respiratory-related brainstem neuronal assemblies in the cat. The Journal of physiology. 2000;525(Pt 2):509–530. doi: 10.1111/j.1469-7793.2000.t01-1-00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Segers LS, Nuding SC, Ott MM, Dean JB, Bolser DC, O'Connor R, Morris KF, Lindsey BG. Peripheral chemoreceptors tune inspiratory drive via tonic expiratory neuron hubs in the medullary ventral respiratory column network. J Neurophysiol. 2015;113:352–368. doi: 10.1152/jn.00542.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]