Abstract
Background
Hyperhomocysteinemia is associated with an increased risk of atherosclerosis. The main cause of elevated levels of homocysteine is 677T allele, the gene encoded by methylenetetrahydrofolate reductase (MTHFR). Carotid atherosclerosis progression, which can be measured by examination of carotid intima-media thickness (C-IMT), is a predictor of recurrent ischemic stroke. The objective of this study was to determine a relationship between MTHFR polymorphism, homocysteine levels, and increased C-IMT in post- ischemic stroke patients.
Methods
This was an epidemiological prospective observational cohort study involving 71 patients with post-ischemic stroke subject of the first (onset 1 month) admitted in the Neurology Clinic of Kariadi Hospital during 2012 to 2013. C-IMT was examined using carotid duplex ultrasound at 1st, 6th, and 12th month after stroke onset. MTHFR gene polymorphism was examined using polymerase chain reaction-restriction fragment length polymorphisms (PCR-RFLP). Homocysteine level was measured using Axis® Homocysteine EIA.
Results
We found 3 categories of MTHFR gene variation, i.e., 677T/T, 677T/C, and 677C/C. The most frequent allele was MTHFR 677C (88.9%), while the MTHFR 677T allele frequency was 11.1%. The majority allele of the subject population was 677C/C, however, there were 3 subjects (4.2%) who had 677T/T allele. The 677T/T allele group had normal homocysteine level and the lowest mean C-IMT among others.
Conclusions
This study supports that the MTHFR 677T allele polymorphism is not associated with hyperhomocysteinemia as well as an increase in C-IMT in post ischemic stroke patients.
Keywords: Methylenetetrahydrofolate reductase (MTHFR), homocysteine, progression of atherosclerosis, intima-media thickness (IMT), post-ischemic stroke
Introduction
The process of atherosclerosis has been known for over 500 years, but its role to pathological condition was unknown for more than 150 years. Definition of atherosclerotic vascular disease has progressed over the last 25 years along with the development of vascular and molecular biology. Atherosclerosis is not a disease that stands alone but is a disease caused by many factors (multifactorial). Currently, atherosclerosis is a major cause of morbidity and mortality both in developed countries, and in developing countries (1).
Hyperhomocysteinemia has been known as one of the risk factors for atherosclerosis that can be modified (2). Some individuals have elevated levels of homocysteine due to a genetic predisposition. Methylenetetrahydrofolate reductase (MTHFR) gene produces an enzyme that helps regulate homocysteine level in the body. If there is a genetic abnormality (mutation) in the MTHFR gene, it can lead to interference in the homocysteine regulatory process. MTHFR genetic mutation is inherited risk factors most often found in elevated levels of homocysteine (3-5).
Metiltetrahidrofolat reductase (MTHFR) plays an important role in the metabolism of folate and homocysteine to catalyze the conversion of 5,10-metiltetrahidrofolat into 5-metiltetrahidrofolat, the major circulating form of folate used in the remethylation process of homocysteine to methionine (5).
Isolation of the cDNA in 1994 has paved the way for the use of molecular genetic examination of research on MTHFR (5). Deficiency cDNA isolation helps to identify the variant sequence similar to the 677 bp (C → T), which generates the code for the form of the enzyme thermolabile. This variant has been known as the most common genetic cause of hyperhomocysteimia and has been widely studied as a risk factor for a number of multifactorial disorders related to homocysteine metabolic disorders (3-5).
Intima-media thickness (IMT), measurement of internal carotid artery using ultrasound, can indicate the presence of atherosclerosis, even at an early stage. Normal IMT value is <0.8 mm, and said to be suspected arterial disease when 0.8 to 0.99 mm and pathological when >1.0 mm. The increasing risk factors will accelerate the increase in IMT. IMT of the carotid artery associated with the occurrence of myocardial infarction and stroke in old age. According to Kuopio Ischemic Heart Disease (KIHD), any change in IMT of 0.1 mm and according to the Atherosclerosis Risk in Communities (ARIC) 0.18 mm will increase the incidence of cardiovascular disease. It was also a predictor of recurrent strokes (6-11).
Many researches have already tried to find an association between MTHFR polymorphism, homocysteine level, and increased carotid IMT (C-IMT), but the result remains controversy. Based on this phenomena, we tried to find whether there were association between MTHFR polymorphism, homocysteine level, and increased C-IMT in Indonesian people that might different from other ethnics, and in post ischemic stroke patient to find the relationship between genetic predisposition, hyperhomocysteinemia as a risk factor of ischemic stroke, and atherosclerosis (C-IMT).
Patients and methods
Research was conducted in the Laboratory of Dr. Kariadi Hospital, Cebior Laboratory of Medical Faculty of Diponegoro University, which both are government owned laboratories and serve the processing of the sample for public. Financial support for this study are borne entirely by the researchers, there were no external financial support. Both sample registry and Gene analyzing were conducted independently. A written informed consent was obtained from all subjects that participating in this study. All data were analyzed using SPSS version 17.
This research has been getting Ethical Clearance of Health Research Ethics Committee of the Faculty of Medicine Diponegoro University and has received permission from the Director of the Dr. Kariadi Hospital, Semarang, Central Java Indonesia.
This was an epidemiological prospective observational cohort study involving 71 patients with post-ischemic stroke subject of the first (onset 1 month) admitted in the Neurology Clinic of Kariadi Hospital during 2012 to 2013.
Inclusion criteria were (I) aged 45-90 years; (II) experiencing atherosclerosis and post ischemic stroke proved by brain CT and carotid Doppler; (III) first stroke event; (IV) no heart disease (ECG within normal limits or IHD without clinical symptoms); (V) stroke onset not more than 1 month; (VI) willing to participate in the study. Exclusion criteria were: (I) bedridden, (II) unconscious. Drop out criteria were: (I) the patient didn’t come in a 2 consecutive IMT examination; (II) suffering from hemorrhagic transformation; (III) patient died; (IV) patient resign.
C-IMT was examined using carotid duplex ultrasound at 1st, 6th, and 12th month after stroke onset, and measured using ultrasound carotid duplex sonography Siemens plane Sonoline Omnia no FBE 0322. C-IMT is defined as the distance between lumen-intima and media-adventitia, both right internal carotid artery and left measured 3 cm proximal and 1 cm distal to the bifurcation. C-IMT was measured at the beginning of the study and measured again after 6 and 12 months. Thickening of the intima media thickness measured using carotid Doppler. It was said to be thicken when >0.1 mm.
MTHFR gene polymorphism was examined using polymerase chain reaction-restriction fragment length polymorphisms (PCR-RFLP) system 9700, an examination conducted by a team from the Laboratory of Molecular Biology (Cebior) Diponegoro University, Semarang, Indonesia.
Homocysteine level was measured using Axis® Homocysteine EIA that was conducted by GAKI laboratory, Diponegoro University, Semarang, Indonesia. The measurement was using enzyme immunoassay to determine Hcy in blood. Protein-bound Hcy is reduced to free Hcy and enzymatically converted to S-adenosyl-L-homocysteine (SAH) in a separate procedure prior to the immunoassay. The enzyme is specific for the L-form of homocysteine, which is the only form present in the blood.
All measurements were performed by board-certified laboratory technicians who were blinded to clinical data.
Results
This research has been carried out within a period of 24 months, from January 2012 to December 2013. In the early stages we obtained 155 subjects who met the inclusion and exclusion criteria. In the 12-month study period there were 83 subjects loss of follow-up so that the remaining 72 subjects who finished the procedure. Loss of follow-up is caused by the patients who died, the people who do not proceed to the examination because they do not get a referral from a family doctor, the patients who did not continue the investigation because they do not want to come to the hospital only for this research, the patients still come to the hospital but refused to continue the examination because it is considered inconvenient or wasting time, patients who do not continue the examination because resettled outside the city or their address were unclear, so that from the 155 study subjects who meet the inclusion and exclusion criteria remaining 72 subjects that met the procedure regularly.
The mean IMT measurement results are based on normal and abnormal IMT division showed an increase of 1, 6, and 12 months examination. On the 1st month examination showed that both the normal and the abnormal group had increased IMT at 6th and 12th month.
Unpaired t-test result showed that there were significant differences between the mean IMT after 6 and 12 months after onset (P≤0.001). The results showed the IMT progression both in the 6 and 12 months examination in which the abnormal group were more progressive than normal group.
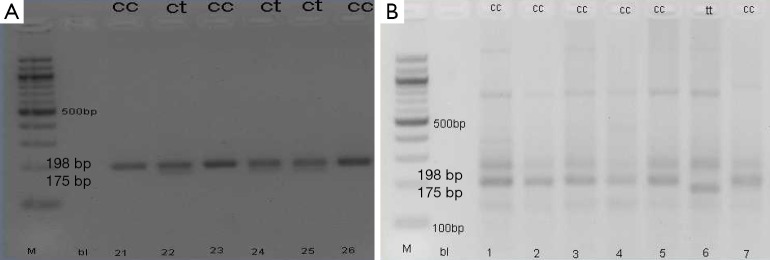
Examination of gene polymorphisms of MTHFR 677C > T performed on all subjects. Research results get three polymorphism categories in the subjects examined, namely; CC, CT, and TT (Figure 1). C allele frequency was 128 (88.9%) and the T allele was 16 (11.1%) (Table 1).
Figure 1.
MTHFR 677T and 677C allele from PCR-RFLP product in electrophorese gel. MTHFR, methylenetetrahydrofolate reductase; PCR-RFLP, polymerase chain reaction-restriction fragment length polymorphisms.
Table 1. The frequency of MTHFR allele and genotype.
| MTHFR allele | Frequency (%) |
|---|---|
| C | 128 (88.9) |
| T | 16 (11.1) |
| CC | 59 (81.9) |
| CT | 10 (13.9) |
| TT | 3 (4.2) |
MTHFR, methylenetetrahydrofolate reductase.
The results showed that the mean homocysteine levels in patients with stroke were lower in TT allele compared to CC and CT alleles. Mann-Whitney test results showed that no significant differences found in mean homocysteine levels between TT alleles group and CC and CT alleles groups (P=0.250) (Table 2).
Table 2. MTHFR polymorphisms and homocysteine.
| Variables | Homocysteine (mg/dL) | P |
|---|---|---|
| TT (n=3) | 12.3±4.5 | 0.250Δ |
| CC and CT (n=69) | 20.1±16.4 |
Δ, Mann-Whitney test. MTHFR, methylenetetrahydrofolate reductase.
Different test on two groups with normal distribution is not done with the Mann-Whitney test between groups TT alleles of genes and gene alleles CC or CT. The results showed that no significant difference in mean C-IMT in the evaluation of 1 month (P=0.979), 6 months (P=0.670) and 12 months (P=0.770) with the Mann-Whitney test (Table 3).
Table 3. MTHFR polymorphisms and C-IMT.
| Variables | C-IMT (mm) |
|||||
|---|---|---|---|---|---|---|
| 1 month | P | 6 months | P | 12 months | P | |
| MTHFR allele | 0.979Δ | 0.670Δ | 0.770Δ | |||
| TT (n=3) | 0.67±0.23 | 0.85±0.20 | 0.92±0.13 | |||
| CC and CT (n=69) | 0.72±0.24 | 0.83±0.26 | 0.95±0.27 | |||
Δ, Mann-Whitney test. MTHFR, methylenetetrahydrofolate reductase; C-IMT, carotid intima-media thickness.
There are three subjects with the MTHFR TT alleles. Patient’s data shows that based on the risk factors there is one subject with a history of smoking, one subject with hypertension, and neither with diabetes mellitus. All subjects had increased IMT thickness on 1, 6, and 12 months examination. However, the subjects with the initials MA increased more progressive than the other two subjects, with delta IMT over 12 months is 0.35 mm. Subjects MA on 3 times homocysteine inspection always had homocysteine levels above the normal range (>15 mol/L), which is 17.46 mol/L on the 1st month, 26.53 mol/L on the 6th month, and 29.65 mol/L on 12th month.
Discussion
C.677C > T polymorphism of the MTHFR gene is the most commonly found in the population. This polymorphism causes the substitution of amino acids alanine into valine. This change causes the condition termolabilitas MTHFR gene that leads to hyperhomocysteinemia and increase the risk of atherosclerosis (12).
This study shows that the frequency of the C allele (88.9%) is more common than the T allele (11.1%) in which the CC allele was found in 59 (81.9%), the CT allele was found in 10 (13.9%) and TT alleles found in 3 (4.2%). In this study the average levels of homocysteine in patients with stroke was lower in TT allele compared with CC alleles and CT.
Results of Mann-Whitney test showed no significant differences in mean homocysteine levels between groups TT allele, CC allele and CT (P=0.250). The study of Frosst et al. (13) also suggests a link between genotype mutant homozygotes TT with hyperhomocysteinemia. It has been known that a T alleles carrier at greater risk for hyperhomocysteinemia compared to C allele.
Increased risk of illness known in hyperhomocysteinemia group when compared with the healthy control group in previous studies. The absence of a comparison group of healthy controls in this study makes us unable to figure hyperhomocysteinemia risk in patients with ischemic stroke. Moreover, polymorphism in the MTHFR gene also is dependent on ethnic background (13-15). The condition of ethnic subjects in this study may be different when compared to the Caucasian group. Several research studied the relationship between MTHFR mutation with venous blood clots, but the result were inconsistent, while some studies indicate a relationship and others found no relationship (3).
Conclusions
MTHFR genetic polymorphisms are not associated with IMT progression. CC alleles associated with the occurrence of hyperhomocysteinemia, allegedly due to ethnic differences.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Palinski W, Napoli C. Pathophysiological events during pregnancy influence the development of atherosclerosis in humans. Trends Cardiovasc Med 1999;9:205-14. [DOI] [PubMed] [Google Scholar]
- 2.Christen WG, Ajani UA, Glynn RJ, et al. Blood levels of homocysteine and increased risks of cardiovascular disease: causal or casual? Arch Intern Med 2000;160:422-34. [DOI] [PubMed] [Google Scholar]
- 3.Varga EA, Sturm AC, Misita CP, et al. Cardiology patient pages. Homocysteine and MTHFR mutations: relation to thrombosis and coronary artery disease. Circulation 2005;111:e289-93. [DOI] [PubMed] [Google Scholar]
- 4.Finkelstein JD. The metabolism of homocysteine: pathways and regulation. Eur J Pediatr 1998;157 Suppl 2:S40-4. [DOI] [PubMed] [Google Scholar]
- 5.Casarini L, Simoni M. Gene polymorphisms in female reproduction. Methods Mol Biol 2014;1154:75-90. [DOI] [PubMed] [Google Scholar]
- 6.Bang OY, Kim JS. Low-molecular-weight heparin in atherosclerotic stroke: a surprising resurrection of anticoagulants? Stroke 2012;43:293-4. [DOI] [PubMed] [Google Scholar]
- 7.Fromm A, Haaland ØA, Naess H, et al. Risk factors and their impact on carotid intima-media thickness in young and middle-aged ischemic stroke patients and controls: the Norwegian Stroke in the Young Study. BMC Res Notes 2014;7:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Groot E, van Leuven SI, Duivenvoorden R, et al. Measurement of carotid intima-media thickness to assess progression and regression of atherosclerosis. Nat Clin Pract Cardiovasc Med 2008;5:280-8. [DOI] [PubMed] [Google Scholar]
- 9.Della-Morte D, Gardener H, Denaro F, et al. Metabolic syndrome increases carotid artery stiffness: the Northern Manhattan Study. Int J Stroke 2010;5:138-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lakka HM, Lakka TA, Tuomilehto J, et al. Hyperinsulinemia and the risk of cardiovascular death and acute coronary and cerebrovascular events in men: the Kuopio Ischaemic Heart Disease Risk Factor Study. Arch Intern Med 2000;160:1160-8. [DOI] [PubMed] [Google Scholar]
- 11.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol 1989;129:687-702. [PubMed] [Google Scholar]
- 12.Sinha PK, Santr G, De D, et al. Carotid intima-media thickness in type 2 diabetes mellitus patients with cardiac autonomic neuropathy. J Assoc Physicians India 2012;60:14-8. [PubMed] [Google Scholar]
- 13.Frosst P, Blom HJ, Milos R, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet 1995;10:111-3. [DOI] [PubMed] [Google Scholar]
- 14.Botto LD, Yang Q. 5,10-Methylenetetrahydrofolate reductase gene variants and congenital anomalies: a HuGE review. Am J Epidemiol 2000;151:862-77. [DOI] [PubMed] [Google Scholar]
- 15.Hustad S, Midttun Ø, Schneede J, et al. The methylenetetrahydrofolate reductase 677C-->T polymorphism as a modulator of a B vitamin network with major effects on homocysteine metabolism. Am J Hum Genet 2007;80:846-55. [DOI] [PMC free article] [PubMed] [Google Scholar]