Abstract
Background
Brain-derived neurotrophic factor (BDNF) plays a critical role in neurodevelopment and plasticity; decreased BDNF functioning may contribute to the pathogenesis of schizophrenia. However, BDNF levels are not static; in animal experiments, brain BDNF increases during spatial learning, and in clinical depression, successful antidepressant treatment raises serum BDNF. We asked: would neuroplasticity-based cognitive training in schizophrenia result in increased serum BDNF?
Methods
Fifty-six schizophrenia outpatients and 16 matched healthy comparison subjects were assessed on baseline cognitive performance and serum BDNF. Schizophrenia subjects were randomly assigned to either 50 hours (10 weeks) of computerized auditory training or a computer game control condition, followed by reassessment of cognition and serum BDNF.
Results
At baseline, schizophrenia participants had significantly lower-than-normal serum BDNF. Schizophrenia subjects who engaged in computerized cognitive training designed to improve auditory processing showed significant cognitive gains and a significant increase in serum BDNF compared with subjects who played computer games. This increase was evident after 2 weeks of training, and after 10 weeks in the active condition, subjects “normalized” their mean serum BDNF levels, whereas the control group showed no change. In the active condition, change in BDNF was significantly associated with improved quality of life.
Conclusions
Serum BDNF levels are significantly increased in clinically stable, chronically ill schizophrenia subjects after neuroplasticity-based cognitive training, but not after computer games. Serum BDNF levels may serve as a peripheral biomarker for the effects of intensive cognitive training and may provide a useful tool for the evaluation of cognitive enhancement methods in schizophrenia.
Keywords: BDNF, biomarker, cognitive enhancement cognitive remediation, cognitive training, neuroplasticity, schizophrenia
Brain-derived neurotrophic factor (BDNF), the most widely distributed neurotrophin in the brain, plays a critical role in neurodevelopment, neuronal function, and plasticity (1,2). Schizophrenia may be related in part to decreases in normal BDNF functioning (3). For example, post-mortem studies show that schizophrenia patients have reduced numbers of BDNF-positive neurons (4), as well as lowered BDNF concentrations in cortical areas and hippocampus (5). Cord blood from infants exposed to obstetric complications who later develop schizophrenia shows lower BDNF than blood from exposed infants who do not develop the disorder (6). Serum BDNF levels in adult schizophrenia patients are generally reduced compared with healthy subjects, including never-medicated, first-episode patients (3,7–11), although results are inconsistent (12–17).
These disparate findings suggest that BDNF signaling may be lacking in schizophrenia, possibly affecting brain plasticity and cognition. In animal experiments, the acquisition and maintenance of spatial memory are impaired when BDNF signaling is decreased (18,19); however, brain BDNF is increased when rodents perform a spatial learning task (18) or are housed in cognitively stimulating environments (20). In clinical depression, successful response to antidepressant treatment is associated with enhanced serum BDNF levels (21–26). We thus asked: would successful neuroplasticity-based cognitive training in schizophrenia result in increased serum BDNF levels, as suggested by the animal data, and in a manner analogous to what is seen during antidepressant treatment in major depression?
We first sought to replicate previous findings of lowered serum BDNF in schizophrenia, studying a well-characterized sample of 56 clinically stable, chronically ill, medicated outpatients compared with 16 healthy subjects matched for age, sex, body mass index (BMI), smoking history, and education. Second, we investigated whether significant changes in serum BDNF were observed at two time points in the 30 schizophrenia subjects randomly assigned to 50 hours of cognitive training versus the 26 subjects assigned to a computer games “placebo” condition. Finally, we explored the association between change in serum BDNF levels and change in cognition, symptoms, and quality of life.
Methods and Materials
Subject Sample
Subjects were obtained from our ongoing randomized controlled trial (RCT) of neuroplasticity-based computerized cognitive training in schizophrenia, registered at http://ClinicalTrials.gov NCT00312962. Behavioral data from the first 55 completed subjects have been reported previously (27,28). Fifty-six clinically stable, chronically ill, volunteer participants with schizophrenia (SZ) were recruited from community mental health centers. Sixteen healthy comparison subjects (HC) were recruited through advertisement to match the SZ subjects at a group level in terms of age, sex, and education. Inclusion criteria were Axis I diagnosis of schizophrenia (determined by the Structured Clinical Interview for DSM-IV [SCID]) (29) or, for HC subjects, no Axis I or Axis II psychiatric disorder (SCID—Nonpatient edition), no substance dependence or current substance abuse, good general physical health, age between 18 and 60 years, and English as first language. Table 1 presents demographics, smoking status, and BMI for all subjects, plus age of onset, baseline clinical characteristics, and medication status of SZ participants. Diagnostic subtypes and medications prescribed in the two SZ subgroups are presented in Supplement 1.
Table 1.
Subject Characteristics, Mean (SD)
| HC Subjects (n = 16) | SZ Subjects (n = 56) | Auditory Training SZ Subgroup (n = 30) | Computer Games SZ Subgroup (n = 26) | |
|---|---|---|---|---|
| Average Subject Age | 44.50 (11.69) | 43.95 (9.30) | 42.13 (9.40) | 46.04 (8.92) |
| Sex (Female/Male) | 6/10 | 14/42 | 8/22 | 6/20 |
| Average Years Education | 13.92 (1.44) | 13.18 (2.07) | 13.10 (2.01) | 13.27 (2.18) |
| Currently Smoking | 50% (5/10) | 47% (24/51) | 50% (14/28) | 43% (10/23) |
| Body Mass Index | 26.87 (4.30) | 29.24 (5.01) | 29.04 (5.47) | 29.49 (4.45) |
| Age of Onset | — | 20.91 (8.99) | 21.00 (9.75) | 20.85 (8.58) |
| Average PANNS Score at Baseline | — | 2.35 (.65) | 2.35 (.75) | 2.35 (.53) |
| Global Assessment of Functioning | — | 48.75 (14.31) | 50.11 (15.52) | 46.59 (12.28) |
| Quality of Life Scale Total | — | 3.10 (1.07) | 3.23 (1.11) | 2.93 (1.02) |
| Chlorpromazine Equivalents | — | 477 (482) | 444 (477) | 515 (495) |
| Serum Anticholinergic Activity (pmol/mL)a | — | 6.1 (7.9) | 5.9 (7.6) | 6.2 (8.5) |
| Number on Antidepressants | — | 20 (36%) | 11 (36%) | 9 (35%) |
Differences between groups are not significant.
HC, healthy comparison; PANNS, Positive and Negative Syndrome Scale; SZ, schizophrenia.
See Vinogradov et al. (28).
Study Procedure
All participants gave written informed consent and then underwent neurocognitive testing over a 2- to 3-week period, followed by a blood draw. At the time this study was initiated, the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery was not yet available, although MATRICS-recommended measures were available and were used. Raw scores were converted to z scores using age-stratified normative data published by the test authors.
After baseline assessments, SZ subjects were stratified by age, education, sex, and symptom severity (mean Positive and Negative Syndrome Scale [PANSS] score < 2.0 and > 2.0) (30), and randomly assigned to either 50 hours of neuroplasticity-based targeted auditory training (AT) or to 50 hours of computer games (CG). All subjects remained on stable doses of medications while in the study (no dose change > 10%). After the intervention, SZ subjects were reassessed on PANSS, Quality of Life Scale (QLS) (31), and cognitive measures by personnel blind to group assignment.
Neuroplasticity-Based Computerized Cognitive Training Exercises
Targeted cognitive training of auditory and verbal processing (AT) was provided by software developed by Posit Science Corporation. In these computerized exercises, which we have described in detail elsewhere (27,28), subjects were driven to make progressively more accurate distinctions about the spectrotemporal fine structure of auditory stimuli and speech under conditions of increasing working memory load and to incorporate and generalize those improvements into language comprehension. In the control condition, CG subjects rotated through a specified series of 16 commercially available computer games for the same number of hours as AT subjects. Both groups rated their experiences as equally enjoyable on the 7-item subscale of Interest/Enjoyment from the Intrinsic Motivation Inventory (32).
Measurement of Serum BDNF Levels
Blood samples were drawn from all participants 2–3 weeks after study entry. SZ subjects had blood samples drawn at two additional points to assess the time course of change: 10 hours (2 weeks) after beginning the computerized intervention (AT or CG) and again after completion of 50 hours (10 weeks) of the intervention. All samples were drawn in the early afternoon (~ 1 PM ± 1 hour).
For BDNF analyses, 10-mL whole-blood samples were left to clot at room temperature for 1 hour. They were then centrifuged, and the separated serum layer was aliquoted into 5 mL polypropylene cryovials and stored at −70°C. Measurements of serum BDNF levels were carried out in the Center for Reproductive Sciences at UCSF by personnel blind to subjects’ group assignment. Serum samples were diluted with diluent included in the R&D Human BDNF Quantikine ELISA kit (Minneapolis, Minnesota) to bring measured levels of BDNF to within the range of the provided standard. A separate control sample was run on each plate to ensure minimal interassay variability (8%–14%). Study samples were run in duplicate. Results are reported in ng/mL.
Statistical Analyses
The distributions of cognitive and BDNF measures were evaluated for missing values and normalcy, and winsorized means were calculated if outliers were present. Three outlying values were adjusted (2 AT, 1 CG) and comprised 1.7% of the total BDNF data. A composite score of global cognition was computed as the average across all the MATRICS-recommended measures. Repeated-measures analysis of variance (ANOVA) was used to test CG and AT group differences in global cognition from baseline to posttraining.
Because age, sex, smoking status, and BMI can affect BDNF levels, independent samples t tests (two-tailed) tested for group differences in these variables between HC and SZ participants, and between AT and CG schizophrenia subgroups. BDNF levels may reflect illness severity in schizophrenia (33), and thus we tested for AT and CG group differences in education, age of onset, and PANNS and QLS Total scores. Pearson correlations (two-tailed) between BDNF level and the demographic and clinical variables were also conducted to identify potential covariates.
Our primary hypotheses were tested via: 1) independent-samples t test to examine group differences in baseline BDNF between the HC and SZ subjects; 2) repeated-measures ANOVA to test CG and AT group differences in BDNF level from baseline, to 2 weeks, to posttraining. Our exploratory analyses consisted of Pearson bivariate correlations (two-tailed) to test the associations in AT subjects between change in BDNF and change on global cognition, PANSS Total score, and QLS Total score.
Results
Baseline Demographic, Clinical, Cognitive, and Serum BDNF Data
There were no significant differences in age, education, sex, smoking status, and BMI between the HC and SZ groups, nor in these variables and clinical or cognitive measures, between the AT and CG schizophrenia subgroups (Table 1 and Supplement 1).
Baseline cognitive data (age-adjusted z score for global cognition) and mean serum BDNF levels (ng/mL) are presented in Table 2 for all subject groups. The SZ subjects showed the expected decrement in cognitive functioning (~ 1 SD below the normal mean). The HC subjects had a significantly higher baseline mean BDNF level of 31.30 (SD = 8.95) ng/mL, compared with a mean of 25.27 ng/mL (SD = 10.34) in SZ subjects, t = 2.11, p < .04. There was no significant difference between AT and CG schizophrenia subgroups in baseline mean BDNF level (see Table 2). There were no significant associations between baseline BDNF and the demographic variables within either the SZ or HC group and no significant associations between baseline BDNF and clinical variables in the SZ group.
Table 2.
Baseline Global Cognition Score (Age Adjusted Z Score)a and Serum BDNF Levels (ng/mL) in Healthy Comparison Subjects and in Schizophrenia Subjects, Mean (SD)
| HC Subjects (n = 15) | Total SZ Sample (n = 56) | HC vs. SZ T test (p) | AT Subgroup (n = 30) | Computer Games Control Subgroup (n = 26) | At vs. CG y test (p value) | |
|---|---|---|---|---|---|---|
| Global Cognition | .07 (.99) | −1.05 (.72) | 4.69 (<.001) | −1.14 (.71) | −.94 (.73) | 1.04 (.30) |
| Serum BDNF Level | 31.30 (8.95) | 25.27 (10.34) | 2.11 (.04) | 25.03 (11.21) | 25.54 (9.44) | .18 (.86) |
AT, auditory training; CG, computer games; BDNF, brain-derived neurotrophic factor; HC, healthy comparison; SZ, schizophrenia.
Global cognition score = mean across all measures: speed of processing (Trails A, Category Fluency, Brief Assessment of Cognition in Schizophrenia Symbol Coding), Working Memory (University of Maryland Letter Number, Wechsler Memory Scale-III Spatial Span), Verbal Learning and Memory (Hopkins Verbal Learning Test—Revised), Visual Learning and Memory (Brief Visuospatial Memory Test—Revised), Problem Solving (BACS Tower of London).
Serum BDNF Levels over the Course of Cognitive Training
The full details of the clinical and neurocognitive changes seen in 55 AT subjects after 50 hours (10 weeks) of cognitive training have been reported elsewhere (27). In sum, AT subjects showed a statistically significant gain in global cognition of .36 SD from baseline to posttraining, compared with CG subjects, who showed no change [.01 SD; repeated-measures ANOVA, F(1,53) = 12.82, p = .001]. There were no differences between AT and CG subjects in PANSS or QLS measures after the intervention.
Repeated-measures ANOVA revealed a significant difference between the AT and CG schizophrenia groups in BDNF change from baseline, to Week 2, to posttraining (Week 10), F(2,53) = 3.47, p = .04. Post hoc contrasts revealed that the AT and CG groups differed significantly in BDNF serum level from baseline to Week 2, F(1,54) = 4.97, p = .03, and from baseline to Week 10, F(1,54) = 6.10, p = .02 (Figure 1). By Week 10, the AT group’s serum BDNF level was comparable to that of the HC subjects (HC: M = 31.30, SD = 8.95; AT: M = 32.23, SD = 15.10). The standardized mean difference in BDNF level between the CG and AT groups (Cohen’s d) was calculated at posttraining and showed a medium effect size of .67.
Figure 1.
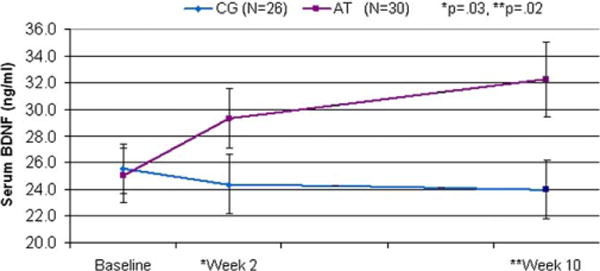
Serum brain-derived neurotrophic factor (BDNF) levels (ng/mL) in schizophrenia subjects participating in 50 hours of computerized auditory training (AT) versus subjects participating in 50 hours of computer games (CG). By posttraining (Week 10), the AT subgroup’s serum BDNF level was comparable to that of age-, sex-, and education-matched healthy comparison subjects (HC: M = 31.88, SD = 9.90; AT: M = 32.23, SD = 15.10; CG: M = 23.97 SD = 11.21).
Association of Change in BDNF to Change in Cognition, Symptoms, and Quality of Life
In the AT subject group (n = 30), there was no significant association between change in BDNF and change in global cognition (posttraining minus baseline z scores) (r = −.22, p = .25). The association of change in BDNF to change in PANSS Total Score was also nonsignificant, but in the expected direction (r = −.26, p = .16). The association of change in BDNF to change in QLS Total ratings was significant (r = .44, p = .01).
Discussion
As predicted, and consistent with prior reports, baseline serum BDNF was significantly lower in our sample of schizophrenia subjects compared with healthy subjects matched for age, sex, smoking history, BMI, and education (7–11). However, schizophrenia subjects who participated in 50 hours (10 weeks) of neuroplasticity-based computerized cognitive training showed a significant increase in serum BDNF compared with carefully matched control subjects who engaged in 50 hours of enjoyable computer games (Figure 1). A significant group difference was observed after only 10 hours (2 weeks) of training, and after 50 hours, the AT participants had achieved mean serum BDNF levels comparable to healthy subjects. In contrast, control subjects who played computer games for the same amount of time showed no change in BDNF levels from baseline at either time point. These data indicate that processes related to the specific demands of the active cognitive training condition induced a sustained increase in serum BDNF, separate from the general factors of computer exposure, contact with laboratory personnel, or engagement with enjoyable games. This increase in BDNF correlated with improved quality of life and is consistent with the positive association seen between increased serum BDNF and successful treatment of depression (25,26).
We know of only one other study that examined serum BDNF in schizophrenia in relation to a behavioral intervention—in this case, caloric restriction (34). Serum BDNF levels were significantly higher in patients put on a hypocaloric diet than those not on a diet. Animal research indicates a relationship between caloric intake and central BDNF signaling (35,36), and diet-induced increases in serum BDNF in patients may reflect similar central processes. The increase in serum BDNF levels seen in treatment-responsive depressed patients but not in nonresponders also suggests a relationship between central processes and BDNF measured peripherally (22,23). Brain and serum BDNF levels change in parallel during maturation and aging; serum and cortical BDNF levels are positively correlated (37); and in a recent study of healthy adults, a positive association was demonstrated between serum BDNF and an in vivo spectroscopic imaging marker of cortical neuronal integrity (38). However, the precise nature of the relationship between serum BDNF levels and BDNF signaling in the brain (if any) is unknown, and nothing in our data directly addresses this question.
We must emphasize that despite the increased serum BDNF levels in AT compared with control subjects, there was no relationship between change in BDNF and improved cognition. Thus, the significance of increased BDNF in relation to treatment success—at least as measured by cognitive change scores—is unclear. Nonetheless, its significant positive association with improved quality of life is intriguing and worthy of further investigation. Although we found no improvements at a group level in symptoms or in quality of life immediately after training in these clinically stable, chronically ill participants (27), we have observed a significant correlation between training-induced cognitive gains and improved QLS measures after a 6-month no-contact follow-up in 22 subjects (39). This finding has led us to propose that successful cognitive training may open a critical window for functional improvement in schizophrenia in the period immediately following the intervention (39). Results from our study suggest that increased serum BDNF levels may contribute to these accruing functional gains.
At present, we do not know whether the observed increase in serum BDNF is due to the unique neuroplasticity-based nature of the training or to some other unaccounted feature of the intervention (e.g., emphasis on auditory stimuli, 80% reward schedule). It is unlikely that a sense of “stress” induced these BDNF changes, for nothing in the enjoyment ratings or in observations by laboratory personnel indicated that the cognitive training (which is gamelike) was experienced as more stressful than the commercial computer games. We also do not know whether increased BDNF would be seen in other forms of cognitive remediation or possibly in response to any successful behavioral (or pharmacologic) cognitive intervention. Finally, we do not have longitudinal data on the HC subjects, so we do not know whether similar changes in serum BDNF would be observed in healthy volunteers as a response to the cognitive training or whether this finding is unique to schizophrenia.
Despite these limitations, our results provide tantalizing early evidence of a neurotrophin response induced by neuroplasticity-based cognitive training—but not computer games—in schizophrenia. Further, this biological response is associated with improved quality of life after 10 weeks of treatment. Our data suggest that serum BDNF levels may serve as a peripheral biomarker for the specific effects of the cognitive training, controlling for the general effects of study participation, engaging computer activities, and participant enjoyment. If indeed this turns out to be the case, serum BDNF may provide a useful tool for the evaluation of cognitive enhancement methods in schizophrenia as well as clues regarding the mechanisms through which cognitive enhancement produces functional benefit.
Supplementary Material
Acknowledgments
This work was supported by National Institute of Mental Health RO 1 Grant No. MH068725-01A1, the San Francisco VA Medical Center, and National Institutes of Health (NIH)/National Center for Research Resources University of California, San Francisco-Clinical Translational Science Institute Grant No. UL1 RR024131. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. We thank Alex Genevsky and Laura Roe for their assistance with this project.
Footnotes
The cognitive training software used in this study was supplied to the first author free of charge by Positscience Corporation. None of the authors have any financial interest in Positscience Corporation. The authors report no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online.
References
- 1.Gorski JA, Zeiler SR, Tamowski S, Jones KR. Brain-derived neurotrophic factor is required for the maintenance of cortical dendrites. J Neurol Sci. 2003;23:6856–6865. doi: 10.1523/JNEUROSCI.23-17-06856.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, et al. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- 3.Buckley PF, Mahadik S, Pillai A, Terry A., Jr Neurotrophins and schizophrenia. Schizophr Res. 2007;94:1–11. doi: 10.1016/j.schres.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 4.Iritani S, Niizato K, Nawa H, Ikeda K, Emson PC. Immunohistochemical study of brain-derived neurotrophic factor and its receptor, TrkB, in the hippocampal formation of schizophrenic brains. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:801–807. doi: 10.1016/S0278-5846(03)00112-X. [DOI] [PubMed] [Google Scholar]
- 5.Durany N, Michel T, Zochling R, Boissl KW, Cruz-Sanchez FF, Riederer P, et al. Brain-derived neurotrophic factor and neurotrophin 3 in schizophrenic psychoses. Schizophr Res. 2001;52:79–86. doi: 10.1016/s0920-9964(00)00084-0. [DOI] [PubMed] [Google Scholar]
- 6.Cannon TD, Yolken R, Buka S, Torrey EF. Decreased neurotrophic response to birth hypoxia in the etiology of schizophrenia. Biol Psychiatry. 2008;64:797–802. doi: 10.1016/j.biopsych.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rizos EN, Rontos I, Laskos E, Arsenis G, Michalopoulou PG, Vasilopoulos D, et al. Investigation of serum BDNF levels in drug-naive patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1308–1311. doi: 10.1016/j.pnpbp.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Grillo RW, Ottoni GL, Leke R, Souza DO, Portela LV, Lara DR. Reduced serum BDNF levels in schizophrenic patients on clozapine or typical antipsychotics. J Psychiatr Res. 2007;41:31–35. doi: 10.1016/j.jpsychires.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Pirildar S, Gonul AS, Taneli F, Akdeniz F. Low serum levels of brain-derived neurotrophic factor in patients with schizophrenia do not elevate after antipsychotic treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:709–713. doi: 10.1016/j.pnpbp.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 10.Tan YL, Zhou DF, Cao LY, Zou YZ, Zhang XY. Decreased BDNF in serum of patients with chronic schizophrenia on long-term treatment with antipsychotics. Neurosci Lett. 2005;382:27–32. doi: 10.1016/j.neulet.2005.02.054. [DOI] [PubMed] [Google Scholar]
- 11.Toyooka K, Asama K, Watanabe Y, Muratake T, Takahashi M, Someya T, et al. Decreased levels of brain-derived neurotrophic factor in serum of chronic schizophrenic patients. Psychiatry Res. 2002;110:249–257. doi: 10.1016/s0165-1781(02)00127-0. [DOI] [PubMed] [Google Scholar]
- 12.Shimizu E, Hashimoto K, Watanabe H, Komatsu N, Okamura N, Koike K, et al. Serum brain-derived neurotrophic factor (BDNF) levels in schizophrenia are indistinguishable from controls. Neurosci Lett. 2003;351:111–114. doi: 10.1016/j.neulet.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Jockers-Scherubl MC, Danker-Hopfe H, Mahlberg R, Selig F, Rentzsch J, Schurer F, et al. Brain-derived neurotrophic factor serum concentrations are increased in drug-naive schizophrenic patients with chronic cannabis abuse and multiple substance abuse. Neurosci Lett. 2004;371:79–83. doi: 10.1016/j.neulet.2004.08.045. [DOI] [PubMed] [Google Scholar]
- 14.Huang TL, Lee CT. Associations between serum brain-derived neurotrophic factor levels and clinical phenotypes in schizophrenia patients. J Psychiatr Res. 2006;40:664–668. doi: 10.1016/j.jpsychires.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Zhang XY, Tan YL, Zhou DF, Cao LY, Wu GY, Xu Q, et al. Serum BDNF levels and weight gain in schizophrenic patients on long-term treatment with antipsychotics. J Psychiatr Res. 2007;41:997–1004. doi: 10.1016/j.jpsychires.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Gama CS, Andreazza AC, Kunz M, Berk M, et al. Serum levels of brain-derived neurotrophic factor in patients with schizophrenia and bipolar disorder. Neurosci Lett. 2007;420:45–48. doi: 10.1016/j.neulet.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Reis HJ, Nicolato R, Barbosa IG, Teixeira do Prado PH, Romano-Silva MA, Teixeira AL. Increased serum levels of brain-derived neurotrophic factor in chronic institutionalized patients with schizophrenia. Neurosci Lett. 2008;439:157–159. doi: 10.1016/j.neulet.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 18.Mizuno M, Yamada K, Olariu A, Nawa H, Nabeshima T. Involvement of brain-derived neurotrophic factor in spatial memory formation and maintenance in a radial arm maze test in rats. J Neurol Sci. 2000;20:7116–7121. doi: 10.1523/JNEUROSCI.20-18-07116.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Linnarsson S, Bjorklund A, Ernfors P. Learning deficit in BDNF mutant mice. Eur J Neurosci. 1997;9:2581–2587. doi: 10.1111/j.1460-9568.1997.tb01687.x. [DOI] [PubMed] [Google Scholar]
- 20.Young D, Lawlor PA, Leone P, Dragunow M, During MJ. Environmental enrichment inhibits spontaneous apoptosis, prevents seizures and is neuroprotective. Nat Med. 1999;5:448–453. doi: 10.1038/7449. [DOI] [PubMed] [Google Scholar]
- 21.Gervasoni N, Aubry JM, Bondolfi G, Osiek C, Schwald M, Bertschy G, et al. Partial normalization of serum brain-derived neurotrophic factor in remitted patients after a major depressive episode. Neuropsychobiology. 2005;51:234–238. doi: 10.1159/000085725. [DOI] [PubMed] [Google Scholar]
- 22.Lee HY, Kim YK. Plasma brain-derived neurotrophic factor as a peripheral marker for the action mechanism of antidepressants. Neuropsychobiology. 2008;57:194–199. doi: 10.1159/000149817. [DOI] [PubMed] [Google Scholar]
- 23.Huang TL, Lee CT, Liu YL. Serum brain-derived neurotrophic factor levels in patients with major depression: Effects of antidepressants. J Psychiatr Res. 2008;42:521–525. doi: 10.1016/j.jpsychires.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Shimizu E, Hashimoto K, Okamura N, Koike K, Komatsu N, Kumakiri C, et al. Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol Psychiatry. 2003;54:70–75. doi: 10.1016/s0006-3223(03)00181-1. [DOI] [PubMed] [Google Scholar]
- 25.Sen S, Duman R, Sanacora G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: Metal-analyses and implications. Biol Psychiatry. 2008;64:527–532. doi: 10.1016/j.biopsych.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brunoni AR, Lopes M, Fregni F. A systematic review and metal-analysis of clinical studies on major depression and BDNF levels: Implications for the role of neuroplasticity in depression. Int J Neuropsychopharmacology. 2008;11:1169–1180. doi: 10.1017/S1461145708009309. [DOI] [PubMed] [Google Scholar]
- 27.Fisher M, Holland C, Merzenich M, Vinogradov S. Neuroplasticity-based auditory training improves verbal memory in schizophrenia. Am J Psychiatry. doi: 10.1176/appi.ajp.2009.08050757. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vinogradov S, Fisher M, Warm H, Holland C, Kirshner MA, Pollock BG. The cognitive cost of anticholinergic burden: Decreased response to cognitive training in schizophrenia. Am J Psychiatry. doi: 10.1176/appi.ajp.2009.09010017. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR. New York: Biometrics Research Department, New York State Psychiatric Institute; 2002. (Axis I Disorders, Research Version, Patient Edition (SCID-I/P)). [Google Scholar]
- 30.Kay SR, Sevy S. Pyramidical model of schizophrenia. Schizophr Bull. 1990;16:537–545. doi: 10.1093/schbul/16.3.537. [DOI] [PubMed] [Google Scholar]
- 31.Bilker WB, Brensinger C, Kurtz MM, Kohler C, Gur RC, et al. Development of an abbreviated schizophrenia quality of life scale using a new method. Neuropsychopharmacology. 2003;28:773–777. doi: 10.1038/sj.npp.1300093. [DOI] [PubMed] [Google Scholar]
- 32.Deci EL, Eghrari H, Patrick BC, Leone DR. Facilitating internalization: The self-determination theory perspective. J Pers. 1994;62:119–142. doi: 10.1111/j.1467-6494.1994.tb00797.x. [DOI] [PubMed] [Google Scholar]
- 33.Rizos EN, Papadopoulou A, Laskos E, Michalopoulou PG, Kastania A, Vasilopoulos D, et al. Reduced serum BDNF levels in patients with chronic schizophrenic disorder in relapse, who were treated with typical or atypical antipsychotics. World J Biol Psychiatry. 2008:1–5. doi: 10.3109/15622970802182733. [DOI] [PubMed] [Google Scholar]
- 34.Guimaraes LR, Jacka FN, Gama CS, Berk M, Leitao-Azevedo CL, et al. Serum levels of brain-derived neurotrophic factor in schizophrenia on a hypocaloric diet. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1595–1598. doi: 10.1016/j.pnpbp.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 35.Lee J, Duan W, Long JM, Ingram DK, Mattson MP. Dietary restriction increases the number of newly generated neural cells, and induces BDNF expression, in the dentate gyrus of rats. J Mol Neurosci. 2000;15:99–108. doi: 10.1385/JMN:15:2:99. [DOI] [PubMed] [Google Scholar]
- 36.Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem. 2002;82:1367–1375. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- 37.Karege F, Schwald M, Cisse M. Postnatal developmental profile of brain-derived neurotrophic factor in rat brain and platelets. Neurosci Lett. 2002;328:261–264. doi: 10.1016/s0304-3940(02)00529-3. [DOI] [PubMed] [Google Scholar]
- 38.Lang UE, Hellweg R, Seifert F, Schubert F, Gallinat J. Correlation between serum brain-derived neurotrophic factor level and an in vivo marker of cortical integrity. Biol Psychiatry. 2007;62:530–535. doi: 10.1016/j.biopsych.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 39.Fisher M, Holland C, Subramaniam K, Vinogradov S. Neuroplasticity-based cognitive training in schizophrenia: What are the effects six months later [published online ahead of print March 5]? Schizophr Bull. 2009 doi: 10.1093/schbul/sbn170. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


