Abstract
The response of non–muscle-invasive bladder cancer (NMIBC) to intravesical immunotherapy with bacillus Calmette-Guérin (BCG) depends on adequate stimulation of an immune response. Although BCG has been used for decades, we lack tools to accurately predict response in individual patients. To address this deficiency, we initiated a clinical trial in patients with intermediate- and high-risk NMIBC. BCG was administered according to the Southwest Oncology Group protocol. Urine samples were collected for cytokine assay at baseline, immediately before and after BCG instillation at 6 wk, and immediately before and after the third BCG instillation of the first maintenance course. Levels of 12 cytokines were measured, and changes from baseline were calculated after treatment. A total of 130 patients were enrolled. Increases in single cytokines correlated with recurrence, but the best predictor of recurrence was changes in a combination of cytokines. A nomogram (CyPRIT) constructed using urinary levels of nine inducible cytokines (IL-2, IL-8, IL-6, IL-1ra, IL-10, IL-12[p70], IL-12[p40], TRAIL, and TNF-α) predicted the likelihood of recurrence with 85.5% accuracy (95% confidence interval 77.9–93.1%). This cytokine panel and nomogram have potential for identifying patients at risk of tumor recurrence during BCG treatment to guide modification of the dose and duration of BCG immunotherapy.
Keywords: Bladder cancer, Bacillus Calmette-Guérin response, Nomogram, Prediction, Urinary cytokines
Intravesical immunotherapy with bacillus Calmette-Guérin (BCG) is the most effective immunologic therapy for non–muscle-invasive bladder cancer (NMIBC) [1]. However, a significant proportion of patients treated with BCG experience recurrence and ultimately require more aggressive treatment. Studies have shown that if intravesical immunotherapy fails and a patient requires radical intervention, survival is better when this intervention is performed within the first 24 mo after diagnosis [2]. Thus, survival rates could be improved by early identification of which patients are at high risk of recurrence and switching those patients promptly to alternative treatment.
Prior attempts to identify predictors of response to BCG focused on tumor markers [3]. However, it is now understood that the most important predictor of response to BCG is a patient's ability to generate an appropriate immune response [4,5]. Thus, we conducted a clinical trial to determine the extent to which the inducible urinary cytokine response to BCG predicts recurrence.
A total of 130 patients with intermediate- and high-risk NMIBC scheduled to undergo intravesical BCG immunotherapy at our center from July 2005 to November 2009 consented to participate in this prospective clinical trial approved by the institutional review board (clinicaltrials.gov identifier NCT01007058) [6]. Pathologic inclusion criteria were similar to the criteria for the European Organization for Research and Treatment of Cancer/International Bladder Cancer Group intermediate- and high-risk categories [7,8]. All patients with high-grade tumors underwent repeat resection 4–6 wk after the initial diagnosis to evaluate occult muscle invasion. Normal imaging results for the upper urinary tract within 6 wk of enrollment were required for eligibility. One intravesical instillation of mitomycin C was administered immediately after surgery when appropriate; Cysview was not used during the study period.
Intravesical BCG (Tice) was administered according to the SWOG protocol [9]; for-cause dose reductions were allowed at the discretion of the treating physician. Patients underwent cystoscopy and cytology at 3-mo intervals for 2 yr and at 3- to 6-mo intervals thereafter. Patient data were analyzed on an intent-to-treat basis. Recurrence was defined as histopathologically confirmed detection of any tumor after initiation of BCG therapy. Progression was defined as an increase in stage to muscle-invasive disease.
Urine samples were collected at baseline before BCG therapy, immediately before and 4 h after BCG instillation at 6 wk (last dose of induction course), and immediately before and after the third instillation of the first maintenance course. Levels of 12 cytokines (IL-6, IL-1ra, IL-2, IL-1β, IL-8, IL-10, IL-12(p40), IL-12(p70), IL-18, IFN-γ, TNF-α, and TRAIL) were measured using a Luminex multiplex platform at Alere (San Diego, CA, USA). Each sample was run in triplicate, and the change from before to after treatment was calculated for each cytokine.
Logistic regression was used to model recurrence or progression for each fold increase in each cytokine. A final model was chosen using backwards selection. A nomogram was then created to facilitate prediction of recurrence (details in supplemental methods). Statistical analyses were performed using Stat/SE version 10.1 (StataCorp, College Station, TX, USA), SAS version 9 (SAS Institute, Cary, NC, USA), and R version 2.13 (R Foundation for Statistical Computing, Vienna, Austria).
Clinicopathologic characteristics are shown in Supplementary Table 1. Most patients (79%) were male and had a history of smoking. Ninety-six tumors (74%) were of high grade. Sixty-two patients had Ta disease, 61 had T1 disease, and 65 had carcinoma in situ as a secondary finding. At a median follow-up of 23.4 mo, 44 patients (34%) had experienced recurrence and 18 (14%) had experienced progression to muscle invasion. Tumor grade was not related to the risk of recurrence or progression (hazard ratio 7.24, 95% confidence interval [CI] 0.95–54.51; p = 0.55).
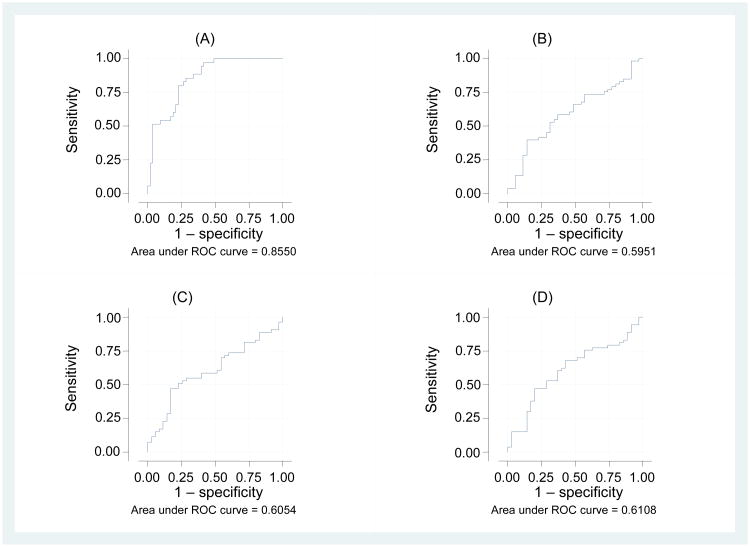
Changes in cytokine levels are summarized in Supplementary Table 2. The nomogram score for changes in the levels of nine cytokines from before to just after the sixth instillation of BCG (Fig. 1) was the best predictor of recurrence (area under the curve, 85.5%; 95% CI 77.9–93.1%; Fig. 2). We designated this nomogram CyPRIT (cytokine panel for response to intravesical therapy; patent application WO2013131093 A1).
Fig. 1.

Nomogram for calculating the risk of recurrence according to changes in urinary cytokine levels from immediately before to 4 h after instillation of bacillus Calmette-Guérin (BCG) at 6 wk (final instillation of induction BCG). This nomogram reflects the complex interaction between the various cytokines. Note that the probability of recurrence increases exponentially for each unit increase in total nomogram points. Cytokine values for point calculations reflect change from before to after BCG therapy.
Fig. 2.
Area under the receiver operating characteristic (ROC) curve for performance in predicting recurrence of non–muscle-invasive bladder cancer after treatment with bacillus Calmette-Guérin (BCG). (A) Model nomogram, (B) change in IL-2 alone, (C) change in IL-6 alone, and (D) change in IL-8 alone. Changes are from immediately before to 4 h after the sixth instillation of BCG (final instillation of induction BCG).
As discussed above, a patient's response to BCG depends on stimulation of an adequate immune response. Zuiverloon and colleagues [3] recently concluded that markers other than clinicopathologic features have limited ability to predict response to BCG immunotherapy. With recognition that involvement of the immune response in BCG antitumor activity is multidimensional, it is evident that effective strategies to monitor and predict response to BCG will require the use of multiple biomarkers reflecting the status of multiple cell types and cytokines. Our nomogram may thus represent a useful tool for predicting a patient's response to intravesical immunotherapy with BCG.
One limitation of our study is that it was conducted in a single center. It will be important to determine whether the predictive value of our panel differs among Ta, T1, and Tis tumors; such analysis will require sufficient numbers of patients in each subcategory. Finally, our nomogram and calculator will need to be externally validated once the worldwide BCG shortage is resolved.
Supplementary Material
Take Home Message.
Changes in a panel of nine inducible urinary cytokines identify patients at risk of tumor recurrence during bacillus Calmette-Guérin (BCG) immunotherapy. This panel (CyPRIT) has potential for use in real-time monitoring to determine whether modification of BCG dose or duration is warranted.
Acknowledgments
Funding/Support and role of the sponsor: This research was supported by the Flight Attendant Medical Research Institute. Cytokine analysis was supported by Alere Inc. The sponsors played a role in the design and conduct of the study; data collection and analysis; and approval of the manuscript.
Footnotes
Trial registration: clinicaltrials.gov NCT01007058.
Author contributions: Ashish M. Kamat had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Kamat, Briggman, Prat.
Acquisition of data: Kamat, Grossman, Anderson.
Analysis and interpretation of data: Kamat, Urbauer, Nogueras González.
Drafting of the manuscript: Kamat, Svatek.
Critical revision of the manuscript for important intellectual content: Dinney, Grossman, Urbauer.
Statistical analysis: Urbauer, Nogueras González.
Obtaining funding: Kamat.
Administrative, technical, or material support: Kamat, Briggman.
Supervision: Kamat.
Other: None.
Financial disclosures: Ashish M. Kamat certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kamat AM, Porten S. Myths and mysteries surrounding bacillus Calmette-Guérin therapy for bladder cancer. Eur Urol. 2014;65:267–9. doi: 10.1016/j.eururo.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herr HW, Sogani PC. Does early cystectomy improve the survival of patients with high risk superficial bladder tumors? J Urol. 2001;166:1296–9. [PubMed] [Google Scholar]
- 3.Zuiverloon TC, Nieuweboer AJ, Vékony H, Kirkels WJ, Bangma CH, Zwarthoff EC. Markers predicting response to bacillus Calmette-Guérin immunotherapy in high-risk bladder cancer patients: a systematic review. Eur Urol. 2012;61:128–45. doi: 10.1016/j.eururo.2011.09.026. [DOI] [PubMed] [Google Scholar]
- 4.Biot C, Rentsch CA, Gsponer JR, et al. Preexisting BCG-specific T cells improve intravesical immunotherapy for bladder cancer. Sci Transl Med. 2012;4:137ra72. doi: 10.1126/scitranslmed.3003586. [DOI] [PubMed] [Google Scholar]
- 5.Kamat AM, Flaig TW, Grossman HB, et al. Expert consensus document: consensus statement on best practice management regarding the use of intravesical immunotherapy with BCG for bladder cancer. Nat Rev Urol. 2015;12:225–35. doi: 10.1038/nrurol.2015.58. [DOI] [PubMed] [Google Scholar]
- 6.Kamat AM, Dickstein RJ, Messetti F, et al. Use of fluorescence in situ hybridization to predict response to bacillus Calmette-Guérin therapy for bladder cancer: results of a prospective trial. J Urol. 2012;187:862–7. doi: 10.1016/j.juro.2011.10.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sylvester RJ, van der Meijden APM, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49:466–75. doi: 10.1016/j.eururo.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 8.Kamat AM, Witjes JA, Brausi M, et al. Defining and treating the spectrum of intermediate risk nonmuscle invasive bladder cancer. J Urol. 2014;192:305–15. doi: 10.1016/j.juro.2014.02.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lamm DL, Blumenstein BA, Crissman JD, et al. Maintenance bacillus Calmette-Guérin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study. J Urol. 2000;163:1124–9. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.