Abstract
Gallbladder cancer is the most common and a highly aggressive biliary tract malignancy with a dismal outcome. The pathogenesis of the disease is multifactorial, comprising the combined effect of multiple genetic variations of mild consequence along with numerous dietary and environmental risk factors. Previously, we demonstrated the association of several candidate gene variations with GBC risk. In this study, we aimed to identify the combination of gene variants and their possible interactions contributing towards genetic susceptibility of GBC. Here, we performed Multifactor-Dimensionality Reduction (MDR) and Classification and Regression Tree Analysis (CRT) to investigate the gene–gene interactions and the combined effect of 14 SNPs in nine genes (DR4 (rs20576, rs6557634); FAS (rs2234767); FASL (rs763110); DCC (rs2229080, rs4078288, rs7504990, rs714); PSCA (rs2294008, rs2978974); ADRA2A (rs1801253); ADRB1 (rs1800544); ADRB3 (rs4994); CYP17 (rs2486758)) involved in various signaling pathways. Genotyping was accomplished by PCR-RFLP or Taqman allelic discrimination assays. SPSS software version 16.0 and MDR software version 2.0 were used for all the statistical analysis. Single locus investigation demonstrated significant association of DR4 (rs20576, rs6557634), DCC (rs714, rs2229080, rs4078288) and ADRB3 (rs4994) polymorphisms with GBC risk. MDR analysis revealed ADRB3 (rs4994) to be crucial candidate in GBC susceptibility that may act either alone (p < 0.0001, CVC = 10/10) or in combination with DCC (rs714 and rs2229080, p < 0.0001, CVC = 9/10). Our CRT results are in agreement with the above findings. Further, in-silico results of studied SNPs advocated their role in splicing, transcriptional and/or protein coding regulation. Overall, our result suggested complex interactions amongst the studied SNPs and ADRB3 rs4994 as candidate influencing GBC susceptibility.
Keywords: genetic susceptibility, polymorphism, gallbladder cancer (GBC), Multifactor-Dimensionality Reduction (MDR), Classification and Regression Tree Analysis (CRT)
1. Introduction
Gallbladder cancer (GBC) is infrequent, however, it is very aggressive, and also the most common biliary tract cancer worldwide with marked geographical, racial and gender-specific orientations [1,2]. The etiology of GBC is multifactorial with gallstone and chronic inflammation as the root of disease [3,4]. Due to the absence of specific symptoms and late presentation, more than 90% of GBC patients are diagnosed at an advanced stage with little treatment alternatives [5]. Owing to unsatisfactory treatment options, the five-year survival rate is less than 5% and neither chemotherapy nor radiotherapy have been shown to improve the overall quality of life [6]. Further, despite recent advancements, the molecular basis of GBC is poorly understood and it still remains a diagnostic and therapeutic challenge for clinicians [7]. Thus, there is always a need to develop novel biomarker for its early diagnosis, and to enhance our understanding of inter-individual variability in vulnerability of GBC.
Previously, we have examined the role of various candidate gene variations in GBC patients from North India. These variants are part of genes involved in various signaling pathways, including apoptosis, cell survival, cell–cell interaction, estrogen metabolism, etc. [8,9,10,11,12]. However, being low penetrance genetic variations, their individual contribution towards GBC is very small and single SNPs cannot exactly account for GBC susceptibility. Further, carcinogenesis is a highly intricate process and polygenic in nature involving multiple gene variations of mild consequence [13]. In addition, gene–gene and gene–environment interactions are believed to be major players in the pathogenesis of GBC and can modulate individual’s susceptibility to cancer [14]. Hence, we have extended our earlier work by simultaneously exploring 14 polymorphisms in nine genes (DR4: A>C (rs20576), G>A (rs6557634); FAS-1377G>A (rs2234767); FASL-844T>C (rs763110); DCC:C >G (rs2229080), A>G (rs4078288), C>T (rs7504990), A>G (rs714); PSCA:C>T (rs2294008), G>A (rs2978974); ADRA2A-1291C>G (rs1801253); ADRB1 1165C>G (rs1800544); ADRB3 190T>C (rs4994); CYP17 T>C (rs2486758)) by using Multifactor-Dimensionality Reduction (MDR) method and classification and regression trees (CRT) to determine possible higher order gene–gene interactions and accomplish a comprehensive appraisal of GBC risk. These are a non-parametric, genetic model-free methodologies [15] having advantage to identify association in studies having small sample sizes and low penetrance of candidate SNPs as compared to previous traditional methods such as logistic regression [16,17].
2. Results
The demographic profile of GBC patients and controls are displayed in Table 1. The mean age of 400 GBC cases and 246 controls were 52.65 ± 10.45 and 47.75 ± 10.65 years, respectively. Most of the GBC patients (~95%) were in stage III and stage IV of cancer.
Table 1.
Characteristic of the Study Subjects.
| Variables | Cases N (%) | Controls N (%) |
|---|---|---|
| Whole Subjects | 400 (100) | 246 (100) |
| Female | 278 (69.5) | 163 (66.3) |
| Male | 122 (30.5) | 83 (33.7) |
| Age ± SD | 52.65 ± 10.45 | 47.75 ± 10.65 |
| Stages | ||
| 0, I | None | NA |
| II | 21 (5.25) | |
| III | 199 (49.75) | |
| IV | 180 (45.0) | |
| Gallstone present | 200 (50.0) | None |
| Gallstone absent | 200 (50.0) | 246 (100) |
| Tobacco | ||
| No | 273 (68.9) | NA |
| Yes | 123 (31.1) |
NA: not available.
2.1. Single Locus Analysis
Table 2 represents the association of all the studied SNPs with GBC risk. The heterozygous genotypes of DR4 (rs20576, rs6557634), and variant genotype of DCC rs4078288 was found to confer significantly increased risk of GBC (adjusted OR > 1; p < 0.05). Further, both hetero- and variant-genotypes of DCC rs714 and ADRB3 rs4994 were associated with the increased susceptibility of GBC, whereas genotype containing at least one variant allele of DCC rs2229080 was found to confer protection against GBC risk.
Table 2.
Single locus analysis of SNPs investigated.
| Pathway | Gene | SNP | MAFcontrols | MAFcases | ORhet a | ORhom a |
|---|---|---|---|---|---|---|
| Death receptor | Dr4 | rs20576 | 8 | 14 | 1.82 (1.18–2.83) | 3.27 (0.93–11.51) |
| rs6557634 | 27 | 33 | 1.61 (1.06–2.44) | 2.05 (0.90–4.70) | ||
| FAS | rs763110 | 39 | 41 | 0.94 (0.66–1.33) | 1.26 (0.78–2.02) | |
| FASL | rs2234767 | 20 | 22 | 0.99 (0.71–1.38) | 1.66 (0.70–4.12) | |
| Tumor suppressor | DCC | rs714 | 37 | 45 | 1.84 (1.29–2.63) | 1.72 (1.08–2.74) |
| rs2229080 | 32 | 24 | 0.64 (0.46–0.89) | 0.32 (0.15–0.68) | ||
| rs7504990 | 32 | 31 | 1.01 (0.72–1.40) | 0.92 (0.51–1.65) | ||
| rs4078288 | 34 | 39 | 0.98 (0.69–1.39) | 1.58 (1.01–2.49) | ||
| Prostate stem cell antigen | PSCA | rs2978974 | 32 | 30 | 0.91 (0.65–1.27) | 0.86 (0.50–1.48) |
| rs2294008 | 42 | 46 | 1.4 (0.97–2.02) | 1.25 (0.77–2.04) | ||
| Adrenergic pathway | ADRa2a | rs1800544 | 45 | 49 | 1.35 (0.92–1.97) | 1.41 (0.87–2.29) |
| ADRB3 | rs4994 | 10 | 21 | 2.58 (1.76–3.78) | 10.61 (1.38–81.92) | |
| ADRB1 | rs1801253 | 22 | 25 | 1.32 (0.95–1.84) | 1.12 (0.46–2.78) | |
| Estrogen metabolism pathway | CYP17 | rs2486758 | 26 | 27 | 1.04 (0.74–1.45) | 1.11 (0.59–2.09) |
Significant values are denoted as bold. a Adjusted for age and gender in logistic regression model; ORhet: odds ratio of heterozygote vs. common homozygote genotypes; ORhom: odds ratio of homozygote vs. common homozygote genotypes, MAF: Minor allele frequency.
2.2. Multifactor Dimensionality Reduction (MDR)
Our MDR analysis demonstrated ADRB3rs4994 polymorphism (testing accuracy = 0.6003, CVC = 10/10, p < 0.0001) as the one-factor model for envisaging the GBC risk. DCCrs2229080, ADRB3rs4994 constitutes the two-factor model with testing accuracy of 0.5658 but CVC = 6/10 (p < 0.0001). The three-factor model, comprising DCCrs714, DCCrs2229080, and ADRB3rs4994 SNPs had the improved testing accuracy of 0.5913 and the CVC of 9/10 (p ≤ 0.0001). Likewise, DCCrs714, DCCrs2229080, PSCArs2978974, and ADRB3rs4994 polymorphisms represents the four-factor interaction model, having a testing accuracy of 0.5353 and CVC = 3/10 with p < 0.0001 (Table 3).
Table 3.
Multifactor dimensionality reduction (MDR) analysis showing association of high-order interactions with GBC.
| No. of Risk Factors | Best Interaction Model | Testing Accuracy | # CVC | X² (p-Value) | OR (95% CI) |
|---|---|---|---|---|---|
| 1 | ADRB3rs4994 | 0.6003 | 10/10 | 28.5717 (p < 0.0001) | 2.7507 (1.8841–4.0158) |
| 2 | DCCrs2229080, ADRB3rs4994 | 0.5658 | 6/10 | 32.5889 (p < 0.0001) | 2.6238 (1.8762–3.6693) |
| 3 | DCCrs714, DCCrs2229080, ADRB3rs4994 | 0.5913 | 9/10 | 44.324 (p < 0.0001) | 3.0155 (2.1684–4.1935) |
| 4 | DCCrs714, DCCrs2229080, PSCArs2978974, ADRB3rs4994 | 0.5353 | 3/10 | 68.7203 (p < 0.0001) | 4.0443 (2.8834–5.6726) |
# The model with maximum testing accuracy and maximum CVC cross was considered as the best model; CVC: cross-validation consistency.
2.3. Classification and Regression Tree Analysis (CRT)
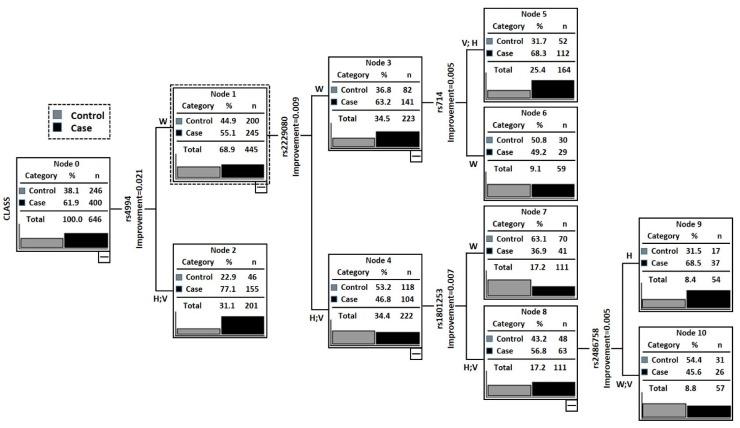
Figure 1 depict the results of CRT analysis, containing all the studied SNPs. The tree comprised of total eleven nodes and six terminal nodes (node that has no child nodes) with ADRB3rs4994 polymorphism lying at the top of tree signifying it as the main contributing factor for GBC. Subjects with ADRB3rs4994 (W), DCCrs2229080 (H + V) and ADRB1rs1801253 (W) genotypes (Node 1) having the lowest case rate (36.94%) was taken as reference.
Figure 1.
Classification and regression tree model for selected 14 SNPs and GBC risk. Terminal nodes at the end. W: Wild type genotype; V: Variant genotype; H: heterozygous.
Table 4 summarizes the risk associated with all the terminal nodes compared with Node 1 (ADRB3rs4994 (W) + DCCrs2229080 (H + V) +ADRB1rs1801253 (W). Subjects having the ADRB3rs4994 (W) + DCCrs2229080 (W) + DCCrs714 (H + V) and ADRB3rs4994 (W) + DCCrs2229080 (H + V) + ADRB1rs1801253 (H + V) + Cyp17rs2486758 (H) genotypes were found to have a significantly increased GBC susceptibility (adjusted OR = 3.7; p = 0.0003 and OR = 3.7; p = 0.0001). Importantly, all the terminal nodes were comprised of ADRB3 rs4994 and DCCrs2229080 polymorphism (Table 4).
Table 4.
Risk estimate based on Classification and Regression Tree Analysis (CRT) terminal nodes.
| Nodes | Genotype of Individuals in Each Node | Case | Control | Total | Case Rate (%) | p-Value | OR (95% CI) a |
|---|---|---|---|---|---|---|---|
| Node 1 | ADRB3rs4994 (W) + DCC rs2229080 (H + V) + ADRB1rs1801253 (W) | 41 | 70 | 111 | 36.94 | – | Reference |
| Node 2 | ADRB3rs4994 (W) + DCC rs2229080 (H + V) + ADRB1rs1801253 (H + V) + Cyp17rs2486758 (W + V) | 26 | 31 | 57 | 45.61 | 0.2836 | 1.43 (0.74–2.75) |
| Node 3 | ADRB3rs4994 (W) + DCCrs2229080 (W) + DCCrs714 (W) | 29 | 30 | 59 | 49.15 | 0.1290 | 1.65 (0.87–3.14) |
| Node 4 | ADRB3rs4994 (W) + DCC rs2229080 (W) + DCCrs714 (H + V) | 112 | 52 | 164 | 68.29 | 0.0003 | 3.66 (2.21–6.12) |
| Node 5 | ADRB3rs4994 (W) + DCCrs2229080 (H + V) + ADRB1rs1801253 (H + V) + Cyp17rs2486758 (H) | 37 | 17 | 54 | 68.52 | 0.0001 | 3.69 (1.86–7.50) |
Case rate: Percentage of cancer patients among all individuals in each node (case/(case + control) × 100); a Adjusted for age and gender.
2.4. In-Silico Analysis
The in-silico analyses of all the studied SNPs are shown in Table 5. MDR and CRT analysis demonstrated ADRB3rs4994 is the key causative factor in gallbladder carcinogenesis. Our in-silico analysis also showed this SNP to alter protein coding, splicing and transcriptional regulation. DCCrs2229080 polymorphism was also shown to change the protein coding and splicing regulation. DCCrs714 and PSCArs2978974 are intronic SNPs with unknown function.
Table 5.
Bioinformatic Analysis.
| SNPs | Result of F-SNP/FAST SNP | |||
|---|---|---|---|---|
| – | FS Score | Functional Category | Prediction Tool | Prediction Result |
| DR4rs20576 | 0 | Protein coding | Ensemble | Nonsynnymous |
| Polyphen | Possible damaging | |||
| DR4rs6557634 | 0.284 | Protein coding | Ensembl | Nonsynonymous |
| Polyphen | Probably damaging | |||
| Splicing regulation | ESE finder | Changed | ||
| ESR Search | Changed | |||
| FASLrs763110 | 0.434 | Protein coding | Ensembl | Frameshift-coding |
| Transcriptional regulation | TF-Search | Changed | ||
| Ensembl-TR | Regulatory region | |||
| FASrs2234767 | 0 | Protein coding | Ensembl | Nonsynnymous |
| DCCrs2229080 | 0.616 | Protein coding | Polyphen | Probably damaging |
| SNPeffect | Deleterious | |||
| LS-SNP | Deleterious | |||
| Missense (non-conservative) Medium–high (3,4) | ||||
| Splicing regulation | ESE finder | Changed | ||
| ESR Search | Changed | |||
| PESX | Changed | |||
| DCCrs4078288 | NA | Intronic enhancer Very low–low (1–2) | ||
| DCCrs7504990 | NA | Intronic with no known function | ||
| DCCrs714 | NA | Intronic with no known function | ||
| Cyp17rs2486758 | 0.176 | Transcriptional regulation | TFSearch | Changed |
| ADRA2Ars1800544 | 0.065 | Transcriptional regulation | Golden path | Exit |
| ADRB3rs4994 | 0.551 | Protein coding | Ensembl | Nonsynonymous |
| SIFT | Damaging | |||
| SNPeffect | Deleterious | |||
| Splicing regulation | ESE finder | Changed | ||
| ESR Search | Changed | |||
| PESX | Changed | |||
| Transcriptional regulation | Golden path | Exit | ||
| ADRB1rs1800544 | 0.774 | Protein coding | Ensembl | Nonsynonymous |
| CYP17rs2486758 | 0.176 | Transcriptional regulation | TFsearch | Changed |
3. Discussion
Recent advancement in molecular biology has suggested extensive interactions amongst various genes or risk alleles (in which effect of single gene variation is influenced by other genetic variation i.e., gene–gene interaction) as the key factor modulating the disease susceptibility. Hence, in this study, we aimed to investigate the synergistic effect of various gene variations to modulate GBC susceptibility instead of their individual effect, by using MDR and CRT. MDR improves the identification of multilocus genotype combinations (higher order gene–gene interactions) predicting the disease vulnerability for common, complex and multifactorial diseases [15]. CRT analysis, which is based on recursive partitioning the data space and fitting a simple prediction model within each partition, is a powerful technique with significant potential and clinical utility [18]. It categorizes the study subjects according to various risk levels on the basis of the various gene polymorphisms [19]. Both MDR and CRT are widely used in large-scale association studies because of their capability to overcome sample size limitations and the curse of dimensionality as compared to case-control studies using logistic regression [16,17].
Our single locus analysis showed ARDB3rs4994 as the important factor enhancing the GBC risk. The MDR analysis also showed ADRB3rs4994 alone as the best candidate with highest testing accuracy and CVC. Further, the three-factor interaction model consisting of DCCrs714, DCCrs2229080, ADRB3rs4994 constitutes the second best SNPs model with testing accuracy of 0.5913 and CVC = 9/10 (p < 0.001). The result of CRT analysis further affirmed ADRB3rs4994 as the major risk factor for GBC advancement. In addition, it corroborated MDR result and showed a complex interaction amongst ADRB3rs4994, DCCrs2229080, DCCrs714 as well as Cyp17rs2486758 attributing increased susceptibility to GBC. These finding suggested some correlation among these genes or proteins.
ADRB3, a member of class of G-protein-coupled receptor family, is abundantly distributed in adipose tissue and regulate lipolysis and thermogenesis [20]. In addition, it has been localized to vascular and nonvascular smooth muscle of human gastrointestinal tract, as well as in gallbladder regulating the blood flow and motility in gastrointestinal tract and gallbladder [21,22]. ADRB3 rs4994 is a missense variation substituting tryptophan with arginine at codon 64. This SNP has been shown to influence fat accumulation and been implicated in the etiology of obesity that may serve as the predisposing factor for GBC [23,24]. It was also shown to alter the susceptibility to colon cancer risk in obese subjects [25]. Moreover, it has been established to increase the risk of gallstone disease, (a precancerous lesion for GBC), suggesting it as a gene marker for increased risk for gallstone [26,27]. In our previous study, we showed that ADRB3rs4994 conferred increased risk of GBC both by gallstone-dependent and -independent mechanisms [10]. Here, our multi-analytical approaches further confirmed the association of this SNP, either alone or in combination, with GBC risk. On the contrary, a recent study failed to show the association of this SNP with pancreatic cancer [28] may be due to different pathology underlying different organs.
DCC (netrin-1), originally discover in colorectal cancer, is characterized as a candidate tumor suppressor gene that encodes the netrin 1 receptor, a member of the immunoglobulin superfamily of cell adhesion molecules [29]. When DCC is present and bound to netrin-1 receptor, it induces cell proliferation and migration, while in the absence of netrin-1, an intracellular domain of DCC is cleaved by a caspase inducing apoptosis in a caspase-9-dependent pathway [30]. In various human cancers, it has been shown to be frequently silenced or inactivated due to loss of heterozygosity at chromosome 18q21 region or epigenetic silencing [29,31]. Loss of DCC gene expression was shown to be an independent prognostic factor in AML [32], colorectal [33] and gastric cancer [34,35] patients. Several studies have demonstrated significant association of DCC polymorphism with colorectal, esophageal, and gastric cancer risk [36,37,38,39]. The deletions at 18q21 loci (containing DCC gene) is an important step in the progression of GBC [40]. A genome-wide association study (GWAS) also suggested DCC as a candidate gene conferring GBC predisposition in a Japanese population [41]. In our previous work, we found no effect of GWAS reported SNPs on GBC risk. On the contrary, we showed significant association of DCC rs714 and rs2229080 with GBC risk [12]. The rs714 has been shown to be associated with loss of heterozygosity (LOH) and decreased expression of DCC in various cancers [42,43]. Further, rs2229080, a missense variation replacing Arg to Gly at DCC codon 201, was reported to increase the risk of colorectal cancer [44] and neuroblastoma [45]. Moreover, this SNP was suggested to be a target of LOH and associated with loss of DCC protein expression indicating that the codon 201 polymorphism may interfere with the DCC transcription or transition [46].
PSCA, originally identified as a prostate cell surface specific marker, was also established to be overexpressed in several other human cancers and suggested to play a role in carcinogenesis by regulating the cell proliferation, adhesion, migration and survival [47]. High expression of PSCA is significantly associated with adverse prognostic features and cancer severity, including; differentiation, invasion, metastasis and decreased overall survival [48,49]. The expression and function of PSCA are tissue specific, i.e., it acts like tumor suppressor gene (TSG) in some organ while as oncogene (OG) in others. In GBC, it was reported to be downregulated and act like TSG by modulating immunological characteristics of GBC cells [50,51,52]. However, a recent study has shown PSCA overexpression in GBC that is associated with invasive potential and prognosis of GBC [49]. Further, several GWAS and case control studies have demonstrated association of PSCA gene polymorphisms rs2294008 and rs2976392 with various cancers, though some controversies also existed [48,53,54,55,56]. The PSCArs2294008, located in exon 1, was found to affect the transcriptional activity [57,58] and the missense allele of rs2294008 was shown to attenuate antitumor activities of PSCA in GBC and consequently it was suggested to be a potential risk for GBC development [51]. The rs2976392G>A positioned in intron 2 is in strong linkage disequilibrium with rs2294008C>T, and its function is unclear till yet [59]. In our previous study, we failed to find the association of PSCA polymorphism with GBC risk, but on gender stratification, Trs2294008-Grs2978974 haplotype was found to confer higher risk of GBC in females (FDR Pcorr = 0.021), while Trs2294008-Ars2978974 haplotype is associated with significantly decreased risk in males (FDR Pcorr = 0.013) suggesting gender specific effect of PSCA haplotypes on GBC susceptibility [11]. Here, we found this SNP to increase GBC risk only in combination with DCC and ADRB3 SNPs, though the CVC is low (3/10, p < 0.0001).
Our in-silico investigation of ADRB3rs4994 and DCCrs2229080 showed alteration in protein coding, splicing regulation and transcriptional regulation. CYP17rs2486758 was also found to alter transcriptional regulation. Other associated SNPs (DCCrs714, PSCArs2978974) are intronic, hence our in silico study did not show any effect of these SNPs. Though, intronic SNPs are reported to be important player in splicing regulation and may affect other SNP lying in linkage disequilibrium.
Smoking/tobacco usage may be an important issue affecting disease susceptibility. However, we did consider smoking data due to non-reliability of collecting such information from controls. In earlier studies, we had therefore carried out case only analysis for modulation of genetic susceptibility by tobacco usage. However, in the present study, tobacco related analysis has not been done due to limited data. Here, we carried out only MDR and CART analysis for higher order gene–gene analysis.
4. Materials and Methods
4.1. Ethics Statement and Study Population
The present study was approved by the ethical committee of Sanjay Gandhi Postgraduate Institute of Medical Sciences (SGPGIMS). (Approval number: IEC code- 2012-170-EMP-66, approval date: 10.01.2013) Written informed consent was collected from all participants involved in the study.
A total of 646 subjects, including 400 GBC patients and 246 healthy control subjects of North Indian Ethnicity were recruited in this study from the department of Surgical Oncology, KGMU and Department of Surgical Gastroenterology SGPGIMS, Lucknow. The inclusion–exclusion criteria for cases and controls, and staging of cancer were same as previously reported in our studies [8,9,10,11,12]. In general, controls were frequency-matched to cancer cases for age, gender, and ethnicity, and were free from any history of malignancy as well as gallstones. For GBC cases, only confirmed subject (by FNAC; fine needle aspirated cell cytology or histopathology) were included in the study, while those already receiving chemotherapy were excluded.
4.2. Selected SNPs and Genotyping
In the present study, we have included DR4:A>C (rs20576), G>A (rs6557634); FAS-1377G>A (rs2234767); FASL-844T>C (rs763110); DCC:C>G (rs2229080), A>G (rs4078288), C>T (rs7504990), A>G (rs714); PSCA:C>T (rs2294008), G>A (rs2978974); ADRA2A-1291C>G (rs1801253); ADRB1 1165C>G (rs1800544); ADRB3 190T>C (rs4994); and CYP17 T>C (rs2486758) SNPs.
Salting out method was used to isolate genomic DNA from 5 mL peripheral blood leukocytes [60]. The genotyping was performed by the PCR restriction fragment length polymorphism and TaqMan® allelic discrimination assays (Applied Biosystems 7500 Fast Real-Time PCR (Thermo Fisher Scientific, Walthan, MA, USA)) method, as described previously [8,9,10,11,12]. PCR mix without DNA sample was taken as negative control and the 10% of random samples were sequenced to confirm the results consistency.
5. Statistical Analysis
5.1. Single Locus Analysis
Mean with standard deviation (SD) and absolute value were used for continuous and categorical measures, respectively. The frequency distributions of SNPs genotype between cases and controls were compared by using the chi-square analysis or two-sided Fisher’s exact test. Unconditional multivariate logistic regression (LR) was used to assess the odds ratios (ORs) and 95% confidence intervals (CIs) to estimate the risk of gallbladder cancer with the polymorphisms. The ORs were adjusted for age and gender. All statistical analyses were performed using SPSS software version 16.0 (SPSS, Chicago, IL, USA) and a p-value of less than 0.05 was considered a statistically significant.
5.2. Multifactor Dimensionality Reduction (MDR)
The MDR analysis was carried out by onine MDR software version 2.0 [61] producing several genotype interaction models. Amongst them, the genotype combination having the highest testing accuracy and the cross-validation consistency (CVC) is taken as the best interaction model [62]. The combined effect of the variables was calculated using LR analysis and a p-value less than 0.05 was considered to be statistically significant.
5.3. Classification and Regression Tree Analysis (CRT)
The SPSS software (version 16.0) was used to accomplish the CRT analysis producing a decision tree. In CRT analysis, starting with the core node comprising of the total sample, each node is divided into two child nodes repetitively by recursive partitioning [19], thus creating a tree like structure. The risk of all genotypes sets was estimated by considering the node with low case rate was as the reference to calculate the ORs and 95% CIs.
5.4. In-Silico Analysis and Functional Prediction of SNPs
Various online prediction tools such as; FASTSNP, F-SNP [63,64,65,66] were used to predict the functional effects of all the studied SNPs.
6. Conclusions
In conclusion, we found ADRB3 as the main SNPs associated with increased GBC susceptibility. In addition, we showed a complex interaction amongst ADRB3, DCC, PSCA and CYP17 increasing GBC risk. Further, our results allow more precise definition of subjects with high or low risk for GBC. Viewing the functional consequence of these SNPs in cancer initiation and progression, it is of great importance to further look into the underlying mechanism of carcinogenesis at gene levels and their interactive pathway. Future studies exploring the panels of the risk allele for GBC susceptibility in a larger sample size may have important implications in GBC management.
Acknowledgments
Partly this work was supported by Department of Biotechnology (DBT) India and Indian Council of Medical Research (ICMR) India.
Author Contributions
Rajani Rai conceived, designed and performed the experiments, wrote the paper; Jong Joo Kim analyzed the data, wrote the paper; Sanjeev Misra and Ashok Kumar contributed materials/analysis tools, Balraj Mittal conceived and designed the experiments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Dwivedi A.N., Jain S., Dixit R. Gall bladder carcinoma: Aggressive malignancy with protean loco-regional and distant spread. World J. Clin. Cases. 2015;3:231–244. doi: 10.12998/wjcc.v3.i3.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eslick G.D. Epidemiology of gallbladder cancer. Gastroenterol. Clin. N. Am. 2010;39:307–330. doi: 10.1016/j.gtc.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 3.Alexander S., Lemmens V.E., Houterman S., Nollen L., Roumen R., Slooter G.D. Gallbladder cancer, a vanishing disease? Cancer Causes Control. 2012;23:1705–1709. doi: 10.1007/s10552-012-0049-0. [DOI] [PubMed] [Google Scholar]
- 4.Rai R., Tewari M., Kumar M., Singh T.B., Shukla H.S. Expression profile of cholecystokinin type-a receptor in gallbladder cancer and gallstone disease. Hepatobiliary Pancreat. Dis. Int. 2011;10:408–414. doi: 10.1016/S1499-3872(11)60069-6. [DOI] [PubMed] [Google Scholar]
- 5.Kanthan R., Senger J.L., Ahmed S., Kanthan S.C. Gallbladder cancer in the 21st century. J. Oncol. 2015;2015:967472. doi: 10.1155/2015/967472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hundal R., Shaffer E.A. Gallbladder cancer: Epidemiology and outcome. Clin. Epidemiol. 2014;6:99–109. doi: 10.2147/CLEP.S37357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rai R., Tewari M., Kumar M., Singh A.K., Shukla H.S. P53: Its alteration and gallbladder cancer. Eur. J. Cancer Prev. 2011;20:77–85. doi: 10.1097/CEJ.0b013e328341e371. [DOI] [PubMed] [Google Scholar]
- 8.Rai R., Sharma K.L., Misra S., Kumar A., Mittal B. CYP17 polymorphism (rs743572) is associated with increased risk of gallbladder cancer in tobacco users. Tumour Biol. 2014;35:6531–6537. doi: 10.1007/s13277-014-1876-2. [DOI] [PubMed] [Google Scholar]
- 9.Rai R., Sharma K.L., Sharma S., Misra S., Kumar A., Mittal B. Death receptor (DR4) haplotypes are associated with increased susceptibility of gallbladder carcinoma in North Indian population. PLoS ONE. 2014;9:e90264. doi: 10.1371/journal.pone.0090264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rai R., Sharma K.L., Misra S., Kumar A., Mittal B. Association of adrenergic receptor gene polymorphisms in gallbladder cancer susceptibility in a north Indian population. J. Cancer Res. Clin. Oncol. 2014;140:725–735. doi: 10.1007/s00432-014-1621-7. [DOI] [PubMed] [Google Scholar]
- 11.Rai R., Sharma K.L., Misra S., Kumar A., Mittal B. Psca gene variants (rs2294008 and rs2978974) confer increased susceptibility of gallbladder carcinoma in females. Gene. 2013;530:172–177. doi: 10.1016/j.gene.2013.08.058. [DOI] [PubMed] [Google Scholar]
- 12.Rai R., Sharma K.L., Tiwari S., Misra S., Kumar A., Mittal B. Dcc (deleted in colorectal carcinoma) gene variants confer increased susceptibility to gallbladder cancer (ref. No.: Gene-d-12-01446) Gene. 2013;518:303–309. doi: 10.1016/j.gene.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 13.Umar M., Upadhyay R., Mittal B. PLCE1 rs2274223 A>G polymorphism and cancer risk: A meta-analysis. Tumour Biol. 2013;34:3537–3544. doi: 10.1007/s13277-013-0932-7. [DOI] [PubMed] [Google Scholar]
- 14.Sharma K.L., Rai R., Srivastava A., Sharma A., Misra S., Kumar A., Mittal B. A multigenic approach to evaluate genetic variants of PLCE1, LXRs, MMPs, TIMP, and CYP genes in gallbladder cancer predisposition. Tumour Biol. 2014;35:8597–8606. doi: 10.1007/s13277-014-2094-7. [DOI] [PubMed] [Google Scholar]
- 15.Hahn L.W., Ritchie M.D., Moore J.H. Multifactor dimensionality reduction software for detecting gene–gene and gene-environment interactions. Bioinformatics. 2003;19:376–382. doi: 10.1093/bioinformatics/btf869. [DOI] [PubMed] [Google Scholar]
- 16.Ritchie M.D., Hahn L.W., Roodi N., Bailey L.R., Dupont W.D., Parl F.F., Moore J.H. Multifactor-dimensionality reduction reveals high-order interactions among estrogen-metabolism genes in sporadic breast cancer. Am. J. Hum. Genet. 2001;69:138–147. doi: 10.1086/321276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lunetta K.L., Hayward L.B., Segal J., van Eerdewegh P. Screening large-scale association study data: Exploiting interactions using random forests. BMC Genet. 2004;5:32. doi: 10.1186/1471-2156-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piper M.E., Loh W.Y., Smith S.S., Japuntich S.J., Baker T.B. Using decision tree analysis to identify risk factors for relapse to smoking. Subst. Use Misuse. 2011;46:492–510. doi: 10.3109/10826081003682222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Srivastava A., Sharma K.L., Srivastava N., Misra S., Mittal B. Significant role of estrogen and progesterone receptor sequence variants in gallbladder cancer predisposition: A multi-analytical strategy. PLoS ONE. 2012;7:e40162. doi: 10.1371/journal.pone.0040162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrer-Lorente R., Cabot C., Fernandez-Lopez J.A., Alemany M. Combined effects of oleoyl-estrone and a β3-adrenergic agonist (cl316,243) on lipid stores of diet-induced overweight male wistar rats. Life Sci. 2005;77:2051–2058. doi: 10.1016/j.lfs.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Anthony A., Schepelmann S., Guillaume J.L., Strosberg A.D., Dhillon A.P., Pounder R.E., Wakefield A.J. Localization of the β(β)3-adrenoceptor in the human gastrointestinal tract: An immunohistochemical study. Aliment. Pharmacol. Ther. 1998;12:519–525. doi: 10.1046/j.1365-2036.1998.00345.x. [DOI] [PubMed] [Google Scholar]
- 22.Krief S., Lonnqvist F., Raimbault S., Baude B., van Spronsen A., Arner P., Strosberg A.D., Ricquier D., Emorine L.J. Tissue distribution of β3-adrenergic receptor mRNA in man. J. Clin. Investig. 1993;91:344–349. doi: 10.1172/JCI116191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ochoa M.C., Marti A., Azcona C., Chueca M., Oyarzabal M., Pelach R., Patino A., Moreno-Aliaga M.J., Martinez-Gonzalez M.A., Martinez J.A., et al. Gene–gene interaction between PPARγ2 and ADRβ3 increases obesity risk in children and adolescents. Int. J. Obes. 2004;28:S37–S41. doi: 10.1038/sj.ijo.0802803. [DOI] [PubMed] [Google Scholar]
- 24.Tan W., Gao M., Liu N., Zhang G., Xu T., Cui W. Body mass index and risk of gallbladder cancer: Systematic review and meta-analysis of observational studies. Nutrients. 2015;7:8321–8334. doi: 10.3390/nu7105387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takezaki T., Hamajima N., Matsuo K., Tanaka R., Hirai T., Kato T., Ohashi K., Tajima K. Association of polymorphisms in the β-2 and β-3 adrenoceptor genes with risk of colorectal cancer in Japanese. Int. J. Clin. Oncol. 2001;6:117–122. doi: 10.1007/PL00012092. [DOI] [PubMed] [Google Scholar]
- 26.Klass D.M., Lauer N., Hay B., Kratzer W., Fuchs M., Group E.S. Arg64 variant of the β3-adrenergic receptor is associated with gallstone formation. Am. J. Gastroenterol. 2007;102:2482–2487. doi: 10.1111/j.1572-0241.2007.01430.x. [DOI] [PubMed] [Google Scholar]
- 27.Srivastava A., Mishra A., Singh R., Rai R., Srivastava N., Mittal B. Multi-analytic approach elucidates significant role of hormonal and hepatocanalicular transporter genetic variants in gallstone disease in north indian population. PLoS ONE. 2013;8:e59173. doi: 10.1371/journal.pone.0059173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuruma S., Egawa N., Kurata M., Honda G., Kamisawa T., Ueda J., Ishii H., Ueno M., Nakao H., Mori M., et al. Case-control study of diabetes-related genetic variants and pancreatic cancer risk in Japan. World J. Gastroenterol. 2014;20:17456–17462. doi: 10.3748/wjg.v20.i46.17456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fearon E.R., Cho K.R., Nigro J.M., Kern S.E., Simons J.W., Ruppert J.M., Hamilton S.R., Preisinger A.C., Thomas G., Kinzler K.W., et al. Identification of a chromosome 18q gene that is altered in colorectal cancers. Science. 1990;247:49–56. doi: 10.1126/science.2294591. [DOI] [PubMed] [Google Scholar]
- 30.Forcet C., Ye X., Granger L., Corset V., Shin H., Bredesen D.E., Mehlen P. The dependence receptor DCC (deleted in colorectal cancer) defines an alternative mechanism for caspase activation. Proc. Natl. Acad. Sci. USA. 2001;98:3416–3421. doi: 10.1073/pnas.051378298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arakawa H. Netrin-1 and its receptors in tumorigenesis. Nat. Rev. Cancer. 2004;4:978–987. doi: 10.1038/nrc1504. [DOI] [PubMed] [Google Scholar]
- 32.Inokuchi K., Yamaguchi H., Hanawa H., Tanosaki S., Nakamura K., Tarusawa M., Miyake K., Shimada T., Dan K. Loss of DCC gene expression is of prognostic importance in acute myelogenous leukemia. Clin. Cancer Res. 2002;8:1882–1888. [PubMed] [Google Scholar]
- 33.Sun X.F., Rutten S., Zhang H., Nordenskjold B. Expression of the deleted in colorectal cancer gene is related to prognosis in DNA diploid and low proliferative colorectal adenocarcinoma. J. Clin. Oncol. 1999;17:1745–1750. doi: 10.1200/JCO.1999.17.6.1745. [DOI] [PubMed] [Google Scholar]
- 34.Yoshida Y., Itoh F., Endo T., Hinoda Y., Imai K. Decreased dcc mRNA expression in human gastric cancers is clinicopathologically significant. Int. J. Cancer. 1998;79:634–639. doi: 10.1002/(SICI)1097-0215(19981218)79:6<634::AID-IJC14>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 35.Bamias A.T., Bai M.C., Agnantis N.J., Michael M.C., Alamanos Y.P., Stefanaki S.V., Razi E.D., Skarlos D.V., Kappas A.M., Pavlidis N.A. Prognostic significance of the deleted in colorectal cancer gene protein expression in high-risk resected gastric carcinoma. Cancer Investig. 2003;21:333–340. doi: 10.1081/CNV-120018219. [DOI] [PubMed] [Google Scholar]
- 36.Toma M., Stavarachi M., Cimponeriu D., Apostol P., Cojocaru M., Belusicaa L., Panduru N., Radu I., Gavrila L. P53 and DCC polymorphisms and the risk for colorectal cancer in Romanian patients—A preliminary study. J. Analele Univ. Oradea Fasc. Biol. 2009;16:162–165. [Google Scholar]
- 37.Djansugurova L., Zhunussova G., Khussainova E., Iksan O., Afonin G., Kaidarova D., Parker M.I. Association of DCC, MLH1, GSTT1, GSTM1, and TP53 gene polymorphisms with colorectal cancer in Kazakhstan. Tumour Biol. 2015;36:279–289. doi: 10.1007/s13277-014-2641-2. [DOI] [PubMed] [Google Scholar]
- 38.Starinsky S., Figer A., Ben-Asher E., Geva R., Flex D., Fidder H.H., Zidan J., Lancet D., Friedman E. Genotype phenotype correlations in israeli colorectal cancer patients. Int. J. Cancer. 2005;114:58–73. doi: 10.1002/ijc.20645. [DOI] [PubMed] [Google Scholar]
- 39.Malik M.A., Gupta A., Zargar S.A., Mittal B. Role of genetic variants of deleted in colorectal carcinoma (DCC) polymorphisms and esophageal and gastric cancers risk in kashmir valley and meta-analysis. Tumour Biol. 2013;34:3049–3057. doi: 10.1007/s13277-013-0870-4. [DOI] [PubMed] [Google Scholar]
- 40.Wistuba II, Albores-Saavedra J. Genetic abnormalities involved in the pathogenesis of gallbladder carcinoma. J. Hepatobiliary Pancreat. Surg. 1999;6:237–244. doi: 10.1007/s005340050113. [DOI] [PubMed] [Google Scholar]
- 41.Cha P.C., Zembutsu H., Takahashi A., Kubo M., Kamatani N., Nakamura Y. A genome-wide association study identifies SNP in DCC is associated with gallbladder cancer in the Japanese population. J. Hum. Genet. 2012;57:235–237. doi: 10.1038/jhg.2012.9. [DOI] [PubMed] [Google Scholar]
- 42.Mattar R., Nonogaki S., Silva C., Alves V., Gama-Rodrigues J.J. P53 and RB tumor suppressor gene alterations in gastric cancer. Rev. Hosp. Clin. 2004;59:172–180. doi: 10.1590/S0041-87812004000400004. [DOI] [PubMed] [Google Scholar]
- 43.Enomoto T., Fujita M., Cheng C., Nakashima R., Ozaki M., Inoue M., Nomura T. Loss of expression and loss of heterozygosity in the DCC gene in neoplasms of the human female reproductive tract. Br. J. Cancer. 1995;71:462–467. doi: 10.1038/bjc.1995.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Minami R., Aoyama N., Honsako Y., Kasuga M., Fujimori T., Maeda S. Codon 201Arg/Gly polymorphism of DCC (deleted in colorectal carcinoma) gene in flat- and polypoid-type colorectal tumors. Dig. Dis. Sci. 1997;42:2446–2452. doi: 10.1023/A:1018839907159. [DOI] [PubMed] [Google Scholar]
- 45.Kong X.T., Choi S.H., Bessho F., Kobayashi M., Hanada R., Yamamoto K., Hayashi Y. Codon 201 (Gly) polymorphic type of the DCC gene is related to disseminated neuroblastoma. Neoplasia. 2001;3:267–272. doi: 10.1038/sj.neo.7900169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang H., Arbman G., Sun X.F. Codon 201 polymorphism of DCC gene is a prognostic factor in patients with colorectal cancer. Cancer Detect. Prev. 2003;27:216–221. doi: 10.1016/S0361-090X(03)00064-3. [DOI] [PubMed] [Google Scholar]
- 47.Kupcinskas J., Gyvyte U., Bruzaite I., Leja M., Kupcinskaite-Noreikiene R., Pauzas H., Tamelis A., Jonaitis L., Skieceviciene J., Kiudelis G. Common genetic variants of PSCA, MUC1 and PLCE1 genes are not associated with colorectal cancer. Asian Pac. J. Cancer Prev. 2015;16:6027–6032. doi: 10.7314/APJCP.2015.16.14.6027. [DOI] [PubMed] [Google Scholar]
- 48.Geng P., Li J., Wang N., Ou J., Xie G., Liu C., Zhao X., Xiang L., Liao Y., Liang H. PSCA rs2294008 polymorphism with increased risk of cancer. PLoS ONE. 2015;10:e0136269. doi: 10.1371/journal.pone.0136269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zou Q., Yang L., Yang Z., Huang J., Fu X. PSCA and OCT-4 expression in the benign and malignant lesions of gallbladder: Implication for carcinogenesis, progression, and prognosis of gallbladder adenocarcinoma. BioMed Res. Int. 2013;2013:648420. doi: 10.1155/2013/648420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saeki N., Ono H., Sakamoto H., Yoshida T. Down-regulation of immune-related genes by PSCA in gallbladder cancer cells implanted into mice. Anticancer Res. 2015;35:2619–2625. [PubMed] [Google Scholar]
- 51.Ono H., Chihara D., Chiwaki F., Yanagihara K., Sasaki H., Sakamoto H., Tanaka H., Yoshida T., Saeki N., Matsuo K. Missense allele of a single nucleotide polymorphism rs2294008 attenuated antitumor effects of prostate stem cell antigen in gallbladder cancer cells. J. Carcinog. 2013;12:4. doi: 10.4103/1477-3163.109030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ono H., Hiraoka N., Lee Y.S., Woo S.M., Lee W.J., Choi I.J., Saito A., Yanagihara K., Kanai Y., Ohnami S., et al. Prostate stem cell antigen, a presumable organ-dependent tumor suppressor gene, is down-regulated in gallbladder carcinogenesis. Genes Chromosomes Cancer. 2012;51:30–41. doi: 10.1002/gcc.20928. [DOI] [PubMed] [Google Scholar]
- 53.Fu Y.P., Kohaar I., Rothman N., Earl J., Figueroa J.D., Ye Y., Malats N., Tang W., Liu L., Garcia-Closas M., et al. Common genetic variants in the PSCA gene influence gene expression and bladder cancer risk. Proc. Natl. Acad. Sci. USA. 2012;109:4974–4979. doi: 10.1073/pnas.1202189109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qiao L., Feng Y. Genetic variations of prostate stem cell antigen (PSCA) contribute to the risk of gastric cancer for eastern Asians: A meta-analysis based on 16792 individuals. Gene. 2012;493:83–91. doi: 10.1016/j.gene.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 55.Wu X., Ye Y., Kiemeney L.A., Sulem P., Rafnar T., Matullo G., Seminara D., Yoshida T., Saeki N., Andrew A.S., et al. Genetic variation in the prostate stem cell antigen gene PSCA confers susceptibility to urinary bladder cancer. Nat. Genet. 2009;41:991–995. doi: 10.1038/ng.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matsuo K., Tajima K., Suzuki T., Kawase T., Watanabe M., Shitara K., Misawa K., Ito S., Sawaki A., Muro K., et al. Association of prostate stem cell antigen gene polymorphisms with the risk of stomach cancer in Japanese. Int. J. Cancer. 2009;125:1961–1964. doi: 10.1002/ijc.24519. [DOI] [PubMed] [Google Scholar]
- 57.Kohaar I., Porter-Gill P., Lenz P., Fu Y.P., Mumy A., Tang W., Apolo A.B., Rothman N., Baris D., Schned A.R., et al. Genetic variant as a selection marker for anti-prostate stem cell antigen immunotherapy of bladder cancer. J. Natl. Cancer Inst. 2013;105:69–73. doi: 10.1093/jnci/djs458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saeki N., Ono H., Yanagihara K., Aoyagi K., Sasaki H., Sakamoto H., Yoshida T. Rs2294008t, a risk allele for gastric and gallbladder cancers, suppresses the PSCA promoter by recruiting the transcription factor YY1. Genes Cells Devoted Mol. Cell. Mech. 2015;20:382–391. doi: 10.1111/gtc.12228. [DOI] [PubMed] [Google Scholar]
- 59.Zhang T., Chen Y.N., Wang Z., Chen J.Q., Huang S. Effect of PSCA gene polymorphisms on gastric cancer risk and survival prediction: A meta-analysis. Exp. Ther. Med. 2012;4:158–164. doi: 10.3892/etm.2012.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miller S.A., Dykes D.D., Polesky H.F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.MDR software. [(accessed on16 October 2015)]. Available online: www.multifactordimensionalityreduction.org.
- 62.Yang C.H., Lin Y.D., Yang C.S., Chuang L.Y. An efficiency analysis of high-order combinations of gene-gene interactions using multifactor-dimensionality reduction. BMC Genom. 2015;16:489. doi: 10.1186/s12864-015-1717-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee P.H., Shatkay H. F-SNP: Computationally predicted functional SNPs for disease association studies. Nucleic Acids Res. 2008;36:820–824. doi: 10.1093/nar/gkm904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yuan H.Y., Chiou J.J., Tseng W.H., Liu C.H., Liu C.K., Lin Y.J., Wang H.H., Yao A., Chen Y.T., Hsu C.N. FASTSNP: An always up-to-date and extendable service for SNP function analysis and prioritization. Nucleic Acids Res. 2006;34:635–641. doi: 10.1093/nar/gkl236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.FASTSNP. [(accessed on16 October 2015)]. Available online: http://fastsnp.ibms.sinica.edu.tw.
- 66.F-SNP. [(accessed on 16 October 2015)]. Available online: http://compbio.cs.queensu.ca/F-SNP/