Abstract
Purpose
To investigate the differences in outcomes among patients with muscle-invasive bladder cancer on 2 NRG Oncology Radiation Therapy Oncology Group protocols who achieved complete response and near-complete response after induction chemoradiation and then completed bladder-preserving therapy with chemoradiation therapy (chemo-RT) to full dose (60-64 Gy).
Patients and Methods
A pooled analysis was performed on 119 eligible patients with muscle-invasive bladder cancer enrolled on 2 NRG Oncology Radiation Therapy Oncology Group trials, who were classified as having a complete (T0) or near-complete (Ta or Tis) response after induction chemo-RT and completed consolidation with a total RT dose of at least 60 Gy. Bladder recurrence, salvage cystectomy rates, and disease-specific survival were estimated by the cumulative incidence method and bladder-intact and overall survivals by the Kaplan-Meier method.
Results
Among the 119 eligible patients, 101 (85%) achieved T0, and 18 (15%) achieved Ta or Tis after induction chemo-RT and proceeded to consolidation. After a median follow-up of 5.9 years, 36 of 101 T0 patients (36%) versus 5 of 18 Ta or Tis patients (28%) experienced bladder recurrence (P=.52). Thirteen patients among complete responders eventually required late salvage cystectomy for tumor recurrence, compared with 1 patient among near-complete responders (P=.63). Disease-specific, bladder-intact, and overall survivals were not significantly different between T0 and Ta/Tis cases.
Conclusions
The bladder recurrence and salvage cystectomy rates of the complete and the near-complete responders were similar. Therefore it is reasonable to recommend that patients with Ta or Tis after induction chemo-RT continue with bladder-sparing therapy with consolidation chemo-RT to full dose (60-64 Gy).
Introduction
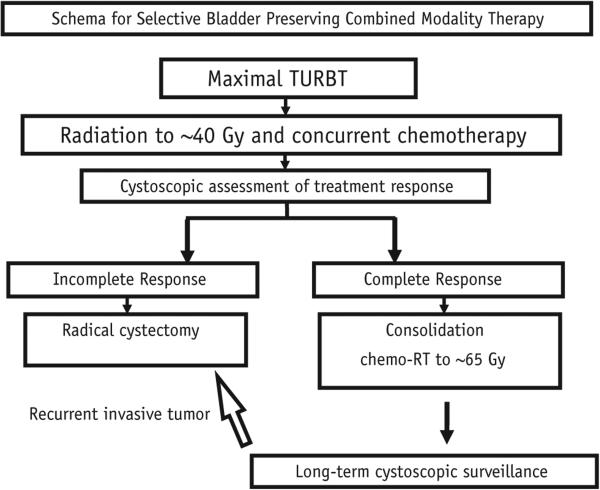
The definitive treatments of localized muscle-invasive bladder cancer (MIBC) can be broadly divided into 2 groups: removal of the bladder and bladder-sparing modalities. Radical cystectomy remains the standard and most common treatment offered to patients with MIBC in the United States. According to contemporary publications (1, 2) cystectomy series for patients with MIBC report 5-year overall survival (OS) rates ranging from 40% to 60%, according to clinical staging. A large body of experience from institutions in North America and Europe suggests that bladder-sparing approaches yield favorable results in appropriately selected patients, with 5-year OS rates of 50% to 60%, and survival rates with an intact bladder of 40% to 45% (3-9). A North American contemporary radiation-based trimodality bladder-sparing therapy algorithm (Fig. 1) for patients with MIBC who are cystectomy candidates is based on results of consecutive Radiation Therapy Oncology Group (RTOG) protocols (10, 11). It consists of maximal transurethral resection of bladder tumor (TURBT), induction external beam radiation therapy (RT) with concurrent chemotherapy, and cystoscopic assessment of treatment response, with prompt cystectomy for nonresponders or consolidation external beam RT with concurrent chemotherapy for complete responders. Active cystoscopic surveillance with salvage cystectomy at first sign of invasive recurrence after the bladder-sparing treatment therapy is paramount to achieving excellent outcomes, as may be cisplatin-based adjuvant chemotherapy, if tolerated.
Fig. 1.
Schema for bladder preservation trimodality therapy in muscle-invasive bladder cancer. Both NRG Oncology Radiation Therapy Oncology Group (RTOG) protocols (9906 and 0233) have included cisplatin-based adjuvant chemotherapy, if tolerated, after consolidation chemoradiation therapy (chemo-RT) for patients with complete response or after early salvage cystectomy for patient with incomplete response. Abbreviation: TURBT = transurethral resection of bladder tumor.
Historically, complete response to the induction chemoradiation, defined as no evidence of primary tumor (T0) on cystoscopic assessment with biopsy, was required for patients to proceed with consolidation chemoradiation. However, many clinicians felt uncomfortable recommending early salvage cystectomy for a small amount of noninvasive bladder cancer, in the form of Ta or Tis at this treatment check point, and this prompted modification of the most recent NRG Oncology RTOG protocols to allow patients with near-complete response (Ta or Tis) after the induction phase to continue with bladder-sparing modality.
To evaluate the appropriateness of this approach, we investigated the differences in the outcomes among patients on 2 NRG Oncology RTOG protocols who achieved complete response and near-complete response after the induction chemoradiation and then completed bladder-preserving therapy with chemo-RT to full dose (60-64 Gy).
Patients and Methods
This analysis was based on patients with MIBC enrolled on 1 of the 2 prospective bladder-sparing NRG Oncology RTOG protocols. Further details of the eligibility criteria, chemotherapy, RT technique, doses and fields, response criteria, and follow-up have been described previously (12, 13).
Patient eligibility and selection
Eligible patients had cT2-T4a cN0 M0 invasive bladder cancer and were judged operable candidates for radical cystectomy, if necessary. After initial evaluation, which included chest radiograph, intravenous pyelogram (if indicated), and abdominal and pelvic computed tomography scan, a thorough TURBT was performed. Subjects were then treated as per protocol with induction chemotherapy and RT. An immediate radical cystectomy was recommended for all patients who had T1 or greater, as determined by cystoscopy, cytology, and tumor-site biopsy 4 weeks after induction chemoradiation. Patients with complete (T0) or near-complete (Ta or Tis) responses received consolidation therapy, which started 7 to 14 days after cystoscopic evaluation. Patients were ineligible for the protocols if there was evidence of distant spread of disease, which included metastases to lymph nodes above the bifurcation of the common iliac vessels, white blood cell count <4000/mL (and absolute neutrophil count <1800/mL), platelet count <100,000/ mL, a serum creatinine level >1.5 to 1.7 mg/mL, and a 24-hour creatinine clearance <60 mL/min. Both protocols excluded patients with tumor-related hydro-nephrosis. All institutional, state, and federal guidelines were followed. All institutions obtained institutional review board approval before patient recruitment, and all patients signed approved informed consent forms before trial enrollment.
Study design and treatment
NRG Oncology RTOG XXXX
After TURBT induction therapy involved 13 days of concomitant-boost RT. In the morning patients were treated with 1.6 Gy to the pelvis for all 13 days, and in the evening they received 1.5 Gy to the bladder for the first 5 sessions, and 1.5 Gy to the tumor for the last 8 sessions (20.8 Gy to the pelvis, 28.3 Gy to whole bladder, and 40.3 Gy to the bladder tumor). Minimal interval between treatments was 4 hours. Patients received paclitaxel 50 mg/m2 on days 1, 8, and 15 and cisplatin 20 mg/m2 on days 1-2, 8-9, and 15-16. Patients who achieved a complete response or near-complete response (defined as T0, Ta, or Tis) received consolidation chemoradiation consisting of 1.5-Gy pelvic RT delivered twice daily for 8 days to 24 Gy (total dose: 64.3 Gy to the tumor and 44.8 Gy to the pelvic lymph nodes) with the same chemotherapy regimen. Patients who were found to have ≥T1 after the induction phase were treated with cystectomy. Adjuvant chemotherapy began 12 weeks after consolidation chemo-RT or 8 weeks after cystectomy. It consisted of gemcitabine 1000 mg/m2 on days 1, 8, and 15 and cisplatin 70 mg/m2 on day 1, repeated every 21 days for 4 cycles, with appropriate dose modification based on toxicity.
NRG Oncology RTOG XXXX
After TURBT, patients were stratified by T stage and randomized to 2 arms. Induction therapy involved 13 days of concomitant-boost RT. In the morning patients were treated with 1.6 Gy to the pelvis for all 13 days, and in the evening they received 1.5 Gy to the bladder for the first 5 sessions, and 1.5 Gy to the tumor for the last 8 sessions (20.8 Gy to the pelvis, 28.3 Gy to whole bladder, and 40.3 Gy to the bladder tumor). Minimal interval between treatments was 4 hours. Patients on arm 1 received paclitaxel 50 mg/m2 on days 1, 8, and 15 and cisplatin 15 mg/m2 in days 1-3, 8-10, and 15-17. Patients on arm 2 received 5-fluorouracil 400 mg/m2 and cisplatin 15 mg/m2 on days 1-3, 8-10, and 15-17. Patients who achieved a complete response or near-complete response (defined as T0, Ta, or Tis) received consolidation chemoradiation consisting of 1.5-Gy pelvic RT delivered twice daily for 8 days to 24 Gy (total dose: 64.3 Gy to the tumor and 44.8 Gy to the pelvic lymph nodes) with the following chemotherapy regimens: on arm 1 paclitaxel 50 mg/m2 on days 1 and 8 and cisplatin 15 mg/m2 on days 1, 2, 8, and 9; on arm 2 5-fluorouracil 400 mg/m2 on days 1-3 and 8-10 with cisplatin 15 mg/m2 on days 1, 2, 8, and 9. Patients who were found to have ≥T1 after the induction phase were treated with cystectomy. Adjuvant chemotherapy began 12 weeks after consolidation chemo-RT or 8 weeks after cystectomy. It consisted of gemcitabine 1000 mg/m2, paclitaxel 50 mg/m2, and cisplatin 35 mg/m2 given on days 1 and 8 and repeated every 21 days for 4 cycles, with appropriate dose modification based on toxicity.
Follow-up
Patients with conserved bladders were actively followed with cystoscopy, biopsy of the tumor site, bimanual examination under anesthesia, and urine cytology every 3 months in the first year, every 3 to 4 months in the second year, every 6 months for 3 years, and then annually. Patients were promptly considered for intravesical therapy for non-MIBC recurrences and salvage radical cystectomy for MIBC recurrence.
Data collection/analysis
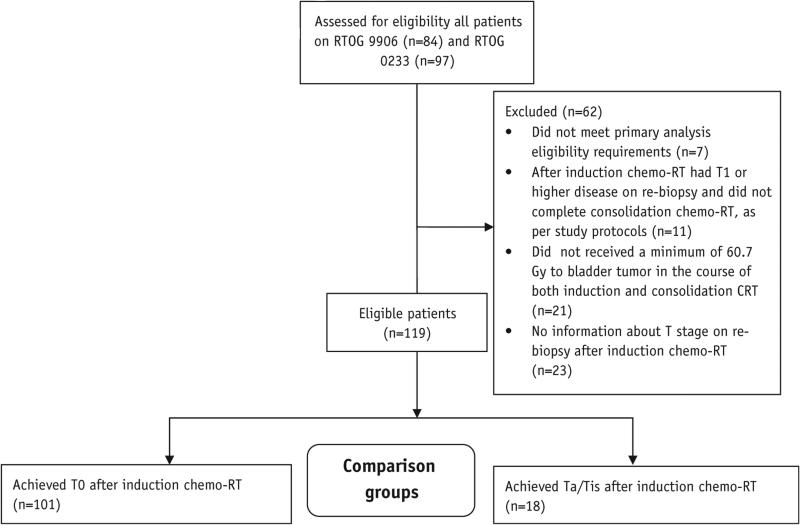
For the purposes of this analysis, patients were included if they met the following criteria: they were eligible for 1 of the 2 protocols, they have achieved a complete (T0) or near-complete (Ta or Tis) responses to induction chemo-RT, and they have received at least 60 Gy to bladder tumor in the course of their RT (Fig. 2).
Fig. 2.
CONSORT diagram. Eligible patients on clinical trials NRG Oncology Radiation Therapy Oncology Group (RTOG) 9906 and 0233 analyzed according to response to induction chemoradiation therapy.
Endpoint definitions
For OS, failure was defined as death due to any cause. For bladder-intact survival, failure was defined as bladder removal (cystectomy) or death due to any cause. For disease-specific survival, failure was defined as death due to bladder cancer. For bladder-intact disease-specific survival, failure was defined as bladder removal (cystectomy) or death due to bladder cancer. For bladder recurrence survival, failure was defined as a tumor recurrence in the bladder after consolidation therapy or death due to any cause. All efficacy endpoints were measured from study entry to date of first failure or last follow-up for censored patients.
Statistical methods
Bladder-intact survival and OS were calculated using the Kaplan-Meier method (14), and T-stage subgroups were compared by way of the log–rank test (15). Disease-specific survival was calculated using cumulative incidence, and subgroups were compared by Gray's test (15, 16). Association between response to induction chemo-RT and subsequent bladder recurrence was evaluated using Fisher's exact test (17, 18).
Results
Pretreatment characteristics
Between 1999 and 2002, 84 eligible patients were entered on NRG Oncology RTOG trial 1 at 26 participating institutions, with 65 patients completing the trial. Between 2002 and 2008, 97 eligible patients were entered on NRG Oncology RTOG trial 2 at 18 participating institutions, with 78 patients completing the trial. Of 181 patients who entered the 2 trials, 143 underwent the entire combined-modality bladder-sparing therapy, and 119 were analyzable, as per the CONSORT diagram in Figure 2. Median age was 65 years (range, 36-90 years); 88% were male, 94% had Zubrod performance score 0, and 93% had clinical T2 stage. Pretreatment characteristics are presented in Table 1.
Table 1.
Pretreatment characteristics and treatment variables of 119 analyzable patients on NRG Oncology Radiation Therapy Oncology Group protocols 9906 and 0233
| Characteristic | T0 (n=101) | Tis/Ta (n=18) | P * |
|---|---|---|---|
| Pretreatment characteristics | |||
| Age (y) | |||
| Median | 65 | 70 | |
| Range | 41-90 | 36-82 | |
| Q1-Q3 | 59-71 | 60-78 | |
| Gender | 1.0 | ||
| Male | 89 (88) | 16 (89) | |
| Female | 12 (12) | 2 (11) | |
| Zubrod performance score | .29 | ||
| 0 | 96 (95) | 16 (89) | |
| 1 | 5 (5) | 2 (11) | |
| Clinical T stage | 1.0 | ||
| cT2 | 94 (93) | 17 (94) | |
| cT3-cT4 | 7 (7) | 1 (6) | |
| Treatment variables | |||
| Maximal complete | .59† | ||
| TURBT | |||
| Yes | 93 (97) | 18 (100) | |
| No | 3 (3) | 0 (0) | |
| Unknown | 5 | 0 | |
| Days elapsed between completing induction chemo-RT and cystoscopic evaluation/biopsy | .47‡ | ||
| Mean | 28.5 | 30.3 | |
| Median | 28 | 28 | |
| Minimum | 15 | 14 | |
| Maximum | 51 | 57 |
Abbreviations: Q1 = first quartile; Q3 = third quartile; RT = radiation therapy; TURBT = transurethral resection of bladder tumor.
Values in parentheses are percentages.
Fisher's exact test is used.
Compares yes versus no only.
t test is used.
Treatment variables
Of 119 patients, 111 (93%) had a visibly complete TURBT before starting the induction chemoradiation phase of the bladder-preservation protocol, with no difference between the 2 groups of patients (P=.59). The median number of days between completing induction chemoradiation and undergoing cystoscopic evaluation with the biopsy was 28 days (range, 14-57 days), with no difference between the groups (P=.47). Treatment variables are presented in Table 1.
Follow-up
Median follow-up for all 119 patients was 5.9 years (range, 0.44-11.3 years) and for patients still alive at the time of analysis 7.0 years (range, 0.55-11.3 years). For 54 patients enrolled on NRG Oncology RTOG trial 1 the median follow-up was 8.0 years (range, 0.44-11.3 years) and for 65 patients enrolled on NRG Oncology RTOG trial 2 the median follow-up was 5.0 years (range, 0.55-8.22 years).
Bladder tumor recurrence
Of the 119 patients, 41 (34%) experienced bladder tumor recurrence. Among these 41 recurrences, 14 were invasive (34%) and required salvage cystectomies. Among 23 patients with initial noninvasive recurrence, 5 patients subsequently had an invasive failure, with the interval between noninvasive and invasive recurrences ranging from 5 to 22 months. Four patients experienced recurrence that was indeterminate for invasiveness. There was no difference between complete responders and near-complete responders in terms of any bladder recurrence rates (P=.52) or invasive bladder recurrence rates (P=.63), as detailed in Table 2.
Table 2.
Rates of bladder tumor recurrence among 119 analyzable patients on NRG Oncology Radiation Therapy Oncology Group protocols 9906 and 0233
| Variable | T0 (n = 101) | Tis/Ta (n = 18) | P * |
|---|---|---|---|
| Any bladder recurrence | .52 | ||
| No | 65 (64) | 13 (72) | |
| Yes | 36 (36) | 5 (28) | |
| Recurrence | T0 (n= 32†) | Tis/Ta (n= 5) | .63 |
| Invasive | 13 (41) | 1 (20) | |
| Noninvasive | 19 (59) | 4 (80) |
Values are presented as number (percentage).
Fisher's exact test is used.
Excluded 4 patients whose recurrence was indeterminate for invasiveness.
Survival
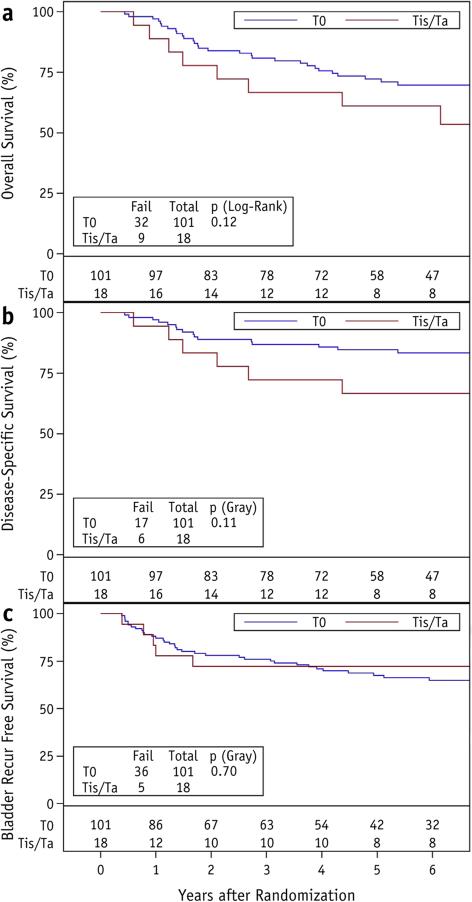
At 5 years OS was 72% (95% confidence interval [CI] 63%-81%) for patients with complete response to induction chemo-RT, versus 61% (95% CI 39%-84%) with near-complete response (P=.12) (Fig. 3a). On univariate analysis, hazard ratio (HR) for death was 1.8 (95% CI 0.9-3.8, P=.125) when analyzed by response completeness. Disease-specific survival at 5 years was 85% (95% CI 78%-92%) among complete responders, versus 67% (95% CI 44%-89%) among non-complete responders (P=.11) (Fig. 3b), with HR of 2.1 (95% CI 0.9-5.3, P=.10) on univariate analysis by response completeness. Bladder recurrence-free survival at 5 years was 68% (95% CI 58%-77%) among complete responders, versus 72% (95% CI 51%-94%) among non-complete responders (P=.70) (Fig. 3c), with HR of 0.8 (95% CI 0.3-2.1, P=.66) on univariate analysis by response completeness. Table 3 summarizes the clinical outcomes at 5 years for patients with complete response and near-complete response to the induction phase of bladder-preservation NRG Oncology RTOG protocols.
Fig. 3.
Overall survival (a), disease-specific survival (b), and bladder recurrence-free survival (c) among 101 patients with T0 and 18 patients with Ta/Tis after induction chemoradiation for muscle-invasive bladder cancer on 2 NRG Oncology Radiation Therapy Oncology Group protocols.
Table 3.
Summary of outcomes at 5 years for 101 patients who achieved complete response (T0) and 18 patients who achieve non-complete response (Ta or Tis) after induction chemoradiation and proceeded to consolidation chemoradiation on NRG Oncology Radiation Therapy Oncology Group protocols 9906 and 0233
| Outcome at 5 years (95% CI) | T0 (n = 101) | Ta/Tis (n = 18) | P |
|---|---|---|---|
| Overall survival (%) | 72 (63-81) | 61 (39-84) | .12 |
| Disease-specific survival (%) | 85 (78-92) | 67 (44-89) | .11 |
| Bladder recurrence-free survival (%) | 68 (58-77) | 72 (51-94) | .70 |
Abbreviation: CI = confidence interval.
Discussion
The key to the success of bladder-preserving trimodality therapy is a close and indefinite cystoscopy surveillance of the bladder, with prompt cystectomy for those who develop invasive recurrence. However, noninvasive recurrences presented a greater management dilemma to patients and clinicians, and broadly speaking, they may be grouped according to their timing. The first group is those who develop Ta, T1, or Tis recurrence months or years after the completion of trimodality therapy. The second group, and the feature of this article, is those who show persistent disease at the mid-trimodality therapy “checkpoint.”
Historically, many have advocated a salvage cystectomy for any bladder recurrence other than low-grade Ta after bladder-sparing treatments for MIBC, although that has not been the case at Massachusetts General Hospital. Of 190 patients treated at Massachusetts General Hospital between 1986 and 1998, 32 (26%) experienced a superficial relapse (19). Salvage cystectomy became necessary in 10 of these 32 patients (31%), either because of additional superficial recurrences (7 patients) or progression to invasive disease (3 patients). Patients with superficial recurrences in this series had similar 5- and 8-year overall and disease-specific survival, but a substantially lower rate of bladder-intact survival. Over time, most institutions have become comfortable with conservative management of superficial recurrences (19-21) and delaying salvage cystectomy until invasive recurrence, on the basis of evidence that survival was not compromised.
The remaining question, and one that has not been previously evaluated until this report, is the consequence on long-term outcomes of persistent noninvasive bladder cancer after the induction phase of chemo-RT. Using data from 2 consecutive NRG Oncology RTOG protocols that allowed patients with near-complete responses to induction chemoradiation to proceed with bladder-sparing approach without immediate cystectomy, no differences were found in bladder recurrence rates and important clinical outcomes such as OS, disease-specific survival, and bladder recurrence-free survival at 5 years, among patients who achieved a complete response (defined as no tumor, or T0) and those who achieved a near-complete response (defined as Ta or Tis) at the time of the cystoscopic evaluation and biopsy after the induction phase of a bladder-sparing tri-modality treatment protocol. These findings suggest that approximately 15% of patients who begin with trimodality therapy, including a maximum TURBT, several weeks after completing the induction chemoradiation phase may have noninvasive bladder tumor present during the cystoscopic evaluation. It seems safe to recommend that these patients continue with bladder-sparing therapy and avoid immediate salvage cystectomy, because their local failure rates are not different from those who achieve complete response to the induction chemo-RT.
It is important to note that many institutions in Europe do not inspect the bladder between the induction and consolidation phases and instead give an uninterrupted course of concurrent chemo-RT. The invasive local failure rate at 2 years on the chemoradiation arm of the BC2001 trial in the United Kingdom was 11%, with patients receiving the full treatment without any mid-course cystoscopic evaluation. This rate is remarkably similar to the finding in this analysis of an invasive recurrence rate of 13% at 5 years, if one considers that the majority of invasive recurrences occur in the first few years.
This analysis is based on prospectively gathered data on formal multi-institutional protocols with a clinical follow-up of >5 years to allow for capture of most local recurrences. The main limitation of this analysis is a small number of patients who achieved an incomplete response after the induction phase of chemo-RT. This significantly limits the strength of the conclusions and requires confirmatory analysis by other investigators. In fact, Figure 3 plots may show a separation of curves, which is not statistically significant, perhaps due to the small number of patients in the near-complete response group. The high rate of cure and bladder preservation in this analysis is only applicable to analyzable patients who have achieved complete or near-complete responses after induction chemo-RT and completed the consolidation phase.
The European Association of Urology accepts bladder preservation for select patients with MIBC (22), and the US National Comprehensive Cancer Network has updated the 2012 guidelines (23) to reflect the growing evidence supporting the use of the bladder-preservation treatment modality in select patients with clinical T2 and T3 disease. Some healthcare systems have embraced bladder-sparing treatment modalities; for example, in the United Kingdom many eligible patients receive multimodality therapy, with surgery reserved as a salvage option. At the same time in other countries, such as the United States, only a minority of patients have been offered this strategy. Detailed analysis of outcomes, such as presented in this article, and stepwise modification of bladder sparing protocols will lead to improved outcomes with fewer unnecessary treatments and better quality of life for patients with muscle-invasive bladder cancer.
In conclusion, this analysis shows that the outcomes are similar between patients who achieve a complete response (T0) or near-complete response (Ta or Tis) after the induction phase of bladder-preservation trimodality therapy, and suggests that it is reasonable to continue with bladder-preservation therapy in patients who achieve near-complete response to the induction phase.
Summary.
Outcomes among 119 patients with muscle-invasive bladder cancer treated on NRG Oncology RTOG trials 9906 and 0233 were analyzed. A total of 101 patients had complete response to induction chemo-RT, and 18 patients had near-complete response; all completed the consolidation chemo-RT. This analysis shows similar outcomes in these 2 groups of patients and suggests that it is reasonable to continue with bladder preservation therapy to full chemo-RT dose in patients who achieve near-complete response to the induction phase.
FURTHER POINTS.
Any (grey) halftones (photographs, micrographs, etc.) are best viewed on screen, for which they are optimized, and your local printer may not be able to output the greys correctly.
If the PDF files contain colour images, and if you do have a local colour printer available, then it will be likely that you will not be able to correctly reproduce the colours on it, as local variations can occur.
If you print the PDF file attached, and notice some ‘non-standard’ output, please check if the problem is also present on screen. If the correct printer driver for your printer is not installed on your PC, the printed output will be distorted.
Acknowledgments
This project was supported by grants U10CA21661, U10CA180868, U10CA180822, U10CA37422, CURE from the National Cancer Institute; this project is also funded, in part, under grant 4100057652 with the Pennsylvania Department of Health and Eli Lilly. The Department specifically disclaims responsibility for any analyses, interpretations, or conclusions.
Conflict of interest: T.M. reports grants from the National Cancer Institute (NCI), during the conduct of the study; and personal fees from UpToDate, Inc and Novocure, Inc, outside the submitted work. A.G. reports support from grants U10CA21661, U10CA180868, U10CA180822, and U10CA37422 from NCI, from the CURE grant from NCI, and from grant 4100057652 with the Pennsylvania Department of Health, during the conduct of the study. R.D. reports personal fees from Roche, Merck, and Dendreon, outside the submitted work; and is on the Member Scientific Advisory Board of the Bladder Cancer Advocacy Network (non-reimbursed). H.M.S. reports personal fees from AstraZeneca and Medivation; grants from Myriad and Janssen; and personal fees from Blue Earth Diagnostics, Ferring, Bayer, and eviti, outside the submitted work. W.U.S. reports ownership of Pfizer stock.
References
- 1.Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–866. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 2.Zehnder P, Studer UE, Skinner EC, et al. Super extended versus extended pelvic lymph node dissection in patients undergoing radical cystectomy for bladder cancer: A comparative study. J Urol. 2011;186:1261–1268. doi: 10.1016/j.juro.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Kaufman DS, Shipley WU, Griffin PP, et al. Selective bladder preservation by combination treatment of invasive bladder cancer. N Engl J Med. 1993;329:1377–1382. doi: 10.1056/NEJM199311043291903. [DOI] [PubMed] [Google Scholar]
- 4.Rodel C, Grabenbauer GG, Kuhn R, et al. Combined-modality treatment and selective organ preservation in invasive bladder cancer: Long-term results. J Clin Oncol. 2002;20:3061–3071. doi: 10.1200/JCO.2002.11.027. [DOI] [PubMed] [Google Scholar]
- 5.Tester W, Porter A, Asbell S, et al. Combined modality program with possible organ preservation for invasive bladder carcinoma: Results of RTOG protocol 85-12. Int J Radiat Oncol Biol Phys. 1993;25:783–790. doi: 10.1016/0360-3016(93)90306-g. [DOI] [PubMed] [Google Scholar]
- 6.Tester W, Caplan R, Heaney J, et al. Neoadjuvant combined modality program with selective organ preservation for invasive bladder cancer: Results of Radiation Therapy Oncology Group phase II trial 8802. J Clin Oncol. 1996;14:119–126. doi: 10.1200/JCO.1996.14.1.119. [DOI] [PubMed] [Google Scholar]
- 7.Shipley WU, Winter KA, Kaufman DS, et al. Phase III trial of neoadjuvant chemotherapy in patients with invasive bladder cancer treated with selective bladder preservation by combined radiation therapy and chemotherapy: Initial results of Radiation Therapy Oncology Group 89-03. J Clin Oncol. 1998;16:3576–3583. doi: 10.1200/JCO.1998.16.11.3576. [DOI] [PubMed] [Google Scholar]
- 8.Kaufman DS, Winter KA, Shipley WU, et al. The initial results in muscle-invading bladder cancer of RTOG 95-06: Phase I/II trial of transurethral surgery plus radiation therapy with concurrent cisplatin and 5-fluorouracil followed by selective bladder preservation or cystectomy depending on the initial response. Oncologist. 2000;5:471–476. doi: 10.1634/theoncologist.5-6-471. [DOI] [PubMed] [Google Scholar]
- 9.Hagan MP, Winter KA, Kaufman DS, et al. RTOG 97-06: initial report of a phase I-II trial of selective bladder conservation using TURBT, twice-daily accelerated irradiation sensitized with cisplatin, and adjuvant MCV combination chemotherapy. Int J Radiat Oncol Biol Phys. 2003;57:665–672. doi: 10.1016/s0360-3016(03)00718-1. [DOI] [PubMed] [Google Scholar]
- 10.Mak R, Hunt D, Shipley WU, et al. Long-term outcomes in patients with muscle-invasive bladder cancer after bladder-preserving combined-modality therapy: A pooled analysis of RTOG 8802, 8903, 9506, 9706, 9906, and 0233. J Clin Oncol. 2014;32:3801–3809. doi: 10.1200/JCO.2014.57.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Housset M, Maulard C, Chretien Y, et al. Combined radiation and chemotherapy for invasive transitional-cell carcinoma of the bladder: A prospective study. J Clin Oncol. 1993;11:2150–2157. doi: 10.1200/JCO.1993.11.11.2150. [DOI] [PubMed] [Google Scholar]
- 12.Kaufman DS, Winter KA, Shipley WU, et al. Phase I-II RTOG study (99-06) of patients with muscle-invasive bladder cancer undergoing transurethral surgery, paclitaxel, cisplatin, and twice-daily radiotherapy followed by selective bladder preservation or radical cystectomy and adjuvant chemotherapy. Urology. 2009;73:833–837. doi: 10.1016/j.urology.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 13.Mitin T, Hunt D, Shipley WU, et al. Transurethral surgery and twice-daily radiation plus paclitaxel-cisplatin or fluorouracilcisplatin with selective bladder preservation and adjuvant chemotherapy for patients with muscle invasive bladder cancer (RTOG 0233): A randomised multicentre phase 2 trial. Lancet Oncol. 2013;14:863–872. doi: 10.1016/S1470-2045(13)70255-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:447–457. [Google Scholar]
- 15.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 16.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1143. [Google Scholar]
- 17.Fisher RA. On the interpretation of χ2 from contingency tables, and the calculation of P. J R Stat Soc. 1922;85:87–94. [Google Scholar]
- 18.Fisher RA. Statistical Methods for Research Workers. Oliver and Boyd; 1954. [Google Scholar]
- 19.Onozawa M, Miyanaga N, Hinotsu S, et al. Analysis of intravesical recurrence after bladder-preserving therapy for muscle-invasive bladder cancer. Jpn J Clin Oncol. 2012;42:825–830. doi: 10.1093/jjco/hys105. [DOI] [PubMed] [Google Scholar]
- 20.Mitin T, Shipley WU, Efstathiou JA, et al. Trimodality therapy for bladder conservation in treatment of invasive bladder cancer. Curr Urol Rep. 2013;14:109–115. doi: 10.1007/s11934-012-0301-x. [DOI] [PubMed] [Google Scholar]
- 21.Heney NM, Kaufman DS, Shipley WU. Surgery: Selective bladder-preserving therapy for muscle-invasive cancer. Nat Rev Clin Oncol. 2009;6:193–194. doi: 10.1038/nrclinonc.2009.21. [DOI] [PubMed] [Google Scholar]
- 22.Gakis G, Efstathiou J, Lerner SP, et al. ICUD-EAU International Consultation on Bladder Cancer 2012: Radical cystectomy and bladder preservation for muscle-invasive urothelial carcinoma of the bladder. Eur Urol. 2013;63:45–57. doi: 10.1016/j.eururo.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 23.National Comprehensive Cancer Network Bladder cancer. Available at: http://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf.