Abstract
In this study, the phytotoxicity of seven metal oxide nanoparticles(NPs)—titanium dioxide (nTiO2), silicon dioxide (nSiO2), cerium dioxide (nCeO2), magnetite (nFe3O4), aluminum oxide (nAl2O3), zinc oxide (nZnO) and copper oxide (nCuO)—was assessed on two agriculturally significant crop plants (maize and rice). The results showed that seed germination was not affected by any of the seven metal oxide NPs. However, at the concentration of 2000 mg·L−1, the root elongation was significantly inhibited by nCuO (95.73% for maize and 97.28% for rice), nZnO (50.45% for maize and 66.75% for rice). On the contrary, minor phytotoxicity of nAl2O3 was only observed in maize, and no obvious toxic effects were found in the other four metal oxide NPs. By further study we found that the phytotoxic effects of nZnO, nAl2O3 and nCuO (25 to 2000 mg·L−1) were concentration dependent, and were not caused by the corresponding Cu2+, Zn2+ and Al3+ ions (0.11 mg·L−1, 1.27 mg·L−1 and 0.74 mg·L−1, respectively). Furthermore, ZnO NPs (<50 nm) showed greater toxicity than ZnO microparticles(MPs)(<5 μm) to root elongation of both maize and rice. Overall, this study provided valuable information for the application of engineered NPs in agriculture and the assessment of the potential environmental risks.
Keywords: phytotoxicity, metal oxide nanoparticles, maize, rice, germination
1. Introduction
The use of engineered nanoparticles (NPs) in medicine, cosmetics, energy, agriculture and machinery, etc. has increased rapidly [1,2]. Considering that engineered NPs can be released to the environment, accidentally or incidentally [3,4], nanotoxicity is receiving increasing attentions currently [5,6,7,8,9]. Despite the fact that more and more researchers have reported nanotoxicity in plants [10,11,12], the studies are still at the emerging stage and knowledge on the effects of NPs in plant systems needs further investigation [13,14], especially in crop plants, since the engineered NPs may be a threat to human health through the food chain [15].
Engineered NPs are divided into four categories according to USEPA: carbon-based materials, metal-based materials, dendrimers and composites. As an important part of metal-based materials, metal oxide NPs are widely used in industry, cosmetics, environmental pollution control, etc. [16,17]. At present, many kinds of metal oxide NPs have been applied in agriculture, specifically in plant protection and fertilization [18]. For instance, silicon dioxide NPs can be used as controlled release carriers in drug delivery and as an active ingredient against insect pests [19]; zinc oxide NPs can be used as insecticides [20].
Indeed, some metal oxide NPs were reported to have positive effects on crop plants. For example, the spraying of nTiO2 at the dose of 0.25‰~4‰ could significantly promote the growth of spinach [21]; root length of green peas treated with nZnO at 125, 250 and 500 mg·kg−1 soil was approximately two times longer than the control [11]. Nevertheless, more and more researchers have reported the phytotoxicity of metal oxide NPs in crop plants. For instance, Asli et al. reported that nTiO2 with mean diameters of 30 nm inhibited leaf growth and transpiration of maize seedlings [22]. Kim et al. proved that both 1000 mg·kg−1 nCuO and nZnO could significantly inhibit the growth of cucumber by 44%, respectively [23]. In this case, it can be seen that opposite conclusions (positive effects or negative effects) can be drawn about the same metal oxide NPs in different plants. More interestingly, the effects on the same plant caused by the same sort of metal oxide NPs also show totally opposite results. For instance, Castiglione et al. reported that nTiO2 at 4‰ could significantly inhibit the seed germination and root elongation of maize [24], while Burke et al. showed that nTiO2 at 200 mg·kg−1 had no significant effects on maize [12]. These opposite conclusions were probably caused by the different synthetic methods of the metal oxide NPs, different concentration and different media (hydroponics or soil culture), etc. Therefore, previous studies of the phytotoxicity of metal oxide NPs are confusing and somewhat controversial, due to the absence of integrated evaluation systems. In addition, the toxicity in crop plants was usually assessed using one or two kinds of metal oxide NPs. Therefore, it is urgent to systematically investigate the phytotoxicity of a wide variety of metal oxide NPs in crop plants.
Maize (Zea mays L.) is one of the three most important food crops worldwide. Rice (Oryza sativa L.), as another important staple food, feeding more than half of the World’s population [25]. Due to their important roles in the food security of humankind, the assessment of the phytotoxicity of metal oxide NPs on these two agriculturally important crop plants is crucial to human health.
In the present study, germination experiments were carried out on maize and rice to evaluate the phytotoxicity of seven metal oxide NPs—titanium dioxide (nTiO2), silicon dioxide (nSiO2), cerium dioxide (nCeO2), magnetite (nFe3O4), aluminum oxide (nAl2O3), zinc oxide (nZnO) and copper oxide (nCuO). Root length and shoot length, which are sensitive to an adverse environment, were chosen as toxicity indicators. Based on the USEPA guidelines [26], a concentration of 2000 mg·L−1 was chosen as the preliminary concentration to assess the phytotoxicity of the seven metal oxide NPs. In addition, we also studied the effects of the exposure concentration and ion release on the phytotoxicity of nCuO, nZnO and nSiO2. The role of particle size in phytotoxicity was also determined for two different sizes of ZnO (50 nm, 5 μm). This study provided valuable information for the application of metal oxide NPs in agriculture and environmental safety assessment.
2. Experimental Section
2.1. Nanomaterials and Seeds
All metal oxide NPs—nTiO2, nSiO2, nCeO2, nFe3O4, nAl2O3, nZnO, nCuO and ZnO microparticles (ZnO MPs, <5 μm)—were purchased from Sigma-Aldrich (St. Louis, MO, USA). The seeds of maize (Zhengdan No. 958) and rice (Jijing No. 6) were purchased from Seed Building of Changchun, China, and stored in a dry place at room temperature. Preliminary studies showed that the average germination rates of these seeds were higher than 95% to ensure the further treatment.
2.2. Preparation and Characterization of Nanoparticle Suspensions
The size and morphology were determined by transmission electron microscope (TEM) at 80 kV (H-7500, Hitachi, Ltd., Tokyo, Japan). For TEM observation, NP suspensions were prepared according to the following method: a certain quantity of metal oxide NPs were suspended in deionized water directly to make a concentration of 100 mg·L−1 and dispersed by ultrasonic vibration (100 W, 40 KHz) for 45 min. Then a drop of metal oxide NPs suspensions was dropped on the copper grid and dried at room temperature overnight. The zeta potential of the particles in the suspensions was estimated by a Zetasizer Nano ZS (Malvern Instruments, Malvern, UK).
2.3. Measurement of the Content of the Dissolved Metal Ions
To investigate the roles of the dissolved metal ions in causing phytotoxicity, total dissolved Cu2+, Zn2+ and Al3+ content of metal oxide NPs suspensions were determined. Firstly, nCuO, nZnO and nAl2O3 suspensions at 2000 mg·L−1 were centrifuged at 15,000 rpm for 30 min after dispersal by ultrasonic vibration (100 W, 40 KHz) for 45 min. Then, the supernatants were filtered through 0.2 μm glass filters, and the content of Cu, Zn and Al elements was analysed by an inductively coupled plasma optical emission spectrometer (ICP-OES, Prodigy, Leeman, Hudson, NH, USA). The wavelengths used were 327.396 nm, 213.856 nm and 309.271 nm for the elements Cu, Zn and Al, respectively.
2.4. Seed Germination
For germination, the seeds were surface sterilized by soaking in 70% ethanol for 2 min. Then the seeds were rinsed with sterile water several times to remove any remaining ethanol [27]. Subsequently, the seeds were immersed in sterile water or metal oxide NPs suspensions for 2 h. After that, every ten seeds were transferred into a Petri dish (100 mm × 15 mm) with a filter paper on the bottom, with the distance among each seeds ≥1 cm. Then 5 mL of test solution was added to each Petri dish. Finally, the Petri dishes were covered and sealed with tape. The germination was conducted in dark at 25 °C. After 5 days for maize and 7 days for rice, respectively, the germination rate, the root length and shoot length were measured [14,28].
2.5. Data Analysis
Each treatment was conducted with three replicates and all of the experiment groups were conducted in triplicates, the dates were showed as mean ± SD (standard deviation). Difference analysis was conducted with ANOVA (analysis of variance, LSD). Statistical significance was based on probabilities of p ≤ 0.05.
3. Results and Discussion
3.1. Characterization of Metal Oxide NPs
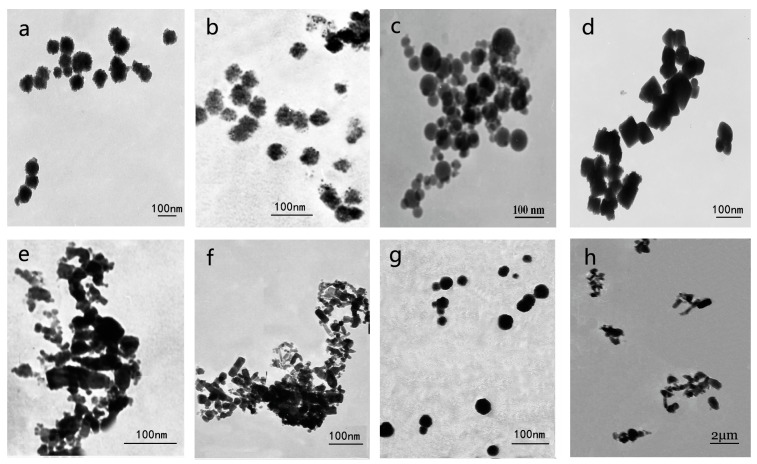
Figure 1 shows TEM images of the seven metal oxide NPs and ZnO MPs, the size and morphology of which was almost the same as that given by the supplier.
Figure 1.
Transmission electron microscopy images of test materials. (a) nTiO2; (b) nSiO2; (c) nCeO2; (d) nFe3O4; (e) nAl2O3; (f) nZnO; (g) nCuO; (h) ZnO MPs.
The data from both the producer and TEM are listed in Table 1. In addition, the purity of all the test particles provided by the supplier was included, except for nAl2O3. The zeta potential and pH values of seven metal oxide NPs and ZnO MPs suspensions (100 mg·L−1 for measuring zeta potential and 2000 mg·L−1 for measuring pH values) were also listed in Table 1.
Table 1.
Test Material Information.
| Particles | Size Sigma-Aldrich | Purity Sigma-Aldrich | Zeta Potential (mV) | pH | Description (TEM) |
|---|---|---|---|---|---|
| nTiO2 | 21 nm | 99.5% | 11.6 ± 1.1 | 7.13 ± 0.03 | Spherical, present in the form of 60–120 nm irregular aggregate in water solution |
| nSiO2 | 5–15 nm (TEM) | 99.5% | −17.6 ± 1.0 | 6.67 ± 0.03 | Spherical, about 5–15 nm, usually gathered into 90 ± 30 nm irregular aggregate |
| nCeO2 | <25 nm (BET) | 99% | 35.1 ± 0.7 | 6.71 ± 0.04 | Spherical, with smooth edge and inhomogenous size, less than 50 nm |
| nFe3O4 | 50–100 nm (TEM) | 97% | 9.12 ± 0.47 | 7.08 ± 0.03 | Keen-edged diamond or square, with the inhomogenous size of 50–100 nm |
| nAl2O3 | 13 nm (TEM) | - | 29.5 ± 0.6 | 7.17 ± 0.02 | Spherical, with the average size of 15 nm, easy to aggregate |
| nZnO | <50 nm (BET) | 97% | −7.63 ± 0.37 | 7.14 ± 0.03 | Clavate or irregular spherical, with inhomogenous size distribution, less than 50 nm |
| nCuO | <50 nm (TEM) | 97% | 20.6 ± 0.6 | 6.36 ± 0.02 | Elliptic or spherical, with the size range of 40–80 nm |
| ZnO MPs | <5 µm | 99.9% | 10.4 ± 1.3 | 6.95 ± 0.03 | Irregular shapes |
3.2. Preliminary Assessment of the Phytotoxicity of Metal Oxide NPs
Concerns about the potential impacts of metal oxide NPs on plants have attracted increasing attention. However, the absence of systematic research methods and evaluation systems has lead to contradictory conclusions. In this study, a simple, rapid and sensitive germination experiment in Petri dishes was conducted to evaluate the phytotoxicity of metal oxide NPs. Germination is the beginning of the physiological process in the life of plants, and it is usually affected by various factors, like temperature, humidity, gas transfer, soil compaction, etc. [29]. In this experiment, the interferences were excluded as the seeds were directly contacted with metal oxide NPs. Both for maize and rice, the germination rates were not affected by the seven metal oxide NPs at 2000 mg·L−1. This result was consistent with previous studies, where seed germination rates were generally insensitive to NP exposure compared with the root elongation. For instance, nCuO, nCeO2 and nSiO2 have been reported to have no effect on germination rates, but significantly inhibit root elongation [9,30,31]. This result was probably due to the protection of the seed coat, which protects the seeds from the NPs [32].
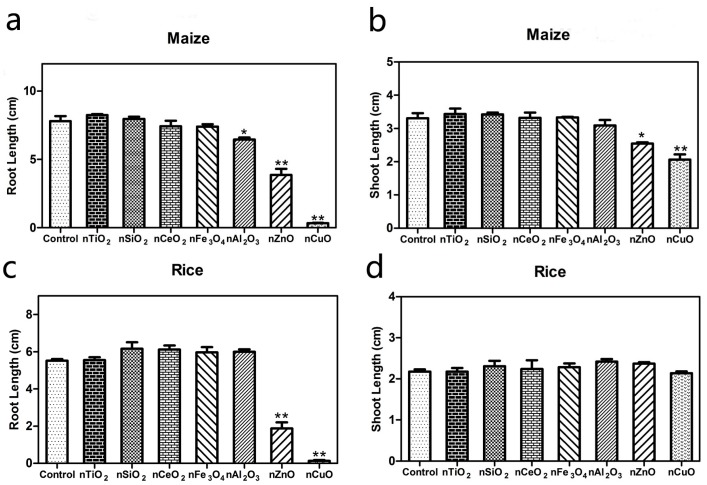
As shown in Figure 2 and Figure S1 (see the Supplementary Information), the effects of seven metal oxide NPs suspensions at 2000 mg·L−1 on root and shoot elongation of maize and rice varied among the NPs. Compared with the control, the growth of maize and rice were not affected by four metal oxide NPs: nTiO2, nSiO2, nCeO2 and nFe3O4. However, nCuO and nZnO reduced the root length of maize by 95.73% and 50.45%, respectively (Figure 2a). Moreover, the shoot length of maize treated with nCuO and nZnO was also reduced by 30.98% and 13.8%, as compared to the control (Figure 2b). As for nAl2O3, minor toxicity was only observed in the root of maize (p < 0.05), while no negative effects were observed in the shoot. As shown in Figure 2c, nCuO and nZnO reduced the root length of rice by 97.28% and 66.75%, respectively. Conversely, no inhibition effects in rice shoots were observed with nCuO and nZnO (Figure 2d). In the case of nAl2O3, no visible toxicity was observed in either the roots or shoots of rice. Due to the direct contact of radicles with the NP suspensions, the results of nCuO and nZnO both demonstrated that the toxic symptoms in roots were more serious than in shoots, which was consistent with previous studies [9,33]. Meanwhile, this result also indicated the possibility of NP uptake in the roots. To date, the uptake of different metal oxide NPs has been confirmed and electron microscopy is the primary tool and a convenient experimental methodology used in intracellular localization of NPs in plants. For instance, Wang et al. reported that nCuO (20–40 nm) were present in the xylem sap of maize [9]. Lin and Xing showed that nZnO (20 ± 5 nm) were present in the apoplast and protoplast of the root endodermis and stele of ryegrass [33].
Figure 2.
Effects of seven metal oxide NPs suspensions on (a) root elongation and (b) shoot elongation of maize at the concentration of 2000 mg·L−1. Effects of seven metal oxide NPs suspensions on (c) root elongation and (d) shoot elongation rice at the concentration of 2000 mg·L−1. The values were given as mean ± SD (standard deviation) of triplicate samples.
The mechanism of metal oxide NP phytotoxicity remains unclear. It was generally reported to be related to the NP species, test plants, concentration, chemical composion, surface modification and particle size [34]. The result of preliminary assessment experiment indicated that the phytotoxicity of the metal oxide NPs varied with the species of NPs. For example, nCuO and nZnO at 2000 mg·L−1 showed significant inhibition on the root elongation of both maize and rice, while no obvious effects were observed with nFe3O4, nSiO2, nTiO2 and nCeO2. Moreover, the different effects of nAl2O3 at 2000 mg·L−1 on the root elongation of maize and rice proved the phytotoxicity of the metal oxide NPs varied with the test plant.
It was well known that the pH values may affect plant growth. The experimental pHs of all the metal oxide NPs were in the range of 6.3–7.2, which should not have any negative effect on root growth [35]. In further study, three toxic NPs, (nCuO, nZnO and nAl2O3) were studied to discern the effects of the potential factors influencing the phytotoxicity: exposure concentration, ion release and particle size.
3.3. Dose Response Relationship of nCuO, nZnO and nAl2O3
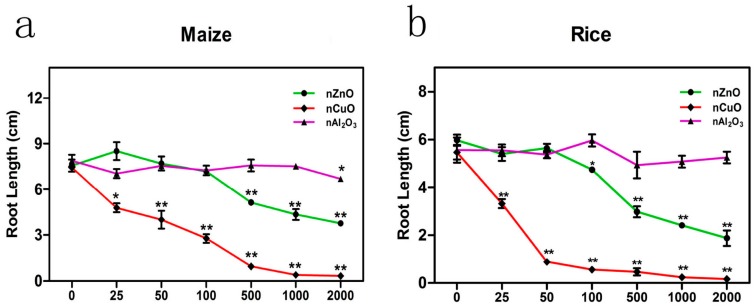
Figure 3 and Figure S2 (see the Supplementary Information) show the effects of three metal oxide NPs (nCuO, nZnO and nAl2O3) at the concentrations of 25–2000 mg·L−1 on both maize and rice. As for nZnO, the dose-response curves were “T” shaped, and no significant inhibition effects were observed at low concentrations (100 mg·L−1 for maize and 50 mg·L−1 for rice). The root growth inhibition effects became visible with increasing concentration. However, as for nCuO, the dose-response curves present an “L” shape. Even at a low concentration of 25 mg·L−1, the root elongation of both maize and rice was significantly inhibited (p < 0.05 and p < 0.01, respectively). At the concentration of 1000 mg·L−1, the growth of maize and rice roots was almost completely terminated. Fifty percent root growth inhibitory concentrations (IC50) of nCuO and nZnO were estimated to be near 60 mg·L−1 and 1500 mg·L−1 for maize, and near 30 mg·L−1 and 400 mg·L−1 for rice, respectively. As for nAl2O3, the phytotoxicity was relatively low as toxicity was only observed at 2000 mg·L−1 in maize. The dose response relationship between metal oxide NPs and plants has been comfirmed by different studies. For example,Wang et al. reported that 2 mg·L−1 nCuO were nontoxic to maize while 10 mg·L−1 and 100 mg·L−1 nCuO significantly inhibited the root elongation [9]; Lin and Xing reported the dose response of nZnO to ryegrass [33].
Figure 3.
Dose-response curves of nZnO, nCuO and nAl2O3 on root growth of (a) maize and (b) rice. The values were given as mean ± SD (standard deviation) of triplicate samples with 10 seeds each.
3.4. Phytotoxicity of Released Metal Ions
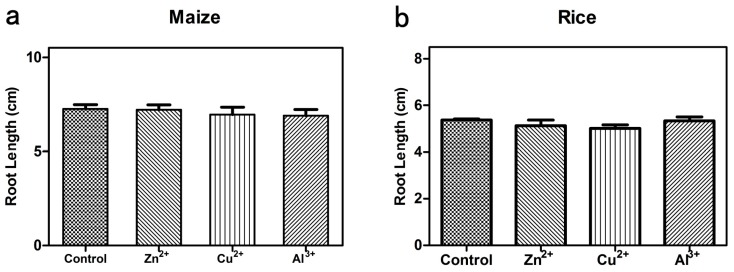
The release of metal ions is inevitable in metal oxide NP suspensions and it is an important interference in any study of nanotoxicity. The ICP-OES results showed that the total content of released Cu2+, Zn2+ and Al3+ in the corresponding metal oxide NPs suspensions were 0.11 ± 0.04 mg·L−1, 1.27 ± 0.03 mg·L−1 and 0.74 ±0.05 mg·L−1, respectively. In fact, these levels are a little higher than the actual released ions content since some small NPs cannot be excluded completely through centrifugation and filtration. Ion solutions were prepared from the corresponding sulfate (CuSO4·5H2O, ZnSO4·7H2O and Al2(SO4)3·16H2O ) in DI-water [36]. As shown in Figure 4, no significant inhibition effects on root growth in both maize and rice were observed in Cu2+, Zn2+ and Al3+ solutions compared with the control.
Figure 4.
Effect of Cu2+, Zn2+ and Al3+ on the root elongation of (a) maize and (b) rice. The values were given as mean ± SD (standard deviation) of triplicate samples with 10 seeds each.
Heavy metals are widely accused of being toxic to plant growth [37,38]. Many previous studies showed that metal ions (Cu2+ and Zn2+, etc.) could inhibit the root and stem growth of plants [39,40]. The release of metal ions is inevitable in metal oxide NP suspensions. However, most researchers have reported that no significant inhibition effects were observed in the corresponding ion solutions. For instance, Wang et al. reported that the phytotoxicity of nCuO mainly depends on the NP itself , but not on the Cu2+ released in the NP suspension [9]. Lin and Xing reported that Zn2+ released from the nZnO suspension did not display any phytotoxicity on radish, rape and ryegrass [41]. Consistent with these observations, our results also indicated that the amounts of metal ions released from the NPs would be negligible, probably due to the low quantity of ions released in the NP suspensions [42].
3.5. Effect of Particle Size on Phytotoxicity
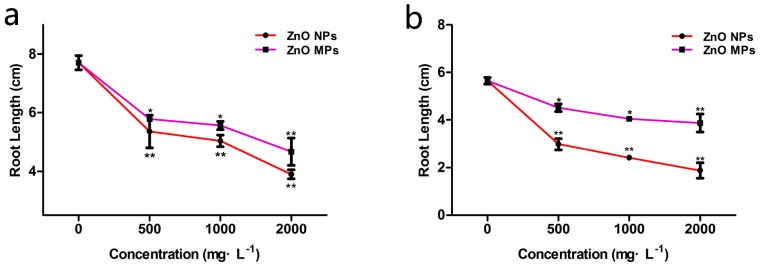
In this study, ZnO NPs (<50 nm) and ZnO MPs (<5 μm) were selected to investigate the effect of particle size on phytotoxicity. Previous dose response relationship experiments with ZnO NPs confirmed that the concentrations of 500, 1000 and 2000 mg·L−1 significantly inhibited the elongation of both maize and rice roots (p < 0.01). Therefore, these three concentrations were selected to assess the different toxicity of ZnO NPs and ZnO MPs. As shown in Figure 5, ZnO MPs also significantly inhibited the elongation of both maize and rice roots, as compared to the controls (p < 0.05). However, compared with the ZnO MPs, the ZnO NPs showed greater toxicity to root elongation of both maize and rice at the three selected concentrations. For instance, compared with the control, ZnO NPs at 2000 mg·L−1 reduced the root length of maize and rice by 50.45% and 66.75%, while ZnO MPs only reduced the root length by 39.5% and 31.44%, respectively. The high surface/volume ratio of NPs with their smaller size make them highly reactive and with better catalytic properties, which increases their toxic potential compared with their bulk counterparts [43]. This result was in agreement with Lee et al. [30], who reported that ZnO NPs (44.4 ± 6.7 nm) were much more toxic than larger ZnO particles (2311 ± 304 nm) at 4000 mg·L−1. In addition, Lee et al. [42] also reported the biomass of buckwheat seedlings was more significantly reduced in response to ZnO NPs than ZnO MPs at concentrations of 10–2000 mg·L−1. In addition, it was reported that NPs with smaller size contained more particles for the same mass concentration and had a greater chance to penetrate through the plant cell membrane [44,45]. To date, despite the fact that various metal oxide NPs have been confirmed to be uptaken by plants [9,32,38,39], more studies are needed to test whether intracellular uptake is a requirement for causing phytotoxicity.
Figure 5.
Effect of ZnO NPs and ZnO MPs on the root elongation of (a) maize and (b) rice. The values were given as mean ± SD (standard deviation) of triplicate samples with 10 seeds each.
4. Conclusions
In summary, we have reported the different phytotoxicity of seven metal oxide NPs—nCeO2, nFe3O4, nSiO2, nTiO2, nAl2O3, nZnO and nCuO—in maize and rice. The seed germination of maize and rice was not affected by all the seven metal oxide NPs, while the root elongation of both maize and rice were significantly inhibited by nCuO and nZnO at 2000 mg·L−1. nAl2O3 was only found to be slightly toxic to the root elongation of maize, while no obvious toxic effects were observed in the other four metal oxide NPs. The toxicity of nCuO, nZnO and nAl2O3 was concentration dependent in both maize and rice. No negative effects were observed in the corresponding Cu2+, Zn2+ and Al3+ solutions, suggesting the phytotoxicity was mainly due to the NPs themselves. In addition, ZnO NPs showed greater toxicity to root elongation of maize and rice than ZnO MPs. Overall, this study provided a unified method to test the phytotoxicity of metal oxide NPs on crop plants. Moreover, this study provided valuable information for the application of engineered NPs in agriculture and the assessment of the potential environmental risks. However, these results are basically concentrated on the phenotypic changes; the underlying molecular mechanism still needs further studies at the physiological, metabolic and genetic levels.
Acknowledgments
The authors sincerely thank the Ministry of Science and Technology (2014DFA31740) and the Department of Science and Technology of Jilin Province, China (20130604037TC) for the financial support provided.
Supplementary Files
Author Contributions
Chuanbin Mao and Li Wang conceived and designed the experiments; Zhongzhou Yang and Jing Chen performed the experiments; Runzhi Dou and Xiang Gao analyzed the data; Zhongzhou Yang and Jing Chen wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Roco M.C. Broader societal issues of nanotechnology. J. Nanopart. Res. 2003;5:181–189. doi: 10.1023/A:1025548512438. [DOI] [Google Scholar]
- 2.Nowack B., Bucheli T.D. Occurrence, behavior and effects of nanoparticles in the environment. Environ. Pollut. 2007;150:5–22. doi: 10.1016/j.envpol.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Service R.F. Science policy: Report faults U.S. strategy for nanotoxicology research. Science. 2008;322 doi: 10.1126/science.322.5909.1779a. [DOI] [PubMed] [Google Scholar]
- 4.Peralta-Videa J.R., Zhao L., Lopez-Moreno M.L., de la Rosa G., Hong J., Gardea-Torresdey J.L. Nanomaterials and the environment: A review for the biennium 2008–2010. J. Hazard. Mater. 2011;186:1–15. doi: 10.1016/j.jhazmat.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 5.Handy R.D., von der Kammer F., Lead J.R., Hassellöv M., Owen R., Crane M. The ecotoxicology and chemistry of manufactured nanoparticles. Ecotoxicology. 2008;17:287–314. doi: 10.1007/s10646-008-0199-8. [DOI] [PubMed] [Google Scholar]
- 6.Dreher K.L. Health and environmental impact of nanotechnology: Toxicological assessment of manufactured nanoparticles. Toxicol. Sci. 2004;77:3–5. doi: 10.1093/toxsci/kfh041. [DOI] [PubMed] [Google Scholar]
- 7.Wiesner M.R., Lowry G.V., Alvarez P., Dionysiou D., Biswas P. Assessing the risks of manufactured nanomaterials. Environ. Sci. Technol. 2006;40:4336–4345. doi: 10.1021/es062726m. [DOI] [PubMed] [Google Scholar]
- 8.Yang L., Watts D.J. Particle surface characteristics may play an important role in phytotoxicity of alumina nanoparticles. Toxicol. Lett. 2005;158:122–132. doi: 10.1016/j.toxlet.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Wang Z., Xie X., Zhao J., Liu X., Feng W., White J.C., Xing B. Xylem- and phloem-based transport of CuO nanoparticles in maize (Zea mays L.) Environ. Sci. Technol. 2012;46:4434–4441. doi: 10.1021/es204212z. [DOI] [PubMed] [Google Scholar]
- 10.Song U., Shin M., Lee G., Roh J., Kim Y., Lee E.J. Functional Analysis of TiO2 Nanoparticle Toxicity in Three Plant Species. Biol. Trace Elem. Res. 2013;155:93–103. doi: 10.1007/s12011-013-9765-x. [DOI] [PubMed] [Google Scholar]
- 11.Mukherjee A., Peralta-Videa J.R., Bandyopadhyay S., Rico C.M., Zhao L., Gardea-Torresdey J.L. Physiological effects of nanoparticulate ZnO in green peas (Pisum sativum L.) cultivated in soil. Metallomics. 2014;6:132–138. doi: 10.1039/C3MT00064H. [DOI] [PubMed] [Google Scholar]
- 12.Burke D.J., Zhu S., Pablico-Lansigan M.P., Hewins C.R., Samia A.C.S. Titanium oxide nanoparticle effects on composition of soil microbial communities and plant performance. Biol. Fertil. Soils. 2014;50:1169–1173. doi: 10.1007/s00374-014-0938-3. [DOI] [Google Scholar]
- 13.Rico C.M., Majumdar S., Duarte-Gardea M., Peralta-Videa J.R., Gardea-Torresdey J.L. Interaction of nanoparticles with edible plants and their possible implications in the food chain. J. Agric. Food Chem. 2011;59:3485–3498. doi: 10.1021/jf104517j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rico C.M., Morales M.I., McCreary R., Castillo-Michel H., Barrios A.C., Hong J., Tafoya A., Lee W.-Y., Varela-Ramirez A., Peralta-Videa J.R., et al. Cerium oxide nanoparticles modify the antioxidative stress enzyme activities and macromolecule composition in rice seedlings. Environ. Sci. Technol. 2013;47:14110–14118. doi: 10.1021/es4033887. [DOI] [PubMed] [Google Scholar]
- 15.Servin A.D., Morales M.I., Castillo-Michel H., Hernandez-Viezcas J.A., Munoz B., Zhao L., Nunez J.E., Peralta-Videa J.R., Gardea-Torresdey J.L. Synchrotron verification of TiO2 accumulation in cucumber fruit: A possible pathway of TiO2 nanoparticle transfer from soil into the food chain. Environ. Sci. Technol. 2013;47:11592–11598. doi: 10.1021/es403368j. [DOI] [PubMed] [Google Scholar]
- 16.Comini E. Metal oxide nano-crystals for gas sensing. Analyt. Chim. Acta. 2006;568:28–40. doi: 10.1016/j.aca.2005.10.069. [DOI] [PubMed] [Google Scholar]
- 17.Hu J.-S., Zhong L.-S., Song W.-G., Wan L.-J. Synthesis of hierarchically structured metal oxides and their application in heavy metal ion removal. Adv. Mater. 2008;20:2977–2982. doi: 10.1002/adma.200800623. [DOI] [Google Scholar]
- 18.Gogos A., Knauer K., Bucheli T.D. Nanomaterials in plant protection and fertilization: Current state, foreseen applications, and research priorities. J. Agric. Food Chem. 2012;60:9781–9792. doi: 10.1021/jf302154y. [DOI] [PubMed] [Google Scholar]
- 19.Li Z.-Z., Xu S.-A., Wen L.-X., Liu F., Liu A.-Q., Wang Q., Sun H.-Y., Yu W., Chen J.-F. Controlled release of avermectin from porous hollow silica nanoparticles: Influence of shell thickness on loading efficiency, UV-shielding property and release. J. Control. Release. 2006;111:81–88. doi: 10.1016/j.jconrel.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 20.Goswami A., Roy I., Sengupta S., Debnath N. Novel applications of solid and liquid formulations of nanoparticles against insect pests and pathogens. Thin Solid Films. 2010;519:1252–1257. doi: 10.1016/j.tsf.2010.08.079. [DOI] [Google Scholar]
- 21.Hong F., Zhou J., Liu C., Yang F., Wu C., Zheng L., Yang P. Effect of nano-TiO2 on photochemical reaction of chloroplasts of spinach. Biol. Trace Elem. Res. 2005;105:269–279. doi: 10.1385/BTER:105:1-3:269. [DOI] [PubMed] [Google Scholar]
- 22.Asli S., Neumann P.M. Colloidal suspensions of clay or titanium dioxide nanoparticles can inhibit leaf growth and transpiration via physical effects on root water transport. Plant Cell Environ. 2009;32:577–584. doi: 10.1111/j.1365-3040.2009.01952.x. [DOI] [PubMed] [Google Scholar]
- 23.Kim S., Sin H., Lee S., Lee I. Influence of metal oxide particles on soil enzyme activity and bioaccumulation of two plants. J. Microbiol. Biotechnol. 2013;23:1279–1286. doi: 10.4014/jmb.1304.04084. [DOI] [PubMed] [Google Scholar]
- 24.Ruffini Castiglione M., Giorgetti L., Geri C., Cremonini R. The effects of nano-TiO2 on seed germination, development and mitosis of root tip cells of Vicia narbonensis L. and Zea mays L. J. Nanopart. Res. 2010;13:2443–2449. doi: 10.1007/s11051-010-0135-8. [DOI] [Google Scholar]
- 25.Kennedy D. The importance of rice. Science. 2002;296:13. doi: 10.1126/science.296.5565.13. [DOI] [PubMed] [Google Scholar]
- 26.U.S. Environmental Protection Agency, 1996 Ecological Effects Test Guidelines (OPPTS 850.4200): Seed Germination/ Root Elongation Toxicity Test. [(accessed on 4 January 2010)]; Available online: http://www.epa.gov/opptsfrs/publications/OPPTS_Harmonized/850_Ecological_Effects_Test_Guidelines/Drafts/850-4200.pdf.
- 27.Pokhrel L.R., Dubey B. Evaluation of developmental responses of two crop plants exposed to silver and zinc oxide nanoparticles. Sci. Total Environ. 2013;452:321–332. doi: 10.1016/j.scitotenv.2013.02.059. [DOI] [PubMed] [Google Scholar]
- 28.Atha D.H., Wang H., Petersen E.J., Cleveland D., Holbrook R.D., Jaruga P., Dizdaroglu M., Xing B., Nelson B.C. Copper oxide nanoparticle mediated DNA damage in terrestrial plant models. Environ. Sci. Technol. 2012;46:1819–1827. doi: 10.1021/es202660k. [DOI] [PubMed] [Google Scholar]
- 29.Moral J., Lozano-Baena M.D., Rubiales D. Temperature and water stress during conditioning and incubation phase affecting Orobanche crenata seed germination and radicle growth. Front. Plant Sci. 2015;6 doi: 10.3389/fpls.2015.00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee C.W., Mahendra S., Zodrow K., Li D., Tsai Y.-C., Braam J., Alvarez P.J.J. Developmental phytotoxicity of metal oxide nanoparticles to Arabidopsis thaliana. Environ. Toxicol. Chem. 2010;29:669–675. doi: 10.1002/etc.58. [DOI] [PubMed] [Google Scholar]
- 31.Ma Y., Kuang L., He X., Bai W., Ding Y., Zhang Z., Zhao Y., Chai Z. Effects of rare earth oxide nanoparticles on root elongation of plants. Chemosphere. 2010;78:273–279. doi: 10.1016/j.chemosphere.2009.10.050. [DOI] [PubMed] [Google Scholar]
- 32.Miralles P., Church T.L., Harris A.T. Toxicity, uptake, and translocation of engineered nanomaterials in vascular plants. Environ. Sci. Technol. 2012;46:9224–9239. doi: 10.1021/es202995d. [DOI] [PubMed] [Google Scholar]
- 33.Lin D., Xing B. Root uptake and phytotoxicity of ZnO nanoparticles. Environ. Sci. Technol. 2008;42:5580–5585. doi: 10.1021/es800422x. [DOI] [PubMed] [Google Scholar]
- 34.Brunner T.J., Wick P., Manser P., Spohn P., Grass R.N., Limbach L.K., Bruinink A., Stark W.J. In vitro cytotoxicity of oxide nanoparticles: Comparison to asbestos, silica, and the effect of particle solubility. Environ. Sci. Technol. 2006;40:4374–4381. doi: 10.1021/es052069i. [DOI] [PubMed] [Google Scholar]
- 35.Murata M.R., Hammes P.S., Zharare G.E. Effect of solution pH and calcium concentration on germination and early growth of groundnut. J. Plant Nutr. 2003;26:1247–1262. doi: 10.1081/PLN-120020368. [DOI] [Google Scholar]
- 36.El-Ghamery A.A., El-Kholy M.A., Abou El-Yousser M.A. Evaluation of cytological effects of Zn2+ in relation to germination and root growth of Nigella sativa L. and Triticum aestivum L. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2003;537:29–41. doi: 10.1016/S1383-5718(03)00052-4. [DOI] [PubMed] [Google Scholar]
- 37.Zhang X., Zhong T., Liu L., Ouyang X. Impact of soil heavy metal pollution on food safety in China. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0135182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Massaquoi L.D., Ma H., Liu X.H., Han P.Y., Zuo S.-M., Hua Z.-X., Liu D.-W. Heavy metal accumulation in soils, plants, and hair samples: An assessment of heavy metal exposure risks from the consumption of vegetables grown on soils previously irrigated with wastewater. Environ. Sci. Pollut. Res. 2015 doi: 10.1007/s11356-015-5131-1. [DOI] [PubMed] [Google Scholar]
- 39.Xu J., Yang L., Wang Z., Dong G., Huang J., Wang Y. Toxicity of copper on rice growth and accumulation of copper in rice grain in copper contaminated soil. Chemosphere. 2006;62:602–607. doi: 10.1016/j.chemosphere.2005.05.050. [DOI] [PubMed] [Google Scholar]
- 40.Mousavi Kouhi S.M., Lahouti M., Ganjeali A., Entezari M.H. Comparative phytotoxicity of ZnO nanoparticles, ZnO microparticles, and Zn2+ on rapeseed (Brassica napus L.): Investigating a wide range of concentrations. Toxicol. Environ. Chem. 2014;96:861–868. doi: 10.1080/02772248.2014.994517. [DOI] [Google Scholar]
- 41.Lin D., Xing B. Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth. Environ. Pollut. 2007;150:243–250. doi: 10.1016/j.envpol.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 42.Lee S., Kim S., Kim S., Lee I. Assessment of phytotoxicity of ZnO NPs on a medicinal plant, Fagopyrum esculentum. Environ. Sci. Pollut. Res. 2013;20:848–854. doi: 10.1007/s11356-012-1069-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nel A., Xia T., Mädler L., Li N. Toxic potential of materials at the nanolevel. Science. 2006;311:622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 44.Tso C.-P., Zhung C.-M., Shih Y.-H., Tseng Y.-M., Wu S.-C., Doong R.-A. Stability of metal oxide nanoparticles in aqueous solutions. Water Sci. Technol. 2010;61:127–133. doi: 10.2166/wst.2010.787. [DOI] [PubMed] [Google Scholar]
- 45.Nair R., Varghese S.H., Nair B.G., Maekawa T., Yoshida Y., Kumar D.S. Nanoparticulate material delivery to plants. Plant Sci. 2010;179:154–163. doi: 10.1016/j.plantsci.2010.04.012. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.