Abstract
Sepsis is considered today a major public health problem. Despite that mortality has been consistently associated with organ compromise, the mechanisms by which sepsis causes multiple organ dysfunction are not well understood, and hence therapy remains reactive and non-specific. Recent studies have challenged previous paradigms by demonstrating that acute kidney injury (AKI) can occur in the setting of normal or even increased renal blood flow, and that it is characterized by tubular injury and not by necrosis or apoptosis. This data suggests that mechanisms other than hypoperfusion may be at play, and that adaptive responses of the tubular epithelial cell (TEC) may be key to understand the origin of organ dysfunction in the setting of sepsis. In this review, we discuss evidence suggesting that the activation of energy regulatory processes and mitochondrial quality control processes may not only be drivers of this response, but also, may alter the course of organ dysfunction during sepsis in clinically relevant ways.
Keywords: Tubular epithelial cell, sepsis, inflammation, AMPK, mitophagy
Introduction
Sepsis is common, frequently fatal, and is considered today a major public health problem.[1] Importantly, mortality and most of the intermediate to long term consequences of sepsis have consistently been associated to organ compromise.[2] As an example, acute kidney injury occurs in as much as 40–50% of septic patients, which increases the risk of death 6–8 fold[3], and also the risk of progression to chronic kidney disease in survivors.[4] However, anticipation, prevention and treatment of organ injury is challenging because the mechanisms by which sepsis causes organ dysfunction are not well understood. This is a major knowledge gap in the field that if resolved, may result in more efficient preventive and therapeutic strategies.
Sepsis-induced AKI is not equivalent to Acute Tubular Necrosis (ATN)
Recent animal and post-mortem human studies of resuscitated sepsis have shown that histologically, sepsis-induced AKI is not characterized by acute tubular necrosis (less than 5% of tubular cells[5]) as previously thought, but rather by a bland, heterogeneous pattern of tubular injury typified by apical tubular cell vacuolization, and loss of brush border.[5] Takasu et al. have reported that although focal coagulative necrosis occurs in up to 44% of patients, this is only seen in less than 5% of TEC, and that apoptosis is only seen in less than 0.3% of tubules studied.[5] Importantly, these changes frequently occur in the setting of normal or even hyperdynamic renal blood flow[6], suggesting that at least in some cases of resuscitated sepsis, macro-hemodynamic hypoperfusion cannot explain the events leading to this histologic or clinical presentation. Taken together these data support the notion that mechanisms other than changes in global or regional perfusion, such as the response of the TEC to inflammation (i.e. Damage and Pathogen Associated Molecular Patterns, or DAMPs, and PAMPs), may play a key role in the development of the clinical phenotype.[7
The tubular epithelial cell response to sepsis
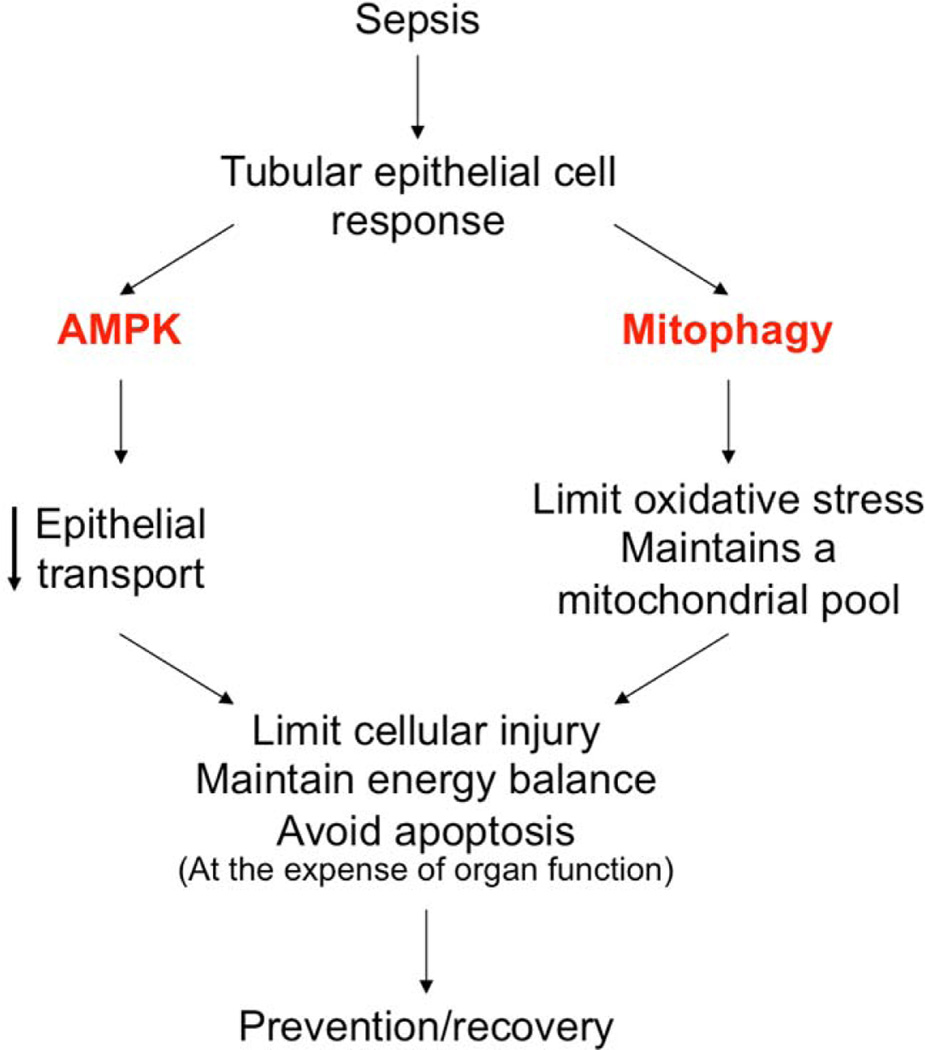
Energy balance dysregulation and mitochondrial injury are two major triggers of apoptosis that occur during sepsis. Yet, with the exception of T lymphocytes and intestinal epithelia, significant necrosis or apoptosis does not occur [5]. This suggests that epithelial cells may respond to early inflammatory injury by triggering energy regulatory pathways that preserve energy balance, and limit oxidative damage from dysfunctional mitochondria. In support of this, TEC have been shown to decrease the expression of ion transporters in response to sterile inflammation[8], which may limit energy expenditure given that ionic transport represents more than 70% of ATP consumption in the TEC[9]. Adenosine monophosphate activated protein kinase (AMPK) is a master sensor of energy status which is activated in response to energy depletion (i.e. increments in AMP:ATP ratio), and limits energy expenditure by decreasing anabolic reactions. Our preliminary data suggests that AMPK is activated within 24 hours of sepsis[10], suggesting it may participate in this early response. Furthermore, Hsiao et al. have shown that mitophagy, a process by which dysfunctional mitochondria are targeted, digested and removed from the cytosol, is activated within hours after experimental sepsis[11]. Based on these observations, it is reasonable to propose, as many have[5], that the acute cellular response to sepsis is adaptive. We further hypothesize that this response may be driven by AMPK and mitophagy activation, which in turn down-regulate metabolism, re-prioritize energy expenditure to functions necessary for survival, limit oxidative damage from dysfunctional mitochondria, and eventually set the stage for re-population of a functional mitochondrial pool by the process of biogenesis, to stabilize energy balance (Figure 1).
Fig. 1.
The role of AMPK and metabolic down-regulation as a survival strategy
In the setting of experimental sepsis, AMPK is activated within 24 hours after cecal ligation and puncture (CLP)[10]. We have also demonstrated that over-activation of AMPK using the AMP analogue, AICAR (5-Aminoimidazole-4-carboxamide ribonucleotide) before CLP is associated with significant protection from AKI, and with decreased circulating inflammatory mediators like IL-6, IL-10 and TNF-a [12], suggesting AMPK may play a protective role in the early response to experimental sepsis. Furthermore, we have shown a temporal association between AMPK activation and a decrease in the epithelial sodium channel (ENaC) expression by immunoblot.[10] These data are reminiscent of the findings by Hsiao et al. who showed a decrease in tubular sodium transport 9–18 hours after CLP.[11] Downregulation of ionic transport in the TEC may be a strategy to avoid energy depletion during sepsis, given the major energy sink ion channel activity represents. In this context the above data suggest that AMPK activation may drive this adaptive response, protecting the TEC by conserving energy homeostasis at the expense of cell function. Several questions still remain and further work is needed to assess whether the association between AMPK and ENaC (or other ion transporters) is indeed causal, if it is actually associated with an energetic benefit, and if it will result in a functional and survival advantage in more chronic models.
The role of Mitophagy as an adaptive mechanism: a halt before death
Dysfunctional mitochondria during sepsis are deleterious for the cell and potential triggers of apoptosis.[13] However, several of this alterations including loss of mitochondrial membrane potential are also triggers of key quality control processes like mitophagy that limit cell injury. In a rodent CLP model, we have found that mitophagy is activated early in the course of sepsis, and that such activation slowly declines within the first 24 hours. These findings are in agreement with previously reported data by Hsiao et al. who showed that mitophagy is activated as early as 3 hours after CLP.[11] This seems to be important because inhibition is associated with worse outcome[11], increased cellular damage and apoptosis in the liver[14], and exogenous activation, improves renal recovery in a rodent model of sterile inflammation with LPS.[15] These data suggest that mitophagy is present early in the course of sepsis, and that modifications in its activation (i.e. activation or inhibition) are associated with cell injury, organ function and outcome. Work is still needed to answer important questions like whether or not these associations are causal in nature, what is the impact of timing of activation on cell function, protection and recovery, and whether early exogenous activation can provide effective protection and functional recovery at later stages of sepsis.
Conclusion
Close examination of the histology of various organs of patients dying from sepsis has dramatically changed the way we think of sepsis-induced organ dysfunction. The recognition that in the case of the kidney, sepsis-induced AKI cannot be entirely explained by the traditional concept of acute tubular necrosis, and that sepsis does not cause overt apoptosis and necrosis in failing organs, has challenged the notion that ischemia is the only mechanism explaining organ dysfunction. Importantly, it has also prompted many to suggest that the acute response to the septic environment may be adaptive in nature. In this review, we have now put forth a conceptual model that cellular energy regulation is fundamental to the adaptive response, and that such regulation is driven at least in part by activation of AMPK and mitophagy. Further work is warranted to understand the role of these energy regulatory pathways in the response of the TEC to sepsis, to determine to what extent this response provides cellular and organ protection, to evaluate if these pathways can yield prognostic or diagnostic biomarkers, and finally, if pharmacologic manipulation can result in organ functional recovery and improved outcome.
Acknowledgements
This work was supported, in part, by National Institutes of Health grants 1K12HL109068-02 (HG).
Footnotes
Contribution from the AKI&CRRT 2015 Symposium at the 20th International Conference on Advances in Critical Care Nephrology, Manchester Grand Hyatt, San Diego, Calif., USA, February 17–20, 2015.
Disclosure Statement
The authors have no conflicts of interest to declare.
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Critical Care Medicine. 2001 Jul;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Yende S, Iwashyna TJ, Angus DC. Interplay between sepsis and chronic health. Trends Mol Med. 2014 Apr;20:234–238. doi: 10.1016/j.molmed.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005 Aug 17;294:813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 4.Murugan R, Kellum JA. Acute kidney injury: what's the prognosis? Nature Publishing Group. 2011 Apr;7:209–217. doi: 10.1038/nrneph.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takasu O, Gaut JP, Watanabe E, To K, Fagley RE, Sato B, et al. Mechanisms of cardiac and renal dysfunction in patients dying of sepsis. Am J Respir Crit Care Med. 2013 Mar 1;187:509–517. doi: 10.1164/rccm.201211-1983OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prowle JR, Ishikawa K, May CN, Bellomo R. Renal blood flow during acute renal failure in man. Blood Purif. 2009;28:216–225. doi: 10.1159/000230813. [DOI] [PubMed] [Google Scholar]
- 7.Gomez H, Ince C, De Backer D, Pickkers P, Payen D, Hotchkiss J, et al. A unified theory of sepsis-induced acute kidney injury: inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock. 2014 Jan;41:3–11. doi: 10.1097/SHK.0000000000000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt C, Höcherl K, Schweda F, Bucher M. Proinflammatory cytokines cause down-regulation of renal chloride entry pathways during sepsis. Critical Care Medicine. 2007 Sep;35:2110–2119. doi: 10.1097/01.ccm.0000281447.22966.8b. [DOI] [PubMed] [Google Scholar]
- 9.Mandel LJ, Balaban RS. Stoichiometry and coupling of active transport to oxidative metabolism in epithelial tissues. Am J Physiol. 1981 May;240:F357–F371. doi: 10.1152/ajprenal.1981.240.5.F357. [DOI] [PubMed] [Google Scholar]
- 10.Jin K, Li H, Volpe J, Emlet D, Pastor-Soler NM, Pinsky MR, et al. Is acute kidney injury in the early phase of sepsis a sign of metabolic downregulation in tubular epithelial cells? Crit Care. 2015 Mar 16;19:286. [Google Scholar]
- 11.Hsiao H-W, Tsai K-L, Wang L-F, Chen Y-H, Chiang P-C, Chuang S-M, et al. The Decline of Autophagy Contributes to Proximal Tubular Dysfunction During Sepsis. Shock. 2012 Mar;37:289–296. doi: 10.1097/SHK.0b013e318240b52a. [DOI] [PubMed] [Google Scholar]
- 12.Escobar DA, Botero-Quintero AM, Kautza BC, Luciano J, Loughran P, Darwiche S, et al. Adenosine monophosphate-activated protein kinase activation protects against sepsis-induced organ injury and inflammation. J Surg Res. 2014 Oct 8; doi: 10.1016/j.jss.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hotchkiss RS, Strasser A, McDunn JE, Swanson PE. Cell death. N Engl J Med. 2009 Oct 15;361:1570–1583. doi: 10.1056/NEJMra0901217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carchman EH, Rao J, Loughran PA, Rosengart MR, Zuckerbraun BS. Heme oxygenase-1-mediated autophagy protects against hepatocyte cell death and hepatic injury from infection/sepsis in mice. Hepatology. 2011 Jun;53:2053–2062. doi: 10.1002/hep.24324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howell GM, Gomez H, Collage RD, Loughran P, Zhang X, Escobar DA, et al. Augmenting autophagy to treat acute kidney injury during endotoxemia in mice. PLoS ONE. 2013;8:e69520. doi: 10.1371/journal.pone.0069520. [DOI] [PMC free article] [PubMed] [Google Scholar]