Abstract
Objective
Asthma and abdominal aortic aneurysms (AAA) both involve inflammation. Patients with asthma have an increased risk of developing AAA or experiencing aortic rupture. This study tests the development of one disease on the progression of the other.
Approach and Results
Ovalbumin sensitization and challenge in mice led to the development of allergic lung inflammation (ALI). Subcutaneous infusion of angiotensin II (Ang-II) into mice produced AAA. Simultaneous production of ALI in AAA mice doubled abdominal aortic diameter and increased macrophage and mast cell content, arterial media smooth muscle cell (SMC) loss, cell proliferation, and angiogenesis in AAA lesions. ALI also increased plasma IgE, reduced plasma IL5, and increased bronchioalveolar total inflammatory cell and eosinophil accumulation. Intraperitoneal administration of an anti-IgE antibody suppressed AAA lesion formation and reduced lesion inflammation, plasma IgE, and bronchioalveolar inflammation. Pre-establishment of ALI also increased AAA lesion size and lesion accumulation of macrophage, mast cell, and media SMC loss, increased plasma IgE, reduced plasma IL5, IL13, and TGF-β, and increased bronchioalveolar inflammation. Consequent production of ALI also doubled lesion size of pre-established AAA and increased lesion mast cell and T cell accumulation, media SMC loss, lesion cell proliferation and apoptosis, plasma IgE, and bronchioalveolar inflammation. In peri-aortic CaCl2 injury-induced AAA in mice, production of ALI also increased AAA formation, lesion inflammation, plasma IgE, and bronchioalveolar inflammatory cell accumulation.
Conclusion
This study suggests a pathologic link between airway allergic disease and AAA. Production of one disease aggravates the progression of the other.
Keywords: asthma, allergic lung inflammation, abdominal aortic aneurysm, risk factor
INTRODUCTION
Asthma and abdominal aortic aneurysms (AAA) are chronic inflammatory diseases that share many biochemical and pathological similarities. Patients or animals with asthma or AAA have elevated plasma IgE levels.1,2 In asthmatic patients, bronchial and alveolar accumulation of eosinophils, mast cells, macrophages, and lymphocytes indicates an important signature of the disease.3 Similarly, in human and experimental AAA lesions, accumulation of macrophages, T cells, mast cells, and neutrophils determines the initiation and progression of this aortic disease and pathology.4 Our recent studies demonstrated an important role of IgE in mouse experimental AAA development. In apolipoprotein E-deficient (Apoe−/−) mice, the absence of IgE receptor FcεR1 protected these mice from angiotensin II (Ang-II)-induced AAA. Anti-IgE antibody treatment also mitigated AAA progression.2 These prior studies suggest an interaction between asthma and AAA.
The Viborg Vascular screening trial of 18,749 men from Denmark demonstrated a 45% AAA risk increase in patients with a record of an asthmatic medication prescription. Although smoking is an important risk factor of human and mouse AAA,5–7 adjustment of cigarette smoking did not attenuate asthma-associated risk of AAA (unpublished observation). The Danish National Registry of Patients population of 15,942 men with AAA revealed a 30% to 100% aortic rupture risk increase in patients with an asthma diagnosis or experience with anti-asthmatic treatments (unpublished observation). These clinical observations suggest that asthma increases the risk of AAA incidence as well as aortic rupture. This current study tested this hypothesis in Apoe−/− mice. The infusion of Ang-II helps produce AAA in Apoe−/− mice.8,9 Ovalbumin sensitization and challenge produce allergic lung inflammation (ALI) in mice.10,11 We produced ALI and AAA simultaneously or sequentially in Apoe−/− mice to test whether the development of ALI at the same time as, before, or after AAA production affects AAA development.
MATERIALS AND METHODS
Materials and Methods are available in the online-only Data Supplement.
RESULTS
Allergic lung inflammation promotes experimental AAA in mice
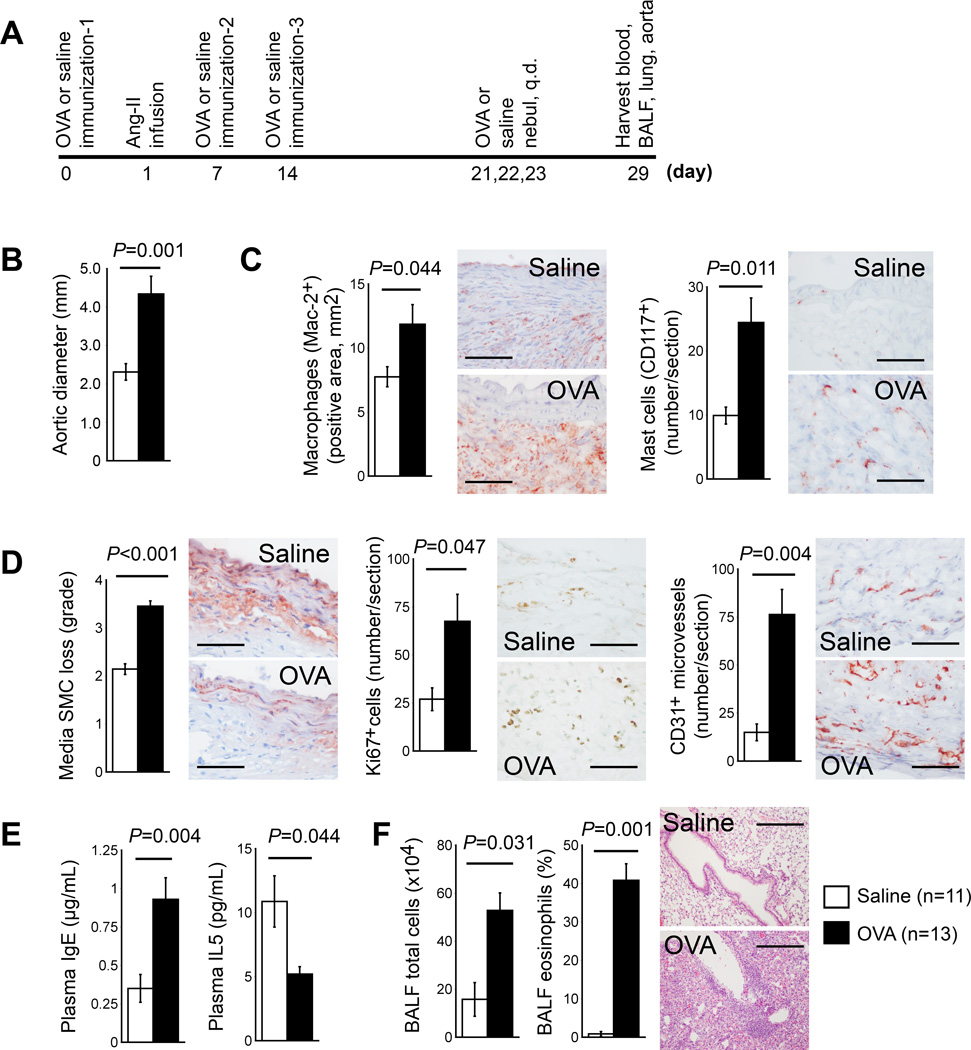
We first concurrently produced ALI together with Ang-II (1,000 ng kg−1min−1) mediated AAA in Apoe−/− mice (Figure 1A). Compared with mock (saline)-sensitized mice, OVA-sensitized mice exhibited significantly enlarged maximal aortic diameters (4.34±0.46 mm vs. 2.31±0.21 mm, P=0.001) (Figure 1B) and aortic cross-section AAA lesion areas (P=0.036), increased lesion Mac-2+ macrophage-positive areas (P=0.044) and mast cell (P=0.011) content (Figure 1C), increased media SMC loss (P<0.001), lesion cell proliferation (P=0.047), and microvascularization (P=0.004) (Figure 1D). Fisher’s exact test did not show a significant difference in post-Ang-II infusion mortality rate between the two groups (Table I in the online-only Data Supplement, left). OVA-sensitized AAA mice showed significantly higher plasma IgE (P=0.004) but reduced plasma IL5 (P=0.044) concentrations (Figure 1E). BALF total inflammatory cells and leukocyte differential counts confirmed production of ALI. OVA-sensitized AAA mice showed significantly higher total BALF inflammatory cell counts (P=0.031), eosinophil (P=0.001) (Figure 1F), and lymphocyte (P=0.015) percentages than mock-sensitized AAA mice, with concurrent reduction of macrophage percentages (P<0.001). In AAA mice with concurrent ALI, elevated AAA lesion sizes may associate with their high plasma IgE levels and high lesion macrophage and mast cell contents. IgE-mediated activation of macrophages and mast cells may contribute to enhanced AAA development in these mice (Figure 1B/1C).2 Allergic inflammation did not significantly affect mouse body weight, blood pressure, plasma lipid contents, or any tested BALF cytokines and chemokines, including IFN-γ, TNF-α, eotaxin, MCP-1, IL4, IL5, IL13, and TGF-β (Table I in the online-only Data Supplement, left). Asthmatic patients or ALI mice often have increased plasma IL5 and IL13.12–14 Human AAA lesions and those from Ang-II-infused Apoe−/− mice also revealed elevated IL5 expression.15,16 Anti-IL5 antibody reduced Ang-II-induced AAA in Apoe−/− mice,16 suggesting a pro-aneurysmogenic role of IL5. Co-development of ALI and AAA may have increased plasma IL5 and IL13. Why these mice showed reduced plasma IL5 remains unknown (Figure 1E). Contrast to the IL5 expression in AAA lesions, plasma IL5 did not differ between AAA and AAA-free patients.17 In Apoe−/− mice, IL5 expression in Ang-II-induced AAA lesions at 28 days after Ang-II infusion did not differ from those of normal mice.16 Therefore, multiple factors may impact the production of IL5 or IL13 in mice with ALI and AAA.
Figure 1.
Concurrent production of ALI increases AAA formation in Apoe−/− mice. A. Experimental protocol. B. Aortic diameters at harvest. C. AAA lesion macrophage and mast cell content. D. AAA lesion SMC loss in grade and lesion cell proliferation (Ki67) and microvascularization (microvessel numbers). Representative data for panels C and D are shown to the right, Scale bar: 50 µm. E. Plasma IgE levels. F. BALF total inflammatory cell number and eosinophil percentage. Representative lung histology data (H&E staining) are shown to the right, scale bar: 200 µm.
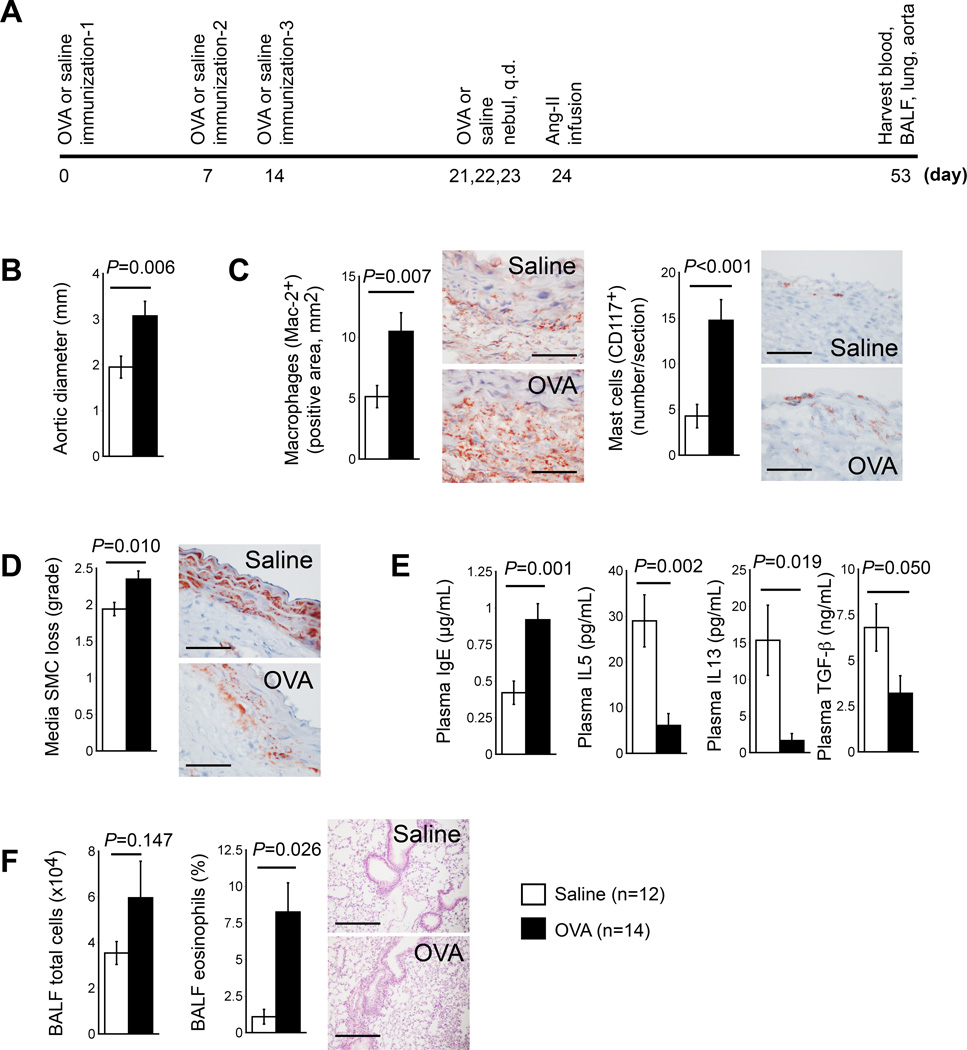
The second group of mice (Figure 2A) first underwent production of ALI, then AAA induction with Ang-II (1,000 ng kg−1min−1). OVA-sensitized mice again developed significantly larger aortic diameters (3.07±0.32 mm vs. 1.95±0.24 mm, P=0.006) (Figure 2B) and cross-section lesion areas (P=0.033) when compared to mock-sensitized mice (Table I in the online-only Data Supplement, right), although aortic rupture post-Ang-II infusion did not differ between the groups. OVA-sensitized mice exhibited higher levels of lesion Mac-2+ macrophage-positive areas (P=0.007), mast cells (P<0.001), and medial SMC loss (P=0.010) than mock-sensitized mice (Figure 2C/2D). Compared with mock-sensitized mice, OVA-sensitized mice had significantly higher plasma IgE levels (P=0.001), but lower anti-inflammatory plasma IL5 (P=0.002), IL13 (P=0.019), and TGF-β (P=0.050) (Figure 2E). OVA-sensitized AAA mice had significantly higher numbers of BALF total inflammatory cells and percentages of BALF eosinophils (P=0.026) (Figure 2F) and lymphocytes (P=0.002), but reduced percentages of macrophages (P=0.018), affirming production of ALI (Figure 2F). OVA-sensitized AAA mice showed an increase in post-AAA diastolic blood pressures (P=0.035) and reduction of BALF IL13 levels (P=0.023), but the other variables measured, including plasma lipoproteins and BALF proteins, did not differ significantly from those of mock-sensitized mice (Table I in the online-only Data Supplement, right).
Figure 2.
OVA-induced ALI increases AAA formation in Apoe−/− mice. A. Experimental protocol. B. Aortic diameters at harvest. C. AAA lesion content of macrophages and mast cells. D. AAA lesion SMC loss in grade. Representative data for panels C and D are shown to the right, Scale bar: 50 µm. E. Plasma levels of IgE, IL5, IL13, and TGF-β. F. BALF total inflammatory cell number and eosinophil percentage. Representative lung histology data (H&E staining) are shown to the right, scale bar: 200 µm.
Allergic lung inflammation exacerbates pre-established AAA in mice
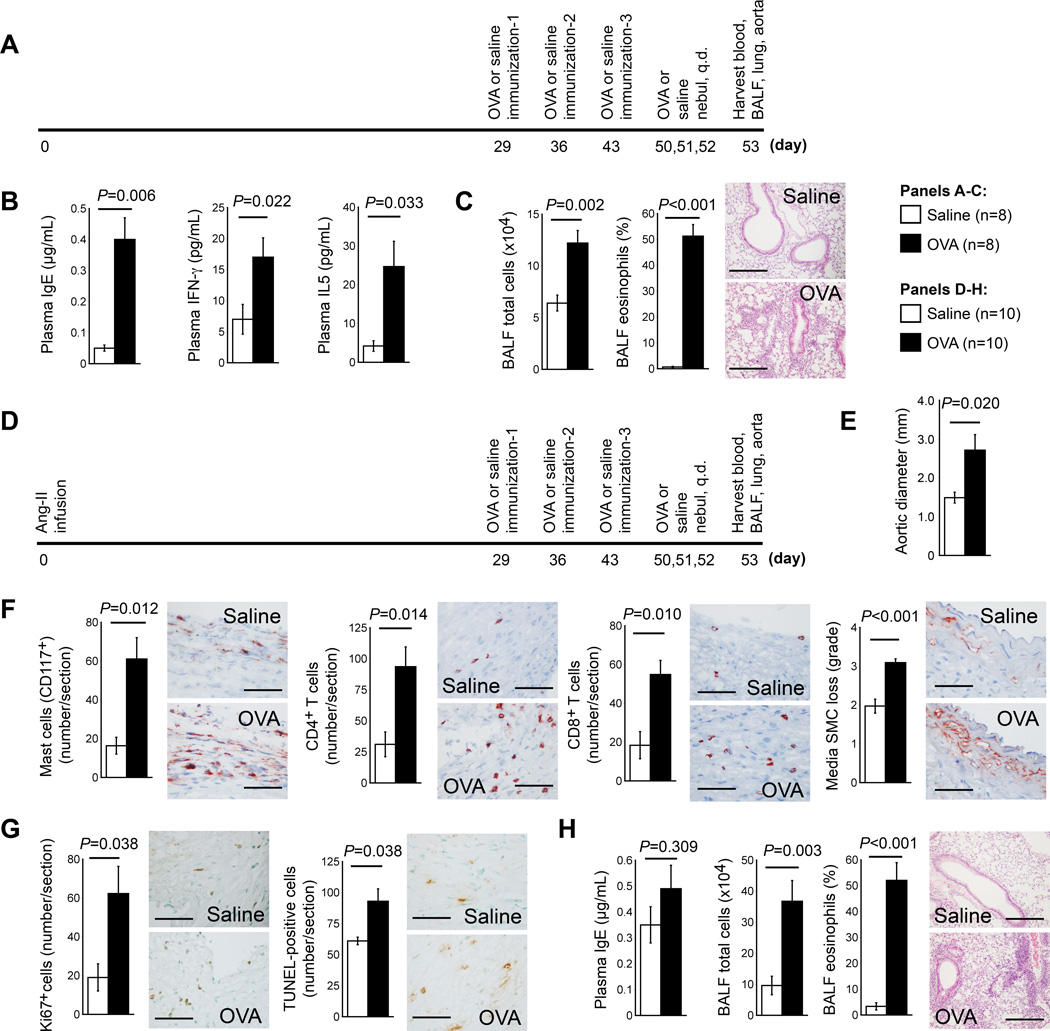
We also tested whether induction of ALI after AAA production exacerbated pre-established AAA. A group of 8~10-week-old male Apoe−/− mice did not undergo AAA induction, but started OVA or mock sensitization on day 29 as controls (Figure 3A). Control mice without AAA did not exhibit significant changes in abdominal aortic diameter (1.000±0.054 mm vs. 1.026±0.046 mm, P=0.727), cross-section areas, or any other arterial wall pathological variables between OVA- and mock-sensitized mice, except collagen content, which was lower in OVA-sensitized mice when compared to mock-sensitized mice (P=0.020). OVA-sensitized mice also had lower plasma total cholesterol (P=0.017) and triglyceride (P=0.040) levels than mock-sensitized mice (Table II in the online-only Data Supplement, left). As anticipated, OVA-sensitized Apoe−/− mice had increased plasma IgE (P=0.006), IFN-γ (P=0.022), and IL5 concentrations (P=0.033) (Figure 3B).10,11 OVA-sensitized mice showed higher BALF levels of total inflammatory cells (P=0.002) and percentages of eosinophils (P<0.001) (Figure 3C) and lymphocytes (P<0.001) than mock-sensitized mice, whereas OVA-sensitized mice revealed reduced macrophage percentages (P<0.001). OVA-sensitized mice had higher BALF concentrations of IgE (P=0.011), TNF-α (P=0.020), MCP-1 (P=0.004), IL5 (P=0.004), and IL13 (P=0.029) than mock-sensitized mice (Table II in the online-only Data Supplement, left).
Figure 3.
OVA-induced ALI promotes progression of pre-established AAA in Apoe−/− mice. A. Experimental protocol of producing ALI alone. B. Plasma levels of IgE, IFN-γ and IL5. C. BALF total inflammatory cell number and eosinophil percentage. D. Experimental protocol of AAA production, followed by ALI production. E. Aortic diameters at harvest. F. AAA lesion contents of macrophages, CD4+ and CD8+ T cells, and media SMC loss in grade. G. AAA lesion numbers of Ki67-positive proliferating cells and TUNEL-positive apoptotic cells. Representative data for panels F and G are shown to the right, Scale bar: 50 µm. H. BALF total inflammatory cell number and eosinophil percentage. Representative lung histology data (H&E staining) in panels C and H are shown to the right, scale bar: 200 µm.
Next, we produced AAA in 8~10-week-old male Apoe−/− mice. Then, at 29 days post-Ang-II infusion, animals underwent OVA or mock immunization to produce airway allergic inflammation (Figure 3D). In Apoe−/− mice that had pre-established AAA, development of ALI did not affect body weight, blood pressure, plasma lipoprotein, BALF cytokines, or blood chemokine concentrations (Table II in the online-only Data Supplement, right). In sharp contrast, OVA-sensitized mice had significantly larger aortic diameters (2.71±0.42 mm vs. 1.49±0.14 mm, P=0.020) (Figure 3E), cross-section AAA lesion areas (P=0.041), lesion mast cells (P=0.012), CD4+ T cells (P=0.014), CD8+ T cells (P=0.010), medial SMC loss (P<0.001), lesional Ki67+ cells (P=0.038), and apoptotic cells (P=0.038) (Figure 3F/3G). Plasma from OVA-sensitized AAA mice had increased TNF-α and reduced TGF-β concentrations. Plasma IgE levels were also higher in OVA-sensitized mice than in mock-sensitized mice, although such a difference did not reach statistical significance (0.49±0.09 µg/mL vs. 0.35±0.07 µg/mL, P=0.309) (Figure 3H). IgE action on mast cells and T cells contributes to AAA formation.2 Action of IgE on elevated lesion mast cells and T cells (Figure 3F) may contribute to enhanced AAA in OVA-sensitized mice (Figure 3E). While plasma IgE concentrations with Ang-II-induced AAA in Apoe−/− mice increased when compared to control mice,2 OVA-sensitized mice (without AAA) had similar plasma IgE concentrations as Ang-II infusion-induced AAA mice (without asthma) (0.40±0.07 µg/mL vs. 0.35±0.07 µg/mL, P=0.726). The OVA-sensitized mice had significantly increased total inflammatory cells and eosinophils (Figure 3H) in BALF, affirming the production of allergic inflammation in OVA-sensitized mice. Two sets of data in Table II in the online-only Data Supplement provide detailed analyses of the AAA lesions and allergic inflammation from mice presented in Figure 3A and 3D.
Pre-established AAA aggravates OVA-sensitized mouse airway allergic responses
The comparison of data from OVA-sensitized mice with and without AAA determined whether pre-established AAA influenced the development of ALI (Figure 3A/3D). Relative to the absence of pre-existing AAA, OVA-sensitized AAA mice showed significantly increased plasma concentrations of IFN-γ (P=0.011), eotaxin (P=0.015), IL4 (P=0.005), IL5 (P=0.048), and TGF-β (P=0.049). OVA-sensitized AAA mice also had enlarged aortic diameters (2.712±0.418 mm vs. 1.000±0.054 mm, P=0.003), increased aortic cross-section lesion areas, and higher lesion macrophages, mast cells, CD4+ and CD8+ T cells, MHC-II-positive areas, media SMC loss, elastin fragmentation, lesion cell proliferation and apoptosis, and microvascularization relative to OVA-sensitized mice without AAA (Table III in the online-only Data Supplement, left). BALF cell analysis showed that pre-established AAA significantly increased BALF total inflammatory cells (P=0.006), macrophages (P=0.004), lymphocytes (P=0.003), and eosinophils (P=0.030) in OVA-sensitized mice. Pre-establishment of AAA did not affect most tested cytokines and chemokines in BALF. Mice with pre-established AAA (Table III in the online-only Data Supplement, left) only showed reduced IL5 (P=0.009) and TGF-β (P=0.011). Pre-established AAA in mock-sensitized mice increased aortic diameters (1.488±0.138 mm vs. 1.026±0.046 mm, P=0.009), lesion areas (P=0.011), lesion macrophages (P<0.001), mast cells (P<0.001), CD4+ T cells (P<0.001), CD8+ T cells (P<0.001), the inflammatory marker MHC-II (P=0.009), medial SMC loss (P<0.001), elastin fragmentation grade (P<0.001), cell proliferation (P<0.001), apoptosis (P<0.001), and microvascularization (P<0.001) (Table III in the online-only Data Supplement, right). Pre-established AAA in mock-sensitized mice also increased plasma IgE (P=0.007), IFN-γ (P=0.001), TNF-α (P=0.004), eotaxin (P=0.003), and TGF-β (P=0.018), as well as lymphocytes (P=0.032). Plasma IL4 (P=0.047) and IL13 (P=0.012) fell, but BALF total inflammatory cells, macrophages, eosinophils, and neutrophils did not change significantly. Pre-established AAA also increased BALF IgE (P=0.005), TNF-α (P=0.001), eotaxin (P=0.006), and IL13 (P=0.028), and reduced BALF TGF-β (P=0.004) in mock-sensitized mice (Table III in the online-only Data Supplement, right), suggesting that AAA augments concentrations of lung inflammatory mediators.
Airway allergic inflammation promotes peri-aortic CaCl2 injury-induced AAA
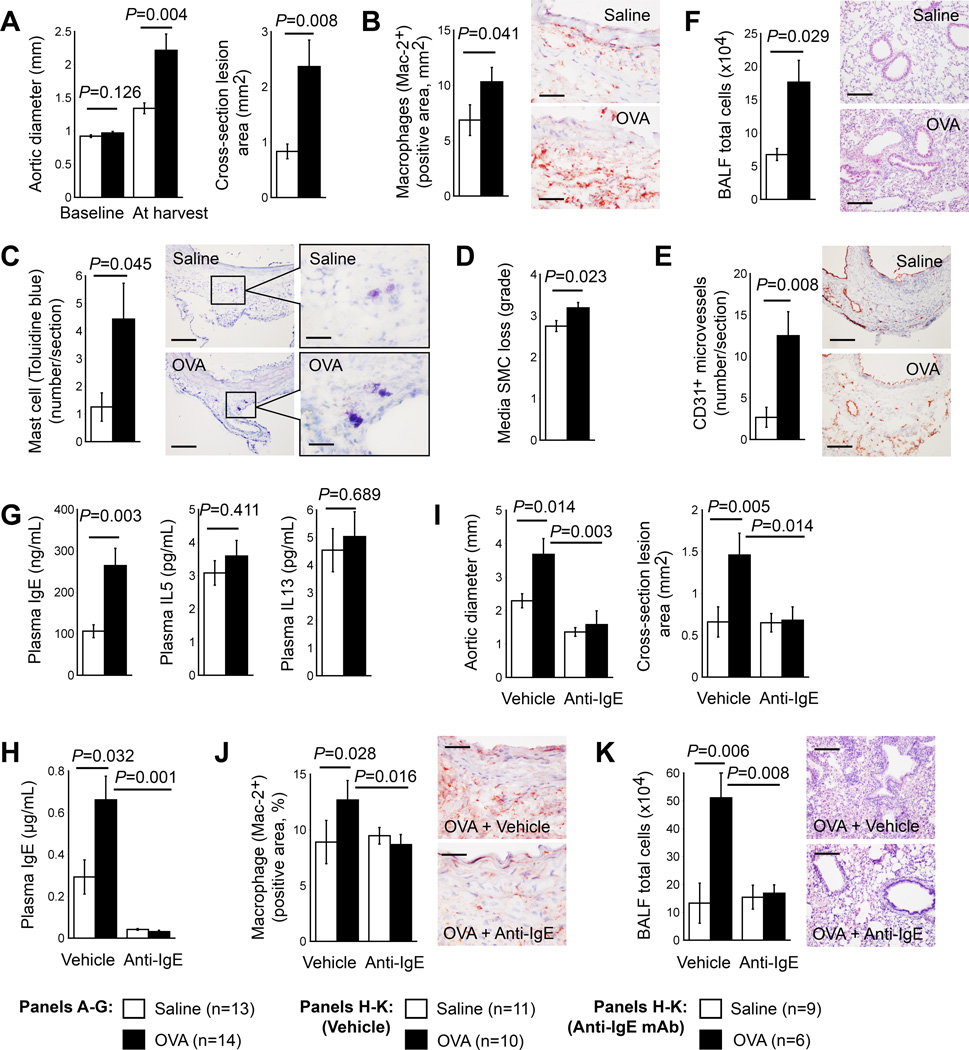
The introduction of ALI to peri-aortic CaCl2 injury-induced AAA in wild-type mice helped test whether the pro-aneurysmogenic activity of ALI applies to other experimental AAA. Production of ALI greatly increased AAA lesion size and cross-sectional lesion area, compared to those of saline-sensitized mice (Figure 4A), with significant increase of lesion macrophages, mast cells, media SMC loss, and lesion microvessel contents (Figure 4B–4E). BALF total inflammatory cell accumulation was also higher in AAA mice with ALI than in mice without ALI (Figure 4F). ELISA detected significantly higher plasma IgE levels in mice with both AAA and ALI than in mice with AAA but sensitized with saline, but plasma IL5 and IL13 levels did not differ significantly between the two groups of mice (Figure 4G). Such insignificant difference in plasma IL5 and IL13 levels may associate with AAA production of the two groups of mice.13–16
Figure 4.
OVA-induced ALI promotes peri-aortic CaCl2-induced AAA in wild-type mice (panels A–G) and anti-IgE antibody mitigates AAA formation in mice with concurrent productions of ALI and Ang-II infusion-induced AAA in Apoe−/− mice (panels H–K). Aortic diameters from baseline and at harvest, and cross-section AAA lesion area (A), AAA lesion Mac-2+ macrophage content (B), mast cell number (C), media SMC loss in grade (D), and CD31+ microvessel number (E), and BALF total inflammatory cells and lung H&E staining from CaCl2-induced AAA mice sensitized and challenged with OVA or saline. Plasma IgE, IL5, and IL13 (G), plasma IgE (H), aortic diameter and cross-section AAA lesion area at harvest (I), AAA lesion Mac-2+ macrophage content (J), and BALF total inflammatory cell number and lung H&E staining (K) from Apoe−/− mice that had Ang-II infusion-induced AAA and sensitized and challenged with OVA or saline. Representative data for panels B, C, E, F, J, and K are shown to the right. Scale bars: B, C-inset, E, and J: 50 µm. C, F, and K: 200 µm.
Anti-IgE antibody mitigates AAA in mice with airway allergic inflammation
Mice with concurrent development of ALI and AAA (Figure 1A) showed significant increase of AAA lesion size (Figure 1B), lesion inflammatory cell accumulation (Figure 1C), and plasma IgE levels (Figure 1E). ALI-induced plasma IgE increase may contribute to increased AAA sizes in mice with concurrent ALI and AAA. The administration of anti-IgE monoclonal antibody to Ang-II-infused mice sensitized with OVA or saline tested this hypothesis. Two doses of intraperitoneal injection of anti-IgE antibody greatly reduced plasma IgE levels from both groups of mice (Figure 4H). In ALI mice, the significant suppression of enlarged AAA sizes and cross-section lesion areas occurred after anti-IgE antibody treatment (Figure 4I), as well as lesion macrophage contents (Figure 4J). As anticipated, the suppression of elevated inflammatory cell accumulation in BALF from ALI mice occurred after anti-IgE antibody treatment (Figure 4K), as previously reported.18,19
DISCUSSION
This study established an association between ALI and AAA in Ang-II-induced AAA mice. Mice with ALI from an inhaled allergen showed significantly enhanced AAA progression, before, during, or after AAA induction, although data did not show significant differences in post-Ang-II infusion superarenal aortic rupture between mice with or without ALI. These observations agree with our preliminary observation from the Viborg Vascular screening trial of 619 AAA patients from 18,749 men. The lack of knowledge in interactions between allergy and AAA may be the cause of incomplete screening information specific for asthma among the AAA populations. The use of anti-asthmatic prescription records helped indicate asthma among this screening population. Patients with such a prescription experienced a 45% higher AAA risk than those without (OR=1.450, 95% C.I.: 1.096–1.918, P<0.01) (unpublished observation).
Allergic asthma20–22 and AAA23–25 involve the T helper cell 2 (TH2) cytokines IL4, IL5, and IL13 and the TH1 cytokines IFN-γ and TNF-α in both humans and animals. Increased allergic airway responses augment BALF IgE and cytokines/chemokines (TNF-α, eotaxin, MCP-1, IL5, IL13).10,11 OVA sensitization- and challenge-induced production of these cytokines in mice with ALI appeared to be aneurysmogenic in these mice. In this study, allergen sensitization and challenge did not change most of the tested BALF cytokines and chemokines regardless whether AIL and AAA were produced sequentially or at the same time, suggesting common mechanisms for the development of type 2 inflammation in the lung and aorta during aneurysmogenesis.
Both allergen and Ang-II infusion induced similar levels of plasma and BALF IgE. We reported previously that Ang-II infusion increased plasma IgE to 0.3 to 0.4 µg/mL.2 This study yielded similar results. While mice without AAA or airway allergic inflammation had plasma IgE concentrations around 0.05 µg/mL, mice with AAA alone without allergic inflammation had plasma IgE levels of 0.35 to 0.42 µg/mL (Table I and II in the online-only Data Supplement). These levels resemble those of mice with allergic inflammation alone without AAA that also had plasma IgE levels at 0.40 µg/mL (Table II in the online-only Data Supplement). Plasma IgE concentrations increased approximately two-fold to 0.93 and 0.92 µg/mL in mice with both ALI and AAA (Table I in the online-only Data Supplement). These data suggest an additive influence on plasma IgE levels of ALI and AAA. We recently reported that IgE participates in experimental AAA by activating mast cells, macrophages, and CD4+ and CD8+ T cells.2 These cells all localize in the aneurysmal mouse aorta. IgE also activates vascular SMCs and endothelial cells, promoting apoptosis of these vascular cells,26 which contribute to aortic aneurysm formation and rupture.27–29 Mice that receive an anti-IgE antibody (e.g. Xolair) biweekly have full protection from AAA formation.2 Here we tested further a role of IgE in AAA. In mice with concurrent ALI and AAA, two doses of biweekly intraperitoneal injection of rat anti-mouse IgE monoclonal antibody depleted plasma IgE and significantly reduced AAA lesion size and macrophage accumulation (Figure 4H–4I). Therefore, the increased plasma IgE (Figure 2E/3E/4H and Table I in the online-only Data Supplement) may link ALI to AAA pathogenesis, although additional mechanisms may also contribute to the activity of ALI in inducing AAA, which requires further investigation. Anti-IgE antibody (e.g. Xolair) may serve as a novel regimen for human AAA, although an appropriate dose and formulation may be required for an optimized efficacy.
This study showed that AAA development in Apoe−/− mice also affected ALI, as reflected by the approximately three-fold increases in BALF total inflammatory cells, macrophages, lymphocytes, and eosinophils (Table III in the online-only Data Supplement). AAA patients may bear a higher risk of developing allergic responses than AAA-free patients, a hypothesis that merits future investigation in a cohort of relevant patients. This study presented experimental mechanistic studies to establish a previously unsuspected association between ALI and AAA. Because allergic inflammation with altered TH2 cytokine levels and elevated plasma IgE may all play detrimental roles, other allergic inflammatory diseases, such as atopic dermatitis, allergic rhinitis, and several ocular allergic diseases might also promote AAA formation. The findings from this study highlight a commonality in the inflammatory pathways involved in AAA and other prevalent, and seemingly unrelated diseases. The results have implications for the development of much-needed advances in the prevention, screening criteria, and treatment of AAA, common conditions for which we currently lack sufficiently effective approaches.
Supplementary Material
SIGNIFICANCE.
Asthma and abdominal aortic aneurysms (AAA) both involve inflammation. Patients with hospital-diagnosed asthma or record of anti-asthmatic medications have a higher risk of AAA and aortic ruptures than those who do not, suggesting an interaction between this airway allergic disease and AAA. In this study, we use ovalbumin sensitization to induce allergic lung inflammation (ALI) and chronic angiotensin-II infusion to produce AAA. Production of ALI before, after, or at the same time as AAA production greatly enlarged AAA size and increased lesion inflammation, along with increasing serum IgE, reducing serum Th2 cytokines, and increasing bronchial and alveolar inflammation. Biweekly administration of anti-IgE antibody depletes plasma IgE and suppresses ALI-induced AAA. Pre-establishment of AAA also augments bronchioalveolar inflammation. ALI also enhances peri-aortic CaCl2 injury-induced AAA in mice. Therefore, experimental ALI promotes AAA formation, furnishing novel links between airway disease and AAA and potential therapeutic strategy of human AAA.
Acknowledgements
The authors thank Henriette Lindholt, Guangli Zhou, and Eugenia Shvartz for technical assistance and Chelsea Swallom for editorial assistance.
Sources of funding:
This study is supported by the mid-region of Denmark and the European Commission Seventh Framework Programme, Health–2007–2.4.2–2 agreement number 200647 (JSL); the Natural Science Foundation of China grants 81400214 (JR) and 81570274 (JYZ); and by grants from the National Institutes of Health HL81090, HL123568 (GPS), HL48743, HL080472 (PL), and HL122531 (BDL).
Abbreviations
- AAA
abdominal aortic aneurysms
- ALI
allergic lung inflammation
- Ang-II
angiotensin II
- TH1 and TH2
T helper 1 and T helper 2
- BALF
bronchioalveolar lavage fluid
- OVA
ovalbumin
- H&E
hematoxylin and eosin
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Disclosure:
None
REFERENCES
- 1.Fanta CH. Asthma. N Engl J Med. 2009;360:1002–1014. doi: 10.1056/NEJMra0804579. [DOI] [PubMed] [Google Scholar]
- 2.Wang J, Lindholt JS, Sukhova GK, Shi MA, Xia M, Chen H, Xiang M, He A, Wang Y, Xiong N, Libby P, Wang JA, Shi GP. Ige actions on cd4+ t cells, mast cells, and macrophages participate in the pathogenesis of experimental abdominal aortic aneurysms. EMBO Mol Med. 2014;6:952–969. doi: 10.15252/emmm.201303811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arm JP, Lee TH. The pathobiology of bronchial asthma. Adv Immunol. 1992;51:323–382. doi: 10.1016/s0065-2776(08)60491-5. [DOI] [PubMed] [Google Scholar]
- 4.Rizas KD, Ippagunta N, Tilson MD., 3rd Immune cells and molecular mediators in the pathogenesis of the abdominal aortic aneurysm. Cardiol Rev. 2009;17:201–210. doi: 10.1097/CRD.0b013e3181b04698. [DOI] [PubMed] [Google Scholar]
- 5.Lee AJ, Fowkes FG, Carson MN, Leng GC, Allan PL. Smoking, atherosclerosis and risk of abdominal aortic aneurysm. Eur Heart J. 1997;18:671–676. doi: 10.1093/oxfordjournals.eurheartj.a015314. [DOI] [PubMed] [Google Scholar]
- 6.Wilmink TB, Quick CR, Day NE. The association between cigarette smoking and abdominal aortic aneurysms. J Vasc Surg. 1999;30:1099–1105. doi: 10.1016/s0741-5214(99)70049-2. [DOI] [PubMed] [Google Scholar]
- 7.Buckley C, Wyble CW, Borhani M, Ennis TL, Kobayashi DK, Curci JA, Shapiro SD, Thompson RW. Accelerated enlargement of experimental abdominal aortic aneurysms in a mouse model of chronic cigarette smoke exposure. J Am Coll Surg. 2004;199:896–903. doi: 10.1016/j.jamcollsurg.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Daugherty A, Manning MW, Cassis LA. Angiotensin ii promotes atherosclerotic lesions and aneurysms in apolipoprotein e-deficient mice. J Clin Invest. 2000;105:1605–1612. doi: 10.1172/JCI7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schulte S, Sun J, Libby P, Macfarlane L, Sun C, Lopez-Ilasaca M, Shi GP, Sukhova GK. Cystatin c deficiency promotes inflammation in angiotensin ii-induced abdominal aortic aneurisms in atherosclerotic mice. Am J Pathol. 2010;177:456–463. doi: 10.2353/ajpath.2010.090381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang B, Huang X, Wolters PJ, Sun J, Kitamoto S, Yang M, Riese R, Leng L, Chapman HA, Finn PW, David JR, Bucala R, Shi GP. Cutting edge: Deficiency of macrophage migration inhibitory factor impairs murine airway allergic responses. J Immunol. 2006;177:5779–5784. doi: 10.4049/jimmunol.177.9.5779. [DOI] [PubMed] [Google Scholar]
- 11.Kuperman DA, Huang X, Koth LL, Chang GH, Dolganov GM, Zhu Z, Elias JA, Sheppard D, Erle DJ. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat Med. 2002;8:885–889. doi: 10.1038/nm734. [DOI] [PubMed] [Google Scholar]
- 12.Garcia G, Taille C, Laveneziana P, Bourdin A, Chanez P, Humbert M. Anti-interleukin-5 therapy in severe asthma. Eur Respir Rev. 2013;22:251–257. doi: 10.1183/09059180.00004013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lama M, Chatterjee M, Nayak CR, Chaudhuri TK. Increased interleukin-4 and decreased interferon-gamma levels in serum of children with asthma. Cytokine. 2011;55:335–338. doi: 10.1016/j.cyto.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 14.Lee YC, Lee KH, Lee HB, Rhee YK. Serum levels of interleukins (il)-4, il-5, il-13, and interferon-gamma in acute asthma. J Asthma. 2001;38:665–671. doi: 10.1081/jas-100107544. [DOI] [PubMed] [Google Scholar]
- 15.Schonbeck U, Sukhova GK, Gerdes N, Libby P. T(h)2 predominant immune responses prevail in human abdominal aortic aneurysm. Am J Pathol. 2002;161:499–506. doi: 10.1016/S0002-9440(10)64206-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu J, Ehrman B, Graham LM, Eagleton MJ. Interleukin-5 is a potential mediator of angiotensin ii-induced aneurysm formation in apolipoprotein e knockout mice. J Surg Res. 2012;178:512–518. doi: 10.1016/j.jss.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forester ND, Cruickshank SM, Scott DJ, Carding SR. Functional characterization of t cells in abdominal aortic aneurysms. Immunology. 2005;115:262–270. doi: 10.1111/j.1365-2567.2005.02157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coyle AJ, Wagner K, Bertrand C, Tsuyuki S, Bews J, Heusser C. Central role of immunoglobulin (ig) e in the induction of lung eosinophil infiltration and t helper 2 cell cytokine production: Inhibition by a non-anaphylactogenic anti-ige antibody. J Exp Med. 1996;183:1303–1310. doi: 10.1084/jem.183.4.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang JY, Kim JW, Kim JS, Kim SJ, Lee SH, Kwon SS, Kim YK, Moon HS, Song JS, Park SH, Lee SY. Inhibitory effects of anti-immunoglobulin e antibodies on airway remodeling in a murine model of chronic asthma. J Asthma. 2010;47:374–380. doi: 10.3109/02770901003801972. [DOI] [PubMed] [Google Scholar]
- 20.Ray A, Cohn L. Altering the th1/th2 balance as a therapeutic strategy in asthmatic diseases. Curr Opin Investig Drugs. 2000;1:442–448. [PubMed] [Google Scholar]
- 21.Ngoc PL, Gold DR, Tzianabos AO, Weiss ST, Celedon JC. Cytokines, allergy, and asthma. Curr Opin Allergy Clin Immunol. 2005;5:161–166. doi: 10.1097/01.all.0000162309.97480.45. [DOI] [PubMed] [Google Scholar]
- 22.Cho SH, Stanciu LA, Holgate ST, Johnston SL. Increased interleukin-4, interleukin-5, and interferon-gamma in airway cd4+ and cd8+ t cells in atopic asthma. Am J Respir Crit Care Med. 2005;171:224–230. doi: 10.1164/rccm.200310-1416OC. [DOI] [PubMed] [Google Scholar]
- 23.Decker D, Springer W, Decker P, Tolba R, Remig J, Strunk H, Hirner A, von Ruecker A. Changes in th1/th2 immunity after endovascular and conventional infrarenal aortic aneurysm repair: Its relevance for clinical practice. Eur J Vasc Endovasc Surg. 2003;25:254–261. doi: 10.1053/ejvs.2002.1834. [DOI] [PubMed] [Google Scholar]
- 24.Galle C, Schandene L, Stordeur P, Peignois Y, Ferreira J, Wautrecht JC, Dereume JP, Goldman M. Predominance of type 1 cd4+ t cells in human abdominal aortic aneurysm. Clin Exp Immunol. 2005;142:519–527. doi: 10.1111/j.1365-2249.2005.02938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan WL, Pejnovic N, Liew TV, Hamilton H. Predominance of th2 response in human abdominal aortic aneurysm: Mistaken identity for il-4-producing nk and nkt cells? Cell Immunol. 2005;233:109–114. doi: 10.1016/j.cellimm.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 26.Wang J, Cheng X, Xiang MX, Alanne-Kinnunen M, Wang JA, Chen H, He A, Sun X, Lin Y, Tang TT, Tu X, Sjoberg S, Sukhova GK, Liao YH, Conrad DH, Yu L, Kawakami T, Kovanen PT, Libby P, Shi GP. Ige stimulates human and mouse arterial cell apoptosis and cytokine expression and promotes atherogenesis in apoe−/− mice. J Clin Invest. 2011;121:3564–3577. doi: 10.1172/JCI46028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walsh K, Smith RC, Kim HS. Vascular cell apoptosis in remodeling, restenosis, and plaque rupture. Circ Res. 2000;87:184–188. doi: 10.1161/01.res.87.3.184. [DOI] [PubMed] [Google Scholar]
- 28.Henderson EL, Geng YJ, Sukhova GK, Whittemore AD, Knox J, Libby P. Death of smooth muscle cells and expression of mediators of apoptosis by t lymphocytes in human abdominal aortic aneurysms. Circulation. 1999;99:96–104. doi: 10.1161/01.cir.99.1.96. [DOI] [PubMed] [Google Scholar]
- 29.Kovacevic M, Jonjic N, Stalekar H, Zaputovic L, Stifter S, Vitezic D. Apoptotic cell death and rupture of abdominal aortic aneurysm. Med Hypotheses. 2010;74:908–910. doi: 10.1016/j.mehy.2009.10.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.