Abstract
Intakes and absorbed organ doses were estimated for 29 303 workers employed at three former US gaseous diffusion plants as part of a study of cause-specific mortality and cancer incidence in uranium enrichment workers. Uranium urinalysis data (>600 000 urine samples) were available for 58 % of the pooled cohort. Facility records provided uranium gravimetric and radioactivity concentration data and allowed estimation of enrichment levels of uranium to which workers may have been exposed. Urine data were generally recorded with facility department numbers, which were also available in study subjects’ work histories. Bioassay data were imputed for study subjects with no recorded sample results (33 % of pooled cohort) by assigning department average urine uranium concentration. Gravimetric data were converted to 24-h uranium activity excretion using department average specific activities. Intakes and organ doses were calculated assuming chronic exposure by inhalation to a 5-µm activity median aerodynamic diameter aerosol of soluble uranium. Median intakes varied between 0.31 and 0.74 Bq d−1 for the three facilities. Median organ doses for the three facilities varied between 0.019 and 0.051, 0.68 and 1.8, 0.078 and 0.22, 0.28 and 0.74, and 0.094 and 0.25 mGy for lung, bone surface, red bone marrow, kidneys, and liver, respectively. Estimated intakes and organ doses for study subjects with imputed bioassay data were similar in magnitude.
INTRODUCTION
The National Institute for Occupational Safety and Health (NIOSH) is currently studying the patterns of cause-specific mortality and cancer incidence in a pooled cohort of workers employed at three former uranium enrichment facilities in the USA. Operation of these types of facilities presents a potential for worker exposures to primarily soluble uranium compounds that are known or suspected to cause adverse human health effects. The purpose of this paper is to describe the methods used to estimate individual organ dose for the workers who had potential for internal exposure to uranium.
Uranium is enriched to increase the abundance of the isotope 235U relative to 238U because 235U is significantly more fissionable than 238U, making it a desirable component for nuclear fuels and weapons. The abundance of 235U (~0.71 % by weight) in naturally occurring uranium is too low for most nuclear applications. Low-enriched uranium contains <20 wt. % 235U, high-enriched uranium contains >20 wt. % 235U and depleted uranium contains <0.71 wt. % 235U. Low-enriched uranium and natural uranium are generally used in fuel for nuclear power reactors and in plutonium production reactors, respectively, whereas high-enriched uranium is generally used in naval nuclear propulsion reactors, research reactors and in nuclear weapons components.
The gaseous diffusion process, which was the preferred process adopted in the USA during the Manhattan Project, involves moving uranium hexafluoride (UF6) gas through a porous membrane (diffusion barrier) in an enclosed system at a pressure that is high relative to the rest of the system, but still below atmospheric pressure. Because the molecular diffusivity of 235UF6 is greater than that of 238UF6 due to the lighter atomic weight, the 235UF6 diffuses more readily through the barrier, resulting in two streams of UF6: one that is enriched in 235U and one that is depleted in 235U.
The Oak Ridge Gaseous Diffusion Plant in Oak Ridge, Tennessee, also known as K-25, was designed and constructed to provide enriched uranium in support of the US nuclear weapons programme and for uranium fuel used in naval, research and commercial nuclear power reactors. Construction of the facility began in June 1943, and the facility became operational in 1945. The facility was further expanded between 1946 and 1954. The plant was placed in standby in 1985 and permanently shut down in 1987(1). Approximately 48 000 workers were employed at the plant between 1945 and 1985; however, about half of those employees were construction workers.
Construction of the Portsmouth Gaseous Diffusion Plant in Piketon, Ohio, began in August 1952, with the plant becoming partially operational by September 1954 and fully operational by March 1956. The plant was originally built as part of a US government expansion programme for the production of high-enriched uranium for naval reactors and nuclear weapons. The facility’s mission changed focus in the mid-1960s from weapons to producing fuel for the nation’s commercial nuclear power plants. In May 2001, enrichment operations ended at the Portsmouth facility. Approximately 13 000 workers were employed in production operations at the Portsmouth facility from 1954 through 2001(2).
The Paducah Gaseous Diffusion Plant in Paducah, Kentucky, which began operation in September 1952, was also part of the US government programme to produce high-enriched uranium for naval nuclear reactors, research reactors and nuclear weapons. Approximately 7000 workers have been employed at the plant since its inception, and ~1200 workers are currently employed. Enrichment levels produced at Paducah were limited to low values (~0.96 %), as the plant was designed to produce feed for the other two gaseous diffusion plants(3). In April 2001, the assay programme at Paducah was upgraded, enabling enrichment levels of up to 5 % of 235U. Uranium enrichment operations ceased at the Paducah facility in 2013.
METHODS
The study cohort was selected from a population of ~68 000 workers from the 3 study facilities. Entry into the cohort was limited to workers with at least 1 y of continuous employment at the K-25 facility between 1 January 1948 and 31 December 1985, the Portsmouth facility between 1 March 1956 and 31 May 2001 or the Paducah facility between 1 September 1952 and 31 December 2003. More than half of the 48 000 workers ever employed at the K-25 facility were hired in 1945 and 1946 and worked <6 months. The purpose of setting the criteria for start of employment in 1948 as opposed to the start of operations was to increase the probability that a study subject would have bioassay/monitoring data available and to minimise biases potentially introduced by the early bioassay/dosimetry techniques.
Individual work histories for each study subject were reconstructed using facility-specific employment and exposure records previously collected by NIOSH and partners in support of earlier studies(4 – 6). Information on start and end dates, facility, job title and department number were available and incorporated into a master work history file.
Uranium concentration data for >600 000 urine samples were abstracted from health physics databases obtained previously from each of the three facilities. Individual urine bioassay records contained data on urine uranium gravimetric concentration (in mg l−1) measured using fluorometric methods and uranium radioactivity concentration (in disintegration per minute per 100 ml) measured using gross alpha counting. There were also records of isotopic analysis of uranium in urine samples, although in most cases, this type of analysis was inconsistent and did not appear to have been done on a routine basis. Sporadic monitoring data were also available for other radionuclides such as 239Pu, 237Np and 99Tc. Assessment of exposure to these radionuclides is beyond the scope of this paper and will be reported elsewhere.
Radiation doses are dependent on the relative abundance of 235U and 238U in the work environment. However, the enrichment level of the uranium to which individual workers were exposed was not reported in bioassay records, and given that many urine samples were only analysed using fluorometric methods, it was necessary to estimate the enrichment of the uranium in the urine sample. For each urine sample for which both non-zero uranium gravimetric and radioactivity concentrations were reported, the radioactivity concentration was first converted to becquerel per liter (1 Bq = 1 disintegration per second), and a specific activity (in becquerel per milligram) was calculated by taking the ratio of the radioactivity concentration (becquerel per liter) to the gravimetric concentration (milligram per litre).
Urine sample analysis results reported in the study subject’s individual bioassay record were generally associated with a department number. All urine samples with calculated uranium specific activities were thus grouped according to department number and facility. This allowed for the estimation of an average enrichment level of uranium in urine samples by department and by facility, which would be used to convert gravimetric concentration data to radioactivity concentration for all workers. Specific activity was converted to enrichment using the following empirical relationship:
| (1) |
where E is the enrichment (%) and SA is the specific activity in becquerel per milligram of the sample(7, 8). This empirical relationship is based on historical measurements of specific activity in uranium of varying enrichments reported by multiple sources. It is only applicable to uranium enriched by the gaseous diffusion method and the relationship deviates from measured data for enrichments of <0.71 % (natural uranium). Urine samples with calculated enrichment of <0.01 % (15 Bq mg−1) or >98 % (2600 Bq mg−1) were not included in the calculation of average department or facility values.
Urine bioassay data were converted from gravimetric concentration (milligram per liter) to 24-h uranium mass excretion (milligram per day) by multiplying by the 24-h urine volume excretion of 1.6 l per day. The urinary excretion rate of 1.6 l per day was assumed to be affected by an uncertainty described by a geometric standard deviation (GSD) equal to 1.6 representing day-to-day variations and inter-individual variability(9).
Uranium 24-h excretion was converted to activity by multiplying by the specific activity of the uranium corresponding to the average department-specific enrichment. If the urine sample was not associated with a department number, the facility-specific enrichment was used.
Each study subject with positive bioassay data (i.e. non-zero) was assigned an effective enrichment. Each line in the subject’s work history contains department number and start/end date of work in that department. The department-specific average enrichment level (Ei) was assigned to each line of work history. If there was no enrichment level associated with a department number or if the department number was missing from that line of work history, the facility average enrichment level was assigned. The assigned enrichment level was then multiplied by the number of days (ti) that the worker was in that department as indicated by the work history record to give a time-weighted enrichment. The time-weighted enrichments were summed over the study subject’s work history and divided by the total number of days worked to get an effective enrichment (Eeff):
| (2) |
Study subjects whose bioassay data (i.e. all bioassay data points in the subject’s bioassay record) were reported as equal to zero or less than the detection limit were assumed to be unexposed and assigned a value of zero for all intakes and organ doses. For study subjects with at least one positive sample (i.e. sample greater than zero or greater than the detection limit for the facility), any bioassay data point recorded as zero was assigned a value, GM, imputed using the following method:
| (3) |
where GM is the assumed geometric mean of the distribution of urine samples below the detection limit, L, and f is the fraction of urine samples below L in the individual’s bioassay data set(10).
For study subjects who had no reported bioassay data, the level of urinary uranium excretion was imputed from averaging the urine uranium concentration in all urine samples associated with a specific department. For each record of a study subject’s work history, the average urine uranium concentration associated with the recorded department number was assigned as a bioassay sample using the ending date of that work history record as the assumed date of the sample. The assumption here is that a steady state level of urine uranium excretion was reached by the end of the work history period in that department.
Intakes and organ doses were evaluated using the Internal Dose Evaluation Program (InDEP; Oak Ridge Center for Risk Analysis, Inc., Oak Ridge, TN, v. 4.1, 2014). The intakes were calculated from bioassay data using least-squares regression techniques. For each study subject, a single chronic exposure by inhalation to a soluble (fast absorption) uranium aerosol with a 5-µm activity median aerodynamic diameter (AMAD) particle size was assumed. The exposure was assumed to begin on the study subjects first day of employment and end on the date of termination. The bioassay data were fit using least-squares regression, assuming a uniform logarithmic error described by a GSD equal to 1.6(11).
Annual absorbed doses were then calculated for five organs selected a priori because of the tendency for these organs to take up uranium (i.e. lung, bone surfaces, red marrow, kidneys and liver)(12). Doses were cumulated until the date of last observation of each study subject (i.e. date of death, date lost to follow-up or the study end date of 31 December 2011). Cumulative dose estimates were summarised using descriptive statistics, and distributions of data were examined using @RISK (@RISK for Excel, v. 6.0.1, Palisade Corporation, ©2012).
RESULTS AND DISCUSSION
The final combined cohort consisted of 29 303 study subjects with minimum employment duration of 1 y at 1 of the 3 study facilities. The average duration of employment for study subjects was 14 y (median 11 y). The combined cohort was 81 % male and 93 % white. Only 1.9 % of study subjects worked at more than one of the gaseous diffusion plants.
Uranium bioassay data were available for ~58 % of the cohort. Of these 58 %, ~29 % were assumed to be unexposed because all bioassay data points were reported as zero or as less than the facility detection/administrative level. Study subjects with at least one reported positive bioassay result had an average (maximum) of 23 (337), 65 (660) and 35 (424) bioassay samples in their individual records for K-25, Portsmouth and Paducah facilities, respectively. The urinalysis results for a few study subjects with only one positive bioassay from K-25 (n = 1) and Paducah (n = 37) were dated prior to the assumed period of exposure for the worker (i.e. before their hire date) and were assumed to be pre-employment urine samples. Because they had no subsequent data, they were assumed to be unexposed. Bioassay data were imputed for an additional 33 % of the cohort, and of these, 13 % were imputed to have all zero bioassay data and were assumed to be unexposed. The remaining 9 % of the cohort had no reported or imputed bioassay data and were also assumed to be unexposed.
Estimated enrichment based on the analysis of uranium specific activities in cohort urinalysis data averaged (range) 3.7 % (0.10–98 %), 6.2 % (0.13–98 %) and 1.9 % (0.14–9.7 %) for the K-25, Portsmouth and Paducah facilities, respectively. Department-specific enrichment levels used for the conversion of urine uranium gravimetric concentration to radioactivity concentration averaged (range) 3.2 % (0.13–22 %), 5.3 % (0.13–39 %) and 2.5 % (0.14–9.7 %) for each of the three facilities. The ranges of these enrichment levels reflect the reported values for facility operations(1, 2, 13).
Descriptive statistics for reported bioassay data used to estimate intakes and organ doses are shown in Table 1. Descriptive statistics for imputed bioassay data are shown in Table 2. Bioassay data were available for 58 % of the combined cohort, and 71 % of that group (41 % of combined cohort) have at least one positive bioassay data point. Bioassay data were imputed for 33 % of the cohort, and 89 % of that group (29 % of the combined cohort) were imputed to have at least one positive bioassay data point. Only 9 % of the cohort had no reported or imputed bioassay data and, in addition to having work history in primarily administrative positions, were assumed to be unexposed. Overall, 29 % of the cohort was determined to be unexposed due to reported, imputed or missing bioassay data in combination with work history records.
Table 1.
Descriptive statistics by facility for individual mean urine uranium bioassay data reported in facility records for study subjects with at least one positive (i.e. >0) bioassay data point.
| K-25 | Portsmouth | Paducah | |
|---|---|---|---|
| No. of bioassay samples | 137 083 | 147 264 | 139 754 |
| Study subjects with bioassay data | 5909 | 2256 | 3959 |
| Uranium concentration (Bq d−1) | |||
| Mean | 0.88 | 0.47 | 0.46 |
| Median | 0.34 | 0.19 | 0.26 |
| 5th Percentile | 0.072 | 0.025 | 0.073 |
| 95th Percentile | 2.1 | 1.7 | 1.1 |
Bioassay data were reported as gravimetric concentration and converted to activity using department-specific or facility-specific enrichment and normalised to 24-h excretion. Values are for study subjects with at least one positive bioassay sample.
Table 2.
Descriptive statistics by facility for individual mean urine uranium bioassay data imputed for study subjects using department average urine uranium concentrations.
| K-25 | Portsmouth | Paducah | |
|---|---|---|---|
| No. of bioassay samples | 31 266 | 4442 | 69 |
| Study subjects with imputed bioassay data | 7615 | 903 | 22 |
| Uranium concentration (Bq d−1) | |||
| Mean | 0.63 | 0.49 | 0.38 |
| Median | 0.39 | 0.39 | 0.32 |
| 5th Percentile | 0.11 | 0.015 | 0.0066 |
| 95th Percentile | 1.2 | 1.7 | 1.2 |
Imputed bioassay data were converted to activity from gravimetric concentration using department-specific or facility-specific enrichment and normalised to 24-h excretion. Values are for study subjects imputed to have at least one positive bioassay sample.
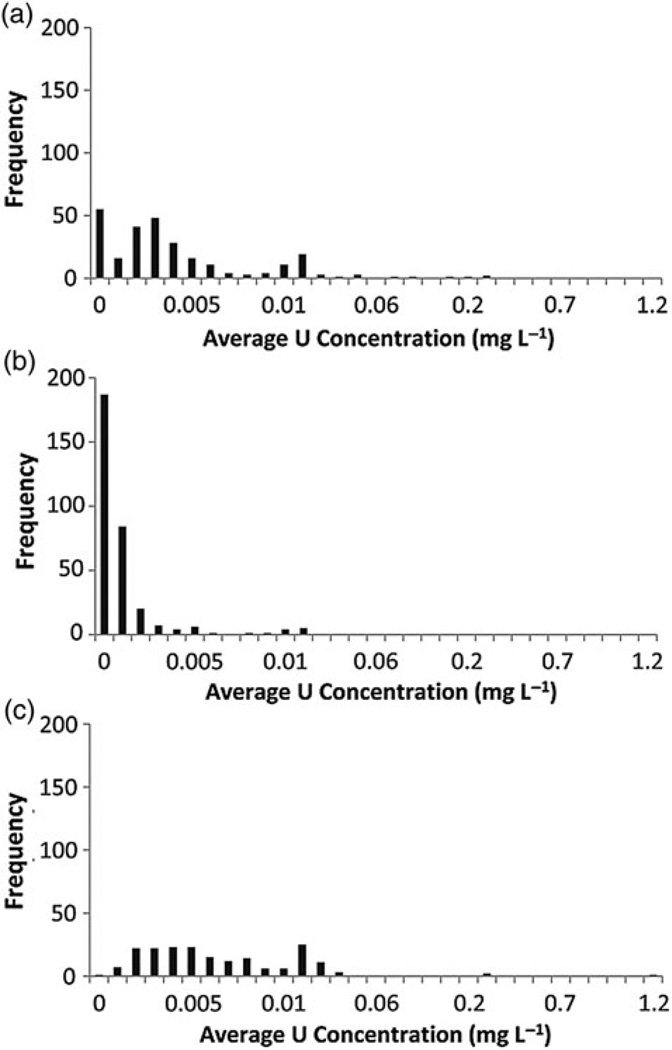
Figure 1a–c shows the frequency distributions of department average urine uranium concentration used to impute bioassay data for study subjects with no reported urinalysis results. The K-25 facility had the highest number of study subjects with imputed data (45 % of the K-25 cohort), and the distribution of average uranium concentrations among departments approximated a somewhat bi-modal distribution. Only 13 % of the Portsmouth cohort had bioassay data imputed, and the average uranium concentrations for the majority of the departments were zero.
Figure 1.
Frequency distributions of department average urine uranium concentration at (a) K-25 (n = 269 departments), (b) Portsmouth (n = 320 departments) and (c) Paducah (n = 193 departments) used to impute bioassay data for study subjects with no reported urinalysis results.
Effective enrichments calculated for individual study subjects with both reported and imputed bioassay data were similar. For those study subjects with reported bioassay data, median effective enrichments (5th–95th percentile range) were 3.7 % (2.4– 4.6 %), 4.4 % (2.1–9.9 %) and 1.9 % (1.9–6.1 %) for K-25, Portsmouth, and Paducah respectively. For study subjects with imputed bioassay data, median effective enrichments (5th–95th percentile range) were 3.6 % (1.7–5.9 %), 5.2 % (1.6–14 %) and 3.9 % (2.3–6.9 %) for K-25, Portsmouth and Paducah, respectively.
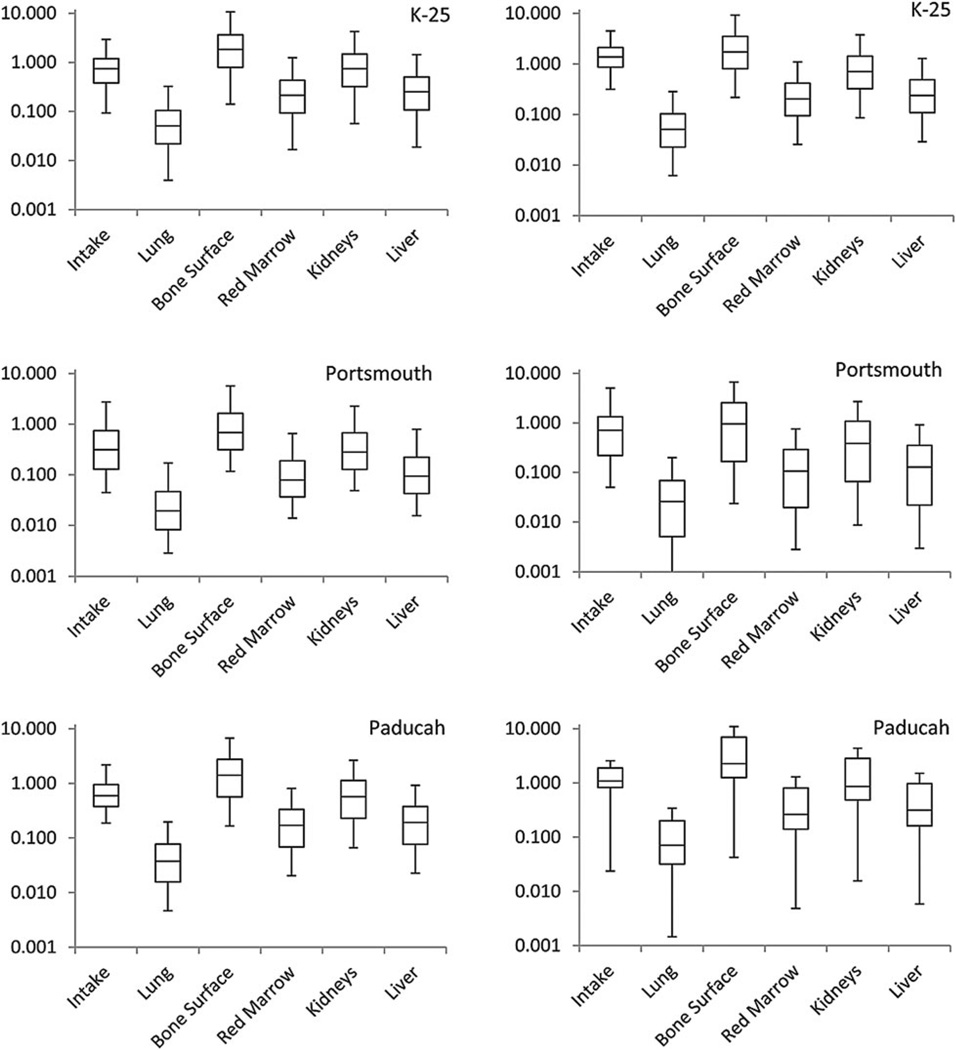
Intakes and organ doses calculated for study subjects with reported and imputed bioassay data are shown in Fig. 2. Median intakes and organ doses calculated for study subjects with reported and imputed data were similar in magnitude, although the interquartile range and 5th–95th percentile range were larger for Portsmouth and Paducah facilities, respectively. For Portsmouth, this is likely because of the large number of departments with average urine uranium concentrations of zero (see Fig. 1b), and the relatively small number of study subjects for whom data were imputed (7615 for K-25 versus 903 for Portsmouth). For the Paducah facility, data were imputed for only 22 study subjects resulting in a larger 5–95 percentile range. Median intakes calculated for study subjects with imputed bioassay data were 45–56 % higher than those calculated for study subjects with reported data. Median organ doses calculated from imputed data were also somewhat biased, although the bias varied from −4 % for K-25 study subjects to 38 % for Paducah study subjects.
Figure 2.
Intakes (becquerel per day) and organ doses (milligray) for the three facilities. Figures on the left represent intakes and organ doses calculated for study subjects with reported bioassay data. Figures on the right represent intakes and organ dose calculated for study subjects with no reported bioassay data using department-specific imputed values. Lines represent data ranges (5th and 95th percentiles), and boxes represent interquartile range, with centre box line representing the median.
Absorbed dose to the red bone marrow was estimated as part of a previous nested case–control study of multiple myeloma mortality in a K-25 cohort(14). In that study, median (interquartile range) calculated absorbed bone marrow dose was 0.060 mGy (0.021– 0.20 mGy), which is almost a magnitude smaller than the median calculated for this study, although the interquartile range is similar. This is due to exclusion, in the current study, of subjects who were employed <1 y at the facility when compared with the criteria of a minimum employment of 30 d in the previous case–control study. Due to the difference in exclusion criteria, only 85 study subjects (with reported bioassay data) were selected for both study cohorts. Median (interquartile range) estimated red bone marrow dose for these two sub-groups were 0.12 mGy (0.042–0.23 mGy) for the multiple myeloma study and 0.28 mGy (0.092–0.61 mGy) for the current combined cohort study.
In another cohort mortality study of uranium workers at the Fernald Feed Materials Production Center (FMPC), intakes and organ doses from exposure to uranium were estimated using methods similar to this study(15). FMPC median (interquartile range) estimated intakes were 0.045 (0.0018–0.81) Bq d−1, and organ absorbed doses were 0.053 (0.0023–0.59), 0.0015 (0.000061–0.018), 0.0044 (0.00019–0.051) and 0.0015 (0.000054–0.018) mGy for lung, red bone marrow, kidney and liver, respectively. Intakes calculated for the FMPC study subjects were about a magnitude lower than the intakes calculated for this current study cohort, likely due to the larger particle size assumed for the FMPC cohort. Median absorbed lung doses were similar, however, due to the assumed exposure to more soluble uranium compounds likely present in the gaseous diffusion plants compared with the relatively insoluble compounds assumed in the exposure scenario at FMPC; there was greater translocation of uranium from the lung to other organs resulting in higher doses to the red bone marrow, kidneys and liver for the current study cohort compared with FMPC cohort.
Uncertainty in particle size and solubility contribute to variability in estimated intakes and organ doses; however, sensitivity analyses performed as part of a previous investigation into uncertainty in intake and organ dose estimates for FMPC uranium workers indicated that the largest source of uncertainty is introduced in the normalisation of spot urine samples to 24-h excretion by volume (i.e. multiplying urine concentration by an uncertain urinary excretion rate of 1.6 l d−1)(16). However, that facility had a rather narrow distribution of enrichment levels to which workers could be exposed. In this current study, work in each of the three facilities presented a potential for exposure to a wider variety of enrichment levels, particularly at the K-25 and Portsmouth facilities where uranium was enriched to up to 98 %. In these cases, sensitivity analyses suggested that variation in enrichment is a larger source of uncertainty in bioassay samples compared with normalisation by urinary output volume.
CONCLUSIONS
As part of the current ongoing study of cause-specific mortality and cancer incidence in a pooled cohort of gaseous diffusion plant workers, NIOSH analysed the results of uranium concentration measurements from >600 000 urine samples estimate intakes and organ doses for workers exposed to uranium. Urine uranium bioassay data were available for 58 % of the cohort and were imputed for an additional 33 %. Overall, 29 % of the combined cohort was determined to be unexposed to uranium internally.
Novel methods were used to estimate effective enrichment to which each individual worker was exposed using urine uranium concentration data combined with department worked obtained from the individual employment records from each study facility. Intakes and doses from internal exposure to uranium estimated in this study were comparable with those assessed using similar methods in a previous study of a uranium facility and an earlier case– control study in one of the current study facilities.
ACKNOWLEDGEMENTS
This study was made possible by the cooperation and support of the DOE and its employees and contractors. The authors thank Dr David Tollerud of University of Louisville and Dr Richard Hornung of University of Cincinnati for assistance with Paducah work history and exposure data. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the NIOSH.
FUNDING
This work was supported through an agreement between the U.S. Department of Energy (DOE) and the U.S. Department of Health and Human Services (DHHS).
REFERENCES
- 1.DOE. Office of Environmental Management. Washington, D.C.: U.S. Department of Energy; 1997. Linking legacies: connecting the cold war nuclear weapons production processes to their environmental consequences. DOE/EM-0319. [Google Scholar]
- 2.United States Enrichment Corporation, I. History: Portsmouth gaseous diffusion plant. [(last accessed on June 2011)];2011 http://www.usec.com/gaseousdiffusion_ports_history.htm. [Google Scholar]
- 3.U.S. Atomic Energy Commission. AEC gaseous diffusion plant operations. 1968 ORO-658. [Google Scholar]
- 4.National Institute for Occupational Safety and Health. National Institute for Occupational Safety and Health. Cincinnati, OH: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2001. Mortality Patterns among uranium enrichment workers at the Portsmouth gaseous diffusion plant. [Google Scholar]
- 5.Yiin JH, Anderson JL, Daniels RD, Seel EA, Fleming DA, Waters KM, Chen PH. A nested case-control study of multiple myeloma risk and uranium exposure among workers at the oak ridge gaseous diffusion plant. Radiat. Res. 2009;171:637–645. doi: 10.1667/RR1607.1. [DOI] [PubMed] [Google Scholar]
- 6.Chan C, Hughes TS, Muldoon S, Aldrich T, Rice C, Hornung R, Brion G, Tollerud DJ. Mortality patterns among paducah gaseous diffusion plant workers. J. Occup. Environ. Med. 2010;52:725–732. doi: 10.1097/JOM.0b013e3181e48ee0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.U.S. Department of Energy. Guide of good practice for occupational radiological protection in uranium facilities. 2001 DOE-STD-1136-2000. [Google Scholar]
- 8.Anderson JL, Spitz HB, Yiin JH. Characterization of internal exposure to enriched uranium at a former gaseous diffusion plant. Health Phys. 2007;93:636–644. doi: 10.1097/01.HP.0000269525.26563.d1. [DOI] [PubMed] [Google Scholar]
- 9.Mueller E, Latini J, Lux M, Stablein U, Brubaker L, Kreder K, Fitzgerald MP. Gender differences in 24-hour urinary diaries of asymptomatic North American adults. J. Urol. 2005;173:490–492. doi: 10.1097/01.ju.0000149947.28100.cd. [DOI] [PubMed] [Google Scholar]
- 10.Anderson JL, Apostoaei AI. Method for analyzing left-censored bioassay data in large cohort studies. J. Expo. Sci. Environ. Epidemiol. 2015 doi: 10.1038/jes.2015.36. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Castellani CM, Marsh JW, Hurtgen C, Blanchardon E, Berard P, Giussani A, Lopez MA. IDEAS Guidelines (Version 2) for the estimation of committed doses from incorporation monitoring data. European Radiation Dosimetry Group e.V. Braunschweig. 2013 doi: 10.1093/rpd/ncv457. EURADOS-2013-01. [DOI] [PubMed] [Google Scholar]
- 12.International Commission on Radiological Protection. Age-dependent doses to members of the public from intake of radionuclides: Part 3 ingestion dose coefficients. 1995 ICRP Publication 69. [PubMed] [Google Scholar]
- 13.United States Enrichment Corporation, I. History: paducah gaseous diffusion plant. [(last accessed on June, 2011)];2011 http://www.usec.com/gaseousdiffusion_pad_history.htm. [Google Scholar]
- 14.Anderson JL, Spitz HB, Yiin JH. Estimating active bone marrow dose from occupational exposure to uranium at a former gaseous diffusion plant. Health Phys. 2007;93:113–119. doi: 10.1097/01.HP.0000261161.20101.36. [DOI] [PubMed] [Google Scholar]
- 15.Anderson JL, Daniels RD, Fleming DA, Tseng CY. Exposure assessment for a cohort of workers at a former uranium processing facility. J. Expo. Sci. Environ. Epidemiol. 2012;22:324–330. doi: 10.1038/jes.2012.20. [DOI] [PubMed] [Google Scholar]
- 16.Anderson JL, Apostoaei AI, Thomas BA. Estimation of internal exposure to uranium with uncertainty from urinalysis data using the InDEP computer code. Radiat. Prot. Dosim. 2013;153:64–73. doi: 10.1093/rpd/ncs097. [DOI] [PMC free article] [PubMed] [Google Scholar]