Abstract
Asymmetric amplification during self-replication is a key feature that is used to explain the origin of homochirality. Asymmetric autocatalysis of pyrimidyl alkanol in the asymmetric addition of diisopropylzinc to pyrimidine-5-carbaldehyde is a unique example of this phenomenon. Crystallization of zinc alkoxides of this 5-pyrimidyl alkanol and single-crystal X-ray diffraction analysis of the alkoxide crystals reveal the existence of tetramer or higher oligomer structures in this asymmetric autocatalytic system.
Keywords: asymmetric amplification, autocatalysis, enantioselectivity, X-ray diffraction, zinc alkoxide
The origin of biological homochirality, such as that found in l-amino acids, is a fundamental question that has attracted the interest of scientists from a wide range of research areas.[1] Although there are several possible origins of homochirality, propagation and amplification of chirality generated from the initial breaking of symmetry are also key topics for the evolution of homochirality. Asymmetric autocatalysis with amplification of chirality has been suggested as a mechanistic model for the evolution of homochirality. In this reaction, a chiral product serves as an asymmetric catalyst to produce more of itself; the process is thus an automultiplication of the chiral compound.
We found asymmetric autocatalysis with amplification of the enantiomeric excess (ee) in a real chemical reaction (Scheme 1).[2–4] When diisopropylzinc (iPr2Zn) is added to pyrimidine-5-carbaldehyde 1 in the presence of a catalytic amount of (S)-pyrimidyl alkanol 2 with a low ee, asymmetric autocatalytic amplification of the ee produces (S)-2 with high ee as the final product. Early autocatalytic work with pyrimidine-5-carbaldehyde[2a] and 2-methylpyrimidine-5-carbaldehyde was succeeded by the use of superior 2-alkynyl analogues[2b] and the amplification efficiency was dramatically increased to obtain product 2 with more than 99.5 % ee after consecutive asymmetric autocatalytic amplification, even when the initial catalyst has only ca. 5×10−5 % ee. This unique property means that, in addition to chiral molecules, a trace imbalance of chirality induced by chiral triggers such as circularly polarized light,[5] crystal chirality,[6] various chiral materials,[7] or isotope chirality[8] can also be amplified to afford enantioenriched alkanol 2 with corresponding absolute configurations. Furthermore, spontaneous absolute asymmetric synthesis can be achieved by using this reaction.[9]
Scheme 1.
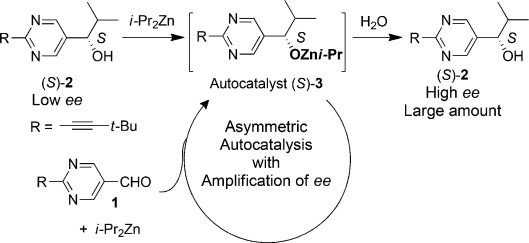
Asymmetric autocatalytic reaction of pyrimidyl alkanol.
Although the theory of asymmetric amplification during self-replication was first proposed in 1953,[10] the above reaction remains the only practical example of a chemical reaction involving asymmetric autocatalysis that leads to high levels of amplification of ee. Several studies have been undertaken to understand the mechanism of this reaction. The large amplification of ee observed in this autocatalytic reaction is often explained by the formation of aggregates, which has been proposed to account for the positive nonlinear effect observed during asymmetric catalysis.[11] Mechanistic studies based on kinetic experiments,[12] reaction modeling,[13] NMR,[14] and DFT calculations[15] have been examined by several research groups. Early analysis of autocatalysis kinetics showed this to be incompatible with the reactive monomer model from non-autocatalytic asymmetric zinc alkylation,[11c] and indicated that a homochiral dimer was the active entity, with heterochiral dimers being inactive.[12b] Dimeric catalysis with a tetramic transition-state cycle was then suggested based on the nearly second-order kinetics with respect to the aldehyde.[12d] NMR spectroscopy also supported the existence of dimer species[14b] and DFT calculations based on this mechanism suggested a difference in catalytic activity between enantiopure and racemic dimers.[15d] However, the observed significant amplification efficiency[12a],[c] cannot be fully explained by these models. The inclusion of tetramers or higher oligomers in the reaction system was proposed.[15c],[e] Recently, NMR studies revealed the existence of tetramers or higher species and kinetics studies showed an inverse temperature dependence of the reaction, which also support the involvement of tetramers or higher oligomers. [14c,d] Furthermore, various possible conformations of oligomers were calculated by DFT method.[15h] Irrespective of whether the active catalyst or resting state is a dimer or higher oligomer(s), the formation of an aggregate seems to be a key feature of this reaction. However, no detailed structural study of the asymmetric autocatalyst has been undertaken by X-ray diffraction analysis.[16]
Herein, we report the single-crystal X-ray analysis of the asymmetric autocatalyst 3, that is, the isopropylzinc alkoxide of 2, and reveal that, depending on the conditions, enantiopure and racemic alkoxides can have completely different structures (Scheme 2).
Scheme 2.
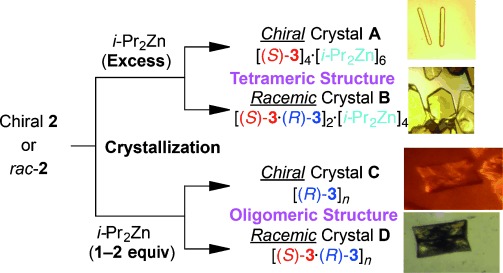
Crystallization of the zinc alkoxide of 5-pyrimidyl alkanol.
Single crystals of enantiopure zinc alkoxide were generated by reacting enantiopure (S)-5-pyrimidyl alkanol 2 with excess neat iPr2Zn (8.4 equiv) at room temperature; the alkanol was dissolved by heating at 80 °C and single crystals were obtained after allowing the solution to stand at room temperature for 1–2 weeks. Racemic zinc alkoxide was obtained similarly by reacting racemic alkanol 2 with an excess of neat iPr2Zn (8.4 equiv).
Single-crystal X-ray diffraction analysis, which was performed at 100 K, revealed that the enantiopure alkoxides form a tetramer structure in the orthorhombic space group P212121 (Crystal A, Figure 1 a), and that racemic alkoxides form a tetramer structure in the monoclinic space group C2/c (Crystal B, Figure 1 b). In both structures, two alkoxides form Zn2O2 square dimers and two dimers coordinate to each other to form a 12-membered macrocycle through nitrogen coordination to the zinc atom in a Zn2O2 square; both structures contain coordinatively unsaturated zinc. In the racemic tetramer B, (R)- and (S)-alkoxides form Zn2O2 square dimers, and macrocycle were also constructed with (R)- and (S)-alkoxides. This Zn2O2 square and macrocycle tetramer structure has good accordance with the proposed stable tetramer structure by DFT calculations including steric hindrance of isopropyl group.16c In the chiral tetramer A, six iPr2Zn moieties coordinate to the nitrogen atoms that do not coordinate to the Zn2O2 square. These coordinated iPr2Zn are activated by nitrogen coordination, with C-Zn-C angles of 156-164°, and with a C–Zn bond length of 1.99–2.01 Å.[16–18] In the racemic alkoxide crystal B, four iPr2Zn moieties coordinate.
Figure 1.
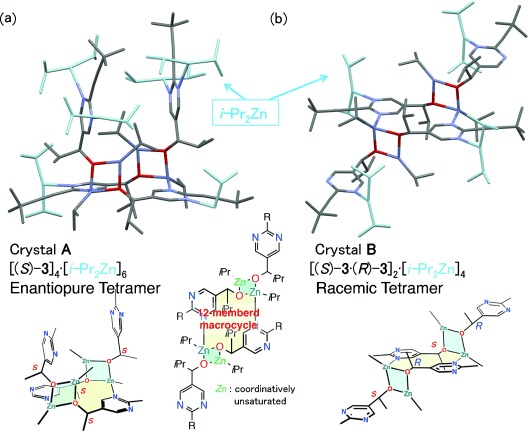
Single-crystal X-ray structures and simplified schematic drawing of alkoxide tetramer crystalized with excess iPr2Zn. a) Enantiopure alkoxide tetramer crystal A. b) Racemic alkoxide tetramer crystal B.
The most remarkable difference between enantiopure and racemic alkoxide is the direction of the outer alkoxide groups. In the racemic tetramer B, two Zn2O2 rings are located on the side opposite to the 12-membered macrocycle and the pyrimidyl alkoxide is also located on the opposite side. In contrast, in the chiral tetramer A, the Zn2O2 rings are located on the same face as the 12-membered macrocycle and the pyrimidyl alkoxides form a face-to-face structure. The Zn–N distance in the 12-membered macrocycle is slightly extended because of this face-to-face structure (Zn–N distance of 2.20 and 2.26 Å for racemic B and enantiopure A crystal structures, respectively). Thus, the enantiopure tetramer seems to be less stable than the racemic tetramer.[19]
Zinc alkoxide with oligomeric structures16c was also observed upon changing the crystallization conditions. Mixing 1–2 equivalents of iPr2Zn with either enantiopure or racemic alkanol afforded Crystals C and D, respectively, with different structures (Figure 2). Similar to the tetramer structure, Zn2O2 dimer structures were bridged through coordination of the zinc atoms to the pyrimidine nitrogen atoms. In these structures, Zn–N coordination led to a one-dimensional (1D) oligomer structure. Although the crystal packing and parameters are different for the enantiopure and racemic oligomer crystals, in contrast to the tetramer structures, the 1D oligomer structures of the alkoxides (for example, the angle of the Zn2O2 square) are quite similar in the enantiopure (C) and racemic (D) versions. These results suggest that higher oligomeric structures of both enantiopure and racemic alkoxides exist in the reaction mixture as an equilibrium that depends on the concentration of the alkoxide and iPr2Zn.
Figure 2.
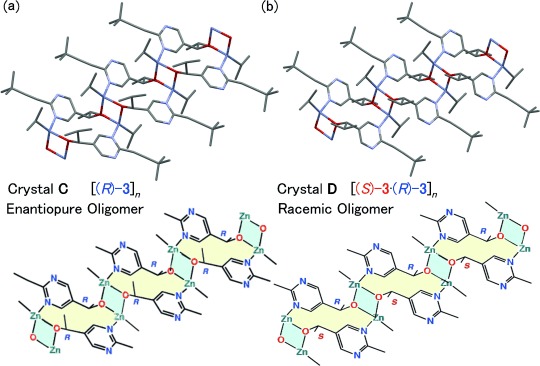
Single-crystal X-ray structures and simplified schematic drawing of alkoxide oligomer crystallized with 1–2 equiv iPr2Zn. a) Enantiopure alkoxide oligomer crystal C. b) Racemic alkoxide oligomer crystal D.
In the tetramer crystal A, the coordinatively unsaturated zinc is considered to play an important role in the coordination and activation of the aldehyde. When enantiopure catalyst is crystallized in the presence of THF, a highly coordinating solvent, THF occupies the coordination site of the tricoordinating zinc atom to form a crystal E of a different structure from crystal A (Figure 3). This result is thus consistent with the fact that the asymmetric autocatalytic reaction of pyrimidyl alkanol is suppressed in the presence of THF.[15b]
Figure 3.
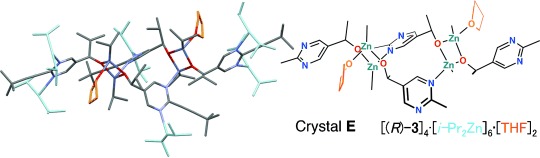
THF-coordinated structure of enantiopure tetramer Crystal E.
In conclusion, we have obtained single crystals of the zinc alkoxide of pyrimidyl alkanol. The X-ray structure analysis of these crystals constitutes the first direct observation of the aggregate structure of an asymmetric autocatalyst. Both enantiopure and racemic alkoxides form either tetramer or higher oligomeric structures, the form of which changes dramatically depending on the enantiopurity, amount of iPr2Zn, and solvent. We believe that these coordinative tetramer structures may be key for delivering the high enantiomeric amplification and spontaneous symmetry breaking in the asymmetric autocatalytic reaction of pyrimidyl alkanol.
Experimental Section
Experimental Details for the preparation of Crystals A–E and X-ray diffraction analysis were described in supporting information. CCDC 1420664 (crystal A), 1420666 (crystal B), 1420663 (crystal C), 1420665 (crystal D), and 1420667 (crystal E) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
Acknowledgments
This work has been financially supported by Grant-in-Aid for Scientific Research from Japan Society for the Promotion of Science (JSPS KAKENHI Grant Numbers 23685012, 26810026 & 15H03781) and MEXT-Supported Program for the Strategic Research Foundation at Private Universities, 2012–2016.
Supplementary material
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
References
- 1a.Weissbuch I, Addadi L, Berkovitch-Yellin Z, Gati E, Lahav M, Leiserowitz L. Nature. 1984;310:161–164. [Google Scholar]
- 1b.Kondepudi DK, Kaufman RJ, Singh N. Science. 1990;250:975–976. doi: 10.1126/science.250.4983.975. [DOI] [PubMed] [Google Scholar]
- 1c.Bolli M, Micura R, Eschenmoser A. Chem. Biol. 1997;4:309–320. doi: 10.1016/s1074-5521(97)90074-0. [DOI] [PubMed] [Google Scholar]
- 1d.Siegel JS. Chirality. 1998;10:24–27. [Google Scholar]
- 1e.Feringa BL, van Delden RA. Angew. Chem. Int. Ed. 1999;38:3418–3438. doi: 10.1002/(sici)1521-3773(19991203)38:23<3418::aid-anie3418>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 1999;111:3624–3645. [Google Scholar]
- 1f.Green MM, Park J-W, Sato T, Teramoto A, Lifson S, Selinger RLB, Selinger JV. Angew. Chem. Int. Ed. 1999;38:3138–3154. doi: 10.1002/(sici)1521-3773(19991102)38:21<3138::aid-anie3138>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 1999;111:3328–3345. [Google Scholar]
- 1g.Ribó JM, Crusats J, Sagués F, Claret J, Rubires R. Science. 2001;292:2063–2066. doi: 10.1126/science.1060835. [DOI] [PubMed] [Google Scholar]
- 1h.Zepik H, Shavit E, Tang M, Jensen TR, Kjaer K, Bolbach G, Leiserowitz L, Weissbuch I, Lahav M. Science. 2002;295:1266–1269. doi: 10.1126/science.1065625. [DOI] [PubMed] [Google Scholar]
- 1i.Mislow K. Collect. Czech. Chem. Commun. 2003;68:849–864. [Google Scholar]
- 1j.Pizzarello S, Weber AL. Science. 2004;303:1151. doi: 10.1126/science.1093057. [DOI] [PubMed] [Google Scholar]
- 1k.Viedma C. Phys. Rev. Lett. 2005;94:065504. doi: 10.1103/PhysRevLett.94.065504. [DOI] [PubMed] [Google Scholar]
- 1l.Soloshonok VA, Ueki H, Yasumoto M, Mekala S, Hirschi JS, Singleton DA. J. Am. Chem. Soc. 2007;129:12112–12113. doi: 10.1021/ja065603a. [DOI] [PubMed] [Google Scholar]
- 1m.Breslow R, Cheng Z-L. Proc. Natl. Acad. Sci. USA. 2010;107:5723–5725. doi: 10.1073/pnas.1001639107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1n.Weissbuch I, Lahav M. Chem. Rev. 2011;111:3236–3267. doi: 10.1021/cr1002479. [DOI] [PubMed] [Google Scholar]
- 1o.Ernst K-H. Phys. Status Solidi B. 2012;249:2057–2088. [Google Scholar]
- 1p.Saito Y, Hyuga H. Rev. Mod. Phys. 2013;85:603–621. [Google Scholar]
- 1q.Ribó JM, Blanco C, Crusats J, El-Hachemi Z, Hochberg D, Moyano A. Chem. Eur. J. 2014;20:17250–17271. doi: 10.1002/chem.201404534. [DOI] [PubMed] [Google Scholar]
- 1r.Olsson S, Björemark PM, Kokoli T, Sundberg J, Lennartson A, McKenzie CJ, Håkansson M. Chem. Eur. J. 2015;21:5211–5219. doi: 10.1002/chem.201406354. [DOI] [PubMed] [Google Scholar]
- 2a.Soai K, Shibata T, Morioka H, Choji K. Nature. 1995;378:767. [Google Scholar]
- 2b.Shibata T, Yonekubo S, Soai K. Angew. Chem. Int. Ed. 1999;38:659–661. doi: 10.1002/(SICI)1521-3773(19990301)38:5<659::AID-ANIE659>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 1999;111:746–748. [Google Scholar]
- 2c.Sato I, Urabe H, Ishiguro S, Shibata T, Soai K. Angew. Chem. Int. Ed. 2003;42:315–317. doi: 10.1002/anie.200390105. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 2003;115:329–331. [Google Scholar]
- 3a.Soai K, Kawasaki T. Top. Curr. Chem. 2007;284:1–31. [Google Scholar]
- 3b.Kawasaki T, Soai K. Bull. Chem. Soc. Jpn. 2011;84:879–892. [Google Scholar]
- 3c.Soai K, Kawasaki T. In: Asymmetric Autocatalysis - Discovery and State of The Art. In The Soai Reaction and Related Topic. Pályi G, Zicchi C, Caglioti L, editors. Modena, Edizioni Artestampa: Academia Nationale di Scienze Lettere e Arti Modena; 2012. pp. 9–34. [Google Scholar]
- 3d.Kawasaki T, Soai K. Isr. J. Chem. 2012;52:582–590. [Google Scholar]
- 3e.Kawasaki T, Sato I, Mineki H, Matsumoto A, Soai K. Yuki Gosei Kagaku Kyokaishi. 2013;71:109–123. [Google Scholar]
- 3f.Soai K, Kawasaki T. Top. Organomet. Chem. 2013;44:261–279. [Google Scholar]
- 3g.Soai K, Kawasaki T, Matsumoto A. Chem. Rec. 2014;14:70–83. doi: 10.1002/tcr.201300028. [DOI] [PubMed] [Google Scholar]
- 3h.Soai K, Kawasaki T, Matsumoto A. Acc. Chem. Res. 2014;47:3643–3654. doi: 10.1021/ar5003208. [DOI] [PubMed] [Google Scholar]
- 4a.Avalos M, Babiano R, Cintas P, Jiménez JL, Palacios JC. Chem. Commun. 2000:887–892. doi: 10.1021/jo000907f. [DOI] [PubMed] [Google Scholar]
- 4b.Blackmond DG. Proc. Natl. Acad. Sci. USA. 2004;101:5732–5736. doi: 10.1073/pnas.0308363101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4c.Podlech J, Gehring T. Angew. Chem. Int. Ed. 2005;44:5776–5777. doi: 10.1002/anie.200501742. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 2005;117:5922–5924. [Google Scholar]
- 4d.Brown JM, Gridnev ID, Klankermayer J. In: Amplification of Chirality, Vol. 284. Soai K, editor. Berlin: Springer; 2008. pp. 35–65. “Topics in Current Chemistry”. [Google Scholar]
- 4e.Gehring T, Busch M, Schlageter M, Weingand D. Chirality. 2010;22:E173–E182. doi: 10.1002/chir.20849. [DOI] [PubMed] [Google Scholar]
- 4f.Barabás B, Tóth J, Pályi G. J. Math. Chem. 2010;48:457–489. [Google Scholar]
- 4g.Lente G. Symmetry. 2010;2:767–798. [Google Scholar]
- 4h.Micheau J-C, Coudret C, Buhse T. In: The Soai Reaction and Related Topic. Pályi G, Zucchi C, Caglioti L, editors. Editioni Artestampa, Modena: Accad. Nazl. Sci. Lett. Arti; 2012. p. 169. [Google Scholar]
- 4i.Bissette AJ, Fletcher SP. Angew. Chem. Int. Ed. 2013;52:12800–12826. doi: 10.1002/anie.201303822. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 2013;125:13034–13061. [Google Scholar]
- 5a.Sato I, Sugie R, Matsueda Y, Furumura Y, Soai K. Angew. Chem. Int. Ed. 2004;43:4490–4492. doi: 10.1002/anie.200454162. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 2004;116:4590–4592. [Google Scholar]
- 5b.Kawasaki T, Sato M, Ishiguro S, Saito T, Morishita Y, Sato I, Nishino H, Inoue Y, Soai K. J. Am. Chem. Soc. 2005;127:3274–3275. doi: 10.1021/ja0422108. [DOI] [PubMed] [Google Scholar]
- 6a.Soai K, Osanai S, Kadowaki K, Yonekubo S, Shibata T, Sato I. J. Am. Chem. Soc. 1999;121:11235–11236. [Google Scholar]
- 6b.Sato I, Kadowaki K, Soai K. Angew. Chem. Int. Ed. 2000;39:1510–1512. doi: 10.1002/(sici)1521-3773(20000417)39:8<1510::aid-anie1510>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 2000;112:1570–1572. [Google Scholar]
- 6c.Kawasaki T, Suzuki K, Hakoda Y, Soai K. Angew. Chem. Int. Ed. 2008;47:496–499. doi: 10.1002/anie.200703634. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 2008;120:506–509. [Google Scholar]
- 6d.Shindo H, Shirota Y, Niki K, Kawasaki T, Suzuki K, Araki Y, Matsumoto A, Soai K. Angew. Chem. Int. Ed. 2013;52:9135–9138. doi: 10.1002/anie.201304284. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 2013;125:9305–9308. [Google Scholar]
- 6e.Mineki H, Kaimori Y, Kawasaki T, Matsumoto A, Soai K. Tetrahedron: Asymmetry. 2013;24:1365–1367. [Google Scholar]
- 6f.Kawasaki T, Uchida M, Kaimori Y, Sasagawa T, Matsumoto A, Soai K. Chem. Lett. 2013;42:711–713. [Google Scholar]
- 6g.Matsumoto A, Ide T, Kaimori Y, Fujiwara S, Soai K. Chem. Lett. 2015;44:688–690. [Google Scholar]
- 7a.Sato I, Kadowaki K, Urabe H, Jung JH, Ono Y, Shinkai S, Soai K. Tetrahedron Lett. 2003;44:721–724. [Google Scholar]
- 7b.Hitosugi S, Matsumoto A, Kaimori Y, Iizuka R, Soai K, Isobe H. Org. Lett. 2014;16:645–647. doi: 10.1021/ol403384q. [DOI] [PubMed] [Google Scholar]
- 7c.Kawasaki T, Araki Y, Hatase K, Suzuki K, Matsumoto A, Yokoi T, Kubota Y, Tatsumi T, Soai K. Chem. Commun. 2015;51:8742–8744. doi: 10.1039/c5cc01750e. [DOI] [PubMed] [Google Scholar]
- 8a.Sato I, Omiya D, Saito T, Soai K. J. Am. Chem. Soc. 2000;122:11739–11740. [Google Scholar]
- 8b.Kawasaki T, Matsumura Y, Tsutsumi T, Suzuki K, Ito M, Soai K. Science. 2009;324:492–495. doi: 10.1126/science.1170322. [DOI] [PubMed] [Google Scholar]
- 8c.Kawasaki T, Shimizu M, Nishiyama D, Ito M, Ozawa H, Soai K. Chem. Commun. 2009:4396–4398. doi: 10.1039/b908754k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8d.Kawasaki T, Okano Y, Suzuki E, Takano S, Oji S, Soai K. Angew. Chem. Int. Ed. 2011;50:8131–8133. doi: 10.1002/anie.201102263. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 2011;123:8281–8283. [Google Scholar]
- 8e.Kawasaki T, Ozawa H, Ito M, Soai K. Chem. Lett. 2011;40:320–321. [Google Scholar]
- 8f.Matsumoto A, Oji S, Takano S, Tada K, Kawasaki T, Soai K. Org. Biomol. Chem. 2013;11:2928–2931. doi: 10.1039/c3ob40293b. [DOI] [PubMed] [Google Scholar]
- 9a.Soai K, Sato I, Shibata T, Komiya S, Hayashi M, Matsueda Y, Imamura H, Hayase T, Morioka H, Tabira H, Yamamoto J, Kowata Y. Tetrahedron: Asymmetry. 2003;14:185–188. [Google Scholar]
- 9b.Singleton DA, Vo LK. Org. Lett. 2003;5:4337–4339. doi: 10.1021/ol035605p. [DOI] [PubMed] [Google Scholar]
- 9c.Kawasaki T, Suzuki K, Shimizu M, Ishikawa K, Soai K. Chirality. 2006;18:479–482. doi: 10.1002/chir.20273. [DOI] [PubMed] [Google Scholar]
- 9d.Suzuki K, Hatase K, Nishiyama D, Kawasaki T, Soai K. J. Syst. Chem. 2010;1:5. [Google Scholar]
- 10.Frank FC. Biochim. Biophys. Acta. 1953;11:459–463. doi: 10.1016/0006-3002(53)90082-1. [DOI] [PubMed] [Google Scholar]
- 11a.Puchot C, Samuel O, Dunach E, Zhao S, Agami C, Kagan HB. J. Am. Chem. Soc. 1986;108:2353–2357. doi: 10.1021/ja00269a036. [DOI] [PubMed] [Google Scholar]
- 11b.Oguni N, Matsuda Y, Kaneko T. J. Am. Chem. Soc. 1988;110:7877–7878. [Google Scholar]
- 11c.Kitamura M, Okada S, Suga S, Noyori R. J. Am. Chem. Soc. 1989;111:4028–4036. [Google Scholar]
- 11d.Satyanarayana T, Abraham S, Kagan HB. Angew. Chem. Int. Ed. 2009;48:456–494. doi: 10.1002/anie.200705241. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 2009;121:464–503. [Google Scholar]
- 12a.Sato I, Omiya D, Tsukiyama K, Ogi Y, Soai K. Tetrahedron: Asymmetry. 2001;12:1965–1969. [Google Scholar]
- 12b.Blackmond DG, McMillan CR, Ramdeehul S, Schorm A, Brown JM. J. Am. Chem. Soc. 2001;123:10103–10104. doi: 10.1021/ja0165133. [DOI] [PubMed] [Google Scholar]
- 12c.Sato I, Omiya D, Igarashi H, Kato K, Ogi Y, Tsukiyama K, Soai K. Tetrahedron: Asymmetry. 2003;14:975–979. [Google Scholar]
- 12d.Buono FG, Blackmond DG. J. Am. Chem. Soc. 2003;125:8978–8979. doi: 10.1021/ja034705n. [DOI] [PubMed] [Google Scholar]
- 13a.Buhse T. Tetrahedron: Asymmetry. 2003;14:1055–1061. [Google Scholar]
- 13b.Islas JR, Lavabre D, Grevy J-M, Lamoneda RH, Cabrera HR, Micheau J-C, Buhse T. Proc. Natl. Acad. Sci. USA. 2005;102:13743–13748. doi: 10.1073/pnas.0503171102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13c.Lente G. J. Phys. Chem. A. 2005;109:11058–11063. doi: 10.1021/jp054613f. [DOI] [PubMed] [Google Scholar]
- 13d.Micskei K, Póta G, Caglioti L, Pályi G. J. Phys. Chem. A. 2006;110:5982–5984. doi: 10.1021/jp0614502. [DOI] [PubMed] [Google Scholar]
- 13e.Barabás B, Caglioti L, Zucchi C, Maioli M, Gal E, Micskei KK, Pályi G. J. Phys. Chem. B. 2007;111:11506–11510. doi: 10.1021/jp072945+. [DOI] [PubMed] [Google Scholar]
- 13f.Micskei K, Rábai G, Gál E, Caglioti L, Pályi G. J. Phys. Chem. B. 2008;112:9196–9200. doi: 10.1021/jp803334b. [DOI] [PubMed] [Google Scholar]
- 13g.Busch M, Schlageter M, Weingand D, Gehring T. Chem. Eur. J. 2009;15:8251–8258. doi: 10.1002/chem.200900634. [DOI] [PubMed] [Google Scholar]
- 13h.Crusats J, Hochberg D, Moyano A, Ribó JM. ChemPhysChem. 2009;10:2123–2131. doi: 10.1002/cphc.200900181. [DOI] [PubMed] [Google Scholar]
- 13i.Dóka É, Lente G. J. Am. Chem. Soc. 2011;133:17878–17881. doi: 10.1021/ja207408y. [DOI] [PubMed] [Google Scholar]
- 13j.Micheau J-C, Coudret C, Cruz J-M, Buhse T. Phys. Chem. Chem. Phys. 2012;14:13239–13248. doi: 10.1039/c2cp42041d. [DOI] [PubMed] [Google Scholar]
- 13k.Ercolani G. Tetrahedron: Asymmetry. 2014;25:405–410. [Google Scholar]
- 13l.Barabás B, Zucchi C, Maioli M, Micskei K, Pályi G. J. Mol. Model. 2015;21:33. doi: 10.1007/s00894-015-2576-6. [DOI] [PubMed] [Google Scholar]
- 14a.Gridnev ID, Serafimov JM, Quiney H, Brown JM. Org. Biomol. Chem. 2003;1:3811–3819. doi: 10.1039/b307382n. [DOI] [PubMed] [Google Scholar]
- 14b.Gridnev ID, Serafimov JM, Brown JM. Angew. Chem. Int. Ed. 2004;43:4884–4887. doi: 10.1002/anie.200353572. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 2004;116:4992–4995. [Google Scholar]
- 14c.Quaranta M, Gehring T, Odell B, Brown JM, Blackmond DG. J. Am. Chem. Soc. 2010;132:15104–15107. doi: 10.1021/ja103204w. [DOI] [PubMed] [Google Scholar]
- 14d.Gehring T, Quaranta M, Odell B, Blackmond DG, Brown JM. Angew. Chem. Int. Ed. 2012;51:9539–9542. doi: 10.1002/anie.201203398. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 2012;124:9677–9680. [Google Scholar]
- 15a.Gridnev ID, Brown JM. Proc. Natl. Acad. Sci. USA. 2004;101:5727–5731. doi: 10.1073/pnas.0308178101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15b.Gridnev ID. Chem. Lett. 2006;35:148–153. [Google Scholar]
- 15c.Klankermayer JJ, Gridnev ID, Brown JM. Chem. Commun. 2007:3151–3153. doi: 10.1039/b705978g. [DOI] [PubMed] [Google Scholar]
- 15d.Schiaffino L, Ercolani G. Angew. Chem. Int. Ed. 2008;47:6832–6835. doi: 10.1002/anie.200802450. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 2008;120:6938–6941. [Google Scholar]
- 15e.Schiaffino L, Ercolani G. ChemPhysChem. 2009;10:2508–2515. doi: 10.1002/cphc.200900369. [DOI] [PubMed] [Google Scholar]
- 15f.Schiaffino L, Ercolani G. Chem. Eur. J. 2010;16:3147–3156. doi: 10.1002/chem.200902543. [DOI] [PubMed] [Google Scholar]
- 15g.Ercolani G, Schiaffino L. J. Org. Chem. 2011;76:2619–2626. doi: 10.1021/jo102525t. [DOI] [PubMed] [Google Scholar]
- 15h.Gridnev ID, Vorobiev AK. ACS Catal. 2012;2:2137–2149. [Google Scholar]
- 16.Lennartson A, Hedström A, Håkansson M. Acta Crystallogr. Sect. E. 2007;63:m123–m125. X-ray crystal structure of another iPr2Zn complex. [Google Scholar]
- 17.Hursthouse MB, Motevalli M, O′Brien P, Walsh JR, Jones AC. J. Mater. Chem. 1991;1:139–140. [Google Scholar]
- 18.Bacsa J, Hanke F, Hindley S, Odedra R, Darling GR, Jones AC, Steiner A. Angew. Chem. Int. Ed. 2011;50:11685–11687. doi: 10.1002/anie.201105099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angew. Chem. 2011;123:11889–11891. [Google Scholar]
- 19. Although the crystal structure cannot be compared directly with the solution structure, this face-to-face conformation of the enantiopure tetramer is quite different from the suggested stable conformation from previous DFT studies (e.g. Ref. [16h]). Preliminary DFT calculations on both tetramer structures based on this crystal structure after removing the coordinating iPr2Zn were performed at the the B3LYP/6–311+G(d,p)//B3LYP/6–31G(d) level. The results of these calculations starting from the observed X-ray structure conformations indicate that the enantiopure alkoxide has a higher energy than the more open racemic tetramer by 4.8 kcal mol−1.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


