Abstract
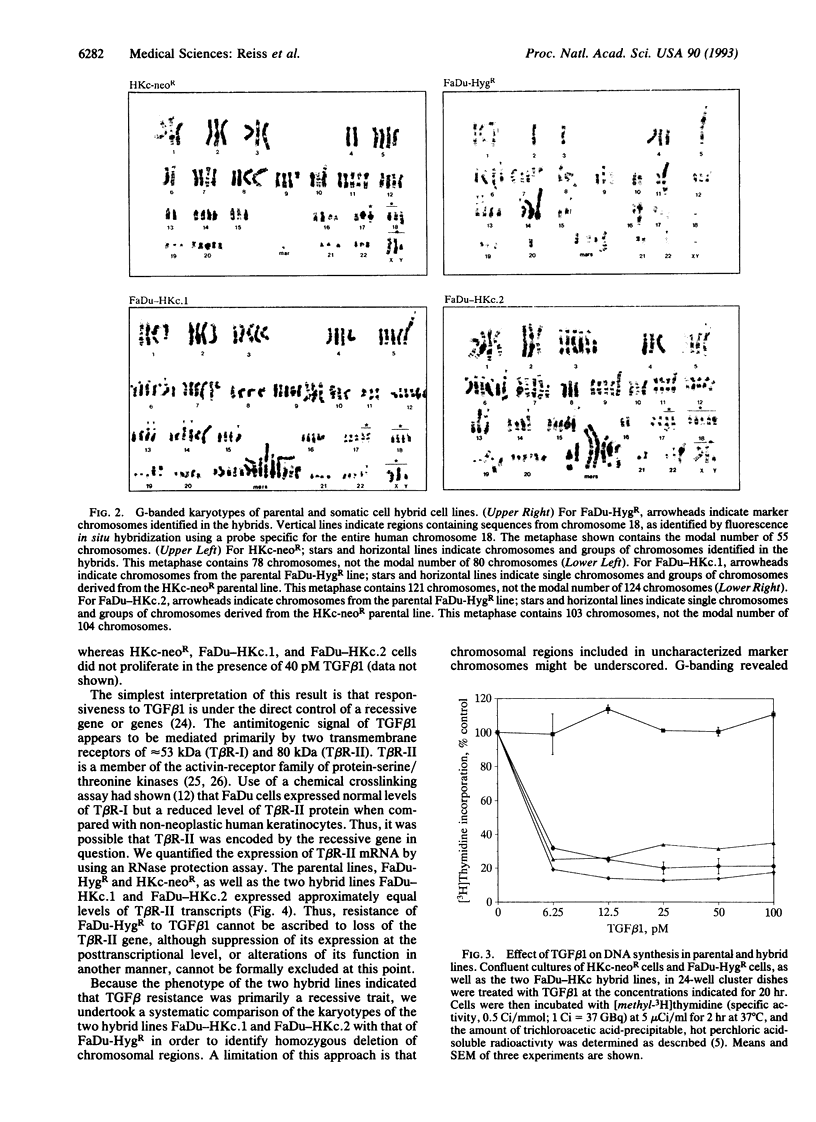
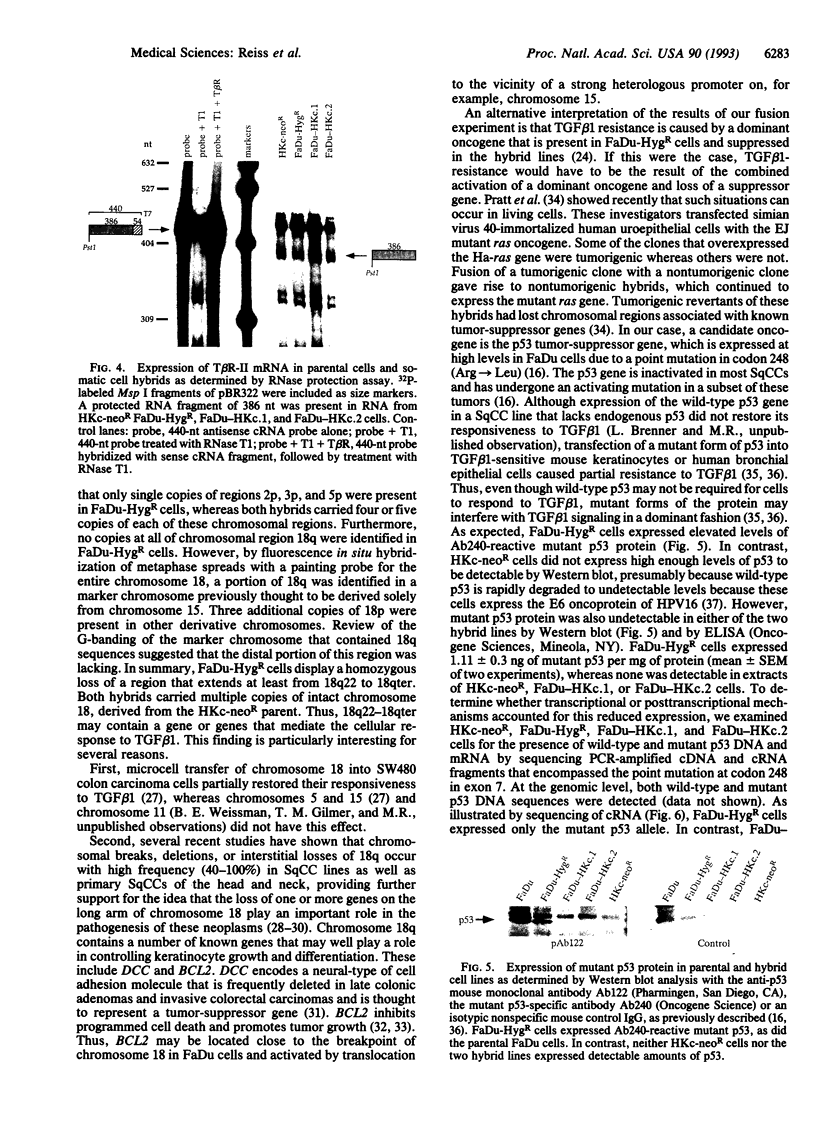
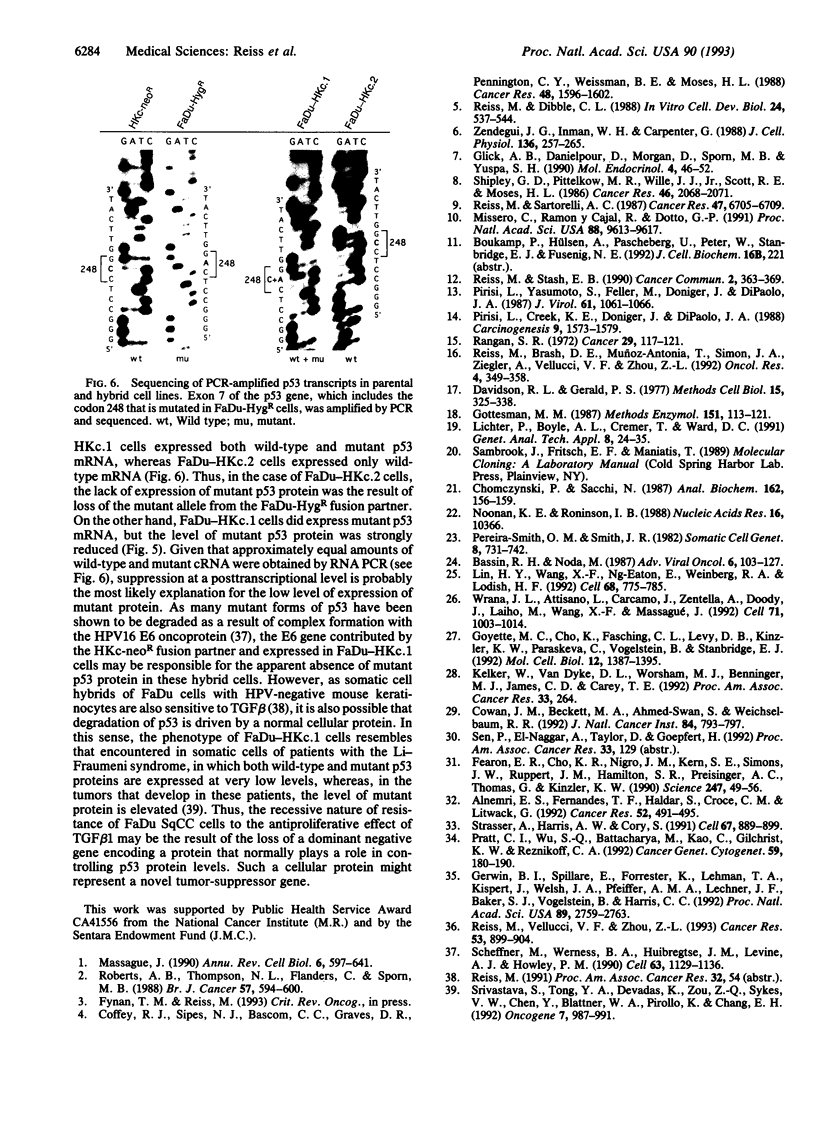
Because most human squamous carcinoma cell lines of the aerodigestive and genital tracts are refractory to the antiproliferative action of transforming growth factor beta 1 (TGF beta 1) in vitro, we have begun to identify the causes for resistance of squamous carcinoma cell lines to TGF beta 1 by using somatic cell genetics. Two stable hybrid cell lines (FaDu-HKc.1 and FaDu-HKc.2) were obtained by fusing a TGF beta 1-resistant human squamous carcinoma cell line, FaDu-HygR, with a human papilloma virus 16-immortalized, TGF beta 1-sensitive, human foreskin keratinocyte cell line, HKc-neoR. Whereas TGF beta 1 did not inhibit DNA synthesis in parental FaDu-HygR cells, it reduced DNA synthetic activity of HKc-neoR, FaDu-HKc.1, and FaDu-HKc.2 cells by 75-85% (IC50, 2-5 pM). Although squamous carcinoma cells express lower than normal levels of TGF beta 1 type II receptors on their cell surface, TGF beta 1 type II receptor mRNA was detected in all four cell lines. Recessive genes involved in TGF beta 1 signaling may be localized to the distal portion of chromosome 18q, as this was the sole chromosomal region of homozygous deletion in parental FaDu-HygR cells. Furthermore, our previous observation that mutant p53 decreases sensitivity of keratinocytes to TGF beta 1 was supported by the finding that the level of the mutant p53 protein expressed by the hybrid cell lines was greatly reduced. In summary, TGF beta 1 resistance of FaDu cells appears to be recessive and is presumably due to the loss of one or more post-receptor elements of the signaling pathway.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alnemri E. S., Fernandes T. F., Haldar S., Croce C. M., Litwack G. Involvement of BCL-2 in glucocorticoid-induced apoptosis of human pre-B-leukemias. Cancer Res. 1992 Jan 15;52(2):491–495. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Coffey R. J., Jr, Sipes N. J., Bascom C. C., Graves-Deal R., Pennington C. Y., Weissman B. E., Moses H. L. Growth modulation of mouse keratinocytes by transforming growth factors. Cancer Res. 1988 Mar 15;48(6):1596–1602. [PubMed] [Google Scholar]
- Cowan J. M., Beckett M. A., Ahmed-Swan S., Weichselbaum R. R. Cytogenetic evidence of the multistep origin of head and neck squamous cell carcinomas. J Natl Cancer Inst. 1992 May 20;84(10):793–797. doi: 10.1093/jnci/84.10.793. [DOI] [PubMed] [Google Scholar]
- Davidson R. L., Gerald P. S. Induction of mammalian somatic cell hybridization by polyethylene glycol. Methods Cell Biol. 1977;15:325–338. doi: 10.1016/s0091-679x(08)60223-x. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Cho K. R., Nigro J. M., Kern S. E., Simons J. W., Ruppert J. M., Hamilton S. R., Preisinger A. C., Thomas G., Kinzler K. W. Identification of a chromosome 18q gene that is altered in colorectal cancers. Science. 1990 Jan 5;247(4938):49–56. doi: 10.1126/science.2294591. [DOI] [PubMed] [Google Scholar]
- Gerwin B. I., Spillare E., Forrester K., Lehman T. A., Kispert J., Welsh J. A., Pfeifer A. M., Lechner J. F., Baker S. J., Vogelstein B. Mutant p53 can induce tumorigenic conversion of human bronchial epithelial cells and reduce their responsiveness to a negative growth factor, transforming growth factor beta 1. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2759–2763. doi: 10.1073/pnas.89.7.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick A. B., Danielpour D., Morgan D., Sporn M. B., Yuspa S. H. Induction and autocrine receptor binding of transforming growth factor-beta 2 during terminal differentiation of primary mouse keratinocytes. Mol Endocrinol. 1990 Jan;4(1):46–52. doi: 10.1210/mend-4-1-46. [DOI] [PubMed] [Google Scholar]
- Gottesman M. M. Drug-resistant mutants: selection and dominance analysis. Methods Enzymol. 1987;151:113–121. doi: 10.1016/s0076-6879(87)51012-6. [DOI] [PubMed] [Google Scholar]
- Goyette M. C., Cho K., Fasching C. L., Levy D. B., Kinzler K. W., Paraskeva C., Vogelstein B., Stanbridge E. J. Progression of colorectal cancer is associated with multiple tumor suppressor gene defects but inhibition of tumorigenicity is accomplished by correction of any single defect via chromosome transfer. Mol Cell Biol. 1992 Mar;12(3):1387–1395. doi: 10.1128/mcb.12.3.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichter P., Boyle A. L., Cremer T., Ward D. C. Analysis of genes and chromosomes by nonisotopic in situ hybridization. Genet Anal Tech Appl. 1991 Feb;8(1):24–35. doi: 10.1016/1050-3862(91)90005-c. [DOI] [PubMed] [Google Scholar]
- Lin H. Y., Wang X. F., Ng-Eaton E., Weinberg R. A., Lodish H. F. Expression cloning of the TGF-beta type II receptor, a functional transmembrane serine/threonine kinase. Cell. 1992 Feb 21;68(4):775–785. doi: 10.1016/0092-8674(92)90152-3. [DOI] [PubMed] [Google Scholar]
- Massagué J. The transforming growth factor-beta family. Annu Rev Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- Missero C., Ramon y Cajal S., Dotto G. P. Escape from transforming growth factor beta control and oncogene cooperation in skin tumor development. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9613–9617. doi: 10.1073/pnas.88.21.9613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan K. E., Roninson I. B. mRNA phenotyping by enzymatic amplification of randomly primed cDNA. Nucleic Acids Res. 1988 Nov 11;16(21):10366–10366. doi: 10.1093/nar/16.21.10366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira-Smith O. M., Smith J. R. Phenotype of low proliferative potential is dominant in hybrids of normal human fibroblasts. Somatic Cell Genet. 1982 Nov;8(6):731–742. doi: 10.1007/BF01543015. [DOI] [PubMed] [Google Scholar]
- Pirisi L., Creek K. E., Doniger J., DiPaolo J. A. Continuous cell lines with altered growth and differentiation properties originate after transfection of human keratinocytes with human papillomavirus type 16 DNA. Carcinogenesis. 1988 Sep;9(9):1573–1579. doi: 10.1093/carcin/9.9.1573. [DOI] [PubMed] [Google Scholar]
- Pirisi L., Yasumoto S., Feller M., Doniger J., DiPaolo J. A. Transformation of human fibroblasts and keratinocytes with human papillomavirus type 16 DNA. J Virol. 1987 Apr;61(4):1061–1066. doi: 10.1128/jvi.61.4.1061-1066.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt C. I., Wu S. Q., Bhattacharya M., Kao C., Gilchrist K. W., Reznikoff C. A. Chromosome losses in tumorigenic revertants of EJ/ras-expressing somatic cell hybrids. Cancer Genet Cytogenet. 1992 Apr;59(2):180–190. doi: 10.1016/0165-4608(92)90213-r. [DOI] [PubMed] [Google Scholar]
- Rangan S. R. A new human cell line (FaDu) from a hypopharyngeal carcinoma. Cancer. 1972 Jan;29(1):117–121. doi: 10.1002/1097-0142(197201)29:1<117::aid-cncr2820290119>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Reiss M., Brash D. E., Muñoz-Antonia T., Simon J. A., Ziegler A., Vellucci V. F., Zhou Z. L. Status of the p53 tumor suppressor gene in human squamous carcinoma cell lines. Oncol Res. 1992;4(8-9):349–357. [PubMed] [Google Scholar]
- Reiss M., Dibble C. L. Reinitiation of DNA synthesis in quiescent mouse keratinocytes; regulation by polypeptide hormones, cholera toxin, dexamethasone, and retinoic acid. In Vitro Cell Dev Biol. 1988 Jun;24(6):537–544. doi: 10.1007/BF02629088. [DOI] [PubMed] [Google Scholar]
- Reiss M., Sartorelli A. C. Regulation of growth and differentiation of human keratinocytes by type beta transforming growth factor and epidermal growth factor. Cancer Res. 1987 Dec 15;47(24 Pt 1):6705–6709. [PubMed] [Google Scholar]
- Reiss M., Stash E. B. High frequency of resistance of human squamous carcinoma cells to the anti-proliferative action of transforming growth factor beta. Cancer Commun. 1990;2(11):363–369. doi: 10.3727/095535490820874029. [DOI] [PubMed] [Google Scholar]
- Reiss M., Vellucci V. F., Zhou Z. L. Mutant p53 tumor suppressor gene causes resistance to transforming growth factor beta 1 in murine keratinocytes. Cancer Res. 1993 Feb 15;53(4):899–904. [PubMed] [Google Scholar]
- Roberts A. B., Thompson N. L., Heine U., Flanders C., Sporn M. B. Transforming growth factor-beta: possible roles in carcinogenesis. Br J Cancer. 1988 Jun;57(6):594–600. doi: 10.1038/bjc.1988.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffner M., Werness B. A., Huibregtse J. M., Levine A. J., Howley P. M. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990 Dec 21;63(6):1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- Shipley G. D., Pittelkow M. R., Wille J. J., Jr, Scott R. E., Moses H. L. Reversible inhibition of normal human prokeratinocyte proliferation by type beta transforming growth factor-growth inhibitor in serum-free medium. Cancer Res. 1986 Apr;46(4 Pt 2):2068–2071. [PubMed] [Google Scholar]
- Srivastava S., Tong Y. A., Devadas K., Zou Z. Q., Sykes V. W., Chen Y., Blattner W. A., Pirollo K., Chang E. H. Detection of both mutant and wild-type p53 protein in normal skin fibroblasts and demonstration of a shared 'second hit' on p53 in diverse tumors from a cancer-prone family with Li-Fraumeni syndrome. Oncogene. 1992 May;7(5):987–991. [PubMed] [Google Scholar]
- Strasser A., Harris A. W., Cory S. bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell. 1991 Nov 29;67(5):889–899. doi: 10.1016/0092-8674(91)90362-3. [DOI] [PubMed] [Google Scholar]
- Wrana J. L., Attisano L., Cárcamo J., Zentella A., Doody J., Laiho M., Wang X. F., Massagué J. TGF beta signals through a heteromeric protein kinase receptor complex. Cell. 1992 Dec 11;71(6):1003–1014. doi: 10.1016/0092-8674(92)90395-s. [DOI] [PubMed] [Google Scholar]
- Zendegui J. G., Inman W. H., Carpenter G. Modulation of the mitogenic response of an epidermal growth factor-dependent keratinocyte cell line by dexamethasone, insulin, and transforming growth factor-beta. J Cell Physiol. 1988 Aug;136(2):257–265. doi: 10.1002/jcp.1041360207. [DOI] [PubMed] [Google Scholar]