Abstract
Objective
Comprehensive understanding of the mechanisms regulating angiogenesis might provide new strategies for angiogenic therapies for treating diverse physiologic and pathologic ischemic conditions. The ETS factor ETV2 (aka ER71) is essential for the formation of hematopoietic and vascular systems. Despite its indispensable function in vessel development, ETV2 role in adult angiogenesis has not yet been addressed. We have therefore investigated the role of ETV2 in vascular regeneration.
Approach and Results
We utilized endothelial Etv2 conditional knockout (CKO) mice and ischemic injury models to assess the role of ETV2 in vascular regeneration. While Etv2 expression was not detectable under steady state conditions, its expression was readily observed in endothelial cells following injury. Mice lacking endothelial Etv2 displayed impaired neovascularization in response to eye injury, wounding, or hindlimb ischemic injury. Lentiviral Etv2 expression in ischemic hindlimbs led to improved recovery of blood flow with enhanced vessel formation. Following injury, Flk1 expression and neovascularization were significantly upregulated by Etv2, while Flk1 expression and VEGF response were significantly blunted in Etv2 deficient endothelial cells. Conversely, enforced Etv2 expression enhanced VEGF mediated endothelial sprouting from embryoid bodies. Lentiviral Flk1 expression rescued angiogenesis defects in endothelial Etv2 CKO mice following hindlimb ischemic injury. Furthermore, Etv2+/−; Flk1+/− double heterozygous mice displayed a more severe hindlimb ischemic injury response compared to Etv2+/− or Flk1+/− heterozygous mice, revealing an epistatic interaction between ETV2 and FLK1 in vascular regeneration.
Conclusion
Our study demonstrates a novel obligatory role for the ETV2 in postnatal vascular repair and regeneration.
Keywords: ETV2/ER71, FLK1/VEGFR2, Tie2-Cre, VEcadherin-Cre, injury-induced neovascularization, vascular regeneration
Introduction
Angiogenesis is an important process for successful embryogenesis and injury-mediated tissue repair and regeneration. ETS transcription factors have emerged as critical regulators of angiogenesis 1, 2. A winged helix-turn-helix motif formed by the ETS domain can bind a consensus sequence (GGAA/T) to regulate target gene expression 3. ETS factors such as Fli1, Erg, Ets1 and Ets2, are abundantly expressed in blood and endothelial cells. Consistently, mice or zebrafish deficient in these Ets factors display differing levels of hematopoietic and vascular defects 4-7. Importantly, there is functional redundancy in these ETS factors in angiogenesis 5, 8, 9. Distinct from these ETS factors, Etv2 (aka Er71, etsrp) is transiently expressed in the primitive streak, yolk sac blood islands and large vessels including the dorsal aorta during embryogenesis 10-12. Remarkably, Etv2 deficient animals die early in gestation due to complete block in blood and blood vessel formation 10-12 . ETV2 positively activates genes critical for hematopoietic and endothelial cell specification 10-14. ETV2 additionally activates other Ets genes and potentiates VEGF signaling through ETV2-FLK1 positive feedback mechanism 14. Studies in zebrafish and Xenopus have also demonstrated the critical function of er71 in blood and vessel formation 15-17. As such, ETV2 performs a non-redundant and indispensable function in hematopoietic and vessel development during embryogenesis 10-17.
Despite the importance of ETV2 in early embryonic hematopoietic and vascular development, Etv2 inactivation after hematopoietic and endothelial cell specification using Tie2-Cre leads to normal development 18, indicating that ETV2 has a transient role in the specification of hematopoietic and endothelial lineages. However, potential functions of ETV2 in adult vascular homeostasis and pathophysiological angiogenesis have not yet been addressed. To directly investigate a role for ETV2 in postnatal vascular regeneration, we generated and characterized conditional knockout (CKO) mice lacking endothelial Etv2 by targeting the floxed allele of Etv2 with Tie2-Cre 19-21 or VECadherin-Cre 22. Consistent with the observations of Kataoka et al. (2013) 18, we found that endothelial Etv2 was not required for embryonic development or maintenance of the adult vasculature; however, mice lacking endothelial Etv2 exhibited significantly impaired new vessel formation in response to tissue injuries including laser-induced eye injury 23, wounding 24, or hindlimb ischemic injury 25, 26, which are models for age-related macular degeneration, wound healing, and peripheral arterial disease, respectively. Moreover, lentiviral delivery of Etv2 into ischemic hindlimbs led to improved recovery of blood flow, augmented angiogenic gene expression and enhanced vascular regeneration. We also identify the FLK1 pathway as a major downstream effector of ETV2 in injury-induced neovascularization. These results highlight a requisite postnatal function of ETV2 in injury-induced neovascularization.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement
Results
Etv2 is upregulated in response to injury and required for neovascularization
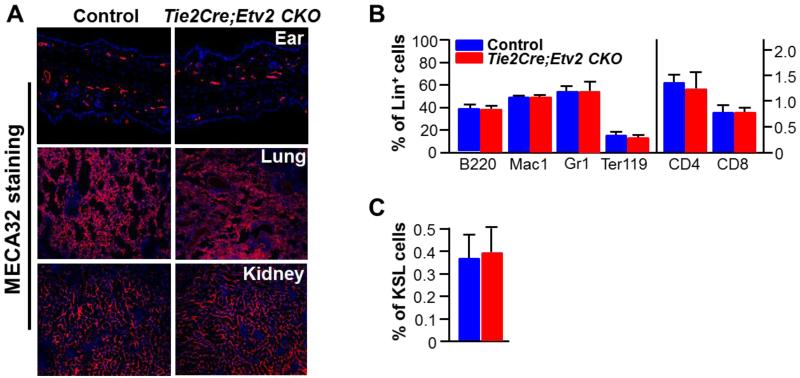
To investigate the role of Etv2 after the hematopoietic and endothelial cell lineage specification, we generated Tie2-Cre; Etv2 (Etv2f/f or Etv2f/−) conditional knockout (CKO) mice. Consistent with the previous report 18, Tie2-Cre; Etv2 CKO mice were born at near expected Mendelian ratios and were viable, healthy, and fertile. Successful deletion of the floxed Etv2 allele was verified in purified lung endothelial cells (CD31+VECADHERIN+) as well as in whole bone marrow (BM) hematopoietic cells from Tie2-Cre; Etv2 CKO mice (Figure IA and IB in the online-only Data Supplement). Etv2 message was not detected in CD31+CD45− endothelial cells isolated from Tie2-Cre; Etv2 CKO adductor muscle (Figure IC in the online-only Data Supplement). In adult Tie2-Cre; Etv2 CKO mice, established vasculature and BM hematopoiesis appeared similar to those of controls as shown by MECA32 or CD31/PECAM1 staining (Figure 1A; Figure II in the online-only Data Supplement) and hematopoietic cell analysis using FACS (Figure 1B and 1C), respectively.
Figure 1. Tie2-Cre; Etv2 CKO mice show no pheotypic defects in vascular and blood development.
A, Control and Tie2-Cre; Etv2 CKO mice were subjected to MECA32 staining on ear, lung and kidney. MECA32 (red) for endothelial cells and DAPI (blue) for nuclear staining. n=3, magnifications: ear = 20 ×, lung and kidney = 10×
B, BM analysis with hematopoietic lineage markers. n=3. B220 for B cells; Mac1 and Gr1 for monocyte and granulocytes; Ter119 for erythrocyte; CD4 and CD8 for T cells.
C, BM analysis with hematopoietic stem cell markers. n=3. KSL indicates c-KIT+Sca1+Lin− cell population enriched for BM hematopoietic stem cells. Error bars indicate standard deviations.
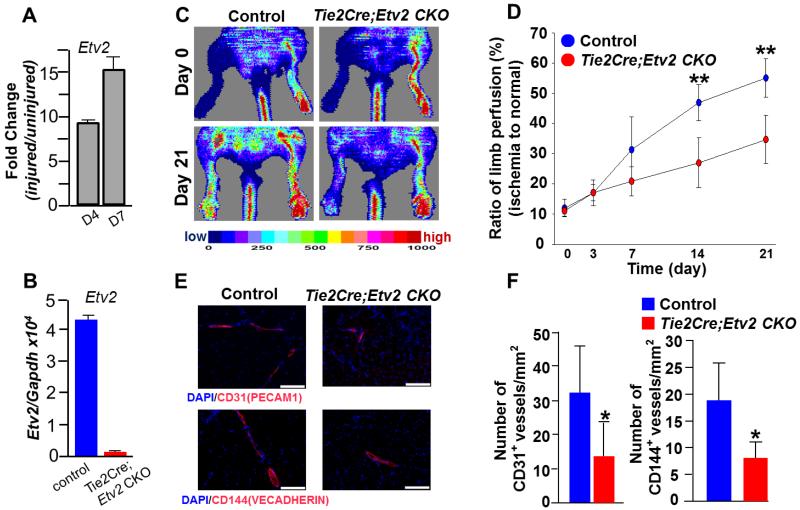
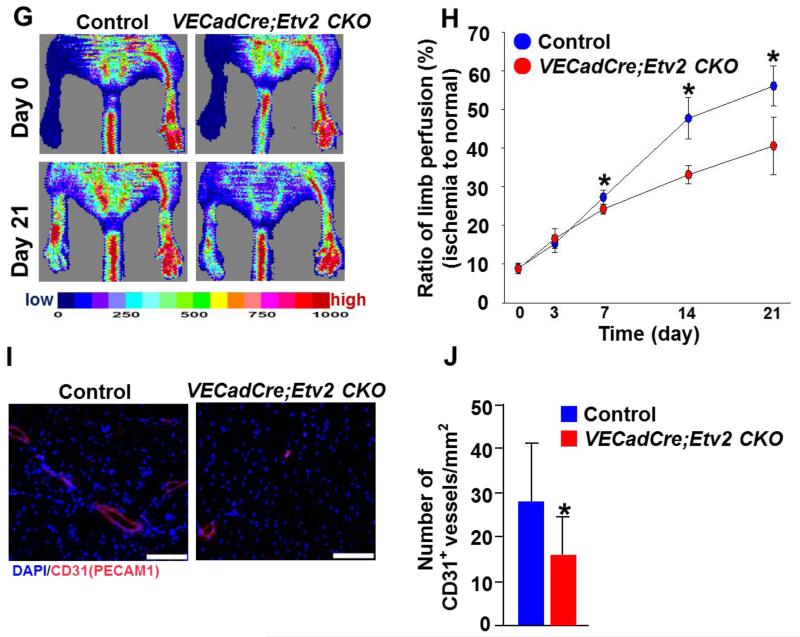
In adult tissue, Etv2 expression is extremely low or undetectable, even in isolated endothelial cells 27 (Figure III in the online-only Data Supplement). However, following hindlimb ischemic injury endothelial Etv2 expression was readily upregulated (Figure 2A; Figure III in the online-only Data Supplement), suggesting its role in neoangiogenesis. To directly determine if Etv2 was required for injury-induced vascular regeneration, we subjected Tie2-Cre; Etv2 CKO mice to hindlimb ischemia 25, 26, 28 and assessed their vascular regeneration ability. As expected, Etv2 expression was almost undetectable in the endothelial cells from Tie2-Cre; Etv2 CKO mice after injury (Figure 2B). Laser Doppler Perfusion Image (LDPI) analysis revealed significantly impaired blood perfusion in Tie2-Cre; Etv2 CKO hindlimbs, compared to control mice (Figure 2C and 2D). Capillary density as determined by CD31 (PECAM1) or VECADHERIN staining was lower in the CKO mice after injury (Figure 2E and 2F). The impaired blood perfusion observed in Tie2-Cre; Etv2 CKO mice was due to Etv2 deficiency as demonstrated by the rescue experiment with lentiviral Etv2 injection (Figure IV in the online-only Data Supplement). To confirm that endothelial Etv2 is responsible for the results of Tie2-Cre; Etv2 CKO mice, we additionally generated VEcadherin-Cre; Etv2 (Etv2f/f or Etv2f/−) CKO mice to specifically delete Etv2 in endothelial cells 22. VEcadherin-Cre; Etv2 CKO mice were also obtained at expected Mendelian ratios and were healthy. Importantly, VECadherin-Cre; Etv2 CKO mice displayed similar angiogenesis defects in hindlimb ischemic injury response as observed in Tie2-Cre; Etv2 CKO mice (Figure 2G-2J). It is well appreciated that both hematopoietic and endothelial cells are required for optimal recovery from hindlimb ischemic injury 29, 30. Thus, we generated and performed hindlimb ischemia studies using Vav-Cre; Etv2 CKO mice (hematopoietic Etv2 deletion) 31, 32 to determine hematopoietic Etv2 contribution to vascular regeneration. As shown in Figure V in the online-only Data Supplement, Vav-Cre; Etv2 CKO mice did not show deficiency in blood perfusion recovery at early time points, day 7 and 14. Only at a later time, day 21, blood perfusion was decreased, compared to controls. These data suggest a key contribution of endothelial Etv2 in ischemia-induced angiogenesis.
Figure 2. Impaired neovascularization in Tie2-Cre; Etv2 CKO and VECadehrin-Cre; Etv2 CKO mice after hindlimb ishcemic injury.
A, qRT-PCR analysis on FACS-sorted CD31+CD45− cells from ischemic hindlimbs. Cells were sorted 4 and 7 dyas after injury. Sorted cells from 4 mice/group were combined and used for gene expression analysis.
B, qRT-PCR analysis on Etv2 expression using FACS-sorted VECADHERIN(VECAD)+ cells from adductor muscle. Cells were sorted from control and Tie2-Cre; Etv2 CKO mice 7 days after ishcemic injury. Sorted cells from 4 mice/group were combined and used for gene expression analysis.
C, Representative images showing physiological status and blood perfusion measured by LDPI of control and Tie2-Cre; Etv2 CKO ischemic hindlimbs.
D, Blood perfusion ratio of ischemic limbs of control and Tie2-Cre; Etv2 CKO mice imaged on day 0, 3, 7, 14 and 21 after ischemic injury is shown. n=5/group, **P < 0.01
E, Representative images of immunohistochemistry. Adductor muscle from control and Tie2-Cre; Etv2 CKO mice were harvested 21 days after injury and subjected to immunohistochemistry using anti-CD31(PECAM1) or CD144 (VECADHERIN) antibody. DAPI (blue) for nuclear staining, Scale bars: 100 μm.
F, Quantification of CD31 (PECAM1) or CD144 (VECADHERIN) positive vessel density in the ischemic region is shown. n=5/group, *P < 0.05
G, Representative images of physiological status and blood perfusion measured by LDPI of control and VECadherin-Cre; Etv2 CKO ischemic hindlimbs.
H, Blood perfusion ratio of ischemic limbs of control and VECadherin-Cre; Etv2 CKO mice imaged on day 0, 3, 7, 14 and 21 after ischemic injury is shown. n=5/group, *P < 0.05
I, Representative photographs of immunohistochemistry are shown. Adductor muscle from control and VECadherin-Cre; Etv2 CKO mice were harvested 21 days after injury and subjected to immunohistochemistry with anti- CD31(PECAM1) antibody. DAPI (blue) for nuclear staining, Scale bars: 100 μm.
J, Quantification of CD31(PECAM1)-positive vessel density in the ischemic region is shown. n=5/group, *P < 0.05. Error bars indicate standard deviations.
In addition to reduced vessel repair ability following hindlimb ischemic injury, Tie2-Cre; Etv2 CKO mice also displayed reduced vascular regeneration ability in a mouse model of choroidal neovascularization (CNV), the major cause of blindness of age-related macular degeneration (AMD) 23, 33. Particularly, there was a significant reduction in the CNV volume in Tie2-Cre; Etv2 CKO mice, compared to controls (5.1×103 ± 7.5×102 μm3 vs 2.3×103 ± 3.7×102 μm3, p=0.001) when mice were analyzed 7 days after the laser injury (Figure 3A and 3B). In an excisional wound healing model 24, new vessel formation, as measured by a MECA32+ vascular area in the wounded back-skin, was impaired in Tie2-Cre; Etv2 CKO mice compared to controls (Figure 3C and 3D). Collectively, these results demonstrate that endothelial ETV2 is required for neovascularization in response to injury.
Figure 3. Tie2-Cre; Etv2 CKO mice display significantly reduced neovascularization in laser-induced eye injury and wounding.
A, Representative images of CNV lesions visualized by FITC-dextran labeling from control and Tie2-Cre; Etv2 CKO mice. Scale bars = 100 μm.
B, CNV lesion volume (expressed as volume of fluorescence) was quantified 7 days after laser treatment. n=5/group. **P <0.01
C, Representative images of MECA32 staining from control and Tie2-Cre; Etv2 CKO back skin obtained 5 days after skin excisional wound (injured). Magnifications = 10 ×, DAPI (blue) for nuclear staining, Ep:Epidermis, Der:Dermis.
D, MECA32 positive area was quantified 5 days after the injury. n=8/group. **P < 0.01. Error bars indicate standard deviations.
Lentiviral Etv2 delivery into ischemic hindlimbs results in enhanced blood perfusion recovery accompanying augmented capillary formation and reduced tissue fibrosis
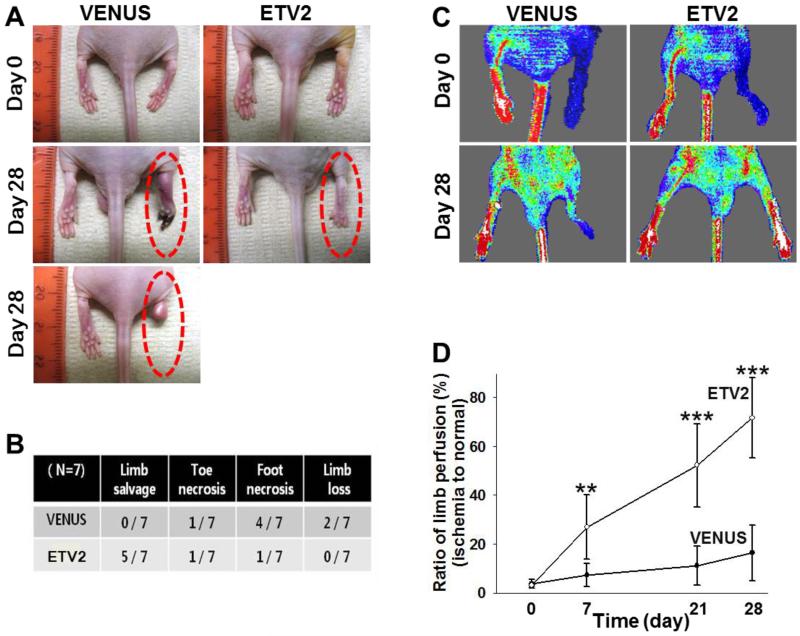
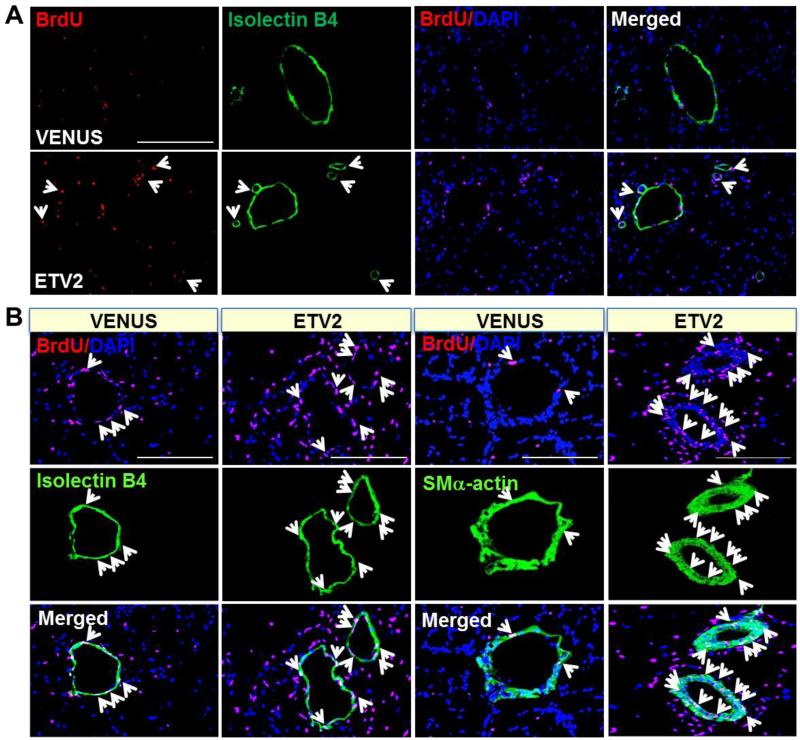
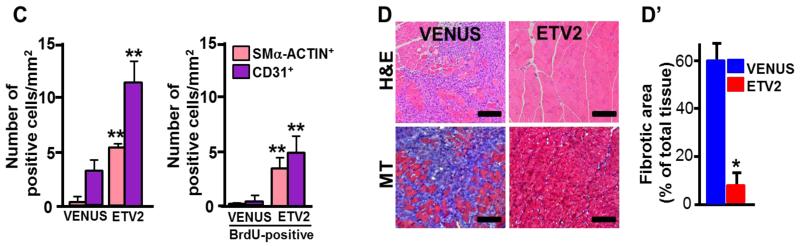
As Etv2 was required for vascular regeneration, we tested if Etv2 gene delivery could augment neovascularization process following injury. Thus, we subjected athymic nude mice to hindlimb ischemic injury followed by lentiviral Etv2 delivery into the adductor muscle. Due to decreased angiogenic recovery in response to injury, athymic nude mice have been instrumental for investigating the efficacy of expressing genes for injury repair 34. Lentivirus expressing Venus/Gfp or Etv2-ires-Venus/Gfp efficiently infected endothelial cells, as shown by CD31+GFP+ cells in the injected areas of the hindlimb (Figure VIA-VIC in the online-only Data Supplement). Etv2 expression was observed at day 4 after the injection, although it was no longer detected after about a month (Figure VID and VIE in the online-only Data Supplement). Whereas control Venus-lentivirus injection had no effect on limb perfusion recovery, Etv2-lentivirus injection protected mice considerably from limb loss or foot necrosis when examined 28 days later (Figure 4A and 4B). Consistently, LDPI analysis showed enhanced blood perfusion recovery in mice injected with Etv2-lentivirus, compared to mice injected with control Venus-lentivirus (Figure 4C and 4D). Similar results were also obtained in C57BL/6J mice, which have strong recovery ability of blood perfusion following ischemic injury (Figure VII in the online-only Data Supplement). Immunohistochemical analysis revealed that Etv2-lentiviral injection led to a greater increase in new vessel formation (Figure 5A-5C). Vascular endothelial cells were proliferating, as determined by the presence of increased number of CD31(PECAM1)+BrdU+ cells, compared to controls (Figure 5C). We additionally found vessels that were 50-100 μm in diameter and augmented numbers of SMα-ACTIN+BrdU+ cells in Etv2-lentivirus injected hindlimbs (Figure 5B and 5C), suggesting that arteriogenesis was also induced. Importantly, Etv2-lentivirus injected muscle were protected from necrotic damage caused by ischemia (Figure 5D, upper panels). Fibrosis, measured by Masson’s trichrome staining, was also markedly attenuated in these animals, compared to controls (Figure 5D, lower panels, D’).
Figure 4. Lentiviral Etv2 expression in ischemic hindlimbs enhances blood perfusion recovery.
A, Representative images of lentiviral Venus or Etv2-injected hindlimbs. Pictures were taken on day 0 and 28 after ischemic injury.
B, Physiological status of ischemic limbs 28 days after the treatment. n=7
C, Representative images of LDPI analysis performed on day 0 and 28 after treatment.
D, Blood perfusion ratio of ischemic limbs measured by laser Doppler imaging on 0, 7, 21, and 28 day after treatment. n=7, **P < 0.01, ***P < 0.001 compared with VENUS injection group. Error bars indicate standard deviations.
Figure 5. lentiviral Etv2 expression in ischemic hindlimbs leads to increased vessel formation and reduced tissue fibrosis.
A, Increased capillary formation in Etv2 injected athymic nude mice after hindlimb ischemia. Twenty eight days after ischemic injury and viral particle injection (VENUS and ETV2), the mice were injected with BrdU (red) and Isolectin B4 (green), and adductor muscles were harvested for immunohistochemistry. Arrows indicate colocalization of cells positive for Isolectin B4 with BrdU and DAPI (blue). Scale bars: 345 μm.
B, Increased arteriogenesis by Etv2. Adductor muscles from the mice (A) were subjected to immunocytochemistry. Arrows indicate colocalization of cells positive for Isolectin B4 or SMα-actin with BrdU and DAPI (blue). Scale bars: 170 μm.
C, Quantification of CD31-positive or SMα-actin-positive vessel density in the ischemic region of the mice is shown. All CD31+ or SMα-actin+ cells regardless of BrdU (left) and CD31+BrdU+ or SMα-actin+BrdU+ cells (right) were counted, respectively. **P < 0.01 compared to VENUS injection group.
D, H & E and Masson’s trichrome staining (MT) staining. Adductor muscles obtained 28 days after ischemic injury were subjected to H & E (upper) and MT staining (lower). Blue region indicates fibrosis. (D’) Quantification of MT staining. n=7/group, Scale bars = 100 μm, *P < 0.05 compared to VENUS injection group. Error bars indicate standard deviations.
Etv2-mediated activation of angiogenic program in vascular regeneration
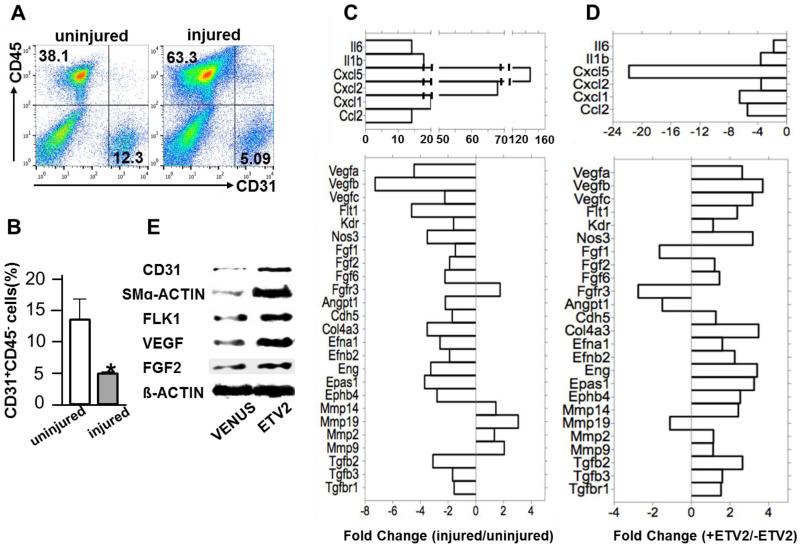
To understand mechanisms by which ETV2 regulates vascular regeneration, we first examined changes in cell population in ischemic hindlimbs. Notably, fewer endothelial cells were present in the ischemic hindlimbs shortly after the injury (4 days later), probably reflecting apoptotic response of endothelial cells to ischemia35. In contrast, there was a significant increase in CD45+ blood cells at this time in the injured hindlimbs, presumably indicating an elevated inflammatory response to injury (Figure 6A and 6B). To further understand molecular events occurring following injury, we examined gene expression profiles in the mouse hindlimb tissues collected 7 days after the ischemic injury. We chose this time point, as vessel remodeling had initiated and inflammation was beginning to subside at this time 35, 36. Angiogenesis PCR array analysis showed that expression of the inflammation-related cytokines, including Il6, Il1β, Cxcl2 and Cxcl5, was highly upregulated at this time point in the injured tissues (Figure 6C; Table I in the online-only Data Supplement). On the other hand, angiogenesis-related genes were generally downregulated. In particular, Vegfs and their receptors, Flk1 and Flt1 expression levels were lower in the injured tissues, which could be partly due to decreased numbers of endothelial cells in the injured tissue. To investigate molecular changes induced by ETV2, we additionally performed an angiogenesis PCR array using RNA obtained from hindlimb tissues that received Etv2 and compared to that of the controls (collected 7 days after injury) (Figure 6D; Table II in the online-only Data Supplement). Upon Etv2-lentivirus injection, inflammatory and chemokine gene expression was down regulated. Conversely, a majority of the angiogenic genes that were downregulated in the ischemic tissue became upregulated by lentiviral Etv2. Notably, Vegfs, Flk1 and Flt1 expression was increased. Western blot analysis confirmed increased CD31, SMα-ACTIN, FLK1 and VEGF protein levels without significant changes in FGF2 production in Etv2-injected hindlimbs (Figure 6E). Collectively, these results suggest that Etv2 expression in ischemic hindlimbs leads to an augmented angiogenic program with concomitant reduction of inflammatory reaction, resulting in endothelial and smooth muscle cell proliferation, neovascularization and tissue repair.
Figure 6. ETV2 Activates angiogenic program in ischemic hindlimbs.
A, FACS analysis using anti-CD45 and anti-CD31(PECAM1) antibody on adductor muscle harvested 7 days after the injury is shown.
B, Quantification of the flow cytometry analysis. n=3/group, *P < 0.05
C, Angiogenesis PCR array using adductor muscle RNA obtained 7 days after ischemic injury is shown. Data is shown as fold change between injured (n=4) and uninjured (n=5) groups.
D, Angiogenesis PCR array using adductor muscle RNA obtained 7 days after ischemic injury of the mice is shown. Data is shown as fold change between Etv2-lentivirus injected and injured control group. n=4/group.
E, Western blot analysis with indicated antibodies on adductor muscles harvested on day 28 after the injection. A representative data from four independent experiments is shown. Error bars indicate standard deviations.
ETV2 regulates VEGF response in vascular regeneration
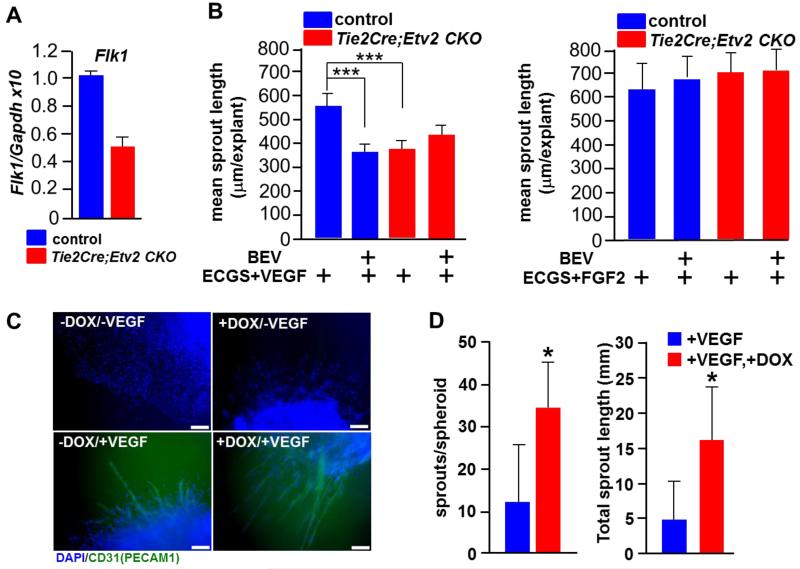
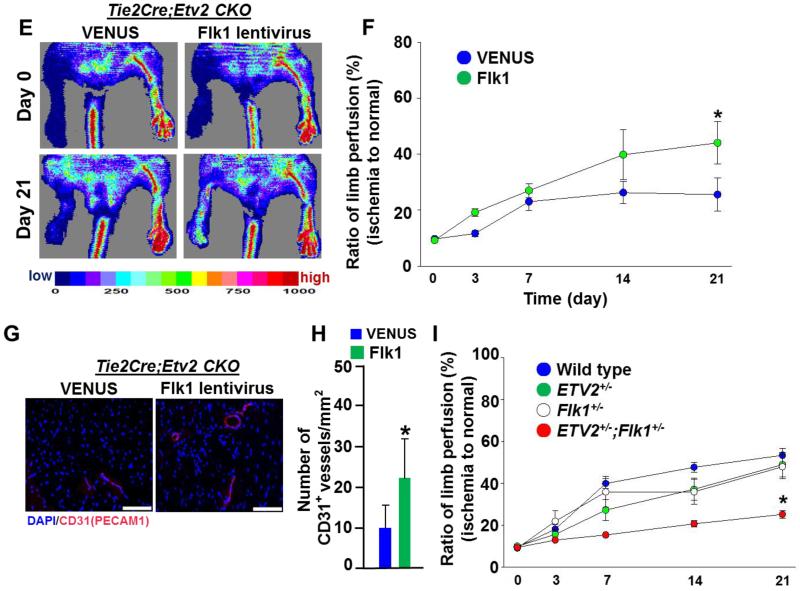
As the VEGF-FLK1 pathway was highly upregulated by lentiviral Etv2 delivery during vessel regeneration, we examined whether the FLK1 pathway, the key downstream target of ETV2 in embryonic vascular development 12-14, could also be a target of ETV2 in neovascularization in the adult. Notably, Flk1 expression levels in endothelial cells isolated from Tie2Cre; Etv2 CKO ischemic hindlimbs were much lower, compared to controls (Figure 7A). To determine if endothelial cells lacking Etv2 show a diminished response to VEGF as a result of blunted Flk1 upregulation, we performed a choroidal explant culture assay, an in vitro quantitative measurement of angiogenesis 37. Tie2-Cre; Etv2 CKO choroidal explants showed impaired endothelial sprouting in the presence of VEGF compared to controls (Figure 7B, left, column 1 vs. 3; Figure VIII in the online-only Data Supplement). While VEGF-mediated sprouting from control explants was suppressed by the VEGF inhibitor Bevacizumab treatment 38 (Figure 7B, left, columns 1 vs. 2), the Tie2-Cre; Etv2 CKO explants were unaffected by Bevacizumab (Figure 7B, left, columns 3 vs. 4), suggesting that impaired sprouting in Etv2 deficient endothelial cells is partly due to a compromised VEGF-FLK1 response. Notably, Tie2-Cre; Etv2 CKO and control choroidal explants showed a similar response to FGF2 (Figure 7B, right, columns 1 vs. 3). Additionally, Bevacizumab did not affect FGF2 responsive vascular sprouting (Figure 7B, right, columns 1 vs. 2, columns 3 vs. 4).
Figure 7. ETV2 regualtes VEGF response in angiogenesis.
A, qRT-PCR analysis of Flk1 expression on FACS-sorted VECADHERIN(VECAD)+ cells from adductor muscle of control and Tie2-Cre; Etv2 CKO mice. Cells were sorted 7 days after ishcemic injury. Sorted cells from 4 mice/group were combined and used for gene expression analysis.
B, Angiogenic sprouting in Tie2-Cre; Etv2 CKO choroidal explants. Choroidal explants of the controls and Tie2-Cre; Etv2 CKO mice were subjected to angiogenic sprouting assay and the mean sprout length was measured 9 days later. BEV; bevacizumab, ECGS; endothelial cell growth supplement, n=4/group, ***P < 0.001.
C, Representative CD31 staining of EB sprouts are shown. DAPI (blue) staining for nucleus is shown. Scale bar: 100 μm.
D, Quantification of the number of sprouts per EB (left) and the total sprout length (right) is shown. n=5/group, *P < 0.05. Error bars indicate standard deviations.
E, Representative images of blood perfusion of Tie2-Cre; Etv2 CKO mice injected with lentiviral Venus or Flk1. Images were obtained on day 0 and 21 after ischemic injury.
F, Blood perfusion ratio of ischemic hindlimbs measured by LDPI in Tie2-Cre; Etv2 CKO injected with lentiviral Venus or Flk1 is shown. n=4/group, *P < 0.05
G, Representative images of CD31 immunohistochemistry. Adductor muscle from Tie2-Cre; Etv2 CKO and Tie2-Cre; Etv2 CKO mice injected with lentiviral Flk1 were harvested 21 days after injury and subjected to immunohistochemistry with anti-CD31(PECAM1) antibody. DAPI (blue) for nuclear staining, Scale bars: 100 μm.
H, Quantification of CD31(PECAM1)-positive vessel density in the ischemic region. n=4/group, *P < 0.05
I, A genetic interation between ETV2 and FLLK in ischemia-induced angiogenesis. Wild type (n=9), Etv2+/− (n=16), Flk1+/−(n=14) and Etv2+/−; Flk1+/− (n=11) mice were subjected to hindlimb ischemia and blood perfusion ratio of the ischemic limbs was measured on day 0, 3, 7, 14 and 21 after the injury using LDPI. *P < 0.05, wt, Etv2+/−, Flk1+/− vs. Etv2+/−; Flk1+/−. Error bars indicate standard deviations.
To further validate the ETV2-FLK1 pathway in vascular regeneration, we utilized in vitro differentiated ES cells (embryoid bodies, EBs), which exhibit robust endothelial differentiation and sprouting angiogenesis on collagen I matrix or matrigel 39-41. Specifically, doxycyclin (DOX) inducible Etv2 EBs 12 were subjected to sprouting angiogenesis on a solidified collagen I matrix in the presence of VEGF and/or DOX. As previously reported, EB sprouting angiogenesis was induced by VEGF 39, 41. Notably, EB sprouting angiogenesis was significantly increased in the presence of both VEGF and DOX (Figure 7C and 7D; Figure IX in the online-only Data Supplement).
If indeed the FLK1 pathway functions downstream of ETV2 in vascular regeneration, it was expected that Flk1 expression would rescue the neovascularization defects observed in Tie2-Cre; Etv2 CKO mice. To test this model, Tie2-Cre; Etv2 CKO mice were subjected to hindlimb ischemic injury followed by lentiviral Flk1 delivery into the adductor muscle. Whereas control lentiviral injection had no effect on limb perfusion recovery, lentiviral Flk1 injection into Tie2-Cre; Etv2 CKO ischemic hindlimbs restored the impaired blood perfusion (Figure 7E and 7F; Figure X in the online-only Data Supplement). Moreover, vessel density of the CKO hindlimbs was augmented by Flk1 lentivirus injection, compared to controls (Figure 7G and 7H).
To further validate that ETV2 mediated vascular regeneration is facilitated through activating the FLK1 pathway, we subjected wild type, Etv2+/− and Flk1+/− single heterozygous mice and Etv2+/−; Flk1+/− double heterozygous mice to hindlimb ischemic injury (Figure 7I). Etv2+/− or Flk1+/− single heterozygous mice showed a trend (not statistically significant) towards impaired recovery in limb perfusion compared to wild type controls, suggesting the potential of a haploinsufficient role for Etv2 or Flk1 in vascular regeneration. Importantly, Etv2+/−; Flk1+/− double heterozygous mice showed significantly (p<0.05) worse blood perfusion recovery compared to either single heterozygous mouse. These data support a role for Etv2 and Flk1 within a common genetic pathway in vessel regeneration.
Discussion
Angiogenesis is fundamental for successful tissue repair and regeneration. It is widely accepted that genetic programs regulating developmental angiogenesis also drive postnatal angiogenesis. For example, VEGF and FLK1 have been extensively studied in the context of embryonic vessel development, vascular regeneration, and pathologic angiogenesis. Notably, Vegfa or Flk1 deficiency leads to embryonic lethality due to defects in vascular and hematopoietic formation 42-44. Importantly, neovascularization in ischemic limbs or myocardial ischemia can be enhanced by VEGF administration, suggesting that a proangiogenic approach may be beneficial for ischemic vascular occlusion 45. Moreover, VEGF targeted therapy is commonly used to correct pathologic angiogenesis such as in AMD and tumors. Tissue injuries or tumors create a hypoxic environment and hypoxia is a key upstream inducer of Vegf activation, which may explain how the VEGF pathway can be activated in various pathologic conditions. However, the upstream events leading to FLK1 activation or inhibition in endothelial cells under pathophysiologic conditions have not been well characterized.
ETV2 function is indispensable for vessel development, as Etv2 deficiency, similar to Vegf or Flk1 deficiency, leads to an absolute block in blood and vessel formation and embryonic lethality. However, Etv2 deletion using Tie2-Cre, VECadherin-Cre or Vav-Cre does not affect normal embryonic development, indicating that Etv2 is only transiently required during vessel and blood specification. Consistently, Etv2 expression becomes silent once the circulatory system is established and is not detectable beyond E11.5 especially in endothelial cells 10-12. These studies raise an important question as to whether Etv2 is dispensable in postnatal life. As mice lacking endothelial Etv2 displayed seemingly normal homeostatic vessel maintenance, we determined in this study whether ETV2 could be involved in injury response. A major unexpected finding of this study is that although ETV2 is not required for steady-state vessel maintenance, Etv2 is necessary for efficient vessel regeneration in response to injury. There was noticeable upregulation of Etv2 in endothelial cells isolated from ischemic tissues. Endothelial Etv2 deficiency led to a poor vascular regeneration capacity. Thus, our findings are consistent with the notion that embryonic signaling either through reactivation or dysregulation can become an important etiology in the pathophysiological events in adults 46-48. As lentiviral Etv2 expression in ischemic hindlimbs led to overall angiogenic gene upregulation including Flk1, we envision that Etv2 reactivation upon injury-mediated signaling may turn on the angiogenic program to facilitate vessel regeneration. Of the angiogenic pathway, we identify the VEGF-FLK1 pathway, as in development 12-14, to be a major target of ETV2 in neovascularization. Specifically, Etv2 deficient endothelial cells displayed blunted Flk1 upregulation and defective VEGF responsive capillary sprouting upon injury. Conversely, ETV2 augmented VEGF response in angiogenic sprouting from EBs. Blood perfusion and vascular regeneration in mice lacking endothelial Etv2 was restored by lentiviral Flk1 delivery. A significant impairment of blood perfusion in Etv2+/−; Flk1+/− mice further suggests a linear relationship between ETV2 and FLK1 in vascular regeneration. While ETV2 functions upstream of Flk1 in development and in injury mediated response as we showed in this study, several papers reported that ETV2 could be induced by VEGF 11, 49. Notably, VEGF is readily induced in hypoxic conditions. It has recently been suggested that there is a positive feedback mechanism between ETV2 and FLK1 signaling in embryogenesis 14. Thus, while injury signals that can lead to Etv2 reactivation remain to be elucidated, it is possible that ETV2-FLK1 feed-forward mechanism might amplify the VEGF response in vascular regeneration.
A single lentiviral Etv2 injection was sufficient to protect mice from limb loss and enhanced blood perfusion in ischemic hindlimbs. Further, Etv2-lentivirus injected muscle was protected from necrotic damage caused by ischemic injury. One simple explanation for the potent function of ETV2 in this process would be that endothelial cells infected with ETV2 proliferate and activate angiogenic program and thus generate more blood vessels, leading to tissue protection. Indeed, we found that endothelial cells were readily infected by lentiviral Etv2 in the injured tissues. Since ETV2 delivery can also induce arteriogenesis, it might be possible that endothelial ETV2 upregulates the expression of paracrine factors for smooth muscle cell recruitment and proliferation. Intriguingly, recent studies show that ETV2 can directly reprogram somatic cells into functional endothelial cells in vitro 50-52. Additionally, ectopic etv2 expression in zebrafish was able to convert skeletal muscle cells into endothelial cells 53. Thus, it is also possible that reprogrammed endothelial cells in the ischemic hindlimbs from other cell types such as skeletal muscle cells, fibroblasts or hematopoietic cells, if any, could also have contributed to the observed protected phenomenon. In agreement with this, we found that hematopoietic cells and presumably skeletal muscles were also infected with lentiviral Etv2 in ischemic hindlimbs. Future studies are warranted whether Etv2 expression could indeed cause in vivo conversion of non-endothelial cells into endothelial cells and if so, then the magnitude of contribution of such in vivo reprogrammed endothelial cells to vascular regeneration. Intriguingly, Etv2 expression was no longer detected once the vascular regeneration was completed in the mouse hindlimb ischemia model. A transient, but not persistent, upregulation of Etv2 in the initial stages of vascular regeneration might therefore be critical. Reminiscent to the transient Etv2 expression pattern during embryogenesis 10-12, there might be a strong pressure to keep this gene off in established vasculature. Indeed, sustained expression of Etv2 in Tie2+ cells leads to abnormal vessel development as manifested by dilated yolk sac vessels 54. Collectively, our findings suggest that Etv2 delivery into injured tissues could be used for therapeutic purpose for conditions requiring vessel regeneration.
A recent study has demonstrated that FGF2, probably through FGFR1/2, can activate Flk1 expression via ERK-ETS 55. However, we found that Tie2-Cre; Etv2CKO and control choroidal explants showed similar vascular sprouting in response to FGF2, suggesting that FGF2 could regulate Flk1 expression by ETV2 independent manner. In the absence of ETV2, other ETS factors such as ETS1 could function downstream of FGF2 in regulating Flk1 expression. Additionally, the observed discrepancy might be due to 1) FGF2 treatment to choroidal explants vs adenoviral delivery of FGFR1 DN to HUVECs or 2) different genetic models; Tie2-Cre; Etv2 CKO vs FGFR1 DN transgenic mice. We propose that ETV2 is critical for regulating VEGF/FLK1 signaling, but dispensable for FGF2 signaling in neoangiogenesis. Combined FGFRs and ETV2 inactivation in endothelial cells is warranted in the future to further understand the interplay between FGF and VEGF/FLK1 signaling with regard to ETV2 in vascular regeneration.
In conclusion, we demonstrated that endothelial Etv2 is critical for vessel regeneration and tissue repair following injury. Additionally, ETV2 has potent efficacy in therapeutic angiogenesis, providing a novel research platform for vascular regeneration therapies. Current therapeutic angiogenic targets such as VEGF have presented severe limitations in specifically targeting vessels undergoing neoangiogenesis, as they are also required for vessel homeostasis. In contrast, Etv2 is activated in endothelial cells upon injury, thus aiming the ETV2 pathway could provide unique opportunity for specifically targeting the activated endothelial cells, while sparing homeostatic vessel integrity.
Supplementary Material
Significance.
Angiogenesis is an important process for successful embryogenesis and injury-mediated tissue repair and regeneration. ETV2, an ETS factor, is an indispensable regulator of embryonic vessel development. Here, we for the first time reveal a novel and unexpected function of ETV2 in injury-induced vascular regeneration. Specifically, we show that while Etv2 expression in endothelial cells is not detectable under steady state conditions, it becomes reactivated and upregulates genes critical for angiogenesis upon injury. Mice lacking endothelial Etv2 display impaired vascular regeneration in response to injuries. Furthermore, enforced Etv2 expression is sufficient to regenerate vessels, as lentiviral Etv2 delivery leads to enhanced endothelial cell proliferation, angiogenesis, vascular regeneration and tissue repair in the mouse hindlimb ischemia model. As such, our study provides compelling necessary and sufficient role of ETV2 in vascular regeneration in adult. Our findings could provide a new research platform for the development of novel therapeutic strategies requiring angiogenic intervention.
Acknowledgements
The authors thank Eun Jae Kim and Ju Young Kim for initial technical support and Emory Children’s Pediatric Research Flow Cytometry Core and Integrated Cellular Imaging Core. C.P. and K.C. conceived and designed experiments, analyzed data and wrote the paper. C.P with the help of T.L., S.H.B., F.L., R.N., S.S.O., I.P-R., B.C., H.S.C., T.M.K., N.U. performed experiments and analyzed data. M.U-F., B-S. K., R.S.A., D.M.O. helped with data interpretation and manuscript preparation. H.M., D.J.L., D.S.L. provided experimental materials.
Sources of Funding
This work was supported by the March of Dimes Foundation 5-FY12-44, the American Heart Association 11SDG7390074 and NIH HL119291 (C.P); NIH R01EY019287, NIH P30EY02687, Carl Marshall Reeves and Mildred Almen Reeves Foundation Inc. Award, Research to Prevent Blindness Inc. Career Development Award, American Health Assistance Foundation, Thome Foundation and a Research to Prevent Blindness Inc. Unrestricted Grant to Washington University (R.S.A); NIH T32 HL07275-34 (S.S.O); HL105732 (D.M.O); HL63736 and HL55337 (K.C).
Nonstandard abbreviations and Acronyms
- ER71/ETV2
Ets related protein 71/ Ets variant 2
- Flk1/VEGFR2
Fetal liver kinase 1/vascular endothelial growth factor receptor 2
- CKO
conditional knockout
- CNV
choroidal neovascularization
- VEGF
vascular endothelial growth factor
- FGF
fibroblast growth factor
Footnotes
Disclosures
None
References
- 1.De Val S, Chi NC, Meadows SM, et al. Combinatorial regulation of endothelial gene expression by ets and forkhead transcription factors. Cell. 2008;135:1053–1064. doi: 10.1016/j.cell.2008.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wythe JD, Dang LT, Devine WP, Boudreau E, Artap ST, He D, Schachterle W, Stainier DY, Oettgen P, Black BL, Bruneau BG, Fish JE. Ets factors regulate vegf-dependent arterial specification. Dev Cell. 2013;26:45–58. doi: 10.1016/j.devcel.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei GH, Badis G, Berger MF, et al. Genome-wide analysis of ets-family DNA-binding in vitro and in vivo. EMBO J. 2010;29:2147–2160. doi: 10.1038/emboj.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spyropoulos DD, Pharr PN, Lavenburg KR, Jackers P, Papas TS, Ogawa M, Watson DK. Hemorrhage, impaired hematopoiesis, and lethality in mouse embryos carrying a targeted disruption of the fli1 transcription factor. Mol Cell Biol. 2000;20:5643–5652. doi: 10.1128/mcb.20.15.5643-5652.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pham VN, Lawson ND, Mugford JW, Dye L, Castranova D, Lo B, Weinstein BM. Combinatorial function of ets transcription factors in the developing vasculature. Dev Biol. 2007;303:772–783. doi: 10.1016/j.ydbio.2006.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abedin MJ, Nguyen A, Jiang N, Perry CE, Shelton JM, Watson DK, Ferdous A. Fli1 acts downstream of etv2 to govern cell survival and vascular homeostasis via positive autoregulation. Circ Res. 2014;114:1690–1699. doi: 10.1161/CIRCRESAHA.1134303145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dejana E, Taddei A, Randi AM. Foxs and ets in the transcriptional regulation of endothelial cell differentiation and angiogenesis. Biochim Biophys ACTA. 2007;1775:298–312. doi: 10.1016/j.bbcan.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Craig MP, Grajevskaja V, Liao HK, Balciuniene J, Ekker SC, Park JS, Essner JJ, Balciunas D, Sumanas S. Etv2 and fli1b function together as key regulators of vasculogenesis and angiogenesis. Arterioscler Thromb Vasc Biol. 2015;35:865–876. doi: 10.1161/ATVBAHA.114.304768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei G, Srinivasan R, Cantemir-Stone CZ, Sharma SM, Santhanam R, Weinstein M, Muthusamy N, Man AK, Oshima RG, Leone G, Ostrowski MC. Ets1 and ets2 are required for endothelial cell survival during embryonic angiogenesis. Blood. 2009;114:1123–1130. doi: 10.1182/blood-2009-03-211391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferdous A, Caprioli A, Iacovino M, Martin CM, Morris J, Richardson JA, Latif S, Hammer RE, Harvey RP, Olson EN, Kyba M, Garry DJ. Nkx2-5 transactivates the ets-related protein 71 gene and specifies an endothelial/endocardial fate in the developing embryo. Proc Natl Acad Sci. USA. 2009;106:814–819. doi: 10.1073/pnas.0807583106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kataoka H, Hayashi M, Nakagawa R, Tanaka Y, Izumi N, Nishikawa S, Jakt ML, Tarui H, Nishikawa S. Etv2/er71 induces vascular mesoderm from flk1+pdgfralpha+ primitive mesoderm. Blood. 2011;118:6975–6986. doi: 10.1182/blood-2011-05-352658. [DOI] [PubMed] [Google Scholar]
- 12.Lee D, Park C, Lee H, Lugus JJ, Kim SH, Arentson E, Chung YS, Gomez G, Kyba M, Lin S, Janknecht R, Lim DS, Choi K. Er71 acts downstream of bmp, notch, and wnt signaling in blood and vessel progenitor specification. Cell Stem Cell. 2008;2:497–507. doi: 10.1016/j.stem.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu F, Kang I, Park C, Chang LW, Wang W, Lee D, Lim DS, Vittet D, Nerbonne JM, Choi K. Er71 specifies flk-1+ hemangiogenic mesoderm by inhibiting cardiac mesoderm and wnt signaling. Blood. 2012;119:3295–3305. doi: 10.1182/blood-2012-01-403766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu F, Li D, Yu YY, Kang I, Cha MJ, Kim JY, Park C, Watson DK, Wang T, Choi K. Induction of hematopoietic and endothelial cell program orchestrated by ets transcription factor er71/etv2. EMBO Rep. 2015;16:654–669. doi: 10.15252/embr.201439939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neuhaus H, Muller F, Hollemann T. Xenopus er71 is involved in vascular development. Dev Dyn. 2010;239:3436–3445. doi: 10.1002/dvdy.22487. [DOI] [PubMed] [Google Scholar]
- 16.Sumanas S, Gomez G, Zhao Y, Park C, Choi K, Lin S. Interplay among etsrp/er71, scl, and alk8 signaling controls endothelial and myeloid cell formation. Blood. 2008;111:4500–4510. doi: 10.1182/blood-2007-09-110569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sumanas S, Lin S. Ets1-related protein is a key regulator of vasculogenesis in zebrafish. PLoS Biol. 2006;4:e10. doi: 10.1371/journal.pbio.0040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kataoka H, Hayashi M, Kobayashi K, Ding G, Tanaka Y, Nishikawa S. Region-specific etv2 ablation revealed the critical origin of hemogenic capacity from hox6-positive caudal-lateral primitive mesoderm. Exp Hematol. 2013;41:567–581. e569. doi: 10.1016/j.exphem.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 19.Dumont DJ, Fong GH, Puri MC, Gradwohl G, Alitalo K, Breitman ML. Vascularization of the mouse embryo: A study of flk-1, tek, tie, and vascular endothelial growth factor expression during development. Dev Dyn. 1995;203:80–92. doi: 10.1002/aja.1002030109. [DOI] [PubMed] [Google Scholar]
- 20.Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M. Tie2-cre transgenic mice: A new model for endothelial cell-lineage analysis in vivo. Dev Biol. 2001;230:230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- 21.Tang Y, Harrington A, Yang X, Friesel RE, Liaw L. The contribution of the tie2+ lineage to primitive and definitive hematopoietic cells. Genesis. 2010;48:563–567. doi: 10.1002/dvg.20654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alva JA, Zovein AC, Monvoisin A, Murphy T, Salazar A, Harvey NL, Carmeliet P, Iruela-Arispe ML. Ve-cadherin-cre-recombinase transgenic mouse: A tool for lineage analysis and gene deletion in endothelial cells. Dev Dyn. 2006;235:759–767. doi: 10.1002/dvdy.20643. [DOI] [PubMed] [Google Scholar]
- 23.Apte RS, Richter J, Herndon J, Ferguson TA. Macrophages inhibit neovascularization in a murine model of age-related macular degeneration. PLoS Med. 2006;3:e310. doi: 10.1371/journal.pmed.0030310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thorey IS, Hinz B, Hoeflich A, Kaesler S, Bugnon P, Elmlinger M, Wanke R, Wolf E, Werner S. Transgenic mice reveal novel activities of growth hormone in wound repair, angiogenesis, and myofibroblast differentiation. J Biol Chem. 2004;279:26674–26684. doi: 10.1074/jbc.M311467200. [DOI] [PubMed] [Google Scholar]
- 25.Limbourg A, Korff T, Napp LC, Schaper W, Drexler H, Limbourg FP. Evaluation of postnatal arteriogenesis and angiogenesis in a mouse model of hind-limb ischemia. Nat Protoc. 2009;4:1737–1746. doi: 10.1038/nprot.2009.185. [DOI] [PubMed] [Google Scholar]
- 26.Liu F, Bhang SH, Arentson E, et al. Enhanced hemangioblast generation and improved vascular repair and regeneration from embryonic stem cells by defined transcription factors. Stem Cell Reports. 2013;1:166–182. doi: 10.1016/j.stemcr.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hollenhorst PC, Jones DA, Graves BJ. Expression profiles frame the promoter specificity dilemma of the ets family of transcription factors. Nucleic Acids Res. 2004;32:5693–5702. doi: 10.1093/nar/gkh906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Couffinhal T, Silver M, Zheng LP, Kearney M, Witzenbichler B, Isner JM. Mouse model of angiogenesis. Am J Pathol. 1998;152:1667–1679. [PMC free article] [PubMed] [Google Scholar]
- 29.Nie L, Guo X, Esmailzadeh L, et al. Transmembrane protein esdn promotes endothelial vegf signaling and regulates angiogenesis. J Clin Invest. 2013;123:5082–5097. doi: 10.1172/JCI67752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silvestre JS, Smadja DM, Levy BI. Postischemic revascularization: From cellular and molecular mechanisms to clinical applications. Physiol Rev. 2013;93:1743–1802. doi: 10.1152/physrev.00006.2013. [DOI] [PubMed] [Google Scholar]
- 31.de Boer J, Williams A, Skavdis G, Harker N, Coles M, Tolaini M, Norton T, Williams K, Roderick K, Potocnik AJ, Kioussis D. Transgenic mice with hematopoietic and lymphoid specific expression of cre. Eur J Immunol. 2003;33:314–325. doi: 10.1002/immu.200310005. [DOI] [PubMed] [Google Scholar]
- 32.Ogilvy S, Elefanty AG, Visvader J, Bath ML, Harris AW, Adams JM. Transcriptional regulation of vav, a gene expressed throughout the hematopoietic compartment. Blood. 1998;91:419–430. [PubMed] [Google Scholar]
- 33.Klein R, Peto T, Bird A, Vannewkirk MR. The epidemiology of age-related macular degeneration. Am J Ophthalmol. 2004;137:486–495. doi: 10.1016/j.ajo.2003.11.069. [DOI] [PubMed] [Google Scholar]
- 34.Couffinhal T, Silver M, Kearney M, Sullivan A, Witzenbichler B, Magner M, Annex B, Peters K, Isner JM. Impaired collateral vessel development associated with reduced expression of vascular endothelial growth factor in apoe-/- mice. Circulation. 1999;99:3188–3198. doi: 10.1161/01.cir.99.24.3188. [DOI] [PubMed] [Google Scholar]
- 35.Paoni NF, Peale F, Wang F, Errett-Baroncini C, Steinmetz H, Toy K, Bai W, Williams PM, Bunting S, Gerritsen ME, Powell-Braxton L. Time course of skeletal muscle repair and gene expression following acute hind limb ischemia in mice. Physiol Genomics. 2002;11:263–272. doi: 10.1152/physiolgenomics.00110.2002. [DOI] [PubMed] [Google Scholar]
- 36.Lee CW, Stabile E, Kinnaird T, Shou M, Devaney JM, Epstein SE, Burnett MS. Temporal patterns of gene expression after acute hindlimb ischemia in mice: Insights into the genomic program for collateral vessel development. J Am Coll Cardiol. 2004;43:474–482. doi: 10.1016/j.jacc.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 37.Kobayashi S, Shinohara H, Tsuneki H, Nagai R, Horiuchi S. N(epsilon)-(carboxymethyl)lysine proliferated cd34(+) cells from rat choroidal explant in culture. Biol Pharm Bull. 2004;27:1382–1387. doi: 10.1248/bpb.27.1382. [DOI] [PubMed] [Google Scholar]
- 38.Presta LG, Chen H, O’Connor SJ, Chisholm V, Meng YG, Krummen L, Winkler M, Ferrara N. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997;57:4593–4599. [PubMed] [Google Scholar]
- 39.Jakobsson L, Franco CA, Bentley K, Collins RT, Ponsioen B, Aspalter IM, Rosewell I, Busse M, Thurston G, Medvinsky A, Schulte-Merker S, Gerhardt H. Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat Cell Biol. 2010;12:943–953. doi: 10.1038/ncb2103. [DOI] [PubMed] [Google Scholar]
- 40.Jakobsson L, Kreuger J, Holmborn K, Lundin L, Eriksson I, Kjellen L, Claesson-Welsh L. Heparan sulfate in trans potentiates vegfr-mediated angiogenesis. Dev Cell. 2006;10:625–634. doi: 10.1016/j.devcel.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 41.Lugus JJ, Chung YS, Mills JC, Kim SI, Grass J, Kyba M, Doherty JM, Bresnick EH, Choi K. Gata2 functions at multiple steps in hemangioblast development and differentiation. Development. 2007;134:393–405. doi: 10.1242/dev.02731. [DOI] [PubMed] [Google Scholar]
- 42.Carmeliet P, Ferreira V, Breier G, et al. Abnormal blood vessel development and lethality in embryos lacking a single vegf allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 43.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the vegf gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 44.Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 45.Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004;56:549–580. doi: 10.1124/pr.56.4.3. [DOI] [PubMed] [Google Scholar]
- 46.Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 47.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Louvi A, Artavanis-Tsakonas S. Notch and disease: A growing field. Semin Cell Dev Biol. 2012;23:473–480. doi: 10.1016/j.semcdb.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rasmussen TL, Shi X, Wallis A, Kweon J, Zirbes KM, Koyano-Nakagawa N, Garry DJ. Vegf/flk1 signaling cascade transactivates etv2 gene expression. PloS One. 2012;7:e50103. doi: 10.1371/journal.pone.0050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ginsberg M, James D, Ding BS, et al. Efficient direct reprogramming of mature amniotic cells into endothelial cells by ets factors and tgfbeta suppression. Cell. 2012;151:559–575. doi: 10.1016/j.cell.2012.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Han JK, Chang SH, Cho HJ, et al. Direct conversion of adult skin fibroblasts to endothelial cells by defined factors. Circulation. 2014;130:1168–1178. doi: 10.1161/CIRCULATIONAHA.113.007727. [DOI] [PubMed] [Google Scholar]
- 52.Morita R, Suzuki M, Kasahara H, Shimizu N, Shichita T, Sekiya T, Kimura A, Sasaki K, Yasukawa H, Yoshimura A. Ets transcription factor etv2 directly converts human fibroblasts into functional endothelial cells. Proc Natl Acad Sci. USA. 2015;112:160–165. doi: 10.1073/pnas.1413234112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Veldman MB, Zhao C, Gomez GA, Lindgren AG, Huang H, Yang H, Yao S, Martin BL, Kimelman D, Lin S. Transdifferentiation of fast skeletal muscle into functional endothelium in vivo by transcription factor etv2. PLoS Biol. 2013;11:e1001590. doi: 10.1371/journal.pbio.1001590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hayashi M, Pluchinotta M, Momiyama A, Tanaka Y, Nishikawa S, Kataoka H. Endothelialization and altered hematopoiesis by persistent etv2 expression in mice. Exp Hematol. 2012;40:738–750. e711. doi: 10.1016/j.exphem.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 55.Murakami M, Nguyen LT, Hatanaka K, Schachterle W, Chen PY, Zhuang ZW, Black BL, Simons M. Fgf-dependent regulation of vegf receptor 2 expression in mice. J Clin Invest. 2011;121:2668–2678. doi: 10.1172/JCI44762. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.