Abstract
Objective
Coronary artery disease (CAD) is the leading cause of excess deaths in RA. However, identification of features denoting those with CAD risk is lacking. The composition of circulating mononuclear cell (PBMC) subsets in RA cases differs markedly from healthy controls in extent of T-cell activation with clonal expansion and differentiation to memory effector status and presence of inflammatory monocytes. We sought to evaluate whether elevations in these subpopulations in RA denote those with increased risk for subclinical CAD, as measured by coronary artery calcium (CAC).
Methods
72 RA patients underwent cardiac computed tomography to assess CAC. PBMC subsets were determined by multiparameter flow cytometry. Multivariable logistic regression was used to determine the associations between PBMC subpopulations and presence of CAC.
Results
33% had CAC and exhibited significant increases of circulating CD4 T cell subsets denoting activation and differentiation to memory effector phenotypes. Analogous increases in CD8 T cell subsets, and intermediate CD14hiCD16+monocytes, were also present compared to those without CAC. The CD4 and CD8 T cell subset increases were highly intercorrelated, while increases in CD14hiCD16+monocytes were independent of the elevated CD4 subsets. After adjustment for relevant confounders, levels of CD4+CD56+CD57+ T cells and CD14hiCD16+monocytes remained associated with the presence of CAC.
Conclusions
These PBMC subsets are markers for CAC and suggest mechanisms of atherogenesis in RA may operate in part through their increases, raising further questions about the mechanisms underlying the presence of these subset alterations in RA and the potential for shared etiologic pathways between RA and CVD.
Introduction
Rheumatoid Arthritis (RA) is associated with a 1.5-3-fold increase in mortality compared with healthy controls and cardiovascular disease (CVD) is the leading cause of these excess deaths. Traditional CV risk factors alone do not account for the increased rates of CVD in RA, suggesting that RA itself is an independent CVD risk factor.(1, 2)
The autoimmune process underlying RA is conceptualized as based on the selection of CD4 T cells by certain MHC allomorphs (3) that bear a sequence motif containing positively charged residues encoding a portion of the P4 pocket structure termed the ‘shared epitope’.(4) Activated CD4 T cells expressing HLA-DR molecules, and dominant clonal expansions in the memory-effector T cell subset, are present in the blood of many individuals with RA at levels far higher than in matched healthy controls, although the different potential factors responsible for this elevation are not satisfactorily understood. (3, 5–8)
In general, activation of antigen-specific T cells, often denoted by HLA-DR expression(5), followed by clonal expansion and progressive T cell differentiation along defined maturation pathways involving transcriptional regulation of various molecules involved in recognition and response, and their patterns of acquisition and loss define functionally different T cell subsets involved in protective memory and immune effector functions.(9) Their differentiation from the naïve state to a memory-effector phenotype is denoted by extinction of the expression of CD28 (CD4+CD28null T cells) and a shift from CD45RA to CD45RO isotype. The pro-inflammatory and autoreactive potential of the CD4+CD28null T cell is well recognized.(10, 11) These activated, expanded and differentiated T cells are widely considered as an intrinsic feature of RA.(3, 7, 8) However, their origin remains poorly understood, and puzzlingly, elevations in the frequency of CD4+CD28null T cells are not correlated with disease activity measurements, exhibit only a weak association with extra articular disease, and are multiple orders of magnitude more frequent than T cells specific for RA antigens such as citrullinated peptides.(7, 8, 12)
The memory-effector subsets also exhibit variable expression of Natural Killer Receptors (NKR) such as CD56, and CD57 that reflect a terminal differentiation phase. CD4 and CD8 T cells characterized by the expression of CD56 and, or CD57 have also been described in some patients with RA. (13–16) The acquisition of an array of NKR enables the CD4+CD28null T cells to be triggered by danger signal ligands expressed by stressed or injured cells(17), providing a new “signal 2” that cooperates with TCR engagement in activating the cell in a site of injury or inflammation.
The intensity of the surface expression of the LPS receptor CD14 and the presence or absence of the FcγIII receptor, CD16, determine three subpopulations of monocytes “classic” CD14hiCD16−, “nonclassic” CD14dimCD16− monocytes, and CD14hiCD16+ “intermediate”(18). The intermediate populations have previously been designated “inflammatory” and are significantly increased in RA, compared with healthy controls.(19) Detailed characterization of the properties of the CD14hiCD16+ intermediate monocyte subset supports their inflammatory potential, including their heightened expression of proinflammatory genes, among them TNF-α (20, 21) and their capacity to induce Th17 cell expansion in vitro(19), although similar to the T cell subset abnormalities, the origin and significance of the intermediate monocyte subset in the immunobiology of RA remain incompletely understood.
Somewhat analogous elevations of CD4, CD8 memory-effector and inflammatory monocyte subsets have been extensively described in otherwise healthy individuals with atherosclerosis, see(22–24). In view of the importance of coronary artery disease (CAD) as a major contributor to the increased mortality evident in RA(1), we sought to explore the hypothesis that the increases in the proportion of the CD14hiCD16+ monocyte subpopulation, together with activation and expansion of the memory-effector CD4+CD28null subset with differentiation to CD56 and CD57 expression that are features of RA would denote those at risk for accelerated development of atherosclerosis independent of conventional CVD risk factors, implying some processes of adaptive immune system activation that appear to be characteristic of RA patients are intertwined with the cellular processes leading to atherosclerotic disease.
Methods
Participants and Enrollment
These cross-sectional analyses derive from baseline data from an ongoing study of the prevalence and characteristics associated with subclinical myocardial dysfunction in RA (the RHeumatoid arthritis mYocardium Study [RHYTHM study]). Enrollment required fulfillment of the 2010 American College of Rheumatology criteria for RA, and age > 18 years old.(25) Individuals with prevalent cardiovascular disease, defined as a self-reported history of physician-diagnosed myocardial infarction, heart failure, coronary artery revascularization, angioplasty, peripheral arterial disease or procedures, pacemaker, or defibrillator devices were excluded. Additional exclusion criteria included an active history of cancer and contraindications to having a PET-CT scan, receiving adenosine, Regadenoson, or fluorodexoyglucose (FDG). Participants were recruited from among the RA patients being followed at the Columbia University Medical Center and from local referring rheumatologists. The study was approved by the Columbia University Institutional Review Board. All participants provided written consent prior to participation
Study Outcome
CAC was ascertained using cardiac multi-detector row computed tomography. CAC was quantified using the Agatston method.(26) For these analyses the presence of CAC was defined by an Agatston score greater than zero.
Clinical Covariates
Information on demographics, smoking, level of education and family history was collected by questionnaire. Resting blood pressure (BP) was measured three times in the seated position, and the average of the last two measurements was used. Hypertension was defined by systolic BP ≥ 140 mmHg, diastolic BP ≥ 90, or antihypertensive medication use. Diabetes was defined as a fasting serum glucose ≥ 126 mg/ dL or use of anti-diabetic medications. The BMI was calculated by dividing weight (in kilograms) by the square of height (in meters).
Measures of RA disease activity (number of tender and swollen joints, and calculation of the 28-joint based disease activity score [DAS28-CRP])(27, 28) and functional capacity (with the health assessment questionnaire (HAQ)) (29) were computed.
Laboratory Covariates
PBMC immunophenotyping
PBMC isolation, staining and multicolor flow cytometry analyses were performed as described.(30) The panel of reagents used to stain each sample in different combinations of four reagents included: From Becton Dickinson (Pharmingen), CD16 FITC, CD14 PE, CD8 PE, CD4 Alexa, CD4PE-CY7, CD27 PE, CD28 PE, CD28 APC, CD45RA FITC, HLA-DR FITC, CD56 APC, NKAT2 PE, NKG2D PE, and CD183 APC; from Miltenyi Biotech, CD3PE-vio770; from eBiosciences, CD8 APC, and CD57 FITC. Each reagent was titrated for use at a concentration that provided optimum plateau discrimination of positive and negative cells and that gave optimal linear fluorescence compensation. Flow cytometry was performed in a Becton-Dickinson Biosciences dual laser FACScaliber, using FlowJo software for subsequent data analysis. Optimal laser alignment and operational paramenters were validated by using fluochrome-bearing beads to achieve reproducible median fluorescence intensity. A minimum of 50,000 events were collected. Lymphocyte and monocyte populations were first identified and gated on forward and orthogonal light scatter signals. Propidium Iodide is routinely added to 2 and 3 fluorochrome stain experiments. Cells taking up Propidium Iodide are used to revise the FSC SSC gates to minimize inclusion of lymphocytes or monocytes taking up Propidium Iodide for all subsequent experiments. The efficiency of this gating strategy yields data based on a population with >98% viable cells. As necessary, subsequent gating was first on CD3, to identify CD3 T cells, followed by gating on either CD4 or CD8 subsets. The Fluorescence Minus One strategy was uniformly used for setting the gating parameters for positive versus negative staining. The CD4 subpopulations are designated as % CD28− (in CD4+), the percentage of the population of CD3+CD4+ T cells that lack expression of CD28; % CD56+ (in CD4+), the percentage of CD3+CD4+ T cells that express CD56; % CD57+ (in CD4+), the percentage of CD3+CD4+ T cells that express CD57; % CD57+ (in CD4+CD56+), the percentage of CD3+CD4+CD56+ T cells that express CD57; %CD28− CD57+ (in CD4+), the percentage of CD3+CD4+ T cells lacking the expression of CD28 that express CD57; %HLA-DR+(in CD4+), the percentage of CD3+CD4+ T cells that express HLA-DR; % HLA-DR+ CD28− (in CD4+), the percentage of CD3+CD4+ T cells that both lack expression of CD28 and are positive for HLA-DR. The CD8 subpopulations are similarly designated.
HLA typing
HLA-DRB1 alleles were determined by sequence-based typing and shared epitope-bearing alleles were identified as described. (31–33)
Lipids and Inflammatory Biomarkers
The remaining analytes including autoantibodies, blood chemistries, CRP and interleukin-6 (IL-6) were measured in the Columbia Clinical and Translational Research Center’s biomarkers laboratory, see http://irvinginstitute.columbia.edu/resources/biomarkers_core.html for details
Statistical Analysis
Variable distributions were examined and compared according to subgroups using t-tests for normally distributed continuous variables, the Kruskal-Wallis test for non-normal continuous variables, and the chi-square or Fisher’s exact test, as appropriate, for categorical variables. Multivariable ordinary logistic regression was used to model the associations of the frequency of PBMC subsets with the presence of any CAC. Variables were transformed to normality as required. Spearman coefficients were calculated to explore the correlations between PBMC subsets. Variance inflation factors were calculated for the covariates of multivariable models to ensure that collinear variables were not co-modeled. To isolate the associations of PBMC subset frequency on CAC, confounders were those that were associated with both the outcome (any CAC) and the PBMC subsets of interest at the p<0.20 level, to account for residual confounding. Non-contributory covariates were excluded using the Likelihood-Ratio Test for nested models. For the final adjusted model, receiver operator characteristics (ROC) were calculated for the various nested model scenarios to explore the adjusted contributions of each covariate to the model, with statistical comparisons of the area under the receiver operator curves (AUCs) between separate models characterized by the addition or exclusion of covariates. Multivariable linear regression was used to explore the associations of the frequency of PBMC subsets with the extent of CAC [as a continuous variable, normally transformed by log(CAC+1)]. Statistical calculations were performed using Intercooled Stata 12 (StataCorp, College Station, TX). A two-tailed α=0.05 was used throughout.
Results
Clinical Characteristics of Participants
Demographic data, RA characteristics and traditional CVD risk factors of the 72 patients are summarized in Table 1 according to the presence or absence of any CAC. One third of patients (24 of 72) demonstrated the presence of CAC. Compared with the group without CAC, RA patients with CAC were significantly older, had higher systolic and diastolic blood pressures, and lower HDL-C, on average. RA patients with CAC also tended to have longer RA duration and were more frequently treated with biologics, although neither of these associations reached statistical significance. The presence of any CAC was not associated with autoantibody status, presence of shared epitope, clinical measures of disease activity, or serological measures of inflammation.
Table 1.
Baseline Characteristics According to Any CAC*
| Total (n=72) |
No CAC (n=48) |
Any CAC (n=24) |
p-value | |
|---|---|---|---|---|
| Age, years | 54 ± 14 | 48 ± 13 | 64 ± 7 | <0.001 |
| Female, n (%) | 62 (86) | 43 (90) | 19 (79) | 0.23 |
| Any college education, n (%) | 44 (62) | 29 (62) | 15 (63) | 0.95 |
| Ever smoking, n (%) | 30 (42) | 18 (38) | 12 (50) | 0.34 |
| Current smoking, n (%) | 6 (8) | 4 (9) | 2 (8) | 0.99 |
| SBP, mm Hg | 117 ± 18 | 112 ± 16 | 127 ± 18 | 0.002 |
| DBP, mm Hg | 70 ± 10 | 69 ± 11 | 74 ± 9 | 0.044 |
| Hypertension, n (%) | 31 (43) | 15 (31) | 16 (67) | 0.004 |
| Anti-hypertensive use, n (%) | 30 (41) | 9 (18) | 16 (67) | <0.001 |
| BMI, kg/m | 28.3 ± 6.2 | 27.7 ± 6.6 | 29.5 ± 5.3 | 0.23 |
| Waist circumference, cm | 90 ± 18 | 88 ± 18 | 94 ± 18 | 0.25 |
| Normal fasting glucose, n (%) | 48 (71) | 34 (76) | 14 (61) | 0.21 |
| Impaired, n (%) | 20 (29) | 11 (24) | 9 (39) | 0.21 |
| Elevated, n (%) | 7 (10) | 5 (11) | 2 (9) | 0.99 |
| Diabetes, n (%) | 8 (12) | 5 (11) | 3 (13) | 0.99 |
| Total cholesterol, mg/dL | 193 ± 40 | 192 ± 39 | 194 ± 43 | 0.85 |
| LDL, mg/dL | 108 ± 35 | 104 ± 34 | 116 ± 38 | 0.21 |
| HDL, mg/dL | 61 ± 20 | 65 ± 23 | 55 ± 13 | 0.030 |
| Triglycerides, mg/dL | 93 (78–139) | 91 (74–138) | 99 (82–140) | 0.15 |
| Lipid lowering medication, n (%) | 8 (11) | 4 (8) | 4 (17) | 0.43 |
| RA duration, years | 6.5 (1.6–15.6) | 5.3 (1.6–13.6) | 11.4 (2.1–23.3) | 0.078 |
| RF or anti-CCP, n (%) | 55 (81) | 36 (80) | 19 (83) | 0.80 |
| Any shared epitope, n (%) | 42 (62) | 27 (59) | 15 (68) | 0.45 |
| Joint Counts | ||||
| Swollen (0–42) | 12 (4––16) | 11 (4–16) | 12 (6–18) | 0.43 |
| Tender (0–44) | 12 (2–20) | 13 (4–20) | 9 (2–22) | 0.58 |
| Deformed+replaced | 4 (1–7) | 3 (0–6) | 5 (2–12) | 0.12 |
| DAS28-CRP, units | 3.9 (2.8–4.6) | 4.0 (2.8–4.6) | 3.4 (2.9–4.5) | 0.53 |
| CRP, mg/L | 2.7 (0.7–6.6) | 2.7 (0.8–6.2) | 2.7 (0.6–8.2) | 0.73 |
| IL-6, pg/mL | 3.2 (1.4–8.8) | 2.8 (1.4–8.7) | 3.7 (1.5–8.8) | 0.82 |
| AM stiffness, min | 20 (5–60) | 20 (5–60) | 20 (5–38) | 0.46 |
| HAQ, units | 1.0 (0.4–1.8) | 1.0 (0.4–1.8) | 1.0 (0.4–1.7) | 0.93 |
| No DMARDs or prednisone, n (%) | 7 (10) | 6 (12) | 1 (4) | 0.41 |
| Non-biologic DMARDs, n (%) | 54 (75) | 33 (69) | 21 (88) | 0.15 |
| MTX | 43 (60) | 27 (56) | 16 (67) | 0.40 |
| Biologics, n (%) | 22 (31) | 12 (25) | 10 (42) | 0.15 |
| TNF inhibitors, n (%) | 18 (25) | 9 (19) | 9 (38) | 0.083 |
| Current prednisone, n (%) | 25 (35) | 17 (35) | 8 (33) | 0.86 |
| Daily dose (users), mg | 5 (4–10) | 5 (2.5–10) | 5 (4–8.75) | 0.73 |
| Any NSAID, n (%) | 16 (22) | 11 (23) | 5 (21) | 0.84 |
| CAC Score, units | 0 (0–29) | 0 | 130 (28–266) | -- |
| CAC=0 units, n (%) | 48 (67) | -- | 0 (0) | -- |
| CAC 1–99 units, n (%) | 11 (15) | -- | 11 (46) | -- |
| CAC 100–399 units, n (%) | 7 (10) | -- | 7 (29) | -- |
| CAC>400 units, n (%) | 6 (8) | -- | 6 (25) | -- |
| Peripheral Cell Counts* | ||||
| White Blood Cells/1000 | 6.5 (5.4–7.7) | 6.7 (5.3–8.3) | 6.7 (5.3–7.4) | 0.70 |
| Lymph% | 34 (23–39) | 33 (23–38) | 30 (23–38) | 0.89 |
| Lymph, absolute/1000 | 1.95(1.36–2.49) | 1.94 (1.29–2.65) | 1.86 (1.31–2.47) | 0.78 |
| Monocyte% | 7 (5–9) | 7 (5–7.6) | 8 (5–10) | 0.13 |
| Monocyte, absolute/1000 | 0.48 (0.31–0.62) | 0.45 (0.24–0.62) | 0.53 (0.38–0.62) | 0.22 |
Mean ± standard deviation or median (interquartile range) depicted, unless otherwise noted Abbreviations: CAC Coronary Artery Calcification, SBP Systolic Blood Pressure, DBP Diastolic Blood Pressure, BMI Body Mass Index, LDL Low Density Lipoproteins, HDL High Density Lipoproteins, RA Rheumatoid Arthritis, RF Rheumatoid Factor, CCP Citrullinated Cyclic Peptide, DAS Disease Activity Score, CRP C reactive Protein, IL-6 Interleukine-6, HAQ Health Assessment Questionnaire, DMARD Disease Modifying Agent Rheumatic Disease, MTX Methotrexate, TNF Tumor Necrosis Factor, NSAID Non Steroidal Anti Inflammatory Drug.
Available in n=56 within ± 4 weeks of study visit
Quantification of PBMC subsets
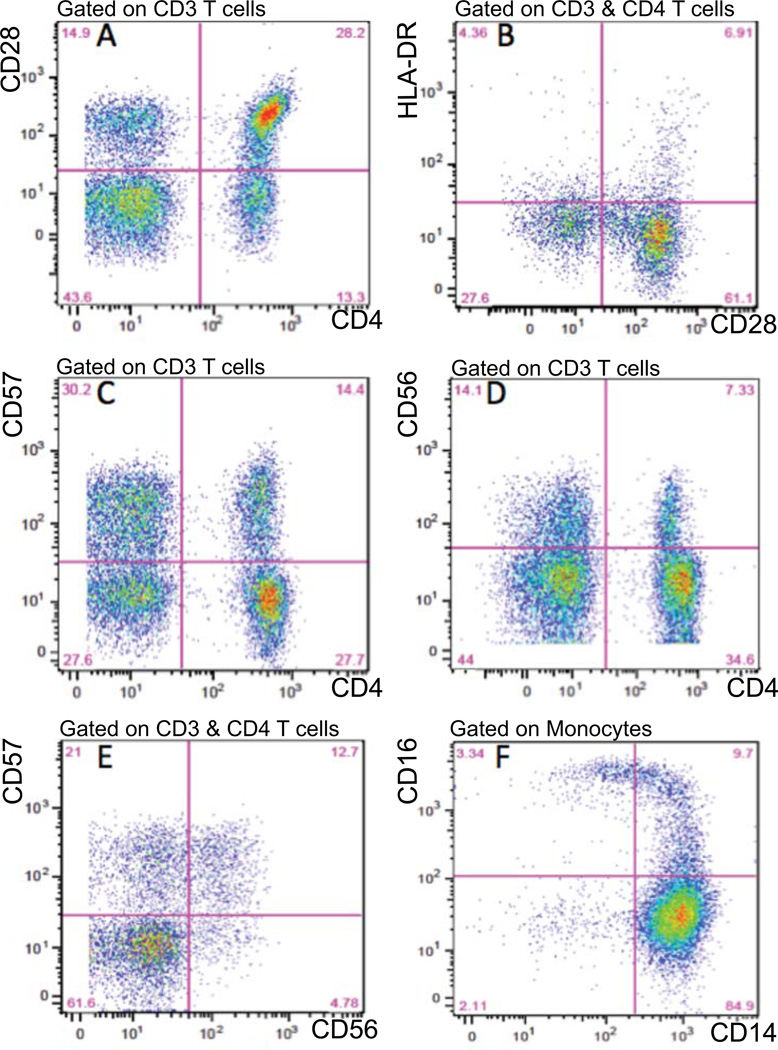
Figure 1 illustrates CD4+ subsets in a representative case, RA54, with elevated proportions of memory effector T cells and a CAC score of 266. The figure shows the quantitation of some of the different CD4 T cell subsets, CD4+ CD28null [% CD28− (in CD4+)] (A), CD4+ CD28− HLA-DR+ CD3+ [%HLA-DR+ CD28− (in CD4+)] (B), CD4+ CD57+, [%CD57+ (in CD4+)] (C), CD4+ CD56+, [%CD57+ (in CD4+)] (D), and CD4+CD57+CD56+, [%CD57+ (in CD4+CD56+)] (E), and that these subsets exhibit considerable overlap. The quantitation of intermediate (“inflammatory”) monocytes, CD14hi CD16+, is illustrated in panel (F).
Figure 1. PBMC subsets in representative case, RA54, with increased memory effector T cells, intermediate monocytes and CAC.
Panel A: distribution of CD28 expression on CD4+ and CD4(CD8) T cells on CD3 T cells. 41.5% (28.2 +13.3) of CD3+ T cells are CD4+, [%CD4+ (in CD3+)] and that 37.0% (100×13.3/(28.2 +13.3)) have extinguished CD28 expression, indicating memory phenotype differentiation [%CD28 (in CD4+)]. Panel B: s distribution of HLA-DR activation marker on CD28+ and CD28 CD4 cells. 10.3%(100×6.91/(6.9 +61.1)) of CD28+ and 13.6% (100×4.36/(4.36 +27.6)) of CD28 cells express HLA-DR, [%HLA-DR+ CD28 (in CD4+)]. Panel C: expression of NKR CD57 on CD4+ and CD4(CD8) T cells. 34.2% (100×14.4/(14.46 +27.7)) of CD4 T cells express CD57, [%CD57+ (in CD4+)]. Panel D: expression of NKR CD56 on CD4+ and CD4(CD8) T cells. 17.5%(100×7.33/(7.33 +34.6)) of CD4 T cells express CD56, [%CD56+ (in CD4+)]. Panel E: extent of coexpression of CD57 and CD56 on CD4 T cells. 72.7% (100×12.7/(12.7 +4.76)) of CD56+CD4+ T cells express CD57, [%CD57+ (in CD4+CD56+)]. Panel F: expression of subset markers, CD14 and CD16, on monocytes. 9.7% of CD14+ monocytes are intermediate CD14hiCD16+ monocytes, Abbreviations: PBMC Peripheral Blood Mononuclear Cells, CAC Coronary Artery Calcification, NKR Natural killer cell receptor.
Multiple PBMC Subsets were Associated with CAC in Univariate Models
Levels of PBMC subsets according to the presence or absence of any CAC are summarized in Table 2. Compared with patients with no CAC, those with CAC had significantly, or near-significant, higher percentages of circulating CD4 T cell subsets denoting activation (%HLA-DR+(in CD4+), %HLA-DR+ CD28+ (in CD4+)), differentiation to memory effector (% CD28− (in CD4+), % HLA-DR+ CD28− (in CD4+), and acquisition of NK receptors (% CD57+ (in CD4+ CD56+), %CD57+ (in CD4+CD56+)). Similar patterns were seen with circulating CD8 T cell subsets – i.e., patients with CAC had higher percentages of CD8 T cell subsets denoting activation (% HLA-DR+ (in CD8+)), differentiation to memory effector (% CD28− (in CD8+)), and acquisition of NK receptors (% CD56+ (in CD8+), % CD57+ (in CD8+), % CD57+ in CD8+ CD56+), and %CD28− CD57+ (in CD8+)), compared to patients with no CAC (Table 2).
Table 2.
PBMC Subsets Reflecting Activation or Differentiation to Memory-Effector phenotype, According to the Presence of Any CAC*
| Total (n=72) |
No CAC (n=48) |
Any CAC (n=24) |
p | |
|---|---|---|---|---|
| CD4 Memory-Effector subsets | ||||
| % CD28 (in CD4+) | 4.7 (1.0–12.1) | 2.9 (0.6–9.9) | 9.5 (4.4–19.4) | 0.019 |
| % CD56+ (in CD4+) | 2.4 (1.1–5.0) | 2.0 (1.0–4.6) | 3.1 (1.4–6.0) | 0.32 |
| % CD57+ (in CD4+) | 8.9 (3.9–15.7) | 6.6 (2.9–13.6) | 11.4 (5.8–22.8) | 0.011 |
| % CD57+ (in CD4+ CD56+) | 41.3 (16.9–73.2) | 28.5 (13.9–64.1) | 60.0 (43.9–80.0) | 0.002 |
| % CD28 CD57+ (in CD4+) | 2.5 (0.2–9.7) | 1.6 (0.1–7.7) | 5.8 (1.5–15.9) | 0.031 |
| CD4 Activation | ||||
| % HLA-DR+ (in CD4+) | 5.5 (3.4–8.0) | 4.7 (3.0–6.7) | 8.0 (4.9–11.2) | 0.001 |
| % HLA-DR+ CD28 (in CD4+) | 0.8 (0.1–2.1) | 0.44 (0.05–1.58) | 1.61 (0.30–4.36) | 0.006 |
| CD8 Memory-Effector subset | ||||
| % CD28 (in CD8+) | 40.9 (15.6–57.5) | 32.4 (13.8–52.6) | 53.0 (37.0–67.2) | 0.005 |
| % CD56+ (in CD8+) | 14.2 (8.4–22.5) | 13.9 (8.4–21.7) | 15.7 (8.4–25.6) | 0.50 |
| % CD57+ (in CD8+) | 45.2 (20.8–57.7) | 33.8 (14.5–51.8) | 55.3 (46.0–61.4) | 0.001 |
| % CD57+ in CD8+ CD56+) | 67.0 (42.0–83.9) | 57.6 (32.4–78.1) | 83.7 (71.7–87.3) | <0.001 |
| %CD28 CD57+ (in CD8+) | 77.2 (57.1–84.4) | 72.4 (49.0–82.5) | 82.1 (70.5–90.2) | 0.004 |
| CD8 Activation | ||||
| % HLA-DR+ (in CD8+) | 17.6 (10.9–28.1) | 14.8 (8.4–22.1) | 27.3 (15.0–30.0) | 0.002 |
| % HLA-DR+ CD28− (in CD8+) | 9.3 (2.5–16.5) | 5.9 (2.0–14.1) | 11.2 (7.3–19.2) | 0.060 |
| All Memory-Effector subsets | ||||
| All %CD3+ CD57+ | 54.6 (28.8–73.1) | 41.5 (18.6–64.9) | 67.1 (56.1–83.3) | 0.001 |
| All %CD3+ CD28 | 51.9 (18.0–68.9) | 38.1 (15.1–60.2) | 60.7 (47.4–78.6) | 0.006 |
| All Activation | ||||
| All %CD3+ HLA-DR+ | 22.8 (16.0–35.2) | 20.0 (12.6–28.3) | 33.9 (21.7–42.4) | 0.001 |
| % CD4 CD8 (in CD3+) | 3.0 (1.8–4.3) | 3.4 (2.4–5.0) | 2.3 (1.7–3.8) | 0.054 |
| Monocyte subsets | ||||
| %CD14+ CD16+ | 7.4 (5.3–10.0) | 6.9 (4.9–9.4) | 8.8 (6.0–12.0) | 0.091 |
| %CD14hi CD16+ | 8.0 (6.1–11.0) | 6.8 (5.0–9.9) | 9.6 (7.8–13.2) | 0.012 |
| %CD16+ | 15.9 (11.7–22.1) | 14.7 (10.6–17.2) | 20.2 (15.0–26.4) | 0.005 |
| %CD14hi CD16 | 75.8 (68.4–80.2) | 77.0 (69.6–81.1) | 72.6 (64.9–72.6) | 0.042 |
Median (interquartile range) depicted
Abbreviations: PBMC Peripheral Blood Mononuclear Cells, CAC Coronary Artery Calcification. p-values are not adjusted for multiple comparisons
The distribution of monocyte subsets was also examined in patients with and without CAC (Table 2). Compared with patients with no CAC, those with CAC had significantly higher percentages of both circulating intermediate CD14hi+CD16+ and CD16+ monocytes as well as lower levels of classic CD14hiCD16− monocytes.
The percentages of each of the CD4+ T cell subsets reflecting activation or differentiation to memory-effector phenotypes were highly and significantly correlated with each other, with correlation coefficients ranging between 0.8055 and 0.4240 (Supplementary table ST1). Similarly, the analogous CD8 T cell subsets were highly correlated with each other, with correlation coefficients ranging between 0.7999 and 0.2113. Interestingly, the elevations in CD4 and CD8 T cell subsets were also highly intercorrelated, with correlation coefficients ranging between 0.6647 and 0.2664. In contrast, the percentages of the CD14hi CD16+ and the CD14hi CD16− monocyte subsets were not correlated with percentages of any of the CD4+ T cell subsets elevations, although some alterations in the percentages of CD8 T cell subsets exhibited weaker positive and sometimes negative correlations with the monocyte subsets. Among those with CAC, none of the PBMC subsets were associated with the extent of CAC as measured by total Agatson units modeled as a continuous variable (data not shown).
T Cells and CD14hiCD16+ Monocytes Were Associated with CAC in Multivariable Models
Although multiple PBMC subsets were significantly associated with the presence of CAC in univariate models, only two, the %CD57+ (in CD4+CD56+) T cells and %CD14hi CD16+ monocytes, remained significantly associated with any CAC when co-modeled and adjusted for age and SBP (Table 3, Reduced Model). Age and SBP were the only two potential confounders associated with either of the PBMC subsets (supplemental tables ST2 and ST3) that were also associated with any CAC, as RA duration was no longer significantly associated with any CAC when co-modeled with age. These supplementary tables also illustrate that the %CD57+ (in CD4+CD56+) T cells and %CD14hi CD16+ monocytes were significantly higher in cases seropositive for RF(rheumatoid factor) or ACPA (anticitrullinated protein antibodies).
Table 3.
Multivariable Associations of PBMC Subsets with the Outcome of Presence of Any CAC***
| Crude Models | Adjusted Models* | Extended Model** | Reduced Model | |||||
|---|---|---|---|---|---|---|---|---|
| OR | p | OR | p | OR | p | OR | p | |
| % CD4 CD8 (in CD3+) | 0.90 | 0.25 | 0.97 | 0.76 | ||||
| log % CD28 (in CD4+) | 1.45 | 0.033 | 1.15 | 0.52 | ||||
| square root % CD57 (in CD4 ) | 1.58 | 0.015 | 1.30 | 0.25 | ||||
| square root% CD57+ (in CD4+ CD56+) | 1.48 | 0.003 | 1.31 | 0.083 | 1.27 | 0.23 | 1.40 | 0.046 |
| square root % CD28 CD57+ (in CD4+) | 1.40 | 0.032 | 1.19 | 0.35 | ||||
| square root % HLA-DR+ (in CD4+) | 3.49 | 0.003 | 2.33 | 0.090 | 1.22 | 0.80 | ||
| square root % HLA-DR+ CD28 (in CD4+)+ | 2.25 | 0.018 | 1.50 | 0.31 | ||||
| log % CD28− (in CD8+) | 3.08 | 0.010 | 1.90 | 0.23 | ||||
| % CD57+ (in CD8+) | 1.76 | 0.002 | 1.37 | 0.17 | ||||
| %CD8+ CD56+that are CD57+ | 1.04 | 0.002 | 1.03 | 0.091 | 1.01 | 0.61 | ||
| % CD57+ in CD8+ CD56+) | 1.05 | 0.008 | 1.02 | 0.31 | ||||
| log % CD57+ in CD8+ CD56+) | 2.38 | 0.014 | 1.58 | 0.31 | ||||
| square root % HLA-DR+ (in CD8+) | 1.84 | 0.006 | 1.53 | 0.11 | 1.12 | 0.79 | ||
| square root% HLA-DR+ CD28− (in CD8+) | 1.43 | 0.055 | 1.41 | 0.19 | ||||
| %CD14hi CD16+ | 1.10 | 0.066 | 1.16 | 0.001 | 1.17 | 0.045 | 1.19 | 0.027 |
| %CD16+ | 1.07 | 0.035 | 1.12 | 0.023 | ||||
| %CD14hi CD16 | 0.95 | 0.079 | 0.96 | 0.25 | ||||
| square root all %CD3+ CD57+ | 1.68 | 0.001 | 1.34 | 0.14 | ||||
| log all %CD3 CD28 | 3.01 | 0.008 | 1.86 | 0.21 | ||||
| square root all %CD3+ HLA-DR+ | 1.89 | 0.003 | 1.56 | 0.081 | ||||
Adjusted Models are three covariate models containing the PBMC variable of interest, age, and SBP
The Extended Model contains all 5 PBMC variables listed in the same model, plus age and SBP
Abbreviations: PBMC Peripheral Blood Mononuclear Cells, CAC Coronary Artery Calcification.
Individually, the areas under the curve (AUCs) for the prediction of any CAC for % CD57+ (in CD4+CD56+) T cells and %CD14hi CD16+ monocytes were significant (i.e. the lower limit of the 95% CI for the AUC was >0.50; Table 4, Models 1 and 2). Combining % CD57+ (in CD4+CD56+) T cells and %CD14hi CD16+ monocytes increased the AUC to 0.755 (Table 4, Model 3). Without the PBMC subsets factored into the model, the AUC for the model including age and SBP only was 0.864 (Table 4, Model 4). The AUC increased to 0.892 with the addition of % CD57+ (in CD4+CD56+) T cells and %CD14hi CD16+ monocytes to the model, which was significantly different from the model including age and SBP only (p=0.007 for the comparison of AUCs; Table 4, Model 5).
Table 4.
ROC Characteristics in the Prediction of Any CAC*
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | p | OR | p | OR | p | OR | p | OR | p | |
| Square root % CD57+ (in CD4+ CD56+), per % | 1.48 | 0.003 | 1.51 | 0.003 | 1.40 | 0.046 | ||||
| %CD14hi CD16+, per % | 1.10 | 0.066 | 1.11 | 0.055 | 1.19 | 0.027 | ||||
| Age, per year | 1.14 | 0.001 | 1.16 | 0.001 | ||||||
| SBP, per mmHg | 1.03 | 0.083 | 1.03 | 0.15 | ||||||
| AUC | 0.722 | 0.682 | 0.755 | 0.864 | 0.892 | |||||
| 95% CI | (0.602–0.843) | (0.544–0.820) | (0.641–0.869) | (0.781–0.947) | (0.821–0.962) | |||||
| p-value comparing AUC of model 3 vs. 5 = 0.12 | ||||||||||
| p-value comparing AUC of model 4 vs. 5 = 0.007 | ||||||||||
Abbreviations: AUC Area Under the Curve, CAC Coronary Artery Calcification. ROC Receiver Operating Characteristics, SBP Systolic Blood Pressure, CI Confidence Interval.
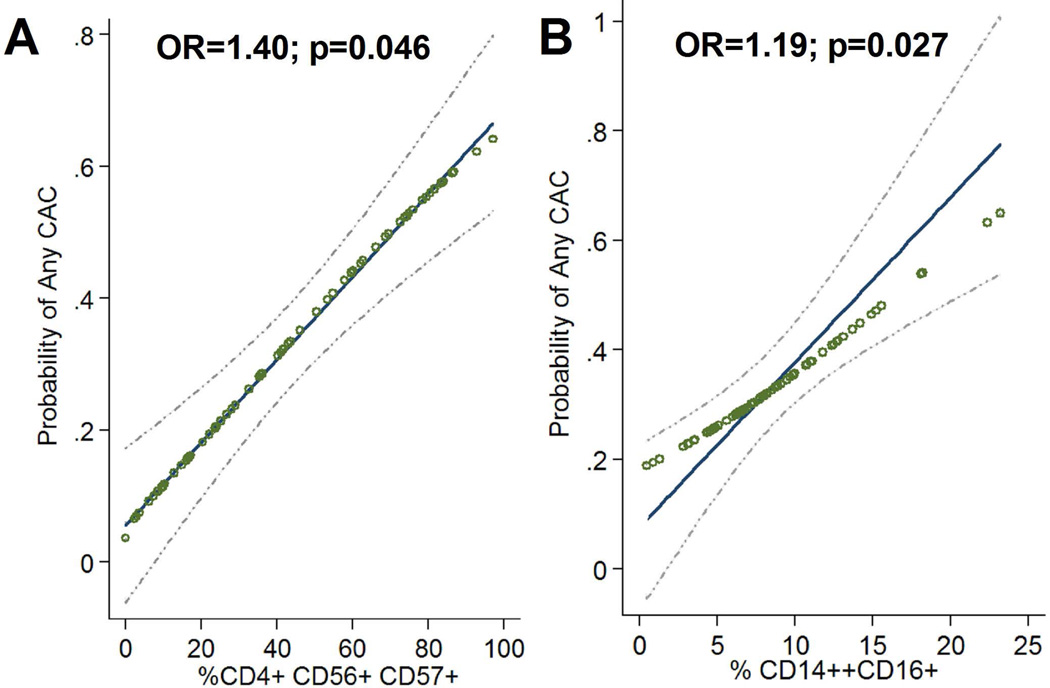
The adjusted associations of each of the PBMC subsets of interest are graphically depicted in Figure 2. Among those with 20% or fewer CD57+ (in CD4+CD56+) T cells, the frequency of any CAC was 9% compared with 44% for those with 80% or more CD57+ (in CD4+CD56+) T cells, after adjusting for age, SBP, and CD14hi CD16+ monocytes (Figure 2.A). Similarly, only 20% of RA patients with 5% or fewer circulating CD14hi CD16+ monocytes (the 25th percentile) had any CAC compared with 41% of those with 11% or more CD14hiCD16+ monocytes (the 75th percentile), after adjustment for age, SBP, and % CD57+ (in CD4+CD56+) T cells (Figure 2.B).
Figure 2. Crude and Adjusted Associations of the Proportions of % CD57+ (in CD4+ CD56+) and CD14hiCD16+ Cells with the Probability of Any CAC.
Crude associations are depicted by open circles. Adjusted associations are depicted with the solid least squares indicator and 95% confidence interval (light dashed line). Each unit increase in the square root of % CD57+ (in CD4+ CD56+) was associated with a 40% higher adjusted odds of any CAC (Panel a; p=0.046) while each unit increase in %CD14hiCD16+ was associated with a 19% higher adjusted odds of any CAC (Panel b.; p=0.027). Adjustments for age, systolic blood pressure, and the other PBMC subset. Other RA and CVD risk factors were not retained in adjusted models as they were not associated with both the PBMC subsets of interest and the presence of CAC. *Abbreviations: PBMC Peripheral Blood Mononuclear Cells, CAC Coronary Artery Calcification, RA Rheumatoid Arthritis, CVD CardioVascular Disease, CD cluster designation, p probabiity, OR odds ratio.
Discussion
The central findings of this study are that development of subclinical atherosclerosis, defined by the presence of CAC, in RA patients is significantly and separately associated with an elevated percentage of circulating CD28−CD57+CD56+ memory effector CD4 T cells, and with an increased proportion of the CD14hiCD16+ monocyte subset bearing an intermediate or ‘inflammatory’ phenotype. These associations are independent of traditional CVD risk factors and other RA clinical characteristics and treatments. These subsets are considered intrinsic features of RA, and in this study were significantly associated with seropositivity for RF or ACPA. However, the extent to which they were associated with atherosclerosis in the setting of RA was surprising. The present findings imply that certain elevations in PBMC subsets found in this cohort are additional biomarkers of the potential development of CAD. While the elevated percentage of the most terminally differentiated CD28−CD57+CD56+ memory effector T cells was the only T cell subset associated with CAC, the correlations among the different CD4 and CD8 T cell subsets indicate that the relationship with the % CD57+CD56+ memory effector T cell subset reflects a functionally broader association with the group of interrelated T cell phenotypes denoting activation and acquisition of memory effector phenotypes that in turn reflect persistent, intense activation of the adaptive immune system.
The strongest association of various T cell subsets with the presence of subclinicial atherosclerosis was with expression by CD4 T cells of two NK receptors, CD56 and CD57, consistent with a root biologic event involving triggering the activation and differentiation of the CD4 T cell to a terminally differentiated phenotype. Moreover, curiously, the activation and differentiation events are not limited to the CD4 T cell lineage, but are also equivalently found in analogous phenotypic changes in the CD8 lineage. The high degree of intercorrelation between changes in the CD4 and CD8 lineages (Table 2 and ST1) suggests that a common mechanism is driving these events in both lineages, despite the absence of a classic paradigm implicating CD8 T cells in the pathogenesis of RA. However, perhaps of relevance, recent genetic studies have associated RA susceptibility with a polymorphism at position 9 of HLA-B(34).
An independent association of CAC with the intermediate, inflammatory subset of monocytes, CD14hi CD16+, was also observed. This subset of monocytes is elevated in RA.(19) Intermediate monocytes have a heightened expression of proinflammatory genes, among them TNF-α (21) and induce Th17 cell expansion in vitro(19). Since the elevation in this subset is not correlated with elevations in the CD28−CD57+CD56+ memory effector CD4 T cells, nor with age, it is likely their elevation in CAC occurs through a mechanism distinct from that inducing elevations of the T cell subsets.
It is likely there is heterogeneity in the RA patient population in terms of the factors contributing to the development of CAC and in the elevations of these subsets, since a variety of different pathogens and antigens can induce the T cell memory-effector phenotype. Similarly, it is presumed that some instances of CAD in RA may primarily reflect operation of conventional CAD-associated genetic and environmental risk factors. However, the reduction in the number of PBMC subsets associated with CAC upon adjusting for age and systolic BP may not necessarily imply that age and systolic BP are variables totally separate from the immune events described in this study, since the development of these T cell subsets in RA and the progressive development of CAD are themselves time-dependent and age-dependent processes. Moreover, there is also a growing literature on the relationship between T cells, elevations in particular T cell subsets, and hypertension.(35, 36)
T cells and macrophages are increasingly recognized to play important roles in atherogenesis.(22) Over two decades ago, IFN-γ-producing T cells that exhibited the CD4+ CD28null memory-effector phenotype were identified in unstable atherosclerotic plaques(37, 38) and in the circulation of individuals with CAD and atherosclerosis in other vascular beds.(23, 39) They interact with macrophages to drive a maladaptive, nonresolving inflammatory response that expands the subendothelial layer.(40) In otherwise healthy individuals with unstable angina, an increase in circulating effector-memory CD4+CD28null T cells predicted subsequent acute myocardial infarction or sudden death.(39) (41) Elevated proportions of circulating effector-memory CD4 T cells also were strongly related to common carotid intima thickness.(23, 42–46) CD4 or CD8 T cell subsets characterized by the expression of CD56 or CD57 have similarly been associated with atherosclerosis and CAD in the general population.(47, 48) Analogously, the intermediate CD14hiCD16+ monocyte subset is increased in otherwise healthy individuals with CAD(24, 49), and is also a significant predictor of major CVD events.(24)
Two earlier reports have proposed a link between CD4+CD28null T cells in RA and accelerated atherosclerosis. Gerli et al. (50) reported that the 20 RA patients with the most elevated CD4+CD28null T cells (≥15%) had higher carotid intima-media thickness and lower flow-mediated vasodilation compared to those with a lower frequency of CD4+CD28null T cells. Warrington et al. noted in a small retrospective series of RA patients that the frequencies of CD4+CD28null T cells were higher in cases of RA with coexistent CAD than in historical RA controls (51). However, both studies were limited in scope and in the determination of potentially confounding conventional risk factors favoring atherosclerosis. The findings of the present study extend these reports, and moreover show that correction for age and hypertension eliminates the significance of the increase in CD4+CD28null T cells. However, this work suggests that the acquisition of NKR such as CD56 and CD57, which indicates a more terminal stage of differentiation in the CD4 T cell population, primarily reflect the proatherogenic character of this response, possibly reflecting their production of cytokines, such as IL17.(19)
The more likely interpretation of these findings is the progressive generation of elevated numbers of memory effector T cell and inflammatory monocyte subsets is an intrinsic element in the immune response mediating RA(3) and this response provisions nascent and clinically unimportant subintimal lipid deposits with effector cells that accelerate the development of advanced atherosclerotic vascular disease. Alternatively, an inflammatory pathway of RA could directly accelerate atherosclerosis, with the memory effector and monocyte subpopulations arising only as a component of the biology of atherosclerosis. The design and results of the present study do not allow distinction between these two interpretations.
Strengths of this study include the extensive clinical characterization and cardiac imaging, along with detailed immunological phenotyping. Weaknesses include the cross-sectional design, so causality cannot be inferred, the lack of a validation cohort, the possibility that CAC can develop in some cases by mechanisms independent from RA, and the relatively small number of patients with CAC. It will be of particular interest to follow the present cohort with repeat CT studies to determine whether the elevation in memory-effector T cells and inflammatory monocytes in those currently without CAC predicts those at risk for developing CAC.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award number AR050026 to JB, and award number AR064473 to RW; by the National Center for Advancing Translational Sciences of the National Institutes of Health, through award number UL1 TR000040, formerly the National Center for Research Resources, award number UL1 RR024156; by an Award from the Rheumatology Research Foundation Disease Targeted Research Grant to JB; and by a Pfizer ASPIRE Investigator Initiated Rheumatology Award WI195003 to RW. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
This study has not had any financial support or other benefits from commercial sources for the work and all of the authors declare they have no potential or real conflicts of interest.
References
- 1.del Rincon ID, Williams K, Stern MP, Freeman GL, Escalante A. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum. 2001;44:2737–2745. doi: 10.1002/1529-0131(200112)44:12<2737::AID-ART460>3.0.CO;2-%23. [DOI] [PubMed] [Google Scholar]
- 2.Wolfe F, Mitchell DM, Sibley JT, Fries JF, Bloch DA, Williams CA, Spitz PW, Haga M, Kleinheksel SM, Cathey MA. The mortality of rheumatoid arthritis. Arthritis Rheum. 1994;37:481–494. doi: 10.1002/art.1780370408. [DOI] [PubMed] [Google Scholar]
- 3.Goronzy JJ, Weyand CM. Developments in the scientific understanding of rheumatoid arthritis. Arthritis Research & Therapy. 2009;11:249. doi: 10.1186/ar2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gregersen P, Silver J, Winchester R. The shared epitope hypothesis: An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 1987;30:1205–1213. doi: 10.1002/art.1780301102. [DOI] [PubMed] [Google Scholar]
- 5.Yu DT, Winchester RJ, Fu SM, Gibofsky A, Ko HS, Kunkel HG. Peripheral blood Ia-positive T cells. Increases in certain diseases and after immunization. Journal of Experimental Medicine. 1980;151:91–100. doi: 10.1084/jem.151.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waase I, Kayser C, Carlson PJ, Goronzy JJ, Weyand CM. Oligoclonal T cell proliferation in patients with rheumatoid arthritis and their unaffected siblings. Arthritis & Rheumatism. 1996;39:904–913. doi: 10.1002/art.1780390606. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt D, Martens PB, Weyand CM, Goronzy JJ. The repertoire of CD4+ CD28− T cells in rheumatoid arthritis. Molecular Medicine. 1996;2:608–618. [PMC free article] [PubMed] [Google Scholar]
- 8.Pawlik A, Ostanek L, Brzosko I, Brzosko M, Masiuk M, Machalinski B, Gawronska-Szklarz B. The expansion of CD4+CD28− T cells in patients with rheumatoid arthritis. Arthritis Research & Therapy. 2003;5:R210–R213. doi: 10.1186/ar766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 10.Appay V. The physiological role of cytotoxic CD4(+) T-cells: the holy grail? Clinical and Experimental Immunology. 2004;138:10–13. doi: 10.1111/j.1365-2249.2004.02605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakajima T, Goek O, Zhang X, Kopecky SL, Frye RL, Goronzy JJ, Weyand CM. De novo expression of killer immunoglobulin-like receptors and signaling proteins regulates the cytotoxic function of CD4 T cells in acute coronary syndromes. Circulation research. 2003;93:106–113. doi: 10.1161/01.RES.0000082333.58263.58. [DOI] [PubMed] [Google Scholar]
- 12.James EA, Rieck M, Pieper J, Gebe JA, Yue BB, Tatum M, Peda M, Sandin C, Klareskog L, Malmstrom V, et al. Citrulline-specific Th1 cells are increased in rheumatoid arthritis and their frequency is influenced by disease duration and therapy. Arthritis Rheumatol. 2014;66:1712–1722. doi: 10.1002/art.38637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fasth AE, Cao D, van Vollenhoven R, Trollmo C, Malmstrom V. CD28nullCD4+ T cells--characterization of an effector memory T-cell population in patients with rheumatoid arthritis. Scand J Immunol. 2004;60:199–208. doi: 10.1111/j.0300-9475.2004.01464.x. [DOI] [PubMed] [Google Scholar]
- 14.Maeda T, Yamada H, Nagamine R, Shuto T, Nakashima Y, Hirata G, Iwamoto Y. Involvement of CD4+,CD57+ T cells in the disease activity of rheumatoid arthritis. Arthritis Rheum. 2002;46:379–384. doi: 10.1002/art.10133. [DOI] [PubMed] [Google Scholar]
- 15.Imberti L, Sottini A, Signorini S, Gorla R, Primi D. Oligoclonal CD4+ CD57+ T-cell expansions contribute to the imbalanced T-cell receptor repertoire of rheumatoid arthritis patients. Blood. 1997;89:2822–2832. [PubMed] [Google Scholar]
- 16.Wang EC, Lawson TM, Vedhara K, Moss PA, Lehner PJ, Borysiewicz LK. CD8high+ (CD57+) T cells in patients with rheumatoid arthritis. Arthritis Rheum. 1997;40:237–248. doi: 10.1002/art.1780400208. [DOI] [PubMed] [Google Scholar]
- 17.Jabri B, Selby JM, Negulescu H, Lee L, Roberts AI, Beavis A, Lopez-Botet M, Ebert EC, Winchester RJ. TCR Specificity Dictates CD94/NKG2A Expression by Human CTL. Immunity. 2002;17:487. doi: 10.1016/s1074-7613(02)00427-2. [DOI] [PubMed] [Google Scholar]
- 18.Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, Leenen PJ, Liu YJ, MacPherson G, Randolph GJ, et al. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116:e74–e80. doi: 10.1182/blood-2010-02-258558. [DOI] [PubMed] [Google Scholar]
- 19.Rossol M, Kraus S, Pierer M, Baerwald C, Wagner U. The CD14(bright) CD16+ monocyte subset is expanded in rheumatoid arthritis and promotes expansion of the Th17 cell population. Arthritis Rheum. 2012;64:671–677. doi: 10.1002/art.33418. [DOI] [PubMed] [Google Scholar]
- 20.Zawada AM, Rogacev KS, Rotter B, Winter P, Marell RR, Fliser D, Heine GH. SuperSAGE evidence for CD14++CD16+ monocytes as a third monocyte subset. Blood. 2011;118:e50–e61. doi: 10.1182/blood-2011-01-326827. [DOI] [PubMed] [Google Scholar]
- 21.Wong KL, Tai JJ, Wong WC, Han H, Sem X, Yeap WH, Kourilsky P, Wong SC. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood. 2011;118:e16–e31. doi: 10.1182/blood-2010-12-326355. [DOI] [PubMed] [Google Scholar]
- 22.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 23.Ammirati E, Cianflone D, Vecchio V, Banfi M, Vermi AC, De Metrio M, Grigore L, Pellegatta F, Pirillo A, Garlaschelli K, et al. Effector Memory T cells Are Associated With Atherosclerosis in Humans and Animal Models. J Am Heart Assoc. 2012;1:27–41. doi: 10.1161/JAHA.111.000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ozaki Y, Imanishi T, Taruya A, Aoki H, Masuno T, Shiono Y, Komukai K, Tanimoto T, Kitabata H, Akasaka T. Circulating CD14+CD16+ monocyte subsets as biomarkers of the severity of coronary artery disease in patients with stable angina pectoris. Circ J. 2012;76:2412–2418. doi: 10.1253/circj.cj-12-0412. [DOI] [PubMed] [Google Scholar]
- 25.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, 3rd, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 26.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 27.Prevoo ML, van 't Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–48. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 28.Wells G, Becker JC, Teng J, Dougados M, Schiff M, Smolen J, Aletaha D, van Riel PL. Validation of the 28-joint Disease Activity Score (DAS28) and European League Against Rheumatism response criteria based on C-reactive protein against disease progression in patients with rheumatoid arthritis, and comparison with the DAS28 based on erythrocyte sedimentation rate. Ann Rheum Dis. 2009;68:954–960. doi: 10.1136/ard.2007.084459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolfe F. Which HAQ is best? A comparison of the HAQ, MHAQ and RA-HAQ, a difficult 8 item HAQ (DHAQ), and a rescored 20 item HAQ (HAQ20): analyses in 2,491 rheumatoid arthritis patients following leflunomide initiation. J Rheumatol. 2001;28:982–989. [PubMed] [Google Scholar]
- 30.Winchester R, Wiesendanger M, O Brien W, Zhang H-Z, Maurer MS, Gillam LD, Schwartz A, Marboe C, Stewart AS. Circulating activated and effector memory T cells are associated with calcification and clonal expansions in bicuspid and tricuspid valves of calcific aortic stenosis. Journal of Immunology. 2011;187:1006–1014. doi: 10.4049/jimmunol.1003521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Winchester R, Minevich G, Steshenko V, Kirby B, Kane D, Greenberg DA, FitzGerald O. HLA Associations Reveal Genetic Heterogeneity in Psoriatic Arthritis and in the Psoriasis Phenotype. Arthritis & Rheumatism. 2012;64:1134–1144. doi: 10.1002/art.33415. [DOI] [PubMed] [Google Scholar]
- 32.Balandraud N, Picard C, Reviron D, Landais C, Toussirot E, Lambert N, Telle E, Charpin C, Wendling D, Pardoux E, et al. HLA-DRB1 genotypes and the risk of developing anti citrullinated protein antibody (ACPA) positive rheumatoid arthritis. PLoS One. 2013;8:e64108. doi: 10.1371/journal.pone.0064108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winchester R. Reshaping Cinderella's slipper: the shared epitope hypothesis. Arthritis Res Ther. 2006;8:109. doi: 10.1186/ar1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raychaudhuri S, Sandor C, Stahl EA, Freudenberg J, Lee HS, Jia X, Alfredsson L, Padyukov L, Klareskog L, Worthington J, et al. Five amino acids in three HLA proteins explain most of the association between MHC and seropositive rheumatoid arthritis. Nat Genet. 2012;44:291–296. doi: 10.1038/ng.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schiffrin EL. The immune system: role in hypertension. Can J Cardiol. 2013;29:543–548. doi: 10.1016/j.cjca.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 36.Harrison DG. The immune system in hypertension. Trans Am Clin Climatol Assoc. 2014;125:130–140. [PMC free article] [PubMed] [Google Scholar]
- 37.Hansson GK, Holm J, Jonasson L. Detection of activated T lymphocytes in the human atherosclerotic plaque. The American journal of pathology. 1989;135:169–175. [PMC free article] [PubMed] [Google Scholar]
- 38.Stemme S, Holm J, Hansson GK. T lymphocytes in human atherosclerotic plaques are memory cells expressing CD45RO and the integrin VLA-1. Arterioscler Thromb. 1992;12:206–211. doi: 10.1161/01.atv.12.2.206. [DOI] [PubMed] [Google Scholar]
- 39.Liuzzo G, Goronzy JJ, Yang H, Kopecky SL, Holmes DR, Frye RL, Weyand CM. Monoclonal T-cell proliferation and plaque instability in acute coronary syndromes. Circulation. 2000;101:2883–2888. doi: 10.1161/01.cir.101.25.2883. [DOI] [PubMed] [Google Scholar]
- 40.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liuzzo G, Biasucci LM, Trotta G, Brugaletta S, Pinnelli M, Digianuario G, Rizzello V, Rebuzzi AG, Rumi C, Maseri A, et al. Unusual CD4+CD28null T lymphocytes and recurrence of acute coronary events. Journal of the American College of Cardiology. 2007;50:1450–1458. doi: 10.1016/j.jacc.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 42.Hansson GK, Zhou X, Tornquist E, Paulsson G. The role of adaptive immunity in atherosclerosis. Ann N Y Acad Sci. 2000;902:53–62. doi: 10.1111/j.1749-6632.2000.tb06300.x. discussion 62–54. [DOI] [PubMed] [Google Scholar]
- 43.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 44.Fasth AE, Snir O, Johansson AA, Nordmark B, Rahbar A, Af Klint E, Bjorkstrom NK, Ulfgren AK, van Vollenhoven RF, Malmstrom V, et al. Skewed distribution of proinflammatory CD4+CD28null T cells in rheumatoid arthritis. Arthritis Research & Therapy. 2007;9:R87. doi: 10.1186/ar2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nature reviews. Immunology. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 46.Olson NC, Doyle MF, Jenny NS, Huber SA, Psaty BM, Kronmal RA, Tracy RP. Decreased naive and increased memory CD4(+) T cells are associated with subclinical atherosclerosis: the multi-ethnic study of atherosclerosis. PLoS One. 2013;8:e71498. doi: 10.1371/journal.pone.0071498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bergstrom I, Backteman K, Lundberg A, Ernerudh J, Jonasson L. Persistent accumulation of interferon-gamma-producing CD8+CD56+ T cells in blood from patients with coronary artery disease. Atherosclerosis. 2012;224:515–520. doi: 10.1016/j.atherosclerosis.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 48.Strioga M, Pasukoniene V, Characiejus D. CD8+ CD28− and CD8+ CD57+ T cells and their role in health and disease. Immunology. 2011;134:17–32. doi: 10.1111/j.1365-2567.2011.03470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schlitt A, Heine GH, Blankenberg S, Espinola-Klein C, Dopheide JF, Bickel C, Lackner KJ, Iz M, Meyer J, Darius H, et al. CD14+CD16+ monocytes in coronary artery disease and their relationship to serum TNF-alpha levels. Thromb Haemost. 2004;92:419–424. doi: 10.1160/TH04-02-0095. [DOI] [PubMed] [Google Scholar]
- 50.Gerli R, Schillaci G, Giordano A, Bocci EB, Bistoni O, Vaudo G, Marchesi S, Pirro M, Ragni F, Shoenfeld Y, et al. CD4+CD28− T lymphocytes contribute to early atherosclerotic damage in rheumatoid arthritis patients. Circulation. 2004;109:2744–2748. doi: 10.1161/01.CIR.0000131450.66017.B3. [DOI] [PubMed] [Google Scholar]
- 51.Warrington KJ, Kent PD, Frye RL, Lymp JF, Kopecky SL, Goronzy JJ, Weyand CM. Rheumatoid arthritis is an independent risk factor for multi-vessel coronary artery disease: a case control study. Arthritis Research & Therapy. 2005;7:R984–R991. doi: 10.1186/ar1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.