Abstract
A novel microfluidic device which consists of two stages for particle focusing and separation using a viscoelastic fluid has been developed. A circular capillary tube was used for three-dimensional particle pre-alignment before the separation process, which was inserted in a polydimethylsiloxane microchannel. Particles with diameters of 5 and 10 μm were focused at the centerline in the capillary tube, and the location of particles was initialized at the first bifurcation. Then, 5 and 10 μm particles were successfully separated in the expansion region based on size-dependent lateral migration, with ∼99% separation efficiency. The proposed device was further applied to separation of MCF-7 cells from leukocytes. Based on the cell size distribution, an approximate size cutoff for separation was determined to be 16 μm. At 200 μl/min, 94% of MCF-7 cells were separated with the purity of ∼97%. According to the trypan blue exclusion assay, high viability (∼90%) could be achieved for the separated MCF-7 cells. The use of a commercially available capillary tube enables the device to be highly versatile in dealing with particles in a wide size range by using capillary tubes with different inner diameters.
I. INTRODUCTION
Microparticle/cell separation is an essential pre-processing step for downstream application such as biochemical analysis, disease diagnosis, and therapeutics.1 Much effort has been invested in the development of point-of-care devices for portable and timely use. To this end, many passive microfluidic techniques have been employed which exploit different characteristic differentiating factors for separation, while negating the need for any external force field to be applied. Inertial microfluidics that relies on channel geometry2–5 and/or flow characteristics6–8 has been utilized to separate particles. Recently, a spiral inertial filtration (SIF) device that can achieve high-throughput (1 ml/min) and high-purity particle separation has been reported.9 The SIF device was applied to cancer cell separation from white blood cells in whole blood at nearly 100% recovery. However, inertial microfluidic methods require an elaborate channel design and high flow conditions for effective particle separation. Therefore, it is still necessary to develop a device for particle separation, which is flow-rate insensitive and user-friendly for operation. More recently, the use of a viscoelastic non-Newtonian fluid has become popular due to its intrinsic nonlinear elastic forces.10 The non-uniform distribution of the first normal stress difference (N1) in viscoelastic flows induces suspended particles to migrate laterally during flowing, which has been applied to particle manipulation technique.11–15
In viscoelastic flow, the lateral migration of particles can be influenced by the cross-sectional shape of the microchannel. When the fluid inertia and elasticity simultaneously affect the lateral migration, suspended particles can be focused at a single position along the centerline in a square channel.11,12 However, to induce such a synergetic effect in a square channel, a relatively narrow range of flow rates should be used. In other words, the synergetic effect would be weakened without having a sophisticated flow control. In contrast, in an elasticity-dominant flow, particles migrate to multiple positions (center and four corners) in the square microchannel, resulting in an inefficient initialization of particles.13 On the other hand, the use of a circular-shaped channel could overcome the limitation found in square channels. All particles can be focused at the centerline by eliminating the particles trapped in the corners of the square channel.14,15 The lateral migration of particles in viscoelastic flow has been further applied to research on particle manipulation. Ahn and coworkers demonstrated a sheathless microfluidic particle separator utilizing the synergetic effect of fluid inertia and elasticity in a square channel.16 However, their separation efficiency was relatively low since the particles were still randomly distributed before the separation process. On the other hand, Lu and Xuan utilized a viscoelastic fluid to enhance the separation performance of pinched flow fractionation.17 However, additional flow injection modules were required for the pre-alignment of all particles, which would not be suitable for multiple, high-throughput processing.11,18
Recently, sheathless size-based particle separation using a non-Newtonian fluid was demonstrated in a simple straight microchannel.19 Even though this method was further applied to biological cell separation, MCF-7 cancer cells were separated only from red blood cells whose sizes distinctly differ from the cancer cells. More recently, our group has developed a microfluidic device for highly efficient, sheathless particle separation using an elasticity-dominant non-Newtonian fluid,20 which suffers from low throughput. A circular channel was fabricated from a rectangular channel to prevent particles from being trapped in the corners of rectangular channels. Thus, the use of the circular channel enhanced the pre-alignment of particles and the separation efficiency over a wide range of flow rates. However, the fabrication process of the circular channel was relatively time-consuming and labor-intensive to ensure the reproducibility of the tube size.
Therefore, in this study, we introduce a novel device to overcome these limitations, which consists of two stages for continuous particle focusing and separation using a non-Newtonian fluid. We used a circular capillary tube for sheathless pre-alignment of particles before separation process in polydimethylsiloxane (PDMS) channel. A capillary-tube integrated PDMS microchannel has been used for emulsion droplet generation.21 However, it has yet to be explored for 3D particle/cell focusing prior to the separation process. The novelty of our device lies in the use of commercially available capillary tubes that could provide the device with versatility in dealing with a wide size-range of target particles simply by inserting capillary tubes with different inner diameters to the microfluidic channel. Moreover, stable separation performance can be achieved in a broad range of flow rates by manual operation, with no external flow injection system such as a syringe pump. In addition, we demonstrated the potential of cancer cell separation from leukocytes by size with high throughput comparable to previous techniques.22
II. MATERIAL AND METHODS
A. Sample preparation
To evaluate the performance of our device, a 5 wt. % of polyvinylpyrrolidone (PVP) aqueous solution was used to ensure the elasticity-dominant flow condition. PVP solution has been widely used for various biological applications, such as rheological measurements of red blood cells (RBCs) with no significant changes in the physical characteristics of RBCs.23–25 Rheological properties such as the viscosity and the relaxation time of the fluid were ∼0.05 Pa-s and 0.0011 s, respectively, which were measured with a rotational rheometer (AR2000-ex, TA Instrument, USA). The fluid relaxation time was estimated from the inverse of the angular frequency at which the trends of the dynamic moduli G′ and G″ (elastic and shear moduli) cross as described in a previous study by D'Avino and coworkers.26 This was obtained from dynamic oscillation tests at 25 °C (see supplementary Figure S-132). Fluorescent polystyrene particles (Fisher Scientific, USA) of 5 μm diameter (green, 7.64 × 107 particles/ml) and 10 μm diameter (red, 9.55 × 106 particles/ml) were suspended in the viscoelastic fluid at the final concentration of 0.1% (v/v). The blockage ratio (; = particle diameter and = inner diameter of capillary tube) was 0.1 and 0.2 for the small and large particles, respectively. The sample solution was injected into the capillary tube with a syringe pump (KDS210, KD Scientific, Holliston, MA) at various flow rates (Q = 0.01–20 μl/min).
To demonstrate high-throughput separation of cancer cells from blood cells, we used a solution of hyaluronic acid (HA) sodium salt (357 kDa, Lifecore Biomedical) in phosphate-buffered saline (PBS) at 0.1 (w/v)%. The high shear viscosity and relaxation time of the 0.1% HA solution are known to be 0.0009 Pa s and 0.00026 s, respectively. These rheological properties of 0.1% HA solution in PBS have been reported in a previous study.27 The zero-shear viscosity was reported to be 230 mPa s, while the power-law index was calculated to be 0.48. All measurements were performed at 25 °C. Fresh human blood with an anti-coagulant (EDTA) was purchased (i-DNA Biotechnology, Singapore). To prepare pure leukocytes, 1-ml whole blood was mixed with 10-ml 1 × RBC lysis buffer (R7757, Sigma Aldrich) and incubated for 15 min at room temperature. Then, the mixed sample was centrifuged at 400 g for 5 min to remove the supernatant. Collected leukocytes were then resuspended in the 0.1 (w/v)% HA solution. Human breast adenocarcinoma (MCF-7) with green fluorescent protein (GFP) staining was used to mimic circulating tumor cells (CTCs), which had been cultured in a low-glucose Dulbecco's modified Eagle's medium (DMEM) (Invitrogen, USA) supplemented with 10% fetal bovine serum (FBS) (Invitrogen, USA) and 1% penicillin/streptomycin (Invitrogen, USA). In the final sample solution, cells suspended in the 0.1 (w/v)% HA solution were fluorescent MCF-7 cells and non-labeled leukocytes for post-analyses after separation. After the cells were resuspended in the viscoelastic solution, cell counting was done with a hemocytometer (C-chip, INCYTO, Korea) and diluted to achieve desired concentrations for the experiment. Final concentrations of cells were ∼2 × 106 MCF-7 cells/ml and ∼5 × 105 leukocytes/ml. The concentration of MCF-7 cells in the final sample solution was set to be higher than that of a clinical sample for demonstration purpose of the device performance.28 For evaluation of separation performance, microscopic images recorded by a high-speed camera (FASTCAM-1024PCI, Photron, USA) as well as a fluorescence camera (PCO Edge Mono, PCO AG, Germany) were analyzed.
B. Microchannel fabrication
The microchannels were fabricated using PDMS (Dow Corning, MI) by standard soft lithography and replica molding procedures. The SU-8 2075 negative photoresist (MicroChem, USA) was spin-coated onto a polished silicon wafer and soft-baked according to the manufacturer's protocol. A patterned film mask was aligned above the SU-8 surface, which was subsequently exposed to UV at a specific dosage. The SU-8 developer was then used to etch away the unexposed SU-8. To fabricate the microchannels, PDMS prepolymer and curing agent were mixed in a 9:1 ratio (v/v) and poured onto the silicon mold before degassing and baking for 2 h at 70 °C. The microchannel was irreversibly bonded to a microscope glass slide by oxygen plasma treatment.
III. WORKING PRINCIPLE
Due to the non-uniform first normal stress difference distribution, particles/cells experience the elastic force that is dependent on rheological conditions. Therefore, to characterize the viscoelastic flows, non-dimensional numbers (Reynolds number (), Weissenberg number (), and Elasticity number ()) were utilized, where is the solution density, is the mean velocity of flow, is the hydraulic diameter, is the characteristic viscosity of solution, is the relaxation time, and is the characteristic shear rate. The hydraulic diameter in the capillary tube was equivalent to the tube diameter = 50 μm. In the second stage of the microchannel, the characteristic length of 104 μm was calculated based on the dimensions of the wetted perimeter (), where is the channel height and is the channel width. The characteristic shear rate was calculated by . The channel width refers to the lateral dimension of the channel while the height is analogous to the channel depth.
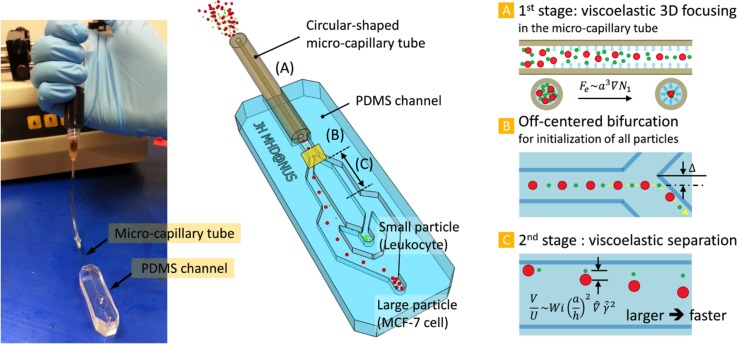
A schematic of the hybrid capillary-inserted microfluidic device using a viscoelastic fluid is described in Figure 1. The proposed device can be divided into two parts: 3-D focusing part (1st stage) and separation part (2nd stage). A detailed description of the fabrication process of the device can be found in supplementary material Figure S-2.32 The inner and outer diameters of the micro-capillary tube were 50 and 360 μm, respectively. For size-based particle separation, the width of the 2nd stage (70 μm) was designed to be slightly bigger than the inner diameter of the capillary tube. The height of the microchannel was 200 μm, and the lengths of the capillary tube and the 2nd stage were 5 and 1 cm, respectively. The required length for each stage was designed by an empirical rule reported previously.14 Briefly, the length is inversely proportional to the blockage ratio. Thus, a longer channel is needed to focus smaller particles. The length of the capillary tube was determined based on the initialization of 5 μm particles at the centerline while the length of the 2nd stage channel was designed to focus only large particles (10 μm diameter).
FIG. 1.
Schematic of our device for sheathless viscoelastic particle focusing and separation. The hybrid capillary-inserted microfluidic device consists of (A) 1st stage micro-capillary tube for viscoelastic 3-D focusing, (B) bifurcation for initialization of all particles, and (C) 2nd stage microchannel for viscoelastic separation. The inset picture is the fabricated device.
At the inlet of the micro-capillary tube (Figure 1(A)), particles are randomly distributed. Particles experience the elastic force () that is proportional to the particle volume (; = radial distance),16,29 so that large particles migrate toward the centerline faster than small particles. Eventually, if the blockage ratio is higher than 0.1,30 particles regardless of their size are focused along the centerline at the outlet of the capillary tube (inlet of the PDMS rectangular channel). Thus, at the first bifurcation in the channel (Figure 1(B)), all the particles are located at the wall, which plays an essential role in the separation process. To enhance the fluidity at the bifurcation, the apex of the bifurcation is slightly deviated from the center of the upstream channel. In the 2nd stage (Figure 1(C)), particles demonstrate different rates of lateral migration depending on their sizes since the migration velocity is affected by the particle size (; = lateral migration velocity, = average flow velocity, and = non-dimensional local shear rate).16,28 Thus, larger particles laterally migrate farther compared to smaller particles while flowing in the 2nd stage. Finally, particles with different sizes can be separated into two outlets.
IV. RESULTS AND DISCUSSION
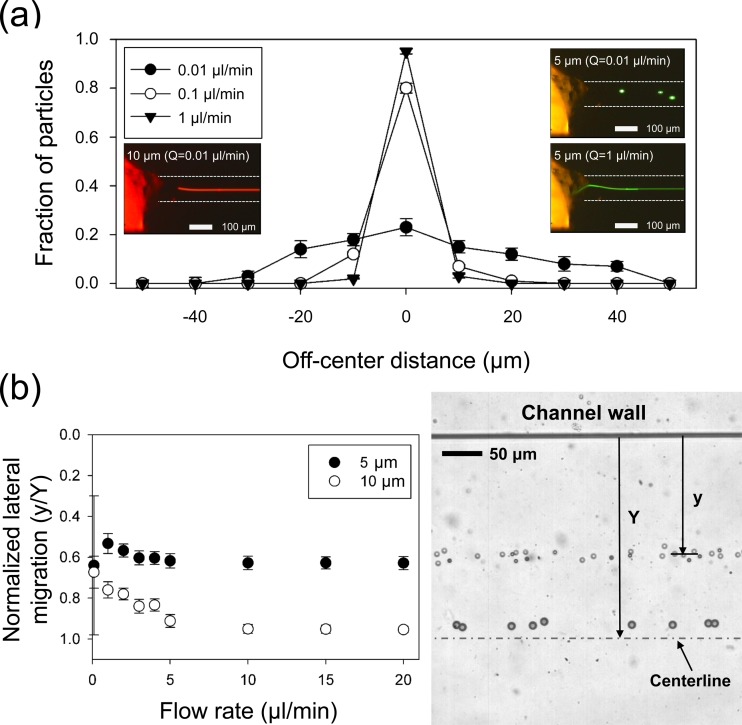
To confirm the 3-D particle focusing in the capillary tube, the lateral distribution of 5 and 10 μm particles was examined at different ranging from 0.01 to 2 μl/min. Under the current experimental conditions, non-dimensional numbers that describe the flow characteristics were 0.00036 ≤ ≤ 0.071, 0.0075 ≤ ≤ 1.5, and = 20.95 (≫1, elasticity-dominant flow). Figure 2(a) shows the distribution of 5 μm polystyrene particles at the interconnection region between the PDMS channel and the capillary tube under different flow conditions. To count the number of particles and evaluate the device performance, the channel width at the interconnection (110 μm) was divided into 11 sections, and particle flow images acquired with a high-speed camera were analyzed with a commercial software package (MATLAB, Mathworks). At 0.01 μl/min, unlike the case of 10 μm particles (inset figure on the left), 5 μm particles were still randomly distributed and unfocused along the centerline. This was expected since 0.01 μl/min was lower than the minimum required for focusing of 5 μm particles based on the empirical design rule.14 According to the design recipe, the required for particle focusing would be dependent upon channel dimension, particle size, and elastic property of the suspending medium.
FIG. 2.
(a) Distribution of 5 μm polystyrene particles at the end of the capillary tube at different flow rates. At 0.01 μl/min, 5 μm particles are not focused at the center, whereas they are tightly focused at the center at 1 μl/min. The inset pictures are typical examples of 5 μm particles at 0.01 and 1 μl/min and 10 μm particles at 0.01 μl/min. (b) Normalized lateral migration distances of 5- and 10 μm polystyrene particles in the expansion region with respect to flow rates: 10 μm particles migrate towards the equilibrium position at the center of the channel while 5 μm particles show shorter lateral displacements. Error bars represent the standard deviation of at least 500 cells sampled from three experiments.
As increased, more particles were entrained in the central area, and at = 1 μl/min, 5 μm particles tightly focused at the center. In the current device, particles that are smaller than 5 μm appear to experience difficulty in being focused along the centerline since the blockage ratio of smaller particles does not satisfy the previously reported threshold value (β ≥ 0.1).29 However, it is of note that by using commercial capillary tubes with smaller inner diameters, the range of focusable particle size can be varied with ease. After the initialization of particles at the first bifurcation, particles experienced the elastic force driving them away from the inner wall. The differentiation in lateral migration of the two different particles could be enhanced in the expansion region so that the particles could be easily separated at the outlet.
Figure 2(b) shows the normalized lateral position of each particle at different . The lateral migration distance from the inner wall ( in Figure 2(b)) was normalized to the half channel width ( = 250 μm) in the expansion region. At = 0.01 μl/min, 5 μm particles seemed to be randomly dispersed, which can be found from a large standard deviation, since the smaller particles were not well focused in the capillary tube in the 1st stage. However, particles with 10-μm diameter appeared to be located closer to one another within a narrow band since the larger particles experienced a bigger elastic force than the smaller. Therefore, at the same flow rate, larger particles showed a greater lateral migration distance from the wall than smaller particles. With increasing , the lateral migration difference between 5 and 10 μm particles became larger, and their migration distances seemed to already reach a plateau at = 10 μl/min. The 10-μm particles seemed to migrate close to the centerline ( = 0.95 ± 0.02), whereas 5 μm particles flowed along the normalized lateral position at = 0.62 ± 0.03. This gap between the lateral migration distances of 5 and 10 μm particles is due to their blockage ratio difference. In addition, the 2nd stage was designed to be shorter than the capillary tube to ensure the difference in lateral migration of the 5 and 10 μm particles. A stacked image of particles in the expansion region is provided in the right panel of Figure 2(b) to demonstrate the differentiation in the lateral migration distance of particles with different sizes.
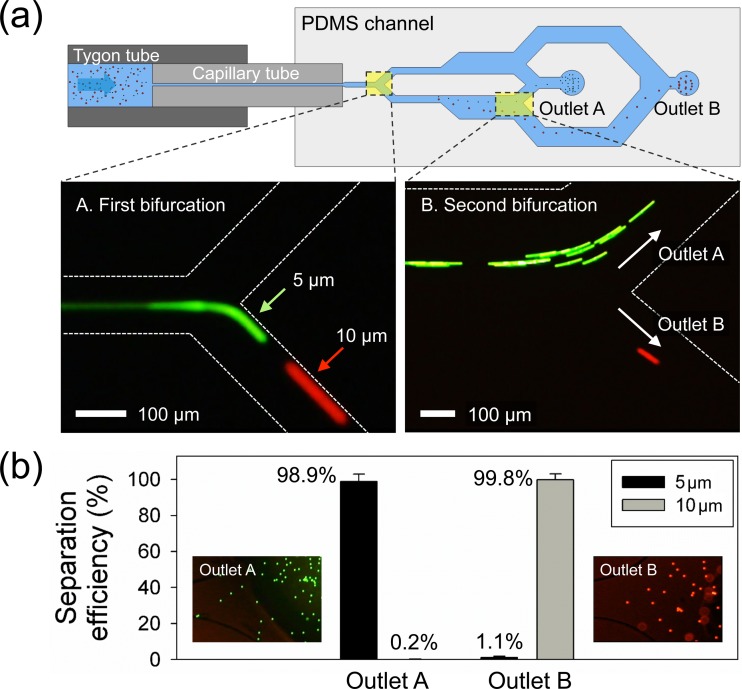
Figure 3 shows the separation process and efficiency for the 5 and 10 μm particles. As shown in Figure 3(a), the location of both particles was well initialized at the apex of the first bifurcation (Figure 3(a-A)). Due to the 5-μm off-centered location of the bifurcating point, all the focused particles flowed only through the bottom channel. In the expansion region, the difference in the lateral migration of 5 (green) and 10 μm particles (red) became more distinct. As seen in Figure 3(a-B), the particles were then separated into two different outlets at = 20 μl/min (see supplementary Video S1).32 Thus, 5 and 10 μm particles could be separated and subsequently collected from the center and side outlets, respectively. Figure 3(b) illustrates the separation efficiency at each outlet, and the inset images in Figure 3(b) show the collected particles at each outlet. After the separation process, the number of particles in the collected sample from each outlet was counted with a flow cytometry analyser (LSR Fortessa, BD) to evaluate the device performance. In the outlet A, 98.9% of 5 μm particles were collected among the total number of the particles while 99.8% of 10 μm particles were separated into the outlet B. The separation purity for each particle was ∼99%. In comparison with a recent study (4 μl/min) that reported a microfluidic device for sheathless size-based particle separation in a viscoelastic fluid,16 the throughput of our device is at least five times greater.
FIG. 3.
Size-based particle separation. (a) At Q = 20 μl/min, 5 μm (green fluorescence), and 10 μm (red fluorescence) polystyrene particles are focused at the first bifurcation and then separated into two different outlets at the second bifurcation. (b) The separation efficiency at each outlet for the collected sample after separation of particles. The inset pictures show the collected particles at each outlet.
To test the potential of our device for clinical application, separation of tumor cells from leukocytes (lysed blood) was performed. A CTC separation device is required to achieve high separation efficiency and throughput due to the extremely low concentration of CTC in blood (∼1–100 CTCs per 109 blood cells).31 This separation, however, is often marred by the overlapping sizes of smaller CTCs and larger granulocytes such as neutrophils and monocytes, hence reducing the separation efficiency of an already rare CTC population. Hence, we demonstrated the ability of our device to distinguish between both cell types by spiking a population of leukocytes with MCF-7 CTCs. To satisfy this condition for CTC separation with no modification of the device, we used a HA solution as the suspending medium, which has been reported as a viscoelastic fluid with low viscosity.27
The 0.1% HA solution has a viscosity 2-order lower than that of the 5% PVP solution, but maintains a sufficiently high elasticity so as to establish cell separation by differential elastic force. This enables the deterministic tuning of the device performance for sorting different cell types with varying cell sizes, by altering the magnitude of the elastic forces exerted on the cells. Additionally, the flow rate can be increased without elevating the inlet pressure in the microchannel, hence allowing for the throughput to be significantly enhanced. However, HA is known to display a large shear thinning effect at higher shear rates, at which inertia effects start being significant. When the inertial effects are equal or less than the elastic effects, the cells will maintain their elasticity-driven focusing position at the channel centerline. Once inertial effects start to dominate, the focusing may become destabilized. In our study, even though El decreased at high flow rates, no defocusing was observed, possibly due to the synergetic interplay between the cell deformability-induced inward migration and the elastic force.
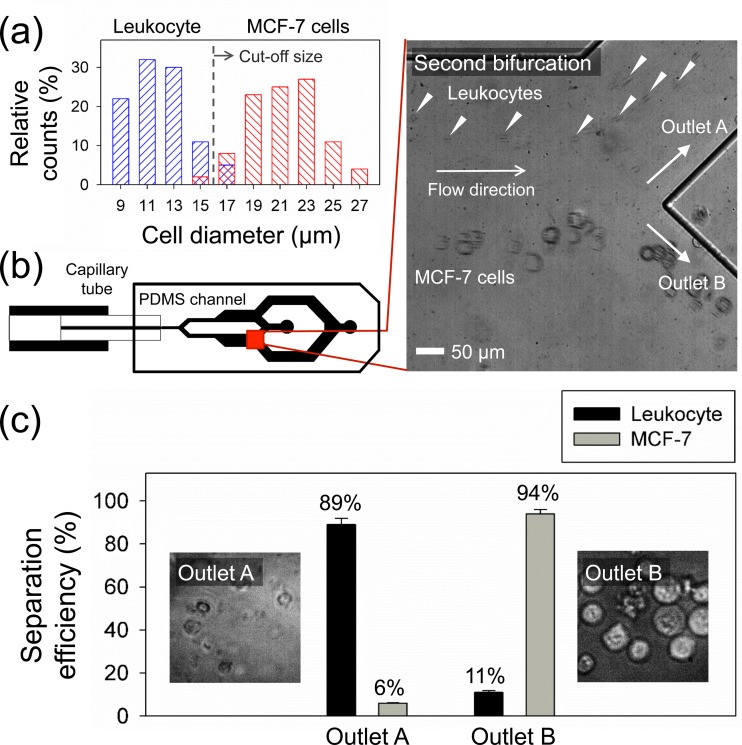
Figure 4(a) shows the cell size distribution in the sample before the separation process. Leukocytes attained after RBC lysis had a diameter range of 9–15 μm, whereas MCF-7 cells were 14–27 μm. The blockage ratio for both cells was higher than 0.1, confirming that the 50 μm capillary was narrow enough for initialization at the first bifurcation. From the size distribution, an approximate size cutoff for size-based separation in our device was determined to be 16 μm. To optimize the separation performance of MCF-7 cells and leukocytes, the lateral migration of 15 μm polystyrene particles was evaluated at = 1, 25, 50, 100, 200, and 300 μl/min to find the flow condition where we can separate MCF-7 cells with no leukocytes flowing in the outlet B (see supplementary Figure S-3 for details).32 Under this flow regime, inertial forces become significant, and the elasto-inertial effect12 accounts for the 3D focusing of all particles at the channel center axis of the capillary tube. Based on the results, the location of 15 μm particles was well initialized at the apex of the bifurcation at ≥ 1 μl/min and appeared to already reach the centerline at = 300 μl/min after the lateral migration. Therefore, flow rates between 1 and 200 μl/min were suitable for MCF-7 cell separation with the connected outlet reservoir using PTFE-tubes for collection.
FIG. 4.
MCF-7 cell separation from leukocytes (a) Initial size distribution of mixed sample of MCF-7 cells and leukocytes (n = 300). Leukocytes have the average diameter of 9–15 μm while the average diameter of MCF-7 cells is 14–27 μm. (b) Separation of cells at Q = 200 μl/min. Solid-white triangles indicate leukocytes at the 2nd bifurcation. (c) The separation efficiency at each outlet for the collected sample. The inset pictures show the collected cells at each outlet. Cells with fluorescence indicate GFP-stained MCF-7 cells, while non-fluorescent cells are leukocytes.
As seen in Figure 4(b), MCF-7 cells and leukocytes were separated and collected into two different outlets (A and B) at = 200 μl/min (see supplementary Video S2)32 with the throughput of 4 × 105 cells/min. Figure 4(c) demonstrates the separation efficiency and the microscopic image of collected cells at each outlet. The separation efficiency () was defined as the ratio of the number of cells collected at the target outlet () to the total number of cells at the outlet (). Based on the hemocytometer analysis, 94% of GFP-stained MCF-7 cells were collected in the outlet B with the purity of ∼97% while 89% of leukocytes were separated into the outlet A. Finally, the trypan blue exclusion assay was used for assessment of the cell viability, and we found the high viability (∼90%) of collected cells from outlet B with minimal cell death (<10%) (see supplementary Figure S-4 for details).32
Most particle separation devices require the use of external pumps to drive a flow, imposing constraints on the portability of the devices. This is not very suitable for a point-of-care purpose. In the particle separation with our device, the wide range of separable flow rates for tumor cells implies an apparent insensitivity of the separation process seems to be to the flow rate. Therefore, with minor modification, it would be highly possible to develop a stand-alone handheld device that can function within a broad pressure range with a manual syringe, hence neglecting the need for any external equipment. The separable particle size can be tuned by changing the size of capillary tube and/or the viscoelastic property of the suspending medium. The throughput of our device can be further enhanced through a parametric study on the height and width of the 2nd stage microchannel, inner diameter of the capillary tube, and rheological property of suspending medium.
V. CONCLUSION
In summary, we have developed a new hybrid capillary-inserted microfluidic device for microparticle focusing and separation via viscoelastic flow. The inner diameter of the capillary tube can be chosen to satisfy the threshold value of the blockage ratio for target particles, confirming versatility of the separation device. The 5 and 15 μm particles were three-dimensionally focused in the capillary tube and separated on the basis of size difference into two different outlets with high efficiency (∼99%) and purity (≥99%). Moreover, this proposed device was further applied to separation of biological particles with different sizes (tumor cells and leukocytes) by using a viscoelastic fluid with low viscosity (HA solution). The separation efficiency and the purity of MCF-7 cells were 94% and 97% at 200 μl/min, respectively. Therefore, this device could be used as a highly versatile tool for clinical/biological applications where biological particles with different sizes need to be sorted.
ACKNOWLEDGMENTS
This work was supported by National Medical Research Council/Cooperative Basic Research Grant No. /0078/2014.
References
- 1. Gossett D. R., Weaver W. M., Mach A. J., Hur S. C., Tse H. T. K., Lee W., Amini H., and Carlo D. D., Anal. Bioanal. Chem. 397, 3249 (2010). 10.1007/s00216-010-3721-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Choi S., Ku T., Song S., Choi C., and Park J. K., Lab Chip 11, 413 (2011). 10.1039/C0LC00148A [DOI] [PubMed] [Google Scholar]
- 3. Huang L. R., Cox E. C., Austin R. H., and Sturm J. C., Science 304, 987 (2004). 10.1126/science.1094567 [DOI] [PubMed] [Google Scholar]
- 4. Kuntaegowdanahalli S. S., Bhagat A. A. S., Kumar G., and Papautsky I., Lab Chip 9, 2973 (2009). 10.1039/b908271a [DOI] [PubMed] [Google Scholar]
- 5. Yamada M., Nakashima M., and Seki M., Anal. Chem. 76, 5465 (2004). 10.1021/ac049863r [DOI] [PubMed] [Google Scholar]
- 6. Hur S. C., Choi S. E., Kwon S., and Carlo D. D., Appl. Phys. Lett. 99, 044101 (2011). 10.1063/1.3608115 [DOI] [Google Scholar]
- 7. Hur S. C., Henderson-MacLennan N. K., McCabe E. R., and Carlo D. D., Lab Chip 11, 912 (2011). 10.1039/c0lc00595a [DOI] [PubMed] [Google Scholar]
- 8. Masaeli M., Sollier E., Amini H., Mao W., Camacho K., Doshi N., Mitragotri S., Alexeev A., and Carlo D. D., Phys. Rev. X 2, 031017 (2012). 10.1103/PhysRevX.2.031017 [DOI] [Google Scholar]
- 9. Burke J. M., Zubajlo R. E., Smela E., and White I. M., Biomicrofluidics 8, 024105 (2014). 10.1063/1.4870399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. D'Avino G., Maffettone P. L., Greco F., and Hulsen M., J. Non-Newtonian Fluid Mech. 165, 466 (2010). 10.1016/j.jnnfm.2010.01.024 [DOI] [Google Scholar]
- 11. Nam J., Lim H., Kim D., Jung H., and Shin S., Lab Chip 12, 1347 (2012). 10.1039/c2lc21304d [DOI] [PubMed] [Google Scholar]
- 12. Yang S., Kim J. Y., Lee S. J., Lee S. S., and Kim J. M., Lab Chip 11, 266 (2011). 10.1039/C0LC00102C [DOI] [PubMed] [Google Scholar]
- 13. Seo K. W., Kang Y. J., and Lee S. J., Phys. Fluids 26, 063301 (2014). 10.1063/1.4882265 [DOI] [Google Scholar]
- 14. D'Avino G., Romeo G., Villone M. M., Greco F., Netti P. A., and Maffettone P. L., Lab Chip 12, 1638 (2012). 10.1039/c2lc21154h [DOI] [PubMed] [Google Scholar]
- 15. Seo K. W., Byeon H. J., Huh H. K., and Lee S. J., RSC Adv. 4, 3512 (2014). 10.1039/C3RA43522A [DOI] [Google Scholar]
- 16. Ahn S. W., Lee S. S., Lee S. J., and Kim J. M., Chem. Eng. Sci. 126, 237 (2015). 10.1016/j.ces.2014.12.019 [DOI] [Google Scholar]
- 17. Lu X. and Xuan X., Anal. Chem. 87, 6389 (2015). 10.1021/acs.analchem.5b01432 [DOI] [PubMed] [Google Scholar]
- 18. Kang K., Lee S. S., Hyun K., Lee S. J., and Kim J. M., Nature Commun. 4, 2567 (2013). 10.1038/ncomms3567 [DOI] [PubMed] [Google Scholar]
- 19. Liu C., Xue C., Chen X., Shan L., Tian Y., and Hu G., Anal. Chem. 87, 6041 (2015). 10.1021/acs.analchem.5b00516 [DOI] [PubMed] [Google Scholar]
- 20. Nam J., Namgung B., Lim C. T., Bae J. E., Leo H. L., Cho K. S., and Kim S., J. Chromatogr. A 1406, 244 (2015). 10.1016/j.chroma.2015.06.029 [DOI] [PubMed] [Google Scholar]
- 21. Zheng B., Tice J. D., and Ismagilov R. F., Adv. Mater. 16, 1365 (2004). 10.1002/adma.200400590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hou H. W., Warkiani M. E., Khoo B. L., Li Z. R., Soo R. A., Tan D. S. W., Lim W. T., Han J., Bhagat A. A. S., and Lim C. T., Sci. Rep. 3, 1259 (2013). 10.1038/srep01259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cha S., Shin T., Lee S. S., Shim W., Lee G., Lee S. J., Kim Y., and Kim J. M., Anal. Chem. 84, 10471 (2012). 10.1021/ac302763n [DOI] [PubMed] [Google Scholar]
- 24. Dobbe J., Streekstra G., Hardeman M., Ince C., and Grimbergen C., Cytometry 50, 313 (2002). 10.1002/cyto.10171 [DOI] [PubMed] [Google Scholar]
- 25. Seo K. W., Ha Y. R., and Lee S. J., Appl. Phys. Lett. 104, 213702 (2014). 10.1063/1.4880615 [DOI] [Google Scholar]
- 26. D'Avino G., Romeo G., Villone M. M., Greco F., Netti P. A., and Maffettone P. L., Lab Chip 12(9), 8 (2012). 10.1039/C2LC21154H [DOI] [PubMed] [Google Scholar]
- 27. Lim E. J., Ober T. J., Edd J. F., Desai S. P., Neal D., Bong K. W., Doyle P. S., McKinley G. H., and Toner M., Nature Commun. 5, 4120 (2014). 10.1038/ncomms5120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Geislinger T. M. and Franke T., Biomicrofluidics 7, 044120 (2013). 10.1063/1.4818907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tehrani M., J. Rheol. 40, 1057 (1996). 10.1122/1.550773 [DOI] [Google Scholar]
- 30. Romeo G., D'Avino G., Greco F., Netti P. A., and Maffettone P. L., Lab Chip 13, 2802 (2013). 10.1039/c3lc50257k [DOI] [PubMed] [Google Scholar]
- 31. Liotta L. A., Kleinerman J., and Saldel G. M., Cancer Res. 36, 889 (1976); available at http://cancerres.aacrjournals.org/content/36/3/889.full.pdf+html. [PubMed] [Google Scholar]
- 32.See supplementary material at http://dx.doi.org/10.1063/1.4938389E-BIOMGB-9-021506 for the fabrication procedure of the capillary-inserted device, lateral migration results of 15-μm polystyrene particles, and results of the Trypan Blue exclusion viability test for the CTC separation.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- See supplementary material at http://dx.doi.org/10.1063/1.4938389E-BIOMGB-9-021506 for the fabrication procedure of the capillary-inserted device, lateral migration results of 15-μm polystyrene particles, and results of the Trypan Blue exclusion viability test for the CTC separation.