Abstract
Background & Aims
Hepatocellular carcinoma (HCC) is the second most common cause of cancer deaths worldwide. The global HCC BRIDGE study was a multiregional, large-scale, longitudinal cohort study undertaken to improve understanding of real-life management of patients with HCC, from diagnosis to death.
Methods
Data were collected retrospectively from January 2005 to September 2012 by chart reviews of eligible patients newly diagnosed with HCC at participating institutions.
Results
Forty-two sites in 14 countries contributed final data for 18 031 patients. Asia accounted for 67% of patients, Europe for 20% and North America for 13%. As expected, the most common risk factor was hepatitis C virus in North America, Europe and Japan, and hepatitis B virus in China, South Korea and Taiwan. The most common Barcelona Clinic Liver Cancer stage at diagnosis was C in North America, Europe, China and South Korea, and A in Taiwan and Japan. Across all stages, first HCC treatment was most frequently transarterial chemoembolization in North America, Europe, China and South Korea, percutaneous ethanol injection or radiofrequency ablation in Japan and resection in Taiwan. Survival from first HCC treatment varied significantly by region, with median overall survival not reached for Taiwan and 60, 33, 31, 24 and 23 months for Japan, North America, South Korea, Europe and China respectively (P < 0.0001).
Conclusions
Initial results from the BRIDGE study confirm previously reported regional trends in patient demographic characteristics and HCC risk factors, document the heterogeneity of treatment approaches across regions/countries and underscore the need for earlier HCC diagnosis worldwide.
Keywords: disease management, epidemiology, global trends, liver cancer, observational study, risk factors, treatment patterns
Key Points
The global HCC BRIDGE (‘Bridge to Better Outcomes in HCC’) study was the first multiregional, large-scale, longitudinal cohort study to document the HCC patient experience from diagnosis to death.
The objective was to provide an improved understanding of global patterns of HCC therapy and associated outcomes across real-world clinical practice.
The study showed the pattern of initial and second recorded treatments in real practice.
These results confirm previously reported regional trends in patient demographic characteristics and HCC risk factors, document treatment heterogeneity across regions/countries, and underscore the need for earlier HCC diagnosis worldwide.
Worldwide, liver cancer is the fifth most common cancer in men and ninth most common in women (1), with hepatocellular carcinoma (HCC) accounting for >90% of primary liver cancer cases (2). In 2012, there were approximately 782 000 new cases and 746 000 deaths from liver cancer worldwide, making it the second most common cause of cancer deaths (after lung cancer) (1). The greatest burden of HCC is in the developing world, with cases in eastern and southeastern Asia, and central and western Africa accounting for more than 80% of the total; 50% of all cases occur in China alone (1).
In most cases, HCC develops in cirrhotic livers, and cirrhosis is the strongest risk factor for the disease (3). The variation in incidence and prevalence of HCC by geographical region is primarily a result of regional differences in exposure to causal factors for cirrhosis, such as hepatitis B virus (HBV) in Asia and sub-Saharan Africa and hepatitis C virus (HCV) in the West and Japan (2–4). Dietary ingestion of fungal aflatoxins has also been recognized as a major risk factor in southern Asia and sub-Saharan Africa (5). Although the incidence of HCC has historically been low in North America and Europe, there is evidence for a significant upward trend in the United States in recent years (6,7), which has been attributed to an increased prevalence of HCV infection (8). The rise in obesity and diabetes worldwide, particularly in North America and Europe, is also leading to recognition of non-alcoholic fatty liver disease as a significant contributor to the aetiology of HCC (8).
Potentially curative treatments for HCC include surgery (resection or transplant), radiofrequency ablation (RFA) and percutaneous ethanol injection (PEI); approximately 30–40% of HCC patients globally who are diagnosed with very early (Stage 0) or early (Stage A) disease are eligible for these procedures (2,9–11). For patients with intermediate stage disease (Stage B), transarterial chemoembolization (TACE) is recommended to establish local control and palliation (3).
Approximately 25–70% of patients with HCC are diagnosed with advanced-stage disease, which is regarded as incurable (6,7,12–14). Patients with advanced HCC have limited treatment options, and chemotherapy provides minimal clinical benefit (14). Sorafenib, a multitargeted kinase inhibitor, is the only systemic agent shown to extend overall survival (OS) compared with placebo in patients with advanced HCC (15). However, the survival benefit with sorafenib is modest (a 2- to 3-month extension in median OS compared with placebo in two phase III trials) (16,17), and there is a pressing need for more effective therapies for patients with advanced HCC.
The outcome for patients with HCC treated in randomized clinical trials is unlikely to fully reflect outcomes in daily clinical practice, as the patient populations in each setting are likely to differ considerably (18). Clinical trials employ strict eligibility criteria and, in the case of HCC, are often limited to patients with good liver function to avoid confounding results. Because patients treated in clinical practice may have less thorough follow-up and patient counselling, compliance may be reduced compared with that in clinical trials. Real-world observational studies are needed to gain better insight into the management of patients with HCC (19).
The global HCC BRIDGE (‘Bridge to Better Outcomes in HCC’) study was the first multiregional, large-scale, longitudinal cohort study to document the HCC patient experience from diagnosis to death, and aimed to include all patients, regardless of treatment received (20). The objective was to provide an improved understanding of global patterns of HCC therapy and associated outcomes across real-world clinical practice, with data collected retrospectively from patient charts. Patients were recruited from Asia, Europe and North America, and data were captured for both systemic and non-systemic treatments with the intent to assess HCC management in the real-world setting and compare it with that recommended by therapeutic guidelines, for example the Barcelona Clinic Liver Cancer (BCLC) guidelines recommended by the American Association for the Study of Liver Diseases (AASLD) (9) and the European Association for the Study of the Liver (EASL) (2).
Here, we describe the methodology of the BRIDGE study and present an overview of the final data from the full cohort of study patients.
Patients and methods
Study design, patients and data collection
The BRIDGE study was a real-world, observational, longitudinal cohort study, with data collected from 1 January 2005 to 30 September 2012. The primary objective was to assess current treatment approaches and associated clinical outcomes in HCC. Secondary objectives were to assess and compare the characteristics of patients with HCC treated with sorafenib or with other therapies in the same time period, and to evaluate the treatment pattern and resource use. The study was done in accordance with ethical principles based on those in the current Declaration of Helsinki, and was consistent with International Conference on Harmonization Good Clinical Practice guidelines, Good Epidemiology Practices and applicable regulatory requirements.
Eligible patients were male or female; aged 18 years or older; newly diagnosed with HCC between 1 January 2005 and 30 June 2011 in accordance with AASLD, EASL or comparable local guidelines (2,9,21,22); and who received or were receiving HCC treatment through a selected study site. Patients whose primary treatment was via participation in a randomized clinical trial were excluded; similarly, patients who, at a later point in time consented to take part in a clinical trial, were withdrawn, with the exception of patients entering single-arm trials or adjuvant treatment trials. Other exclusion criteria were unknown date of HCC diagnosis or unknown date of first visit for HCC at a given study site.
Sites were instructed to enrol all eligible patients on a sequential basis, with data to be extracted on a rolling basis from patient charts by personnel at the study site. A selection scheme was employed to cap enrolment at specific sites when the number of eligible patients exceeded the number allowed by power calculation. Seasonality was avoided by distributing the number of patients entered in a given cohort year based on month of diagnosis (e.g. if 120 patients were entered as part of a cohort, the first 10 eligible patients diagnosed each month would be entered). Study data were entered into a web-based, electronic data-capture system developed by Outcome Sciences, Inc. (Cambridge, MA, USA), and subject to rigorous monthly monitoring and cleaning. Key data collected included patient demographics; HCC risk factors; selected laboratory values required to stage patients; tumour characteristics; HCC-directed therapy; and outcomes. Data on resource use in addition to treatment (e.g. physician visits, type and date of assessments) were also collected.
Study sites
Criteria for site selection included tertiary referral centre providing surgical and routine follow-up care of HCC; oncology centres treating patients with HCC; patient population with HCC aetiologies consistent with the national average (by type and proportion); and centres utilizing HCC screening practices in accordance with national standards. Centres with a patient population previously used to represent the national population for other research purposes (i.e. development of staging systems, or determination of national incidence or prevalence rates) were also considered. Study sites are listed in Table S1.
Data analysis
The results reported here were based on the final data set, including all available data as of September 30, 2012. All eligible patients enrolled in the study were included in the analysis population, with information from patients who did not complete follow-up included in the analyses, unless the patient requested otherwise. The primary measure of treatment outcome was OS, as measured from date of first HCC treatment to death (to be consistent with clinical trial data). Secondary measures of treatment outcome included evidence of disease progression (yes/no), systemic treatment-limiting adverse event and systemic treatment failure, as well as the time to each of these events (also measured from date of starting treatment). Patient follow-up was defined as date of HCC diagnosis or first date on record at the site where the patient was seen for HCC, whichever was earlier, until death or end of study, whichever came first. An ongoing effort to limit the amount of missing data was made by alerting sites to missing data identified during monthly monitoring and cleaning. No missing value imputation technique was applied in the present analysis.
Results are presented as descriptive statistics, based on patients for whom data were available; results for which data are missing for >30% of patients are noted. OS was estimated using Kaplan-Meier methods, with analyses by BCLC stage and by region reported here. Data are available to perform analyses on OS by treatment type; however, initial results suggested the need for further study, which was considered to be beyond the scope of this initial report. Cox proportional hazards models were used to test for significance and all reported P-values are two-sided.
Results
Patient demographics and clinical status at diagnosis
As of September 30, 2012, a total of 42 sites in 14 countries had participated in the study (Fig.1). Data were available for a total of 18 031 patients treated for HCC [Asia: 15 sites, n = 12 031 (67% of patients); Europe: 23 sites, n = 3673 (20%) and North America: four sites, n = 2326 (13%)]. Because of the large percentage of patients from China, and also because of substantial differences in risk factors and treatment patterns between all four Asian countries included, results for the Asian countries are presented separately (either by all four countries separately or by China, separately from grouped Taiwan, South Korea and Japan). The study included a total of 8683 patients from China (72% of Asian patients and 48% of all patients); 1587 patients from Taiwan (13% of Asian patients); 1227 patients from South Korea (10% of Asian patients); and 534 patients from Japan (4% of Asian patients).
Fig. 1.
Distribution of sites participating in the HCC BRIDGE study by country.
The most common risk factor for HCC was HCV in North America, Europe and Japan, and HBV in China, South Korea and Taiwan (Table1). Alcoholic liver disease was a substantially higher risk factor in North America and Europe than in any Asian country. In North America, Europe and South Korea, at least 40% of patients reported past or current alcohol abuse, and more than 50% of patients reported past or current tobacco use; these rates were lower in China, Taiwan and Japan. Median alpha-fetoprotein (AFP) varied greatly between the regions. Patients from North America, Europe, Taiwan and Japan had median AFP in the range of 17–25 ng/ml, while median AFP was 101 ng/ml for South Korean patients and 219 ng/ml for Chinese patients.
Table 1.
Patient demographics and clinical characteristics at diagnosis (N = 18 031)
| Variable/group* | North America n = 2326 | Europe n = 3673 | China n = 8683 | Taiwan n = 1587 | South Korea n = 1227 | Japan n = 534 |
|---|---|---|---|---|---|---|
| Age, mean (SD) | 62 (11) | 65 (11) | 52 (12) | 61 (12) | 57 (10) | 69 (9) |
| Gender (male), n (%) | 1786 (77) | 2860 (78) | 7497 (86) | 1143 (72) | 1021 (83) | 340 (64) |
| Comorbidities, n (%) | ||||||
| Tobacco use† | 1187 (61) | 1759 (54) | 3042 (36) | 531 (34) | 802 (69) | 173 (39) |
| Alcohol abuse† | 759 (40) | 1459 (44) | 2034 (24) | 287 (18) | 779 (67) | 7 (2) |
| HCC risk factors, n (%)‡ | n = 2243 | n = 3466 | n = 8538 | n = 1580 | n = 1172 | n = 446 |
| HBV | 522 (23) | 362 (10) | 6575 (77) | 987 (63) | 884 (75) | 64 (14) |
| HCV | 876 (39) | 1590 (46) | 255 (3) | 489 (31) | 112 (10) | 284 (64) |
| ALD | 471 (21) | 1290 (37) | 416 (5) | 66 (4) | 110 (9) | 59 (13) |
| NASH | 275 (12) | 334 (10) | 53 (1) | 84 (5) | 68 (6) | 9 (2) |
| AFP, ng/mL | n = 2023 | n = 2922 | n = 8048 | n = 1572 | n = 1169 | n = 445 |
| Median | 24 | 17 | 219 | 25 | 101 | 18 |
| Child-Pugh status, n (%) | n = 2051 | n = 2513 | n = 7859 | n = 1559 | n = 1164 | n = 442 |
| A | 1458 (71) | 1801 (72) | 6819 (87) | 1439 (92) | 911 (78) | 390 (88) |
| B | 469 (23) | 627 (25) | 960 (12) | 115 (7) | 228 (20) | 49 (11) |
| C | 124 (6) | 85 (3) | 80 (1) | 5 (<1) | 25 (2) | 3 (1) |
| BCLC stage, n (%) | n = 1588§ | n = 2261§ | n = 6501 | n = 1461 | n = 1152 | n = 433 |
| 0 | 107 (7) | 84 (4) | 192 (3) | 213 (15) | 82 (7) | 107 (25) |
| A | 474 (30) | 582 (26) | 1973 (30) | 810 (55) | 290 (25) | 206 (48) |
| B | 157 (10) | 253 (11) | 591 (9) | 176 (12) | 149 (13) | 62 (14) |
| C | 673 (42) | 1158 (51) | 3606 (55) | 250 (17) | 605 (53) | 53 (12) |
| D | 177 (11) | 184 (8) | 139 (2) | 12 (1) | 26 (2) | 5 (1) |
| Tumour diameter, cm¶ | n = 2081 | n = 3163 | n = 6984 | n = 1467 | n = 1160 | n = 433 |
| Median | 3.8 | 3.5 | 6.7 | 3.5 | 4.4 | 2.5 |
| Range | 0.8–28 | 0.1–35 | 0.5–28 | 0.5–22 | 0.2–25 | 0.7–18 |
| Multiple tumours | n = 2198 | n = 3324 | n = 7131 | n = 1535 | n = 1160 | n = 433 |
| Yes/no (%) | 39/61 | 44/56 | 29/71 | 26/74 | 49/51 | 34/66 |
| Any portal vein invasion or thrombosis | n = 2199 | n = 3290 | n = 7828 | n = 1561 | n = 1162 | n = 439 |
| Yes/no (%) | 19/81 | 14/86 | 23/77 | 10/90 | 29/71 | 10/90 |
| Any extrahepatic spread | n = 2200 | n = 3302 | n = 7888 | n = 1558 | n = 1162 | n = 439 |
| Yes/no/not assessed (%) | 8/90/2 | 4/85/11 | 8/62/31 | 2/97/1 | 10/90/<1 | 3/95/3 |
| ECOG/WHO performance status grade, n (%)** | n = 1736 | n = 3051 | n = 8363 | n = 1565 | n = 1169 | n = 443 |
| 0 | 907 (52) | 1328 (44) | 3445 (41) | 1286 (82) | 734 (63) | 403 (91) |
| 1 | 621 (36) | 1325 (43) | 4663 (56) | 238 (15) | 414 (35) | 33 (7) |
| >1 | 208 (12) | 398 (13) | 255 (3) | 41 (3) | 21 (2) | 7 (2) |
| Karnofsky score, n (%)†† | n = 1430 | n = 1670 | n = 8327 | n = 1563 | n = 1169 | n = 2 |
| <50 | 59 (4) | 12 (1) | 59 (1) | 5 (<1) | 0 (0) | 0 (0) |
| 50–70 | 238 (17) | 200 (12) | 352 (4) | 40 (3) | 239 (20) | 0 (0) |
| 80–100 | 1133 (79) | 1458 (87) | 7916 (95) | 1518 (97) | 930 (80) | 2 (100) |
AFP, alpha-fetoprotein; ALD, alcoholic liver disease; BCLC, Barcelona Clinic Liver Cancer; ECOG/WHO, Eastern Cooperative Oncology Group/World Health Organization; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; NASH, non-alcoholic steatohepatitis; SD, standard deviation.
Statistics based on patients with known values.
Past or current.
Percentages were calculated among patients evaluated for HCC risk factors; patients who were not evaluated had missing data and were not included in the calculations.
Data missing in >30% of patients.
Includes patients with missing number of measurable lesions who had values for ‘largest diameter in liver.’
A greater ECOG/WHO performance status grade indicates worse health status (5 = death; 0 = asymptomatic).
A greater Karnofsky score indicates better health status (100 = normal, no complaints, and no evidence of disease; 0 = death).
Among patients with known staging information, the most common BCLC stage at diagnosis was stage C in North America, Europe, China and South Korea, and stage A in Taiwan and Japan (see Table1). In Taiwan and Japan, approximately 70% of patients were diagnosed with HCC at BCLC stage 0 or A, and less than 20% were diagnosed at BCLC stage C or D. In all other regions or countries (North America, Europe, China and South Korea), more than 50% of HCC cases were stage C or D at diagnosis. Using the Child-Pugh scoring system, the most common status at diagnosis was A across all regions and countries, although the proportion of A was much higher in China, Taiwan and Japan (∼90%) compared with North America and Europe (∼70%). Median tumour diameter at diagnosis ranged from 2.5–6.7 cm, with the largest median tumour diameter observed in Chinese patients. The highest incidences of portal vein invasion or thrombosis and extrahepatic spread occurred in South Korea (29 and 10%, respectively), followed by China and North America. Across regions/countries, most patients had Eastern Cooperative Oncology Group/World Health Organization (ECOG/WHO) performance status grade (23) of 0 or 1 (≥87% per region) and Karnofsky scores (24) of 80–100 (≥79% per region).
First and second recorded treatments
First recorded HCC treatment varied substantially between regions (Fig.2A). Across all disease stages, TACE was most frequently used first in North America, Europe, China and South Korea, while PEI or RFA were most frequently used first in Japan; in Taiwan, resection was the most common first treatment (see Fig.2A). For patients with BCLC stage 0–C at diagnosis, resection, TACE and PEI or RFA were the most frequently used first treatments, while palliative care was most frequently used in patients with stage D disease (Fig.2B).
Fig. 2.

First recorded HCC treatment by country/region (A) and BCLC stage (B). *Percentages are based on percent of population with known values. †Any systemic therapy other than sorafenib, e.g., doxorubicin, gemcitabine, cisplatin, or other cytotoxic or biological agent. ‡Any locoregional therapy not clearly PEI/RFA or TACE, e.g., transarterial radioembolization (TARE) or cryoablation. §Percentages are based on number of patients with data available; total may add up to >100% if more than one treatment was started concurrently. PEI, percutaneous ethanol injection; RFA, radiofrequency ablation; TACE, transarterial chemoembolization.
The most common second treatment following first treatment with resection, TACE or PEI/RFA varied by region, but was most often another non-systemic therapy (Fig.3). After resection, TACE was the most frequently recorded second treatment for HCC in all analysis groups apart from Europe, where PEI/RFA were used more frequently. TACE was also the most common second treatment for HCC after PEI/RFA in all regions apart from North America, where liver transplant was more common. Second treatments showed greatest variation by region after first-line TACE; transplant was most frequently used in North America, PEI/RFA in grouped Taiwan, South Korea and Japan, sorafenib in Europe, and palliative care in China.
Fig. 3.

Second recorded HCC treatment after first recorded resection, TACE, or PEI/RFA. *Combination therapy was not defined in the BRIDGE data; however, patients treated with either PEI or RFA were pooled together. †Percentages are based on percentage of population with known values. ‡Includes grouped patients from Taiwan (n = 1587; 47%), South Korea (n = 1227; 37%), and Japan (n = 534; 16%). PEI, percutaneous ethanol injection; RFA, radiofrequency ablation; TACE, transarterial chemoembolization.
Survival analyses
OS from first HCC treatment by BCLC stage is shown in Fig.4A. Median OS was not reached for BCLC stage 0, and was 80, 27, 15 and 4 months for BCLC stages A, B, C and D respectively (P < 0.0001). Median OS was 34 months among patients who could not be staged by the BCLC staging system. There was significant variation in survival from first HCC treatment by region (Fig. 4B). Median OS was not reached for Taiwan and 60, 33, 31, 24 and 23 months for patients from Japan, North America, South Korea, Europe and China respectively (P < 0.0001).
Fig. 4.
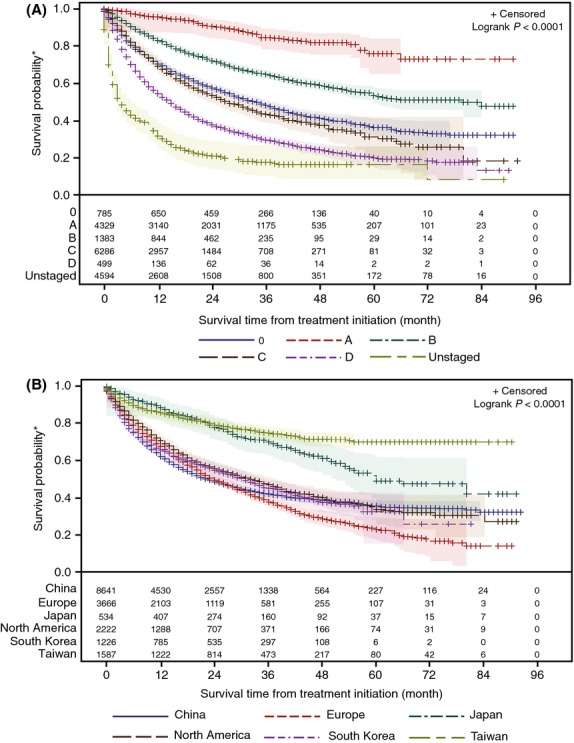
Survival estimates from first HCC treatment by BCLC stage (A) and country/region (B), with number of subjects at risk and 95% Hall-Wellner bands (shaded colours). *Results shown are unadjusted and impacted to unknown degrees by lead-time and selection bias, as well as by censoring that decreases reliability with increasing time.
Discussion
This report describes the first global and largest-to-date observational study in patients with HCC, providing a valuable opportunity to learn from real-world practice in managing this disease. The study data also allow some assessment of the merit of treatment guidelines based on the results of rigorously conducted clinical trials.
Demographic characteristics and HCC risk factors at diagnosis in the BRIDGE study appeared to confirm well-known trends. The high prevalence of male patients in this study support previous findings that HCC is up to four times more commonly diagnosed in males than in females (1,25,26)]. Similarly, the predominant risk factors identified in the BRIDGE study are consistent with those previously reported (25,27), comprising HBV infection in Asian countries (excluding Japan) and HCV infection in Europe, North America and Japan. The younger mean age at diagnosis in China (and, to some extent, also in South Korea) may reflect vertical transmission of HBV from mother to infant (4). Although HBV was also the most common risk factor in Taiwan, the proportion of affected patients (63%) was lower than in China and South Korea (77 and 75%, respectively), and the mean age at diagnosis in Taiwan was older and similar to that in North America. These observations suggest that the universal HBV vaccination program in Taiwan, which has led to a significant decline in HBsAg carrier rates in the Taiwanese population, may also be influencing the epidemiology of HCC in this country (28,29).
The BCLC staging system, which incorporates patient performance status, number and size of nodules, cancer symptoms and liver function, has been proposed as a standard method to determine prognosis and guide treatment selection among patients with HCC by the AASLD and EASL clinical practice guidelines (2,9). In the BRIDGE study, the predominant BCLC stage at diagnosis was stage C in all regions or countries apart from Japan and Taiwan. The high rate of advanced-stage disease at diagnosis underscores the need for earlier diagnosis of HCC, and suggests that surveillance efforts for HCC could be improved in many countries. Although Japan, Taiwan and South Korea have all introduced surveillance programs (30–33), government-funded or national surveillance programs have yet to be established in China (32,33), or in countries in Europe or North America.
The observed differences in disease stage at diagnosis (later in South Korea compared with Taiwan and Japan) and median OS (31 months for South Korea compared with not reached in Taiwan and 60 months in Japan) suggest that surveillance programs in Japan and Taiwan may have been more effective than the program in South Korea, although data from the single site in South Korea may not well reflect the general outcome for the country-wide program. These results could also reflect differences in surveillance program design (with respect to screening tools used, frequency of testing and target population), in implementation and uptake (affected by the proportion of population funded by the government and commitment of clinicians) or a mixture of these factors. Whether patients were diagnosed during surveillance was a data point captured for each patient in this study. However, the way this question was understood by personnel entering data appears to have differed across the participating sites, precluding our ability to clearly assess the influence of surveillance on the overall study results. Evidence from one of the participating sites in the United States does suggest that surveillance can have a positive effect on patient outcomes. In a retrospective analysis of all patients diagnosed with HCC from 2007–2009 (n = 460) at the Mayo Clinic in Rochester, Minnesota, patients diagnosed during surveillance had less advanced disease, were more likely to be eligible for potentially curative treatments and had increased survival times (after 40 months of follow up, median OS not reached in patients diagnosed during surveillance vs. 12.5 months in patients not diagnosed during surveillance, P < 0.001) (34).
While widely recognized to be essential for the effective treatment of patients with the disease, surveillance-supported early diagnosis of HCC also results in lead-time bias that increases OS (35). This is consistent with our finding of superior OS (not adjusted for lead-time bias) for patients in Taiwan and Japan when compared with China, Europe, South Korea and North America (see Fig. 4B). However, there were also differences in survival between regions or countries where the distribution of stage at diagnosis was similar; for example, median OS in North America and South Korea was better than that observed in Europe or China. This may reflect variation in data collection, patient populations (other than stage at diagnosis) or variation in management including treatment patterns. As expected, median OS decreased with progressing BCLC stage (see Fig.4A), supporting the prognostic utility of the BCLC staging system. Our estimates of 27, 15 and 4 months for median OS for BCLC stages B, C and D, respectively, are somewhat higher than those reported for clinical trials in the literature: ∼20 months for BCLC stage B (36), ∼10 months for BCLC stage C treated with sorafenib in the SHARP trial (16) and 3 months for BCLC stage D (36).
The BRIDGE study showed differences in choice of first treatment by region and stage at diagnosis. In early-stage disease (stage 0 or stage A), for which resection, liver transplant or RFA/PEI are recommended by the AASLD/EASL guidelines as potentially curative treatments (2,9), PEI/RFA, resection and TACE were the most frequently used first treatments. The use of TACE in these patients is inconsistent with the AASLD/EASL guidelines. However, some of these cases may be explained by the use of TACE as neoadjuvant therapy, highlighting a possible limitation in the way the data were collected. Indeed, pre-operative TACE has been used extensively in patients with resectable HCC, although a systematic review of the literature has concluded that TACE in this setting does not improve disease-free survival (37). TACE is also frequently used prior to liver transplantation, and approximately 30% of patients receiving TACE in North America subsequently underwent liver transplantation (see Fig.3). An additional explanation for TACE in BCLC stage A is that these patients are either unfit or partial responders to curative therapies. In North America, a fair number of patients who were candidates for transplantation instead received TACE or RFA as first treatment, which may have also resulted in down-staging. Although combination therapy with TACE or RFA plus transplantation was the actual intention, TACE/RFA and transplantation were counted separately given the observational nature of the BRIDGE study.
Despite the known efficacy of liver transplantation, only a minority of patients received this procedure. Possible reasons include ineffective or non-existent identification and surveillance of transplant-suitable candidates, as well as limited availability of organ donors. However, data on whether patients were placed on a transplant registry were not collected as part of the BRIDGE study, limiting its ability to further inform on this issue. It should also be recognized that the treatment patterns observed were also likely influenced by substantial regional differences in practice related to access and cost. While all participating centres likely reflect the best care that can be delivered in each country, what constitutes best care in a resource-rich country (e.g. the United States) will differ from best care offered in less resource-rich countries (e.g. China). Given this difference, it is perhaps not surprising that transplant and systemic therapy with sorafenib, two resource-intensive treatments, were used more frequently in North America and Europe than elsewhere. In addition, in countries with nationalized healthcare and limited resources, the use of transplant and high-cost/low-benefit treatments like sorafenib are likely to be constrained by public policies aimed at delivering benefits broadly across the population in need.
The BCLC treatment algorithm, adopted by AASLD/EASL guidelines, recommends the use of sorafenib for stage C disease (2). However, in the real-world BRIDGE study, use of sorafenib as first recorded treatment was low, despite the high number of patients diagnosed with BCLC stage C disease. Since sorafenib efficacy is considered modest, many physicians may have tried to reduce the tumour burden directly by locoregional therapies, which could be expected to result in survival gain. Alternatively, the use of first-line sorafenib may have been affected by intercountry variations in access, as noted above. Rather than sorafenib, TACE and resection were the most frequent first recorded treatments for patients with stage C disease. This finding underscores the unmet need for evaluating the outcome of surgical resection vs. locoregional therapy vs. systemic therapy in advanced stage HCC (14,38). In patients with BCLC stage D disease, the proportion who received palliative care was surprisingly low across these real-world settings, and the proportion who received liver transplant was surprisingly high. This may have reflected liver transplantation of patients with Child-Pugh class C or ECOG/WHO performance status greater than 2 who otherwise have liver disease meeting Milan criteria. It is unclear, however, what proportion of stage D patients received best supportive care [as recommended by the BCLC treatment algorithm and by the Asian Pacific Association for the Study of the Liver (APASL) consensus guidelines (2,35)], which may not always have been captured in our data collection under ‘palliative care’. In our analysis, any patient who did not receive a definitive therapy was assumed to have received best supportive care only.
Analysis of first recorded treatment by region shows that sorafenib was used more frequently in North America, Europe and South Korea, compared with China, Taiwan and Japan, possibly reflecting later regulatory approval as well as reduced access to sorafenib in Asia. Additionally, resection and TACE were the most frequently recorded first treatments in China, Taiwan and South Korea, compared with TACE and PEI/RFA in Europe and Japan, and TACE, PEI/RFA and resection in North America. This variation in first recorded treatment by region may be because of real differences in the accepted management strategies in Western countries, where the BCLC treatment algorithm is most widely used, compared with Asian countries, where other guidelines predominate. The latter include Japanese guidelines for HCC first compiled in 2005 (21,39), South Korean guidelines initially published in 2003 and updated in 2009 (22), and the APASL consensus guideline for treatment of HCC (35). Further analysis of how such regional guidelines might have influenced the practice patterns reported here, particularly in China, is ongoing.
The primary strengths of the BRIDGE study are the large patient population, wide geographical spread and the capture of real-world clinical practice data from patient charts. However, as with all observational cohort studies, the BRIDGE study also has many limitations. Because it is a medical chart-review study, the robustness of the data depends on the thoroughness of each site's understanding and documentation of medical history, treatment and response. Missing data and loss of patients to follow-up are additional limitations of observational studies and may affect the results and their interpretation. Although substantial effort was made to encourage complete collection of all requested data at the participating sites, there are some results reported here (as noted in Table1) for which the proportion of missing data was >30%. In addition, it is possible that the results are not generalizable because the study was performed at tertiary referral centres, which might be expected to provide the best care available in each country. In an attempt to maximize generalization of results across a particular country, study sites were selected where patient populations had HCC etiologist consistent with previously reported national patterns. In the case of single sites within one country (Taiwan, South Korea and Japan), sites were additionally chosen to be representative of the practice of other centres in the country. However, it is possible that more treatable patients with better liver function and good performance status may have been enrolled, thereby introducing a selection bias. In particular, the lack of enrolled BCLC stage D patients in China (2%), as well as the low proportion with Child-Pugh stage B and C (12 and 1%, respectively), suggest that such patients were not seen at the participating sites and may therefore not have been included in the study. Similarly, the relatively low rates of patients reporting alcohol abuse in China, Taiwan and Japan suggest that such patients also may not have been treated at the participating sites for some reason (for example, such patients might be expected to be less likely to seek and receive care at a tertiary centre, and less likely to be supported in doing so). These low rates could also, however, reflect under-reporting because of the possible stigma associated with admitting such abuse in some countries. WHO estimates of per capita alcohol consumption (litres of pure alcohol) for 2011 were 9.4 for the United States, 12.2 for Europe, 5.9 for China, 14.8 for South Korea and 8.0 for Japan (40). Given these figures and the known strong interaction between alcohol abuse and other risk factors for HCC, the reported rates of alcohol abuse for China (24%), Taiwan (18%) and especially Japan (2%) seem unexpectedly low. These conjectures regarding potentially ‘missing’ advanced-stage patients may help to explain the possibly higher-than-expected median OS reported here for China. They could also contribute to the superior OS seen here for Taiwan and Japan, but this more likely can be attributed to the aforementioned lead-time bias, as well as, particularly in the case of Taiwan, artificial inflation because of the effects of censoring, which makes the OS reported here increasingly less reliable with time. Finally, interactions between variables in such a large sample size as the population in this study are hard to control, and our results must accordingly be interpreted with caution.
In conclusion, these real-world findings from the BRIDGE study provide a broad overview of the current state of HCC treatment and document the heterogeneity of treatment approaches across regions and in different countries. Results from the study confirm previously reported regional trends in patient demographic characteristics and HCC risk factors, underscore the need for earlier diagnosis of HCC worldwide, and also suggest that treatment guidelines may benefit from re-evaluation. The data from Taiwan and Japan, in particular, suggest it may be possible to improve outcomes by focusing on identifying high-risk individuals and then following them with surveillance to achieve early detection. It is hoped that information obtained from the BRIDGE study will help identify unmet clinical needs and contribute to the development of new treatment paradigms that ultimately improve outcomes in patients with HCC. The study has generated a very large dataset which could potentially be used to address unanswered research hypotheses and is available for further analysis by interested investigators. Additional analyses of potential value could include the aforementioned survival by treatment type, including systemic vs. non-systemic therapy, assessment of regional practice vs. regional guidelines, as well as exploratory identification of possible predictors of survival, such as changes in tumour size or AFP levels over time.
Acknowledgments
The authors wish to acknowledge Baisong Huang of Outcome Sciences, in Cambridge, MA, for his contributions in statistical analysis, as well as James W. Shaw and Ying Zhang at Bristol-Myers Squibb in Princeton, NJ, for valuable comments during the manuscript preparation. Professional medical writing and editorial assistance was provided by StemScientific, funded by Bristol-Myers Squibb.
Glossary
- AASLD
American Association for the Study of Liver Diseases
- AFP
alpha-fetoprotein
- ALD
alcoholic liver disease
- APASL
Asian Pacific Association for the Study of the Liver
- BCLC
Barcelona Clinic Liver Cancer
- EASL
European Association for the Study of the Liver
- ECOG/WHO
Eastern Cooperative Oncology Group/World Health Organization
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- NASH
non-alcoholic steatohepatitis
- OS
overall survival
- PEI
percutaneous ethanol injection
- RFA
radiofrequency ablation
- SD
standard deviation
- TACE
transarterial chemoembolization
Financial support
Professional medical writing and editorial assistance was provided by StemScientific, funded by Bristol-Myers Squibb.
Conflict of interest
J.-W. Park does not have any disclosure to report. M. Chen does not have any disclosure to report. M. Colombo: grant and research support Merck, Roche, Bristol-Myers Squibb, Gilead Sciences; science advisory committees Merck, Roche, Novartis, Bayer, Bristol-Myers Squibb, Gilead Sciences, Tibotec, Vertex, Janssen Cilag, Achillion, Lundbeck, Abbott, Boehringer Ingelheim, GlaxoSmithKline, GenSpera, AbbVie; speaking and teaching Tibotec, Roche, Novartis, Bayer, Bristol-Myers Squibb, Gilead Sciences, and Vertex. L. R. Roberts: grant and research support Bristol-Myers Squibb, Gilead Sciences, Inova Diagnostics, and Wako Diagnostics. M. Schwartz: has been a consultant/advisor for Bayer and Onyx and has received research funding from Bristol-Myers Squibb. P.-J. Chen: steering committee meeting GlaxoSmithKline; advisory board Bayer, Bristol-Myers Squibb, GlaxoSmithKline, and Roche; research grants to his institution Bristol-Myers Squibb; speakers bureau Bristol-Myers Squibb; travel/accommodations/meeting expenses to international symposiums Bayer, Bristol-Myers Squibb, and Gilead Sciences. M. Kudo does not have any disclosure to report. P. Johnson: personal fees for lecture honoraria from Bayer Health Care. S. Wagner: employee of and owns stock in Bristol-Myers Squibb. L. S. Orsini: employee of and owns stock in Bristol-Myers Squibb. M. Sherman: has been a consultant/advisor and given expert testimony for Bristol-Myers Squibb.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Table S1. Study sites.
References
- 1.Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0. Estimated cancer incidence, mortality, and prevalence worldwide. Available at http://globocan.iarc.fr. Accessed 28 March 2014.
- 2.European Association for The Study Of The Liver; European Organisation for Research And Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–43. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–17. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 4.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–73. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–76. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 6.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485–91. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2010, National Cancer Institute. Bethesda, MD, Available at http://seer.cancer.gov/csr/1975_2010/, based on November 2012 SEER data submission, posted to the SEER web site, 2013. Accessed: 12 March 2015.
- 8.Dhanasekaran R, Limaye A, Cabrera R. Hepatocellular carcinoma: current trends in worldwide epidemiology, risk factors, diagnosis, and therapeutics. Hepat Med. 2012;4:19–37. doi: 10.2147/HMER.S16316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruix J, Sherman M American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–2. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shiina S, Tateishi R, Arano T, et al. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am J Gastroenterol. 2012;107:569–77. doi: 10.1038/ajg.2011.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shiina S, Tateishi R, Imamura M, et al. Percutaneous ethanol injection for hepatocellular carcinoma: 20-year outcome and prognostic factors. Liver Int. 2012;32:1434–42. doi: 10.1111/j.1478-3231.2012.02838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sloane D, Chen H, Howell C. Racial disparity in primary hepatocellular carcinoma: tumor stage at presentation, surgical treatment and survival. J Natl Med Assoc. 2006;98:1934–9. [PMC free article] [PubMed] [Google Scholar]
- 13.Carrilho FJ, Kikuchi L, Branco F, Goncalves CS, Mattos AA Brazilian HCC Study Group. Clinical and epidemiological aspects of hepatocellular carcinoma in Brazil. Clinics (Sao Paulo) 2010;65:1285–90. doi: 10.1590/S1807-59322010001200010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas MB, Jaffe D, Choti MM, et al. Hepatocellular carcinoma: consensus recommendations of the National Cancer Institute Clinical Trials Planning Meeting. J Clin Oncol. 2010;28:3994–4005. doi: 10.1200/JCO.2010.28.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei Z, Doria C, Liu Y. Targeted therapies in the treatment of advanced hepatocellular carcinoma. Clin Med Insights Oncol. 2013;7:87–102. doi: 10.4137/CMO.S7633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 17.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 18.Welker MW, Lubomierski N, Gog C, et al. Efficacy and safety of sorafenib in advanced hepatocellular carcinoma under daily practice conditions. J Chemother. 2010;22:205–11. doi: 10.1179/joc.2010.22.3.205. [DOI] [PubMed] [Google Scholar]
- 19.Lencioni R, Chen XP, Dagher L, Venook AP. Treatment of intermediate/advanced hepatocellular carcinoma in the clinic: how can outcomes be improved? Oncologist. 2010;15(Suppl 4):42–52. doi: 10.1634/theoncologist.2010-S4-42. [DOI] [PubMed] [Google Scholar]
- 20.Park J-W, Sherman M, Colombo M, et al. Observations of hepatocellular carcinoma (HCC) management patterns from the global HCC BRIDGE study: first characterization of the full study population. Poster 4033. Presented at the 2012 Annual Meeting of the American Society of Clinical Oncology (ASCO); June 1-5, 2012; Chicago, Illinois.
- 21.Kudo M, Okanoue T Japan Society of Hepatology. Management of hepatocellular carcinoma in Japan: consensus-based clinical practice manual proposed by the Japan Society of Hepatology. Oncology. 2007;72(Suppl 1):2–15. doi: 10.1159/000111702. [DOI] [PubMed] [Google Scholar]
- 22.Korean Liver Cancer Study Group and National Cancer Center, Korea. Practice guidelines for management of hepatocellular carcinoma 2009. Korean J Hepatol. 2009;15:391–423. doi: 10.3350/kjhep.2009.15.3.391. [Article in Korean] [DOI] [PubMed] [Google Scholar]
- 23.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–55. [PubMed] [Google Scholar]
- 24.Mor V, Laliberte L, Morris JN, Wiemann M. The Karnofsky Performance Status Scale. An examination of its reliability and validity in a research setting. Cancer. 1984;53:2002–7. doi: 10.1002/1097-0142(19840501)53:9<2002::aid-cncr2820530933>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 25.Venook AP, Papandreou C, Furuse J, de Guevara LL. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist. 2010;15(Suppl 4):5–13. doi: 10.1634/theoncologist.2010-S4-05. [DOI] [PubMed] [Google Scholar]
- 26.Yang JD, Roberts LR. Epidemiology and management of hepatocellular carcinoma. Infect Dis Clin North Am. 2010;24:899–919. doi: 10.1016/j.idc.2010.07.004. viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bosch FX, Ribes J, Díaz M, Cléries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127(5 Suppl 1):S5–16. doi: 10.1053/j.gastro.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 28.Su FH, Huang HY, Chang HJ, et al. Forecasting the declining rate of chronic hepatitis-B carrier status at a Taiwanese university: twenty years after implementation of an universal HBV vaccination program in Taiwan. Chang Gung Med J. 2007;30:521–8. [PubMed] [Google Scholar]
- 29.Ni YH, Chen DS. Hepatitis B vaccination in children: the Taiwan experience. Pathol Biol (Paris) 2010;58:296–300. doi: 10.1016/j.patbio.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 30.Chen CJ, You SL, Lin LH, Hsu WL, Yang YW. Cancer epidemiology and control in Taiwan: a brief review. Jpn J Clin Oncol. 2002;32(Suppl):S66–81. doi: 10.1093/jjco/hye138. [DOI] [PubMed] [Google Scholar]
- 31.Yoo KY. Cancer control activities in the Republic of Korea. Jpn J Clin Oncol. 2008;38:327–33. doi: 10.1093/jjco/hyn026. [DOI] [PubMed] [Google Scholar]
- 32.Kudo M, Han KH, Kokudo N, et al. Liver Cancer Working Group report. Jpn J Clin Oncol. 2010;40(Suppl 1):i19–27. doi: 10.1093/jjco/hyq123. [DOI] [PubMed] [Google Scholar]
- 33.Song P, Gao J, Inagaki Y, et al. Biomarkers: evaluation of screening for and early diagnosis of hepatocellular carcinoma in Japan and China. Liver Cancer. 2013;2:31–9. doi: 10.1159/000346220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang JD, Harmsen WS, Slettedahl SW, et al. Factors that affect risk for hepatocellular carcinoma and effects of surveillance. Clin Gastroenterol Hepatol. 2011;9:617–23. doi: 10.1016/j.cgh.2011.03.027. [DOI] [PubMed] [Google Scholar]
- 35.Omata M, Lesmana LA, Tateishi R, et al. Asian pacific association for the study of the liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010;4:439–74. doi: 10.1007/s12072-010-9165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Llovet JM, Di BisceglieAM, Bruix J, et al. Panel of Experts in HCC-Design Clinical Trials. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698–711. doi: 10.1093/jnci/djn134. [DOI] [PubMed] [Google Scholar]
- 37.Chua TC, Liauw W, Saxena A, et al. Systematic review of neoadjuvant transarterial chemoembolization for resectable hepatocellular carcinoma. Liver Int. 2010;30:166–74. doi: 10.1111/j.1478-3231.2009.02166.x. [DOI] [PubMed] [Google Scholar]
- 38.Kim HY, Park JW, Nam BH, et al. Survival of patients with advanced hepatocellular carcinoma: sorafenib versus other treatments. J Gastroenterol Hepatol. 2011;26:1612–8. doi: 10.1111/j.1440-1746.2011.06751.x. [DOI] [PubMed] [Google Scholar]
- 39.Makuuchi M, Kokudo N, Arii S, et al. Development of evidence-based clinical guidelines for the diagnosis and treatment of hepatocellular carcinoma in Japan. Hepatol Res. 2008;38:37–51. doi: 10.1111/j.1872-034X.2007.00216.x. [DOI] [PubMed] [Google Scholar]
- 40.WHO. Global Status Report on Alcohol and Health. World Health Organization; 2011. Available at http://www.who.int/substance_abuse/publications/global_alcohol_report/en/index.html. Accessed 28 March 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Study sites.



