Abstract
Zebrafish can provide a valuable animal model to screen potential cognitive enhancing and anxiolytic drugs. They are economical and can provide a relatively quick indication of possible functional efficacy. In as much as they have a complex nervous system and elaborate behavioral repertoire, zebrafish can provide a good intermediate model between in vitro receptor and cell-based assays and classic mammalian models for drug screening. In addition, the variety of molecular tools available in zebrafish makes them outstanding models for helping to determine the neuromolecular mechanisms for psychoactive drugs. However, to use zebrafish as a translational model we must have validated, sensitive and efficient behavioral tests. In a series of studies, our lab has developed tests of cognitive function and stress response, which are sensitive to drug effects in a similar manner as rodent models and humans for cognitive enhancement and alleviating stress response. In particular, the three-chamber task for learning and memory was shown to be sensitive to the cognitive enhancing effects of nicotine and has been useful in helping to determine neural mechanisms crucial for nicotinic-induced cognitive enhancement. The novel tank diving test was shown to be a valid and efficient test of stress response. It is sensitive to the reduction of stress-related behaviors of the anxiolytic drugs diazepam and buspirone but not chlordiazepoxide. Nicotine also causes stress alleviating effects which can be interpreted as anxiolytic effects. Zebrafish models of behavioral pharmacology can be useful to efficiently screen test compounds for drug development and can be useful for helping to determine the mechanisms crucial for new therapeutic treatments of neurobehavioral impairments.
Keywords: anxiety, buspirone, diazepam, learning, memory, nicotine, zebrafish
Introduction
Zebrafish offer an outstanding model to provide a screen for neurobehavioral disorders (Stewart et al., 2010) as well as potential psychotherapeutic drugs (Rihel et al., 2010) and the crucial mechanisms for the psychotherapeutic effects. Recently, zebrafish larvae motility assays have been found to be rather useful for an initial drug screen for psychotherapeutic drug candidates (Rihel et al., 2010). Zebrafish have long been used as an important model for studying developmental biology in general and neurobiology in particular (Schier, 1997). Molecular mechanisms of behavioral function can be studied in zebrafish with the use of the great variety of mutant lines available. In addition, morpholinos can be used to reversibly suppress the expression of individual genes during early development. Specific reporter systems and continuous visual access during development provides considerable availability of the developmental process. The same molecular tools useful for studying neurodevelopment are key for the use of zebrafish for screening psychotherapeutic drugs and determining crucial neuromolecular and cellular mechanisms. Much more opportunity remains to be pursued in the study of molecular mechanisms of drug actions on behavioral function in zebrafish.
This paper reviews a series of studies we have done over the past decade to develop tests of cognitive and emotional function in zebrafish and use those tests to determine the effects of drugs known to affect these functions in mammals. The goal of this research is to develop the zebrafish model to test effects of drug candidates as a precursor to later research in mammals. It is envisioned that zebrafish characterized in these behavioral tests and a variety of others developed in the field would provide crucial information in the drug development process after initial characterization using receptor and cell-based assays and before classic rodent assays. It is hoped that by providing proof of concept information concerning drug effects on complex neurobehavioral function in an economical rapid test system, zebrafish can help identify promising candidates from the hundreds of compounds that clear the receptor and cell-based assays and provide the rodent assay effort with a more manageable smaller number of candidates.
Crucial to constructing a zebrafish model, which is useful for development of psychotherapeutic drugs is the use of behavioral tests that are reliable, valid and efficient. Over the past decade, we have developed such tests for assessment of learning and memory in the three-chamber task as well as stress response in the novel tank diving test and have used these tests to assess the efficacy of drug treatments as described below. Both of these tests have proven to be useful in our studies. However, there are a variety of ways to measure these behavioral functions as has been demonstrated in the important work of many other investigators.
Other labs have contributed greatly to the effort to develop the zebrafish model for investigation of the neural bases of cognitive function and stress response as well as for screening efficacy of potential therapeutic drugs. A variety of cognitive tasks of various types have been developed. Using a T-maze with an enriched environment at the end of one choice arm it was shown that zebrafish would readily learn spatial discrimination (Darland and Dowling, 2001). A T-maze has also been used to demonstrate color discrimination of zebrafish for appetitive reinforcement (Colwill et al., 2005; Zhdanova et al., 2008). A delayed spatial alternation paradigm for appetitive reinforcement has been used to index spatial working memory in zebrafish (Williams et al., 2002) and ethanol has been shown to produce dose-dependent choice accuracy impairments (Carvan et al., 2004). Zebrafish have been shown to have significant learning using olfactory cues (Braubach et al., 2009). Interestingly, the sight of conspecifics can be used as reward in associative learning in zebrafish (Al -Imari and Gerlai, 2008 ; Gómez-Laplazaa and Gerlai b, 2009). Several studies have shown the efficacy of classic two-chamber shuttle box active-avoidance learning (Pradel et al., 1999, 2000; Xu et al., 2007; Pather and Gerlai, 2009). This is rather efficient at rapidly determining acquisition of a response, but does have the drawback of not having a differential measure of response speed and choice accuracy. That is in an active response task hyperactivity would be advantageous, whereas in a passive avoidance task sedation would be advantageous. We have used a three-chamber task with a central start chamber and two side chambers presenting choice alternatives with equal swimming needed for each choice to index learning and memory in zebrafish.
Pharmacological studies investigating the case of nicotinic involvement in cognitive function were conducted. Nicotinic receptors of several types have been identified in zebrafish (Zirger et al., 2003). Nicotinic agonist and antagonist effects on learning and memory, dose- and time-effect functions and the relationship of nicotinic effects to changes in monoaminergic neurotransmitter systems were determined.
Stress response and by inference anxiety have been widely studied in zebrafish in recent years and a variety of well-designed test methods have been developed [see recent reviews: (Cachat et al., 2010; Gerlai, 2010; Maximino et al., 2010)]. Useful test paradigms include shoaling response, predator avoidance and light-dark environment choice (Engeszer et al., 2004 Miller and Gerlai, 2007; Bass and Gerlai, 2008; Egan et al., 200 ; Gerlai et al., 2009; Champagne et al., 2010 Sackerman et al., 2010) in addition to the novel tank diving task described in this article. Anxiety is a description of an adverse subjective state in people. As such, it cannot be directly measured in experimental animal models. However, behavioral tests of stress response, which are sensitive to anxiogenic and anxiolytic environmental and pharmacological manipulations in people can be used in animal models to help identify the neural bases of anxiety and possible new anxiolytic treatments. Predatory avoidance can be measured in several ways: fleeing, freezing, shoaling (grouping together of fish) and thigmotaxis (dwelling near a wall or bottom of the tank). Several studies have used shoaling behavior, or gathering together, that zebrafish show when threatened as an index of stress response vs. dispersal, which is more optimal for feeding (Miller and Gerlai, 2008). Predator threat paradigms have been developed in which the zebrafish responds by increasing swimming in reaction to a displayed image of a predator (Blaser and Gerlai, 2006). Drug withdrawal anxiogenesis has been demonstrated in zebrafish. Cocaine withdrawal caused hyperactive swimming and motor stereotypy indicative of anxiety (Lopez - Patino et al., 2008). Recently, Kalueff and co-workers (Egan et al., 2009) used a very similar assessment of the diving response that we have used in studies reviewed below. They also found that zebrafish reliably make a diving response in a novel environment and that the duration of this diving response can be used to study the neurobehavioral bases of stress response. They showed that acute exposure to alarm pheromone or caffeine increased stress-related behaviors effects and that chronic exposure to the antidepressant drug fluoxetine decreased them.
In this article, we present a series of studies we have conducted to develop and optimize methods for assessing cognitive functions such as learning and memory as well as stress response. We have developed a three-chamber task, which is rather flexible and can be used to index spatial learning, reversal learning, response alternation learning and memory. Pharmacological characterization has been made for nicotinic cholinergic systems using nicotinic agonist and antagonist treatments and neurochemical characterization has been done for the monoaminergic transmitters. A method assessing stress response behavior has been developed taking advantage of the tendency of prey fish to dive to the bottom of a novel environment and then over time as they become more familiar with the safety of the environment, they swim more to the upper levels of the environment. This diving response has been shown to be specific to a novel vs. familiar environment. Classic and atypical anxiolytic drugs as well as nicotine and nicotinic antagonist treatments were used to pharmacologically characterize the test. These tests add to our ability to screen novel cognitive enhancing and anxiolytic drug candidates and the crucial mechanisms for these effects. This review presents the work we have done over the past 10 years to develop, validate and use zebrafish behavioral tests of cognition and emotion for use in drug development research for cognitive enhancing and anxiolytic drugs.
Results
The three-chamber task
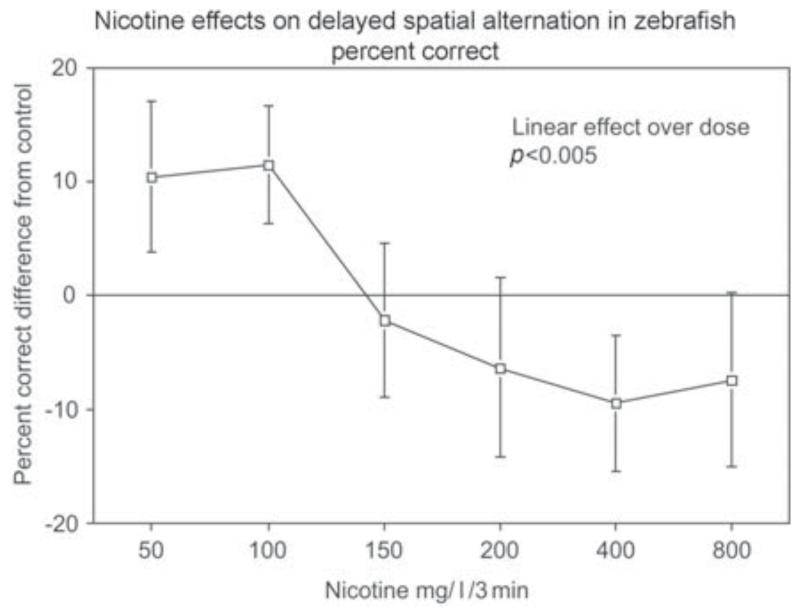
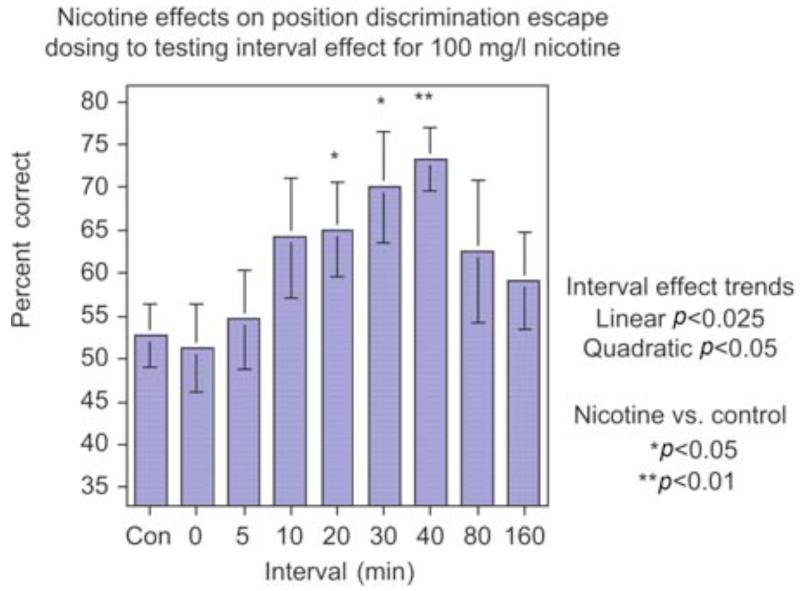
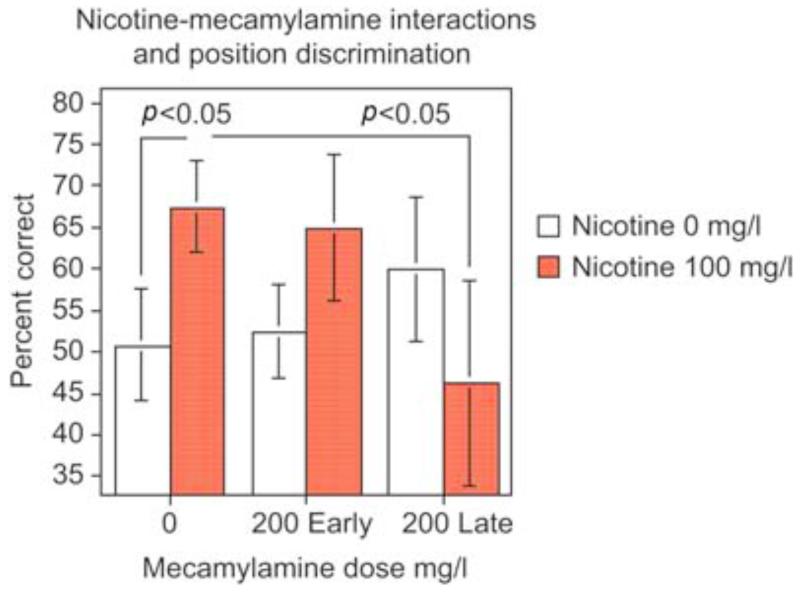
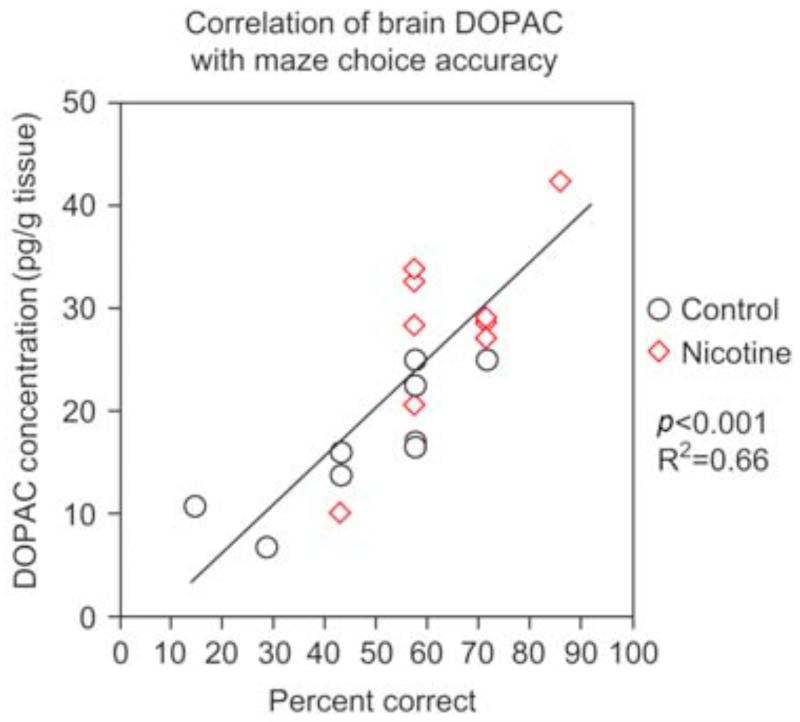
Nicotine administration at low to moderate doses increased delayed spatial alternation accuracy in the three-chamber task, indicating improved spatial working memory (Levin and Chen, 2004). As shown in Figure 1, the dose-effect function for nicotine effects on memory in zebrafish showed an inverted J-shaped dose-response curve with a significant (p < 0.005) linear effect over dose with nicotine treatment improving choice accuracy relative to control performance at lower nicotine doses and this effect trailing off with higher doses. The 50 and 100 mg/l doses each caused a significant (p < 0.05) improvement in accuracy relative to control. Nicotine also increased accuracy in simple spatial discrimination learning (Levin et al., 2006a ; Eddins et al., 2009). This is a fairly rapid test with only a single session of training the fish to go to its non-preferred side of the tank. In this task nicotine caused significant improvement in choice accuracy with 100 (p < 0.01) and 200 mg/l (p < 0.05) for 3 min immersion; however, 50 mg/l was not effective with dosing 40 min before testing. A detailed time-effect function was assessed for the benchmark of 100 mg/l nicotine dose. As shown in Figure 2, nicotine had a delayed time-effect function for improving spatial learning (Levin et al., 2006a). Improvement was not seen immediately after dosing but only became significant starting with a delay of 20 min post-dosing (p < 0.05) and persisting until 40 min post-dosing (p < 0.01). Clearly no differences in choice accuracy were seen immediately after dosing or 5 min later. A suggestion of improvement was apparent 10 min post-dosing but this was not significant. The nicotine-induced improvement dissipated after 40 min post-dosing with no improvement detected at 80 or 160 min after dosing. This time-effect function for choice accuracy contrasted with the quicker nicotine effect on choice latency, which showed significant nicotine-induced increases at 5 (p < 0.025) and 20 (p < 0.001) min post-dosing but not before or after. The non-competitive nicotinic antagonist mecamylamine (200 mg/l for 3 min) reversed the nicotine-induced improvement when it was administered after nicotine exposure just before testing but not when administered together with nicotine 40 min before testing (Figure 3) showing that nicotinic receptor blockade did not antagonize the induction of the nicotine-induced learning improvement, whereas it did antagonize the expression of the effect (Levin et al., 2006a). This is consistent with the hypothesis that the nicotine-induced learning improvement was not due to nicotinic receptor activation. This would have been blocked by concurrent mecamylamine. Rather, the nicotine-induced learning improvement could have been due to the nicotine-induced receptor desensitization, which would not have been blocked by concurrent mecamylamine and subsequent recovery from desensitization prior to testing, which would have been blocked by mecamylamine given shortly before testing. This sequence of events would also be consistent with the delayed induction of the nicotine-induced learning improvement. Dopamine appears to be important for spatial learning in the zebrafish and for nicotine effects on that learning. In the same way that mecamylamine blocks the expression of nicotine- induced learning improvement when given after nicotine exposure, the same dose and timing of mecamylamine blocks nicotine-induced increase in the dopamine metabolite DOPAC (Eddins et al., 2009). In addition, there is a robust positive correlation of spatial discrimination choice accuracy in the three-chamber task and brain DOPAC concentration (Figure 4) regardless of nicotine exposure (Eddins et al., 2009). We did not see relationships between nicotine effects on learning in the three-chamber task and serotonin and norepinephrine systems.
Figure 1.
Nicotine dose-effect function for improving memory in the three-chamber spatial alternation task (mean ± sem) (Levin and Chen, 2004). Relative to control percent accuracy (percent correct after nicotine treatment-percent correct after vehicle) on the delayed spatial alternation task there was a significant (p < 0.005) decrease in accuracy with increasing acute nicotine doses from improvement with lower dose to impairment with higher doses (n = 12). The 50 and 100 mg/l doses each caused a significant (p < 0.05) improvement in accuracy relative to control.
Figure 2.
Nicotine delayed time-effect function for improving spatial discrimination (mean ± sem) (Levin et al., 2006a). There was a delayed improvement in simple spatial discrimination in the three-chamber task, which became significant 20 min after the end of nicotine exposure and continued to be significant up to 80 min after the end of exposure.
Figure 3.
Blockade of nicotine effect with the nicotinic antagonist mecamylamine (mean ± sem) (Levin et al., 2006a). This shows a replication of the significant improvement in choice accuracy in the simple spatial discrimination in the three-chamber task caused by 100 mg/l nicotine given by immersion for 3 min ending 40 min before testing. Mecamylamine given concurrently with nicotine (200 Early) was not effective in reversing this improvement. The same mecamylamine dose given 5 min before testing (200 Late) did successfully reverse the improvement caused by nicotine given 40 min before testing, even though administration of mecamylamine alone at this point did not impair performance.
Figure 4.
Correlation of learning spatial discrimination in the three chamber task and whole brain DOPAC levels (pg/g tissue). There was a significant ( p < 0.001) increase in accuracy on the simple spatial discrimination in the three-chamber task associated with increases in whole brain concentrations of the dopamine metabolite DOPAC (Eddins et al., 2009 ). The nicotine exposure concentration was 100 mg/l for 3 min immersion ending 20 min before testing. Sacrifice occurred just after testing.
The novel tank diving test
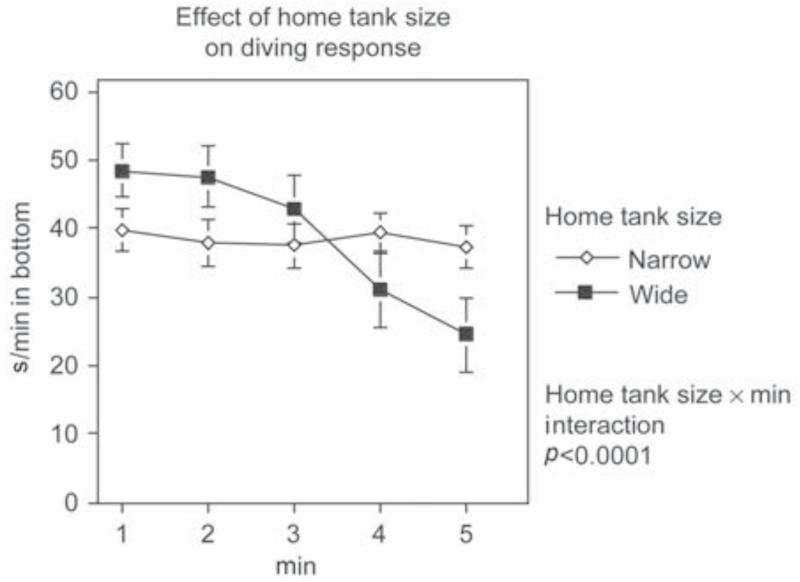
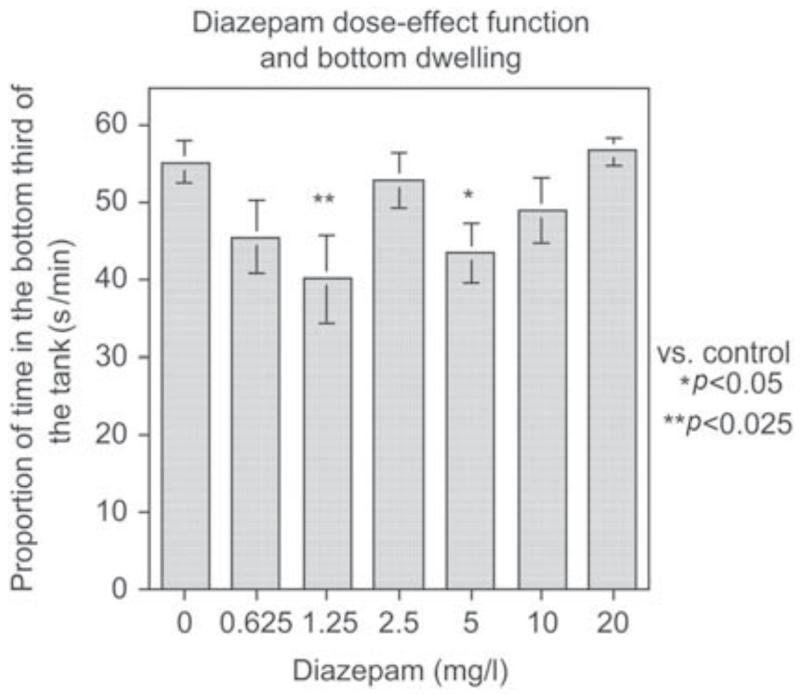
The novel tank diving test (Levin and Cerutti, 2008) was used to assess anxiolytic effects of drugs. Behavioral validation of the novel tank diving test was conducted to determine the impact of novelty on the diving response. Figure 5 shows that even with testing in identical test tanks, fish transferred from a different tank vs. the same type of tank the fish which had been living in the larger tank showed a diving response when initially placed in the test tank, whereas the fish which had been living in the same-sized tank as the test tank did not show this response when transferred into the test tank. The fish transferred from the wider home tank to the narrow test tank showed a greater initial diving response followed over the next 5 min by greater exploration of the higher levels of the test tank. By contrast, the fish which had been hosed in an identically sized tank as the test tank showed less diving response and no change over the 5-min test session (Figure 5). This resulted in a very significant (p < 0.0001) interaction of housing condition × minute of the test (Bencan et al., 2009). It can be posited that it is not the novelty but the stress of being transferred from the wide to the narrow tank that provoked the diving response. However, it does seem that it is the novelty which induces the diving response. Recently we have determined the effect of repeated testing the novel tank diving task and found that the diving response is only seen on the first 5-min session of testing (session mean = 30.1 ± 6.3 s/min in the bottom third of the tank) with significantly (p < 0.005) less diving on the second test (session mean = 8.6 ± 3.5 s/min in the bottom third of the tank) (Levin et al., 2010 ). The test also proved to be sensitive to anxiolytic drugs. The classic anxiolytic drug diazepam produced a significant anxiolytic-like effect in the novel tank diving test (Figure 6) (Bencan et al., 2009). The effect was non-monotonic with moderate but not high or low doses being effective. The effective doses of diazepam for significantly reducing the diving response were 1.25 mg/l (p < 0.05) and 5 mg/l (p < 0.05). Curiously, the intermediate dose of 2.5 mg/l was not found to cause a significant reduction. This could have been the result of beta error in which a true effect is not detected because of variability of response. Analysis of variance effectively protects against alpha error (mistakenly concluding that there is an effect when in fact there is none) but not beta error. The atypical anxiolytic buspirone also produced an anxiolytic-like effect in this test (Figure 7) (Bencan et al., 2009). The buspirone effect was monotonic. Both of these anxiolytic effects were seen at doses that did not cause sedation as indicated by measurements of locomotor speed. By contrast, the anxiolytic drug chlordiazepoxide was not investigators (Hicks et al., 2006), computerized cognitive testing for zebrafish is the goal for future research to make the three-chamber task automated for greater economy and higher throughput.
Figure 5.
Behavioral validation of the novel tank diving test demonstrating the effect of novelty (mean ± sem) (Bencan et al., 2009 ). Zebrafish housed in 3-l wide tanks when tested in the novel 1.5-l narrow tanks showed the novel tank diving response and recovery over the 5-min session in which they spent the great majority of time in the bottom of the tank shortly after introduction to the tank and then spent progressively less time at the bottom as they explored the upper levels with longer experience in the new tank. By contrast, the zebrafish housed in the narrow 1.5-l tanks did not show a change in position across the 5-min session.
Figure 6.
Diazepam decreases bottom dwelling in the 5-min novel tank diving test (mean ± sem) (Bencan et al., 2009 ). The numbers were 12 – 17 per treatment condition. There was a nonmonotonic dose-effect function with intermediate doses of 1.25 ( p < 0.05) and 5 mg/l ( p < 0.05) causing significant reductions in bottom dwelling.
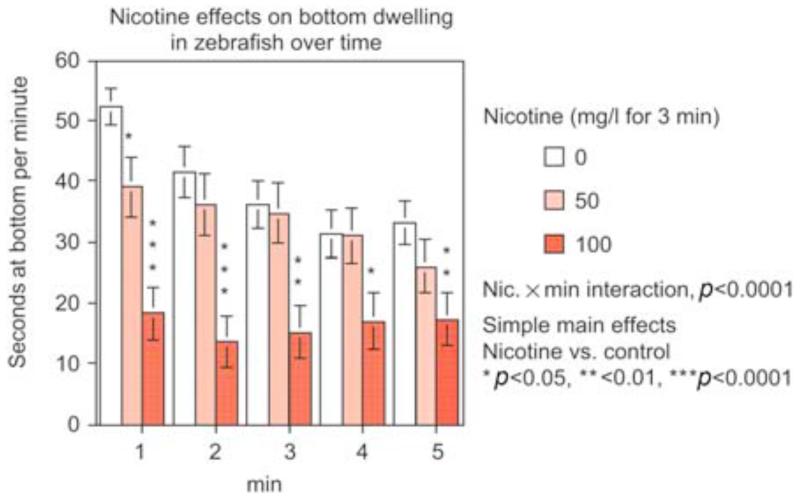
Figure 7.
Nicotine reduces bottom dwelling in the 5-min novel tank diving test (mean ± sem) (Levin et al., 2007). The numbers were 10 per treatment condition. The 50 mg/l nicotine dose only caused a significant (p < 0.05) attenuation of initial diving during the first minute, whereas the 100 mg/l dose caused a decrease in bottom dwelling during the entire test period (p < 0.05 to p < 0.0001).
This line of research showed the utility of the three-chamber task for indexing zebrafish learning of spatial discrimination, color discrimination, color reversal and delayed spatial alternation test of working memory function. The delayed spatial alternation memory task was useful in demonstrating the non-monotonic dose-effect function with nicotine. This task showed the same low-dose cognitive facilitation with nicotine as is seen with mammals such as rats, monkeys and humans (for review see: Levin et al., 2006b). Delayed spatial alternation is a good technique for assessing memory function, but it takes considerable training in zebrafish with a slow rate of acquisition (Levin and Chen, 2004). Simple spatial discrimination is more suited to screening with only a single session of training.
Simple spatial discrimination learning provided a higher throughput assessment that permitted detailed investigation of the time-effect function of nicotine impacts on learning. The delayed onset of the nicotine-induced learning improvement suggested that the short-term receptor activation caused by nicotine might not be the mechanism underlying the learning improvement. This possibility was supported by the demonstration that concurrent application of the nicotinic antagonist mecamylamine did not block nicotine-induced cognitive improvement. Rather, it was later application of mecamylamine that blocked expression of the nicotine effect. This suggested that nicotinic receptor activation was not crucial for the induction of the effect but it was necessary for the expression of the effect. Our hypothesis is that it is the nicotinic receptor desensitization, which is not blocked by mecamylamine followed by later recovery of sensitivity for activation by acetylcholine, which is blockable by mecamylamine, that underlies nicotine-induced learning improvement. Dopamine systems have been in zebrafish from early development onward (Holzschuh et al., 2001). They have been shown to be important for locomotor activity (Anichtchik et al., 2004). The current studies also demonstrate the importance of dopamine systems in the zebrafish brain for cognitive function. Dopamine metabolite levels were found to have a robust positive linear relationship with accuracy in the spatial learning task. Nicotine-induced increase in dopamine metabolite levels corresponded with improved accuracy in the three-chamber task and the nicotinic antagonist reversed both the expression of the dopaminergic effect and improvement in maze choice accuracy.
The novel tank diving task proved useful for determining stress response. The test was validated with a comparison of fish housed in the same-sized tanks as the test tank vs. other fish housed in tanks larger than the test tank. Those fish being transferred from the larger tanks into the novel tank did show the diving response. There were mixed responses with benzodiazepine anxiolytic drugs. Diazepam did produce a significant reduction of the novel tank diving response, but the effect was non-monotonic with intermediate but not higher doses being effective. The doses of diazepam, which were effective in reducing the diving response, did not produce sedation, thus the decreased diving could not be attributed merely to slower response. The biphasic dose-response curve seen with diazepam in zebrafish with moderate but not higher or lower doses being effective has also been seen in rats on an elevated plus maze where only moderate diazepam significantly increased time rats spent on the open arms of the maze (Ruarte and Alvarez, 1999 ).
By contrast, another benzodiazepine chlordiazepoxide was not effective in reducing the novel tank diving response over a broad dose range from low doses that did not affect swimming speed all the way up to doses that caused substantial sedative response. The reason why chlordiazepoxide was not effective whereas diazepam was effective is not immediately clear. Both benzodiazepines facilitate GABA-A receptor actions via actions on benzodiazepine receptors (Atack, 2005). These two drugs also have similar potencies at inhibiting high affinity choline uptake (Miller and Richter, 1985). It could be that the differential effects of these drugs at glycine receptors might be relevant with the greater than sevenfold greater potency of diazepam at the glycine receptor than chlordiazepoxide (Young et al., 1974). Also, chlordiazepoxide is more effective than diazepam at inhibiting thyrotropinreleasing hormone (Drummond, 1985). Further research is necessary to determine whether these mechanisms or others are responsible for the differential effects of diazepam and chlordiazepoxide on the novel tank diving task. The atypical antipsychotic buspirone was fully effective in reducing the novel tank diving response at doses that did not produce sedation showing that its efficacy was not merely due to slower swimming. Finally nicotine, which is not a classic anxiolytic drug, but has been found under certain conditions in humans and mammalian models to reduce anxiety (Jarvik and Pomerleau, 1986; Picciotto et al., 2002), significantly reduced the novel tank diving response. The effective dose of 100 mg/l was also effective in significantly improving learning and memory in the three-chamber task. Thus, the nicotine-induced attenuation of the novel tank diving response was not merely due to disorientation.
The zebrafish model can be very useful for the study of the neurobiological bases of a variety of behaviors as shown in the studies in our lab as well as many others in the field. Our studies have found that zebrafish show cognitive and stress-related behaviors that have important similarities to mammals. These can be studied in zebrafish both to help determine the mechanisms underlying these functions and how they can go awry, but also to help screen for drug treatments to help reverse cognitive and emotional impairments.
Materials and Methods
Subjects
Zebrafish (Danio rerio) were kept at approximately 28.5° C on a 12:12-h light/dark cycle in an automated flow-through continuously filtered water system by Aquatic Habitats (Apopka, FL, USA). Behavioral testing of drug effects took place during the light phase between 8:00 h and 17:00 h. The fish were adults approximately 2 – 4 months of age. In each study the fish were randomly sorted into treatment groups and vehicle-treated controls. The tank water was made by mixing deionized H2O and sea salts (Instant Ocean, 1.2 g/20 l of H2O). The tanks with the groups of adult fish were maintained with constant filtration and aeration. Fish were fed twice daily with brine shrimp and flake fish food.
The three-chamber task for assessing learning and memory
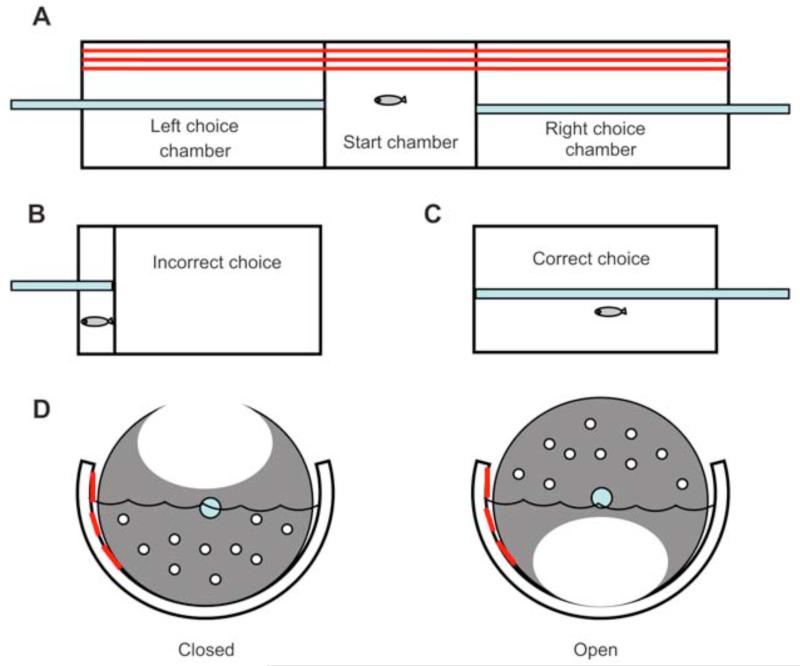
The three-chamber task has a simple apparatus, which can be used to assess a variety of different types of learning as well as memory. The advantage of the three-chamber test over the two-chamber shuttle box is that the activity of the fish is not confounded with choice accuracy. The same amount of locomotion is needed for correct as for incorrect responses. This is not true for either active or passive avoidance in the two-chamber shuttle box paradigm. Figure 9 shows the most recent version of the three-chamber task apparatus (Eddins et al., 2009). Earlier studies used a larger version in a rectangular fish tank (Arthur and Levin, 2001; Levin et al., 2003, 2006a). In brief, the apparatus has a central chamber with movable partitions on either side. The partitions were mounted on Plexiglas rails so that they could be moved to within 1 – 2 cm from the end wall of the tank. Dark strips were placed along the rear side of the tank to provide an axis of orientation for position discrimination. The partitions can be opened to permit access for the fish to each of the two side chambers. Once the fish swims to one side or the other (a choice can be forced by bringing together the partitions), the gate can be closed. The fish is confined to the end of the choice chamber for 10 s as punishment for incorrect choices. The fish is put back into the center start chamber by opening the partition after the end of each trial. The differential reinforcement in the three-chamber task is punishment by swimming space restriction for incorrect choices and being left alone in the full-sized choice chamber for correct choices. To decrease the initial trial choice accuracy gives the subjects greater opportunity to improve with training. We have found that with this training against initial bias procedure fish show accuracy below random choice accuracy (50 % in the two-choice task).
Figure 9.
The three-chamber task for assessing learning and memory (Eddins et al., 2009). (A) To start a trial the fish is in the central chamber. (B) After making an incorrect choice the fish is punished by moving the partition close to the end wall of the apparatus. (C) After a correct choice the fish is left alone with the full-size choice chamber. (D) The partitions on either side can be opened or closed by rotating the access opening to be above or below the water surface. The smaller holes opposite the access opening are present to allow water to pass when the partitions are moved to constrict or enlarge the choice chamber.
The simplest paradigm is spatial discrimination learning, where the fish is consistently reinforced for choosing one side vs. the other. An initial assessment is made with each fish to determine its preferred side. Each fish is then trained against its preference, increasing the sensitivity of the task. The three-chamber task can also be used to assess color discrimination with the use of colored inserts at the ends of each side chamber. The locations of these can be randomly switched so that the fish is consistently rewarded for choosing one color regardless of the location. With both spatial and nonspatial discrimination, reversal learning can be assessed by training the fish to go to one side or one color and then switching the contingencies to the opposite choice being correct. Delayed spatial alternation can be tested as an index of memory. In this paradigm the spatial location of the correct choice is always opposite to the location of the previous response.
We have found in a series of studies that zebrafish will learn simple spatial discrimination, color discrimination, reversal learning as well as delayed spatial alternation (Arthur and Levin, 2001; Levin et al., 2003, 2006a). Zebrafish will quickly learn spatial discrimination. We have found that a series of seven trials in a single session is sufficient to demonstrate spatial learning. Zebrafish will also quickly learn nonspatial color discrimination with the use of colored stimuli placed at each choice side. The side of placement of these color stimuli is randomly switched to make their position location balanced over the course of a test session. Over the course of four sessions of training zebrafish will learn nonspatial color discrimination. We have shown that zebrafish will also quickly learn reversal of color discrimination within a four-session period. Zebrafish learn delayed spatial alternation more slowly but they will reliably show this behavior such that drug effects on memory can be assessed.
As presented below, we have used the spatial discrimination and alternation procedures to assess the effects of nicotinic acetylcholine receptor manipulations on learning and memory in zebrafish.
The novel tank diving test
Zebrafish show a reproducible behavior of diving to the bottom when initially introduced into a novel environment and then over time increasing swimming to higher levels. This diving response by the fish would be adaptive to avoid predation from below. By diving to the bottom of the environment the zebrafish would have no open space below them and thus be protected from the approach of predators approaching from below. It is similar to the thigmotaxis that land animals, such as rodents, show when they stay near the walls when introduced into a novel open field environment to eliminate a path of approach for predators. We have formalized observation of this behavior with the novel tank diving test to assess the effects of anxiolytic drugs.
The zebrafish were placed for testing in a 1.5-l plastic tank from the Aquatic Habitats system. The tanks were backlit and had a white sheet of plastic serve as a background for the imaging system (Figure 10, left panel). The test tanks were filled with home tank water 11.5 cm deep. The real time digital image of the fish swimming in the novel test tank was fed into the Noldus Image Analysis program, EthoVision (Figure 10, right panel). Seconds per minute of the test spent in each third of the tank sectioned horizontally as well as swim path length per minute (log cm/min) were the dependent measures. The log of activity was taken to normalize the data. Because activity data are typically skewed it is appropriate that they are analyzed as the log of the raw score. The time spent in the bottom third of the tank was considered the index of anxiety. Choice of dwelling on the bottom was near a position of safety similar to the position choice of closed vs. open arms in the elevated plus maze and positions near the wall (thigmotaxis) vs. the center of an open field with rodents (Karl et al., 2003). Similar to the elevated plus maze and open field in rodents, there was also a separate total activity measure. A study was conducted of the impact of home tank housing tank size on the dynamics of diving response during the novel tank diving test. The size of the housing tanks for the fish were 1.5 l and 3 l so that the effect of home tank size on the novel tank diving behavioral test could be compared. The tank used in the diving test was always the same sized 1.5-l tanks. In the studies of drug effects on diving, all of the fish were housed in 3-l tanks.
Figure 10.
Novel tank diving test for assessment of anxiolytic effects of drugs (Levin and Cerutti, 2008). (A) The test tanks are 1.5-l thin tanks from the Aquatic Habitats system. The digital video camera images the fish location and conveys the images to the Noldus computer image analysis software. (B) The digitized swimming track of a fish over the course of the 5-min session.
Drug administration
The behaviorally relevant dose ranges of nicotine and the other drugs tested were determined by pilot studies, which tested the effects of concentrations ranging from ineffective to those having acutely toxic effects. Detailed pharmacokinetics of nicotine and a variety of other drugs have not yet been characterized in zebrafish. Nicotine ditartrate was administered by immersing the zebrafish in a dosing tank with concentrations of 50 – 800 mg/l nicotine for 3 min. The dose of nicotine was calculated on weight of the ditartrate salt. After nicotine exposure the fish were placed singly into a 3-l holding tank without nicotine for the interval between exposure and testing. A delay of 20 min was imposed between the end of dosing and the beginning of the test session. In the delay-response study the interval between the end of dosing and the beginning of testing ranged from 0 to 160 min. In the nicotine + mecamylamine experiment, nicotine (0 or 100 mg/l) was administered for 3 min, and then after 15 min in the recovery tank the fish were exposed to the nicotinic antagonist mecamylamine (0 or 200 mg/l) for 5 min. There was no nicotine exposure in either the home tank or the test chamber. All the fish were drug naive and each fish was tested only once in the spatial discrimination learning studies. In the delays spatial alternation study the fish underwent training and were then administered the nicotine doses on a repeated measures counterbalanced design with at least 2 days between doses. For the novel tank diving test several anxiolytic and nicotinic drugs were tested for their acute effects on the zebrafish diving response. All of the drugs were administered by immersing the zebrafish in a beaker with fixed drug concentrations for 3 min. The drugs were buspirone HCl (0, 3.125, 6.25, 12.5, 25 and 50 mg/l), diazepam HCl (0, 0.625, 1.5, 2.5, 5, 10 and 20 mg/l), chlordiazepoxide HCl (0, 0.625, 1.25, 2.5, 5, 10 and 20 mg/l), nicotine ditartrate (0, 50 and 100 mg/l), mecamylamine HCl (0 and 200 mg/l), dihydro-β -erythroidine HCl (DH β E, 0, 50, 100 and 200 mg/l), a nicotinic α 4 β 2 nicotinic receptor antagonist, and methyllycaconitine HCl (MLA, 0, 50, 100 and 200 mg/l), a nicotinic α 7 receptor antagonist. The doses of the drugs were calculated from the weight of the salt. A delay of 5 min was imposed between the end of dosing and the start of the trial. Tank water was used as the vehicle for all of the drugs except for diazepam which had tank water with 5% DMSO as the vehicle to facilitate solution of the drug. The fish were exposed to the drug in a separate beaker and then were put into a holding tank without drug for the interval between exposure and testing.
Neurochemical analysis
After completing the behavioral task, neurochemical levels were assayed in homogenization tissue samples with HPLC. Briefly, fish were anesthetized by submersion in 4° C aquarium water and euthanized by decapitation. The brains were rapidly excised and homogenized (25 Å~ vol/wt) in 0.1 n perchloric acid/100 m m EDTA:mobile phase (1:10). After column purification, to remove solid cellular particulate, samples were diluted in mobile phase (1:10) and 20 μ l were analyzed for monoamine levels with HPLC. The HPLC system consisted of an isocratic pump (model LC1120, GBC Separations, Hubbardston, MA, USA), a Rheodyne injector (model 7725i) with a 20 μ l PEEK loop, and an INTRO amperometric detector (Antec Leyden, Zoeterwoude, Netherlands). The electrochemical flow cell (model VT 03, Antec Leyden) had a 3-mm glassy carbon working electrode with a 25 μ m spacer, and a Ag/AgCl reference electrode. The cell potential was set at 700 mV. The signal was filtered with a low pass in-line noise killer, LINK (Antec Leyden) set at a 14-s peak width and a cut-off frequency of 0.086 Hz. The signal is integrated using the EZChrom elite chromatography software (Scientific Software Inc., Lincolnwood, IL, USA). The injector, flow cell and analytical column were placed in the Faraday-shielded compartment of the detector where the temperature is maintained at 30 °C. The stationary phase was a reverse phase BDS Hypersil C18 column 100 mm Å~ 2.1 mm, with 5 μ m particle size and 120 Å pore size (Keystone Scientific, Waltham, MA, USA). The mobile phase was commercially purchased (ESA, MD-TM mobile phase, Chelmsford, MA, USA). The mobile phase was continually degassed with a Degasys Populaire on-line degasser (Sanwa Tsusho Co. Ltd., Tokyo, Japan) and delivered at a flow rate of 0.5 ml/min. Data analysis Repeated-measures analysis of variance tests were used to assess the effects of the drugs and their interactions. Planned comparisons were made between treatments and control conditions. The final threshold for all effects was p < 0.05 (two-tailed).
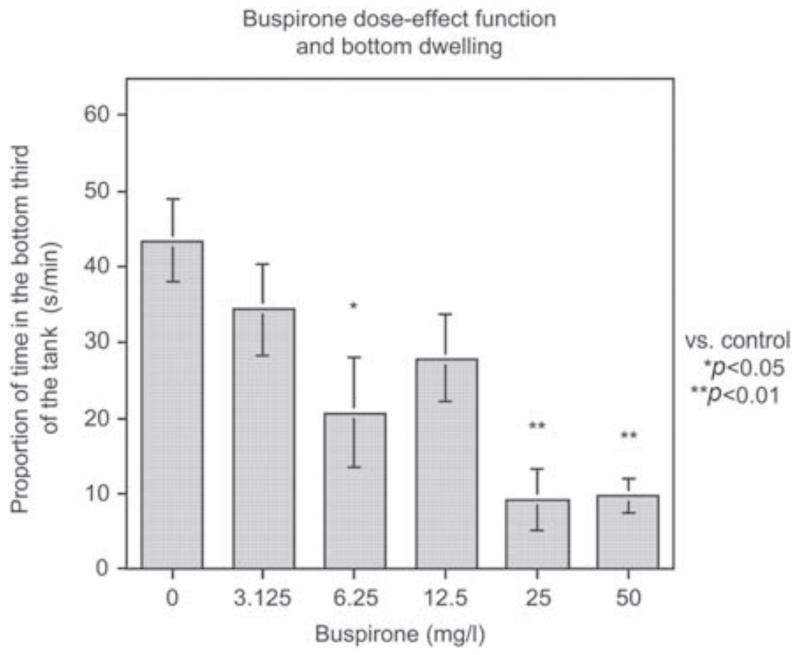
Figure 8.
Buspirone decreases bottom dwelling in the 5-min novel tank diving test (mean ± sem) (Bencan et al., 2009). The numbers were 10 per treatment condition. Over the dose range tested there was a monotonic dose-effect function with 6.25 (p < 0.05), 25 (p < 0.01) and 50 mg/l p < 0.01) doses causing a significant decrease in bottom dwelling.
Acknowledgement
This research was supported by the Duke University Superfund Basic Research Center (NIH ES10356).
References
- Al-Imari L, Gerlai R. Sight of conspecifics as reward in associative learning in zebrafish ( Danio rerio ) Behav. Brain Res. 2008;189:216–219. doi: 10.1016/j.bbr.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Anichtchik OV, Kaslin J, Peitsaro N, Scheinin M, Panula P. Neurochemical and behavioural changes in zebrafish Danio rerio after systemic administration of 6-hydroxydopamine and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. J. Neurochem. 2004;88:443–453. doi: 10.1111/j.1471-4159.2004.02190.x. [DOI] [PubMed] [Google Scholar]
- Arthur D, Levin ED. Spatial and non-spatial discrimination learning in zebrafish ( Danio rerio) Anim. Cogn. 2001;4:125–131. [Google Scholar]
- Atack JR. The benzodiazepine binding site of GABAA receptors as a target for the development of novel anxiolytics. Expert Opin. Invest. Drugs. 2005;14:601–618. doi: 10.1517/13543784.14.5.601. [DOI] [PubMed] [Google Scholar]
- Bass SL, Gerlai R. Zebrafish (Danio rerio) responds differentially to stimulus fish: the effects of sympatric and allopatric predators and harmless fish. Behav. Brain Res. 2008;186:107–117. doi: 10.1016/j.bbr.2007.07.037. [DOI] [PubMed] [Google Scholar]
- Bencan Z, Levin ED. The role of alpha7 and alpha4-beta2 nicotinic receptors in the nicotine-induced anxiolytic effect in zebrafish. Physiol. Behav. 2008;95:408–412. doi: 10.1016/j.physbeh.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bencan Z, Sledge D, Levin ED. Buspirone, chlordiazepoxide and diazepam effects in a zebrafish model of anxiety. Pharmacol. Biochem. Behav. 2009;94:75–80. doi: 10.1016/j.pbb.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser R, Gerlai R. Behavioral phenotyping in zebrafish: comparison of three behavioral quantification methods. Behav. Res. Methods. 2006;38:456–469. doi: 10.3758/bf03192800. [DOI] [PubMed] [Google Scholar]
- Braubach OR, Wood HD, Gadbois S, Fine A, Croll RP. Olfactory conditioning in the zebrafish (Danio rerio) Behav. Brain Res. 2009;198:190–198. doi: 10.1016/j.bbr.2008.10.044. [DOI] [PubMed] [Google Scholar]
- Cachat JM, Canavello PR, Elegante MF, Bartels BK, Elkhayat SI, Hart PC, Tien AK, Tien DH, Beeson E, Mohnot S, et al. Modeling stress and anxiety in zebrafish. In: Kalueff AV, Cachat JM, editors. Zebrafish Models in Neurobehavioral Research. Cambridge University Press; Cambridge: 2010. [Google Scholar]
- Carvan MJ, 3rd, Loucks E, Weber DN, Williams FE. Ethanol effects on the developing zebrafish: Neurobehavior and skeletal morphogenesis. Neurotoxicol. Teratol. 2004;26:757–768. doi: 10.1016/j.ntt.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Champagne DL, Hoefnagels CC, de Kloet RE, Richardson MK. Translating rodent behavioral repertoire to zebrafish ( Danio rerio ): relevance for stress research. Behav. Brain Res. 2010;214:332–342. doi: 10.1016/j.bbr.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Colwill RM, Raymond MP, Ferreira L, Escudero H. Visual discrimination learning in zebrafish (Danio rerio) Behav. Process. 2005;70:19–31. doi: 10.1016/j.beproc.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Darland T, Dowling JE. Behavioral screening for cocaine sensitivity in mutagenized zebrafish. Proc. Natl. Acad. Sci. USA. 2001;98:11691–11696. doi: 10.1073/pnas.191380698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond AH. Chlordiazepoxide is a competitive thyrotropin-releasing hormone receptor antagonist in GH3 pituitary tumour cells. Biochem. Biophys. Res. Commun. 1985;127:63–70. doi: 10.1016/s0006-291x(85)80126-1. [DOI] [PubMed] [Google Scholar]
- Eddins D, Petro A, Williams P, Cerutti DT, Levin ED. Nicotine effects on learning in zebrafish: the role of dopaminergic systems. Psychopharmacology. 2009;202:103–109. doi: 10.1007/s00213-008-1287-4. [DOI] [PubMed] [Google Scholar]
- Egan RJ, Bergner CL, Hart PC, Cachat JM, Canavello PR, Elegante MF, Elkhayat SI, Bartels BK, Tien AK, Tien DH, et al. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav. Brain Res. 2009;205:38–44. doi: 10.1016/j.bbr.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engeszer RE, Ryan MJ, Parichy DM. Learned social preference in zebrafish. Curr. Biol. 2004;14:881–884. doi: 10.1016/j.cub.2004.04.042. [DOI] [PubMed] [Google Scholar]
- Gerlai R. Zebrafish antipredatory responses: a future for translational research ? Behav. Brain Res. 2010;207:223–231. doi: 10.1016/j.bbr.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlai R, Fernandes Y, Pereira T. Zebrafish ( Danio rerio ) responds to the animated image of a predator: towards the development of an automated aversive task. Behav. Brain Res. 2009;201:318–324. doi: 10.1016/j.bbr.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Laplazaa LM, Robert Gerlaib R. Latent learning in zebrafish (Danio rerio) Behav. Brain Res. 2009;208:509–515. doi: 10.1016/j.bbr.2009.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks C, Sorocco D, Levin M. Automated analysis of behavior: a computer-controlled system for drug screening and the investigation of learning. J. Neurobiol. 2006;66:977–990. doi: 10.1002/neu.20290. [DOI] [PubMed] [Google Scholar]
- Holzschuh J, Ryu S, Aberger F, Driever W. Dopamine transporter expression distinguishes dopaminergic neurons from other catecholaminergic neurons in the developing zebrafish embryo. Mech. Dev. 2001;101:237–243. doi: 10.1016/s0925-4773(01)00287-8. [DOI] [PubMed] [Google Scholar]
- Jarvik ME, Pomerleau OF. Nicotine as a psychoactive drug: anxiety and pain reduction, arousal, and appetite regulation. Psychopharmacol. Bull. 1986;22:863–864. [PubMed] [Google Scholar]
- Karl T, Pabst R, von Horsten S. Behavioral phenotyping of mice in pharmacological and toxicological research. Exp. Toxicol. Pathol. 2003;55:69–83. doi: 10.1078/0940-2993-00301. [DOI] [PubMed] [Google Scholar]
- Levin ED, Chen E. Nicotinic involvement in memory function in zebrafish. Neurotoxicol. Teratol. 2004;26:731–735. doi: 10.1016/j.ntt.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Levin ED, Cerutti DT. Behavioral neuroscience of zebrafish. In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. CRC Press; New York: 2008. pp. 293–310. [Google Scholar]
- Levin ED, Chrysanthis E, Yacisin K, Linney E. Chlorpyrifos exposure of developing zebrafish: effects on survival and long-term effects on response latency and spatial discrimination. Neurotoxicol. Teratol. 2003;25:51–57. doi: 10.1016/s0892-0362(02)00322-7. [DOI] [PubMed] [Google Scholar]
- Levin ED, Limpuangthip J, Rachakonda T, Peterson M. Timing of nicotine effects on learning in zebrafish. Psychopharmacology. 2006a;184:547–552. doi: 10.1007/s00213-005-0162-9. [DOI] [PubMed] [Google Scholar]
- Levin ED, McClernon FJ, Rezvani AH. Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification and anatomic localization. Psychopharmacology. 2006b;184:523–539. doi: 10.1007/s00213-005-0164-7. [DOI] [PubMed] [Google Scholar]
- Levin ED, Bencan Z, Cerutti DT. Anxiolytic effects of nicotine in zebrafish. Physiol. Behav. 2007;90:54–58. doi: 10.1016/j.physbeh.2006.08.026. [DOI] [PubMed] [Google Scholar]
- Levin ED, Sledge D, Roach S, Donerly S, Linney E. Methylphenidate-induced developmental neurobehavioral toxicity in zebrafish. Neurobehavioral Teratology Society Annual Meeting, Louisville, KY, USA. Neurotoxicol. Teratol. 2010;32:500. [Google Scholar]
- Lopez-Patino MA, Yu L, Cabral H, Zhdanova IV. Anxiogenic effects of cocaine withdrawal in zebrafish. Physiol. Behav. 2008;93:160–171. doi: 10.1016/j.physbeh.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Maximino C, de Brito TM, da Silva Batista AW, Herculano AM, Morato S, Gouveia A., Jr. Measuring anxiety in zebrafish: a critical review. Behav. Brain Res. 2010;214:157–171. doi: 10.1016/j.bbr.2010.05.031. [DOI] [PubMed] [Google Scholar]
- Miller JA, Richter JA. Effects of anticonvulsants in vivo on high affinity choline uptake in vitro in mouse hippocampal synaptosomes. Br. J. Pharmacol. 1985;84:19–25. [PMC free article] [PubMed] [Google Scholar]
- Miller N, Gerlai R. Quantification of shoaling behaviour in zebrafish ( Danio rerio ) Behav. Brain Res. 2007;184:157–166. doi: 10.1016/j.bbr.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Miller NY, Gerlai R. Oscillations in shoal cohesion in zebrafish (Danio rerio) Behav. Brain Res. 2008;193:148–151. doi: 10.1016/j.bbr.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pather S, Gerlai R. Shuttle box learning in zebrafish ( Danio rerio ) Behav. Brain Res. 2009;196:323–327. doi: 10.1016/j.bbr.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Brunzell DH, Caldarone BJ. Effect of nicotine and nicotinic receptors on anxiety and depression. Neuroreport. 2002;13:1097–1106. doi: 10.1097/00001756-200207020-00006. [DOI] [PubMed] [Google Scholar]
- Pradel G, Schachner M, Schmidt R. Inhibition of memory consolidation by antibodies against cell adhesion molecules after active avoidance conditioning in zebrafish. J. Neurobiol. 1999;39:197–206. [PubMed] [Google Scholar]
- Pradel G, Schmidt R, Schachner M. Involvement of L1.1 in memory consolidation after active avoidance conditioning in zebrafish. J. Neurobiol. 2000;43:389–403. [PubMed] [Google Scholar]
- Rihel J, Prober DA, Arvanites A, Lam K, Zimmerman S, Jang S, Haggarty SJ, Kokel D, Rubin LL, Peterson RT, et al. Zebrafish behavioral profiling links drugs to biological targets and rest/wake regulation. Science. 2010;327:348–351. doi: 10.1126/science.1183090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruarte MB, Alvarez EO. Behavioral profiles displayed by rats in an elevated asymmetric plus-maze: effects of diazepam. Braz. J. Med. Biol. Res. 1999;32:99–106. doi: 10.1590/s0100-879x1999000100015. [DOI] [PubMed] [Google Scholar]
- Sackerman J, Donegan JJ, Cunningham CS, Nguyen NN, Lawless K, Long A, Benno RH, Gould GG. Zebrafish behavior in novel environments: effects of acute exposure to anxiolytic compounds and choice of Danio rerio line. Int. J. Comp. Psychol. 2010;23:43–61. [PMC free article] [PubMed] [Google Scholar]
- Schier AF. Genetics of neural development in zebrafish. Curr. Opin. Neurobiol. 1997;7:119–126. doi: 10.1016/s0959-4388(97)80129-8. [DOI] [PubMed] [Google Scholar]
- Stewart A, Kadri F, DiLeo J, Chung KM, Cachat J, Goodspeed J, Suciu C, Roy S, Gaikwad S, Wong K, et al. The developing utility of zebrafish in modeling neurobehavioral disorders. Int. J. Comp. Psychol. 2010;23:104–120. [Google Scholar]
- Williams FE, White D, Messer WS. A simple spatial alternation task for assessing memory function in zebrafish. Behav. Process. 2002;58:125–132. doi: 10.1016/s0376-6357(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Xu X, Scott-Scheiern T, Kempker L, Simons K. Active avoidance conditioning in zebrafish (Danio rerio) Neurobiol. Learn. Mem. 2007;87:72–77. doi: 10.1016/j.nlm.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Young AB, Zukin SB, Snyder SH. Interaction of benzodiazepines with central nervous glycine receptors: possible mechanism of action. Proc. Natl. Acad. Sci. USA. 1974;71:2246–2250. doi: 10.1073/pnas.71.6.2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhdanova IV, Yua L, Lopez-Patino M, Shang E, Kishi S, Guelin E. Aging of the circadian system in zebrafish and the effects of melatonin on sleep and cognitive performance. Brain Res. Bull. 2008;7:433–441. doi: 10.1016/j.brainresbull.2007.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirger JM, Beattie CE, McKay DB, Boyd RT. Cloning and expression of zebrafish neuronal nicotinic acetylcholine receptors. Gene Expr. Patterns. 2003;3:747–754. doi: 10.1016/s1567-133x(03)00126-1. [DOI] [PubMed] [Google Scholar]