Abstract
Objective
To explore non-HIV related healthcare service(s) (NHRHS) utilization, demographic, clinical, and laboratory factors associated with timely initial ‘retention’ in HIV care among individuals ‘linked’ to HIV care in British Columbia (BC), Canada.
Methods
We conducted a Weibull time-to-initial retention analysis among BC STOP HIV/AIDS cohort participants linked in 2000–2010, who had ≥1 year of follow-up. We defined ‘linked’ as the first HIV-related service following diagnosis and ‘retained’ as having, within a calendar year, either: i) ≥2 HIV-related physician visits/diagnostic tests or ii) ≥2 ART dispensations, ≥3 months apart. Individuals were followed until they were retained, died, their last contact date, or December 31st, 2011.
Results
Of 5231 linked individuals (78% male, median age 39 [Q1–Q3:32–46] years), 4691(90%) were retained (median time-to-initial retention:9[5–13] months) by the end of follow-up and 540(10%) were not. Eighty-four percent of not retained and 96% of retained individuals used ≥1 types of NHRHS during follow-up. Individuals who saw a specialist for NHRHS during follow-up had a shorter time-to-initial retention than those who did not (adjusted Hazard Ratio (aHR)=2.79; 95%CI:2.47–3.16). However, those who saw a GP for NHRHS (aHR=0.79; 95%CI:0.74–0.84) and those admitted to the hospital for NHRHS (aHR=0.60; 95%CI:0.54–0.67), versus those who did not, respectively, had longer times to initial retention; along with females, PWID, and individuals <40 years old.
Conclusions
Overall, 84% of not retained individuals used some type of NHRHS during follow-up. Given that 71% of not retained individuals used GP NHRHS, our results suggest GP-targeted interventions may be effective in improving time-to-initial retention.
Keywords: Retention in HIV care, Time to Retention, Missed Opportunities, Retention, Non-HIV related healthcare service utilization, HIV
INTRODUCTION
Retaining HIV-diagnosed individuals in HIV care is crucial for optimizing HIV-related health outcomes. Studies have shown that individuals retained in HIV care are more likely to timely initiate antiretroviral therapy (ART)(1), have higher CD4 cell counts (2–5), and have increased survival rates (3, 6, 7). They also have a decreased likelihood of developing HIV opportunistic infections(8), being hospitalized (9–11), having high viral loads(2), and developing ART resistance (2, 3, 12).
Furthermore, the importance of retaining individuals in care is emphasized in the context of Treatment as Prevention (TasP). (TasP refers to the timely manner and sustained use of HAART to simultaneously virtually eliminate the risk of disease progression to AIDS and premature death, as well as HIV transmission (13–21).) This idea is demonstrated by the results of a mathematical model, which estimated the proportion of HIV transmissions occurring at each stage of the HIV care cascade (22). The authors estimated that individuals who were diagnosed but not retained in HIV care accounted for the greatest proportion of transmissions in the USA in 2009, 61%, as well as the second highest transmission rate, 5.3 per 100 person-years, following HIV-infected but undiagnosed individuals. These findings further highlight the importance of understanding factors associated with timely retention in HIV care (referred to as ‘retention’ hereafter), and the need to uncover opportunities for engagement in HIV care, both of which are crucial for constructing targeted retention interventions.
Unfortunately, achieving adequate retention remains an ongoing challenge. A recent longitudinal analysis of the HIV cascade of care, from 1996–2011, in British Columbia (BC) found that consistently, the greatest cascade attrition occurred between the stages ‘linked to HIV care’ and ‘retained in HIV care’ (23). However, there was a gap in understanding why this was the case, since BC is a Canadian province with universal free access to ART, laboratory monitoring, and care, as well as a well-established TasP program (24). Thus, we sought to determine factors associated with delayed time-to-initial retention among the HIV-positive population in BC. Specifically, we were interested in exploring if individuals not retained in HIV care (referred to as ‘retained’ hereafter) continued to seek out non-HIV related healthcare and how non-HIV related healthcare utilization was associated with the time-to-initial retention.
METHODS
Study Data
Data used in this analysis came from the BC STOP HIV/AIDS database which has been described in detail elsewhere (25, 26). Briefly, the database is the result of a series of linked provincial datasets. These include: i) The BC Centre for Disease Control, which is the provincial agency that centralises all HIV testing data and new HIV diagnoses from the BC Public Health Microbiology and Reference Laboratory (which performs all confirmatory testing in BC). HIV reporting (non-nominal and nominal) became mandatory in BC in 2003; ii) The BC Centre for Excellence in HIV/AIDS, which has a centralized system which captures all antiretroviral distribution, all plasma viral load testing (pVL), all resistance testing, and approximately 85% of CD4 cell count measurements in BC; iii) The Medical Services Plan (MSP) physician billing database, which captures non-HIV related and HIV-related physician services; iv) The provincial Discharge Abstract Database (DAD), which records all hospitalizations in BC and; v) The BC Pharma Net database, which captures non-ART related drug dispensations.
Eligibility
This analysis was restricted to HIV-positive individuals who were linked to HIV care (referred to as ‘linked’ hereafter) between January 1st, 2000 and December 31st, 2010 and had at least one calendar year of follow-up. Individuals were followed until they were retained. Individuals who were never retained were followed until death, their last contact date, or December 31st, 2011.
Outcome Variable
We undertook the present analysis to assess the time-to-initial retention among linked individuals. We defined linked and retained using previously defined and validated definitions (23): ‘Linked’: the first instance of an HIV-related service (i.e., having a pVL, CD4 cell count, HIV-related physician visit, or ART dispensed): i) after HIV diagnosis for individuals with a confirmed HIV test or ii) ≥30 days after the derived HIV diagnosis date for individuals without a confirmed HIV test.; ‘Retained’: among linked individuals, having either: i) ≥2 HIV-related physician visits or diagnostic tests (CD4 or pVL) or ART drug dispensations, ≥3 months apart within a calendar year. Since retention is defined by two retention events/dates, the retained date for this analysis was defined as the first retention event date.
Explanatory Variables
Of primary interest was exploring the use of non-HIV related healthcare services stratified by retention status. To achieve this, we used five non-HIV related healthcare utilization variables, measured from the time of linkage to the end of study follow-up: i) non-HIV related MSP services provided by a general practitioner (GP), ii) non-HIV related MSP services provided by a specialist, iii) non-HIV related laboratory work, iv) non-HIV related DAD hospital admissions, and v) non-HIV related Pharma Net prescription dispensations. Given the high correlation between non-HIV related healthcare services (for example, in BC, to see a specialist one requires a referral from a GP), we assigned a value of 1 if an individual accessed a specific non-HIV related healthcare service and 0 otherwise. Thus, if an individual saw a GP for non-HIV related reasons, regardless of any other type of non-HIV related healthcare service used, that individual would be classified as ‘1’ for seeing a non-HIV related GP. After creating our binary non-HIV related healthcare service utilization variables we tested them for correlation and found the variable non-HIV related laboratory work was highly correlated with non-HIV related MSP services provided by a specialist (Spearman’s correlation coefficient=0.98). Thus, we only included the variable ‘non-HIV related MSP services provided by a specialist’ in our final Weibull model and in the creation of the variable ‘number of types of non-HIV related healthcare services’, which is the number of different types of non-HIV related healthcare services used by an individual, categorized as zero, one, two, three, and four.
We also considered the following baseline covariates (i.e., characteristics measured during the linkage year): sex; history of testing Hepatitis C antibody (Hepatitis C) positive; AIDS diagnosis; HIV risk group: categorized as people who use injection drugs (PWID), men who have sex with men (MSM), heterosexual, and other (blood transfusion, accidental puncture, or perinatal exposure)/unknown (i.e., no reported HIV risk group)); age; CD4; pVL; patient residence in an urban area, defined by the first three digits of a patient’s postal code; and the regional health authority (HA) where the individual received HIV care (there are five regional HAs in BC: Northern, Vancouver Coastal, Vancouver Island, Fraser, and Interior). In BC, HAs are administratively responsible for health care delivery within their respective regions. Since our retention definition includes the use of ART and ART initiation guidelines have changed over time to recommend initiating ART at higher CD4 cell counts (27–30), we controlled for the covariate ‘year of diagnosis’ to account for this cohort effect. We categorized cohorts as <2000, 2000–2007, 2008–2009 and ≥2010.
Statistical Analyses
Time-to-initial retention was estimated using Kaplan-Meier methods and through Weibull accelerated failure-time model (31). For clarity, Kaplan-Meier curves are displayed up to the first 20 months since most individuals were retained within this time period, though longer-term follow-up was obtained for some individuals and was included in the Weibull Model. Event-free individuals were right-censored as of December 31st, 2011. Individuals included in this analysis were not followed after this date and those lost to follow-up were censored at the date of last contact.
Statistical tests for the dependence between categorical variables were performed using Fisher’s exact test or the Chi-square test, while continuous variables were tested using the Wilcoxon rank-sum test. The Weibull model was built in order to estimate the effects of a number of variables and their influence on initial time-to-retention among linked individuals. Backward stepwise selection based on Akaike Information Criteria (AIC) and type III p-values were used in the final model variable selection (32). In addition, to minimize the potential of collinearity, the correlation between pairs of independent variables was calculated using the Spearman correlation test and highly correlated variables were excluded from further analysis. All analyses were performed using SAS software version 9.3 (SAS, Cary, NC).
RESULTS
In total, 5231 individuals were linked between 2000 and 2010 and had at least one calendar year of follow-up. Of these, 78% were male, 19% were <30 years old, 34% were 30–39 years old, 31% were 40–49 years old, and 17% were ≥50 years old. Also, 36% were PWID, 28% were MSM, 14% were heterosexual, and 22% were in the other/unknown risk group (Table 1).
Table 1.
Non-HIV related healthcare utilization and baseline characteristics associated with initial retention in HIV care.
| Total N=5231 |
Not Retained N=540 |
Retained N=4691 |
P | |
|---|---|---|---|---|
|
| ||||
| Sex, n (%) | ||||
| Female | 1127 (22) | 175 (32) | 952 (20) | <0.001 |
| Male | 4104 (78) | 365 (68) | 3739 (80) | |
| Age (years), n (%) | 5231 | |||
| <30 | 978 (19) | 113 (21) | 865 (18) | 0.023 |
| 30–39 | 1770 (34) | 174 (32) | 1596 (34) | |
| 40–49 | 1607 (31) | 144 (27) | 1463 (31) | |
| ≥50 | 876 (17) | 109 (20) | 767 (16) | |
| Non-HIV related MSP services provided by a general practitioner, n (%) | ||||
| Yes | 3538 (68) | 381 (71) | 3157 (67) | 0.132 |
| No | 1693 (32) | 159 (29) | 1534 (33) | |
| Non-HIV related MSP services provided by a specialist, n (%) | ||||
| Yes | 4733 (90) | 381 (71) | 4352 (93) | <0.001 |
| No | 498 (10) | 159 (29) | 339 (7) | |
| Non-HIV related laboratory test(s) performed, n (%) | ||||
| Yes | 4618 (88) | 336 (62) | 4282 (91) | <0.001 |
| No | 613 (12) | 204 (38) | 409 (9) | |
| Non-HIV related hospital admission(s), n (%) | ||||
| Yes | 431 (8) | 85 (16) | 346 (7) | <0.001 |
| No | 4800 (92) | 455 (84) | 4345 (93) | |
| Non-HIV related drugs prescriptions filled, n (%) | ||||
| Yes | 4084 (78) | 338 (63) | 3746 (80) | <0.001 |
| No | 1147 (22) | 202 (37) | 945 (20) | |
| Number of types of non-HIV related healthcare services used, n (%) | ||||
| None | 250 (5) | 85 (16) | 165 (4) | <0.001 |
| One | 478 (9) | 63 (12) | 415 (9) | |
| Two | 1567 (30) | 130 (24) | 1437 (31) | |
| Three | 2570 (49) | 186 (34) | 2384 (51) | |
| Four | 366 (7) | 76 (14) | 290 (6) | |
| On ART in the retention year, n (%) | ||||
| Yes | 2160 (41) | 0 (0) | 2160 (46) | <0.001 |
| No | 3071 (59) | 540 (100) | 2531 (54) | |
| Hepatitis C, n (%) | ||||
| Positive | 1585 (30) | 43(8) | 1542 (33) | <0.001 |
| Negative | 2467 (47) | 73(14) | 2394 (51) | |
| Unknown | 1179 (23) | 424(79) | 755 (16) | |
| AIDS diagnosed, n (%) | ||||
| Yes | 746 (14) | 13(2) | 733 (16) | <0.001 |
| No | 4485 (86) | 527(98) | 3958 (84) | |
| HIV-risk factors, n (%) | ||||
| Heterosexual | 751 (14) | 47 (9) | 704 (15) | <0.001 |
| PWID | 1885 (36) | 138 (26) | 1747 (37) | |
| MSM | 1452 (28) | 54(10) | 1398 (30) | |
| Other/Unknown | 1143 (22) | 301 (56) | 842 (18) | |
| Year of Diagnosis, n (%) | ||||
| <2000 | 365 (7) | 55 (10) | 310 (7) | 0.001 |
| 2000–2007 | 3779 (72) | 359 (66) | 3420 (73) | |
| 2008–2009 | 781 (15) | 84 (16) | 697 (15) | |
| 2010 | 306 (6) | 42 (8) | 264 (6) | |
| Patient’s Residing Health Authority, n (%) | ||||
| Fraser | 1216 (23) | 154 (29) | 1062 (23) | <0.001 |
| Interior | 304 (6) | 42 (8) | 262 (6) | |
| Vancouver Coastal | 2781 (53) | 212 (39) | 2569 (55) | |
| Vancouver Island | 592 (11) | 53 (10) | 539 (11) | |
| Vancouver Northern | 214 (4) | 23 (4) | 191 (4) | |
| Unknown | 124 (2) | 56 (10) | 68 (1) | |
| Patient’s residence in urban area, n (%) | ||||
| Yes | 4629 (88) | 320 (59) | 4309 (92) | <0.001 |
| No | 185 (4) | 5 (1) | 180 (4) | |
| Unknown | 417 (8) | 215 (40) | 202 (4) | |
| Earliest available pVL during the linkage year (log10 copies/ml) | ||||
| Median (25th–75th percentile) | 3.70 (1.69–4.66) | 3.88 (2.16–4.82) | 3.69 (1.69–4.65) | 0.104 |
| Earliest available CD4 cell count (copies/mm3) during the linkage year | ||||
| Median (25th–75th percentile) | 360 (230–520) | 460 (225–600) | 360 (230–520) | 0.110 |
| Follow-up time from linkage to the end of study follow-up (months) | ||||
| Median (25th–75th percentile) | 48 (27–85) | 8 (5–12) | <0.001 | |
All variables in the table were measured during the year an individual was linked to HIV care except for non-HIV related care service variables. These variables were measured from the time of linkage to the end of study follow-up. HCV=history of testing antibody positive for Hepatitis C; MSM=men who have sex with men; PWID: people with a history of injection drug use; other/unknown= other (blood transfusion, accidental puncture or perinatal exposure)/unknown (i.e., no reported HIV risk group); MSP=medical services plan; ART=antiretroviral therapy; pVL.
Overall, 90% (N=4691) of the study population was retained during study follow-up and had a median time-to-initial retention of 9 (25th–75th percentile (Q1–Q3): 5–13) months (Table 1). Thirty percent and 70% of the study population were retained 6 and 12 months after being linked, respectively. Of retained individuals, 46% were on ART in the year they were considered retained. The remaining 10% (540) of the study population was never retained and had a median study follow-up time of 48 (Q1–Q3: 27–85) months. Bivariable analysis results showed that males, having an AIDS diagnosis, having a known risk factor, being HIV-diagnosed between 2000 and 2007, being on ART, residing in the Vancouver Coastal HA and Vancouver Island HA, and living in an urban area were associated with being retained. All of these variables were included in our final Weibull model except for “patient residence in an urban area”, since only five individuals were both not retained and did not reside in an urban area. We also did not include the variables “history of HCV” since this was highly collinear with PWID, and “year of diagnosis” since this was highly negatively correlated with “follow-up time”. Finally, we did not include “on ART” since this was a part of our retention definition.
Non-HIV related Healthcare Use and Retention in Care Status
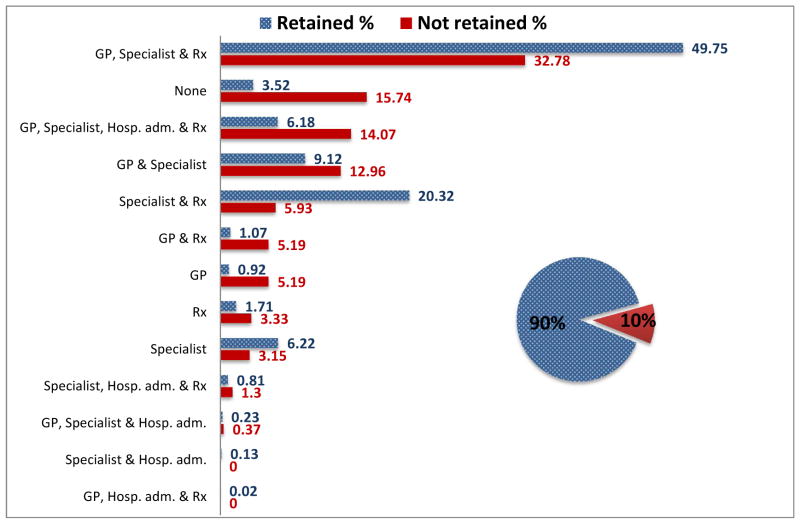
Use of non-HIV related services in the study population was high with approximately 84% (455) of not retained and 96% (4526) of retained individuals using some type of non-HIV related healthcare service during study follow-up. All five of the ‘non-HIV related healthcare utilization’ variables and the ‘number of different types of non-HIV related healthcare services used’ variable were associated with being retained (Table 1). Figure 1 shows the distribution of those retained (patterned bar) and the distribution of those not retained (solid bar), by the types of non-HIV related service utilization variables (see Table 2). The top three non-HIV related service combinations used by retained individuals were: 1. Seeing a non-HIV related GP and a non-HIV related specialist and having a non-HIV related prescription filled, 2. Seeing a non-HIV related specialist and having a non-HIV related prescription filled, and 3. Seeing both a non-HIV related GP and a non-HIV related specialist. The top three non-HIV related service combinations used by not retained individuals were: 1. Seeing a non-HIV related GP and a non-HIV related specialist and having a non-HIV related prescription filled, 2. Using zero non-HIV related services, and 3. Using all four types of non-HIV related services.
Figure 1.
Non-HIV related healthcare utilization by retention status among 5231 individuals linked to HIV care between 2000 and 2010 in BC, Canada.
The distribution of the 90% of study individuals retained in HIV care (shown as a patterned bar) and the distribution of the 10% of study individuals not retained in HIV care (shown as a solid bar) by the type of non-HIV related healthcare service used. GP= billed Medical Services Plan non-HIV related general practitioner visit; Hosp. adm. = non-HIV related hospital admission; Rx=non-HIV related hospital prescription; Specialist= billed Medical Services Plan non-HIV related specialist visit.
Table 2.
Adjusted Hazard Ratios relating non-HIV related healthcare utilization and baseline characteristics associated with time-to-initial retention among 5231 individuals linked to HIV care between 2000 and 2010 in British Columbia, Canada.
|
Model 1 Adjusted Hazard Ratio (95% CI) |
Model 2 Adjusted Hazard Ratio (95% CI) |
|
|---|---|---|
|
| ||
| Number of types of non-HIV related healthcare services used | ||
| None | 1.00 | Not included |
| One | 1.99 (1.65–2.40) | |
| Two | 2.57 (2.16–3.04) | |
| Three | 2.72 (2.30–3.22) | |
| Four | 1.70 (1.39–2.08) | |
| Non-HIV related MSP-services provided by a general practitioner (yes vs. no) | Not included | 0.79 (0.74–0.84) |
| Non-HIV related MSP-services provided by a specialist (yes vs. no) | 2.79 (2.47–3.16) | |
| Non-HIV related hospital admissions (yes vs. no) | 0.60 (0.54–0.67) | |
| Non-HIV related drug prescriptions (yes vs. no) | 1.28 (1.18–1.38) | |
| Sex | ||
| Male | 1.00 | 1.00 |
| Female | 0.83 (0.77–0.90) | 0.84 (0.78–0.91) |
| Age (years) | ||
| <30 | 0.92 (0.85–1.00) | 0.93 (0.86–1.01) |
| 30–39 | 1.00 | 1.00 |
| 40–49 | 1.15 (1.07–1.24) | 1.16 (1.08–1.24) |
| ≥50 | 1.15 (1.05–1.26) | 1.13 (1.03–1.23) |
| AIDS diagnosed | ||
| No | 1.00 | 1.00 |
| Yes | 1.32 (1.22–1.43) | 1.32 (1.22–1.43) |
| HIV-risk factors | ||
| MSM | 1.00 | 1.00 |
| PWID | 0.71 (0.66–0.77) | 0.73 (0.68–0.79) |
| Heterosexual | 1.02 (0.92–1.12) | 1.09 (0.99–1.20) |
| Other/Unknown | 0.39 (0.35–0.42) | 0.42 (0.39–0.46) |
| Patient’s Residing Health Authority | ||
| Vancouver Coastal | 1.00 | 1.00 |
| Fraser | 0.88 (0.82–0.95) | 0.92 (0.86–0.99) |
| Interior | 0.88 (0.78–1.00) | 0.97 (0.85–1.10) |
| Vancouver Island | 1.04 (0.95–1.15) | 1.12 (1.02–1.24) |
| Northern | 1.00 (0.86–1.16) | 1.08 (0.93–1.25) |
| Other/Unknown | 0.62 (0.48–0.80) | 0.64 (0.50–0.82) |
Model 1 includes the variable ‘the number of different types of non-HIV related services used’ but does not includes the four types of ‘non-HIV related healthcare services’ variables. Model 2 includes the four ‘types of non-HIV related healthcare services’ variables but does not include the ‘number of different types of non-HIV related services used’ variable. All variables in Weibull Model 1 and Model 2 were measured during the year an individual was linked to HIV care except for the four types of non-HIV related healthcare services variables. These variables were measured from the time of linkage to the end of study follow-up. GP=general practitioner. MSP=medical services plan (British Columbia’s medical billing system).
Non-HIV Related Healthcare Use and Time to Retention in Care
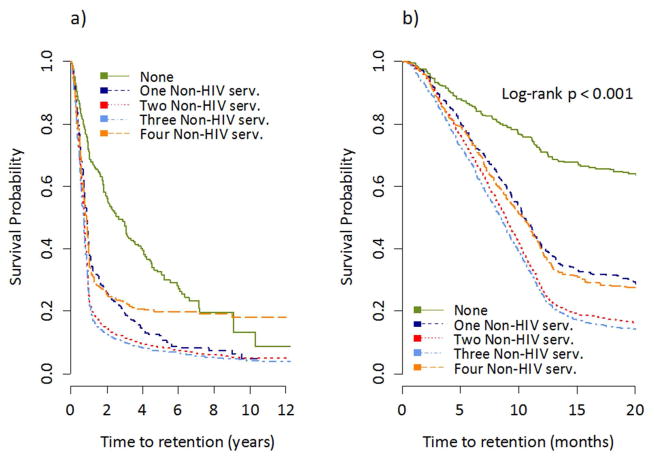
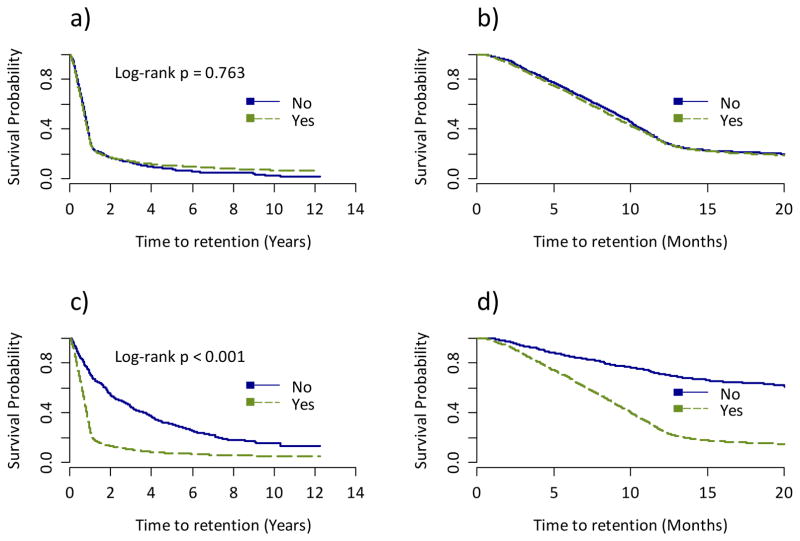
Figure 2 shows Kaplan-Meier time-to-initial retention plots by the number of different types of non-HIV related healthcare services used. We observed that individuals who used zero non-HIV related services had a statistically significantly longer time-to-initial retention than those who used one or more different types of non-HIV related healthcare services (log-rank p-value<0.001). Figure 3 shows Kaplan-Meier survival plots by non-HIV related GP and specialist use. There was no significant difference in the time-to-initial retention between those who saw a non-HIV related GP and those who did not (log-rank p-value=0.7634). However, those who saw a non-HIV related specialist had a significantly shorter time-to-initial retention than those who did not (log-rank p-value<0.001).
Figure 2.
Kaplan Meier time-to-initial retention in HIV care estimates by the number of types of non-HIV related services used, a) shows the x-axis as the full study follow-up and b) shows the x-axis as the first 20 months of study follow-up.
Figure 3.
Kaplan Meier time-to-initial retention in HIV care estimates by a) non-HIV related general practitioner use (during the full study follow-up); b) non-HIV related general practitioner use (in the first 20 months of study follow-up); c) non-HIV related specialist use (during the full study follow-up) d) non-HIV related specialist use (in the first 20 months of study follow-up).
Results from our two final Weibull models are displayed in Table 2. Model 1 included the variable ‘number of different types of non-HIV related services used’, and we found that after controlling for all other covariates, compared with using zero types of non-HIV related services, the adjusted Hazard Ratio (aHR) for using one, two, three, and four types of non-HIV related services was 1.99 (95% Confidence Interval (CI): 1.65–2.40), 2.57 (95% CI: 2.16–3.04), 2.72 (95% CI: 2.3–3.22), and 1.70 (95% CI: 1.39–2.08), respectively.
Model 2 included all of the different types of non-HIV related healthcare services variables explored, but did not include the ‘number of different types of non-HIV related services used’. We found that after controlling for all other covariates, those who saw a specialist for non-HIV related healthcare (versus those who did not) (aHR=2.79; 95% CI: 2.47–3.16) and those who had a non-HIV related prescription filled (versus those who did not) (aHR=1.28; 95% CI: 1.18–1.38) had a shorter time-to-initial retention. However, those who saw a GP for non-HIV related healthcare (versus those who did not) (aHR=0.79; 95% CI: 0.74–0.84) and those who were admitted to the hospital for non-HIV related healthcare (versus those who were not) (aHR=0.6; 95% CI: 0.54–0.67) had a longer time-to-initial retention. Also, females (compared with males) (aHR=0.84; 95% CI: 0.78–0.91), PWID (aHR=0.73; 95% CI: 0.68–0.79), and individuals with other/unknown HIV risk factor (aHR=0.42; 95% CI: 0.39–0.46) (compared with MSM), had a longer time-to-initial retention. In contrast, individuals with an AIDS diagnosis (compared with those without an AIDS diagnosis) and those 40–49 (aHR=1.16; 95% CI: 1.08–1.24) and ≥50 years of age (aHR=1.13; 95% CI: 1.03–1.23) (compared with 30–39 year olds) had a shorter time-to-initial retention. Little variation was observed by HA with only those living in the Vancouver Island HA having a shorter time-to-initial retention than those in the most densely populated HA, Vancouver Coastal (aHR=1.12; 95% CI: 1.02–1.24).
DISCUSSION
We found that that among BC residents linked between 2000 and 2010, 90% were retained by the end of study follow-up and 70% within one year of being linked. The median time-to-initial retention among those retained by the end of study follow-up, was 9 (5–13) months. Additionally, we found high proportions of both retained (96%) and not retained (84%) individuals used some type of non-HIV related healthcare service during study follow-up. The time-to-initial retention decreased (i.e. improved) in a dose-response fashion, with the use of non-HIV related healthcare services for up to three different types of such services but was longer for those who used four non-HIV related services. When considering the types of non-HIV related services used, we observed that individuals who saw a specialist for non-HIV related healthcare were nearly three times more likely to be retained than those who did not. In contrast, individuals who saw a GP for non-HIV related healthcare or had a non-HIV related hospital admission during study follow-up, were less likely to be retained than those who did not see a GP or were not admitted to the hospital for non-HIV related healthcare, respectively. Also, we found that males, having an AIDS diagnosis, having a known risk factor, being HIV-diagnosed between 2000 and 2007, being on ART, residing in the Vancouver Coastal HA and Vancouver Island HA, and living in an urban area were all associated with being retained by the end of study follow-up. These results were observed in the context of a universally free healthcare system.
Comparing our observed proportion achieving retention to previously published work is challenging due to varying published study retention definitions (which likely reflects the lack of a gold standard to measure retention). Published reports have defined retention using missed pVL or CD4 testing or medical appointments over a six month or one year time period (1, 2, 6, 12, 33–35). In contrast, our definition of retained was relatively flexible as it included having an HIV-related physician visit (as demonstrated by a billed service to the MSP), having laboratory work performed, or having ART dispensed (almost half of our retained population was on ART). Furthermore, we measured time-to-initial retention over a longer time period than previous studies.
Individuals who used four non-HIV related services were found to have a longer time-to-initial retention than those who used three or fewer non-HIV related services. We hypothesize that the frequent use of non-HIV related services is an indicator of underlying complexities, and co-morbidities (e.g., homelessness, mental health, substance use) which can make achieving engagement in HIV care very challenging. However, we lack social determinants of health data to assess this hypothesis. Although, a sub-analysis found that females and PWID were more likely to use four non-HIV related services (data not shown).
Our observation that individuals who saw a GP or were admitted to the hospital for non-HIV related healthcare had a longer time-to-initial retention than those who did not was of concern, since 71% of not retained individuals saw a non-HIV related GP during the follow-up period. These results suggest that there were frequent missed opportunities to engage individuals in appropriate HIV care. (Of note, non-HIV related hospital admissions were relatively uncommon, with only 16% of not retained individuals being admitted to the hospital for non-HIV related healthcare.) The reason for this observed association between individuals who saw GPs for non-HIV related healthcare and having a lower likelihood of being retained cannot be elucidated from these analyses. We speculate that this observation may be due to the nature of GP visits and their patterns of use in our midst. In general, GP visits tend to be problem-driven, very focused, and not require a follow-up visit. Also, some patients may see GPs through walk-in clinics and would not necessarily see the same GP at every visit.
However, our results do suggest that non-HIV related GP-targeted interventions should be considered as a means to improve initial retention. To date, few retention improvement strategies have targeted physician-level interventions (36), though Gardner et al. found a low-cost intervention of displaying posters and brochures in six HIV clinics, alongside practitioner-communicated reminders to patients on the importance of attending all clinic visits significantly improved clinic retention rates (37). Based on our results, a similar intervention in a non-HIV specific GP setting may warrant being tested as a method for improving time-to-initial retention in our setting. Alternatively, electronic medical records (EMRs) may be a method for improving retention. In 2009, the state of Louisiana (USA) implemented an EMR-based notification system after a 2007 study found that approximately 1100 individuals who did not receive CD4 or pVL monitoring for >12 months, used at least one non-HIV related service during this period(38). The notification system alerted medical providers providing non-HIV related healthcare when they saw HIV-positive individuals who were without HIV healthcare for >12 months and delivered 488 alerts and identified 345 HIV-positive individuals within a two-year evaluation period. These alerts resulted in 82% of individuals having ≥1 CD4 or pVL test over the evaluation period.
In addition, consistent with previously published work, we found that women, PWID, those with other/unknown HIV transmission risk, and individuals under 40 years of age were less likely to be retained after being linked (39–44). Increasing efforts and evidence-based interventions are urgently needed to retain these sub-groups in HIV care in order to maximize individual health outcomes as well as reduce the risk of forward HIV transmission.
There were several limitations to our study. First, we did not have access to medical charts. Thus, we cannot comment on whether or not a non-HIV related GP or specialist was aware of or inquired about a patient’s HIV status. Second, physicians may not bill a visit as an HIV visit despite a visit involving HIV care, although this is unlikely in our setting where physicians receive higher remuneration for providing HIV care than most other illnesses. Third, risk category data was unknown for a high proportion of our population. Improved risk data collection would allow for improved understanding of retention by population sub-groups. Finally, as this is an observational cohort study, we tried to adjust for several important demographic and clinical characteristics, however, residual confounding may persist.
In summary, we found the median time-to-initial retention was nine months among those retained and that 70% of individuals were retained within 12 months of being linked in a setting with universal access to HIV care and treatment. Also, the majority, 84%, of not retained individuals used some type of non-HIV related healthcare service during the study period. Furthermore, we observed that individuals who saw non-HIV related specialists during the study follow-up period were nearly three times more likely to be retained than those who did not; however, those who saw a GP for non-HIV related healthcare were less likely to be retained than those who did not. This missed opportunity for engagement in HIV care suggests a potential for non-HIV related GP-targeted interventions to improve time-to-initial retention in HIV care.
Acknowledgments
We thank the participants that make up the Seek and Treat for Optimal Prevention in HIV/AIDS cohort and the physicians, nurses, social workers and volunteers who support them. Linkage and preparation of the de-identified individual-level database was facilitated by the BC Ministry of Health. L.L, A.N., D.S., V.D.L., G.C., B.N. and J.S.G.M. contributed to the conception and design of the study. A.N. and D.S. performed all statistical analyses. L.L. drafted the manuscript. V.D.L. and J.S.G.M. advised on all aspects of the study. All authors revised the manuscript critically and approved the final version submitted for publication. VDL is supported by a Scholar Award from the Michael Smith Foundation for Health Research, a New Investigator Award from CIHR and two grants from the Canadian Institutes of Health Research (MOP-125948) and the US National Institute on Drug Abuse (R03DA033851-01). JSGM is supported by the British Columbia Ministry of Health and by the US National Institutes of Health (R01DA036307). He has also received limited unrestricted funding from Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck, and ViiV Healthcare. BN is supported by a Scholar Award from the Michael Smith Foundation for Health Research. The funders had no role in the design, data collection, data analysis, data interpretation, or writing of the report. The remaining authors report no disclosures.
Footnotes
Meetings:
Some of the results described here were presented as an oral presentation at the 9th International Conference on HIV Treatment and Prevention Adherence, Miami, Floridaon June 8th–10th, 2014 (Abstract #408).
References
- 1.Giordano TP, White AC, Sajja P, Graviss EA, Arduino RC, Adu-Oppong A, et al. Factors associated with the use of highly active antiretroviral therapy in patients newly entering care in an urban clinic. Jaids-J Acq Imm Def. 2003 Apr 1;32(4):399–405. doi: 10.1097/00126334-200304010-00009. [DOI] [PubMed] [Google Scholar]
- 2.Berg MB, Safren SA, Mimiaga MJ, Grasso C, Boswell S, Mayer KH. Nonadherence to medical appointments is associated with increased plasma HIV RNA and decreased CD4 cell counts in a community-based HIV primary care clinic. AIDS Care. 2005 Oct;17(7):902–7. doi: 10.1080/09540120500101658. [Research Support, N.I.H., Extramural Research Support, U.S. Gov’t, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 3.Giordano TP, Gifford AL, White AC, Suarez-Almazor ME, Rabeneck L, Hartman C, et al. Retention in care: A challenge to survival with HIV infection. Clinical Infectious Diseases. 2007 Jun 1;44(11):1493–9. doi: 10.1086/516778. [DOI] [PubMed] [Google Scholar]
- 4.Rastegar DA, Fingerhood MI, Jasinski DR. Highly active antiretroviral therapy outcomes in a primary care clinic. Aids Care-Psychological and Socio-Medical Aspects of Aids/Hiv. 2003 Apr;15(2):231–7. doi: 10.1080/0954012031000068371. [DOI] [PubMed] [Google Scholar]
- 5.Mugavero MJ, Amico KR, Westfall AO, Crane HM, Zinski A, Willig JH, et al. Early Retention in HIV Care and Viral Load Suppression: Implications for a Test and Treat Approach to HIV Prevention. Jaids-J Acq Imm Def. 2012 Jan 1;59(1):86–93. doi: 10.1097/QAI.0b013e318236f7d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mugavero MJ, Lin HY, Willig JH, Westfall AO, Ulett KB, Routman JS, et al. Missed Visits and Mortality among Patients Establishing Initial Outpatient HIV Treatment. Clinical Infectious Diseases. 2009 Jan 15;48(2):248–56. doi: 10.1086/595705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Metsch LR, Pereyra M, Messinger S, del Rio C, Strathdee SA, Anderson-Mahoney P, et al. HIV transmission risk behaviors among HIV-infected persons who are successfully linked to care. Clinical Infectious Diseases. 2008 Aug 15;47(4):577–84. doi: 10.1086/590153. [DOI] [PubMed] [Google Scholar]
- 8.Bani-Sadr F, Bedossa P, Rosenthal E, Merrien D, Perre P, Lascoux-Combe C, et al. Does Early Antiretroviral Treatment Prevent Liver Fibrosis in HIV/HCV-Coinfected Patients? Jaids-J Acq Imm Def. 2009 Feb 1;50(2):234–6. doi: 10.1097/QAI.0b013e31818ce821. [DOI] [PubMed] [Google Scholar]
- 9.Horstmann E, Brown J, Islam F, Buck J, Agins BD. Retaining HIV-infected patients in care: Where are we? Where do we go from here? Clin Infect Dis [Review] 2010 Mar 1;50(5):752–61. doi: 10.1086/649933. [DOI] [PubMed] [Google Scholar]
- 10.Gill JM, Mainous AG, Nsereko M. The effect of continuity of care on emergency department use. Arch Fam Med. 2000 Apr;9(4):333–8. doi: 10.1001/archfami.9.4.333. [DOI] [PubMed] [Google Scholar]
- 11.Fleishman JA, Moore RD, Conviser R, Lawrence PB, Korthuis PT, Gebo KA. Associations between outpatient and inpatient service use among persons with HIV infection: A positive or negative relationship? Health Serv Res. 2008 Feb;43(1):76–95. doi: 10.1111/j.1475-6773.2007.00750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sethi AK, Celentano DD, Gange SJ, Moore RD, Gallant JE. Association between adherence to antiretroviral therapy and human immunodeficiency virus drug resistance. Clinical Infectious Diseases. 2003 Oct 15;37(8):1112–8. doi: 10.1086/378301. [DOI] [PubMed] [Google Scholar]
- 13.Montaner JS, Lima VD, Barrios R, Yip B, Wood E, Kerr T, et al. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet. 2010 Aug 14;376(9740):532–9. doi: 10.1016/S0140-6736(10)60936-1. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lima VD, Hogg RS, Montaner JS. Expanding HAART treatment to all currently eligible individuals under the 2008 IAS-USA Guidelines in British Columbia, Canada. PLoS One. 2010;5(6):e10991. doi: 10.1371/journal.pone.0010991. [Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fang CT, Hsu HM, Twu SJ, Chen MY, Chang YY, Hwang JS, et al. Decreased HIV transmission after a policy of providing free access to highly active antiretroviral therapy in Taiwan. The Journal of infectious diseases. 2004 Sep 1;190(5):879–85. doi: 10.1086/422601. [Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- 16.Das M, Chu PL, Santos GM, Scheer S, Vittinghoff E, McFarland W, et al. Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PLoS One. 2010;5(6):e11068. doi: 10.1371/journal.pone.0011068. [Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jia Z, Mao Y, Zhang F, Ruan Y, Ma Y, Li J, et al. Antiretroviral therapy to prevent HIV transmission in serodiscordant couples in China (2003–11): a national observational cohort study. Lancet. 2013 Oct 5;382(9899):1195–203. doi: 10.1016/S0140-6736(12)61898-4. [Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- 18.Wood E, Kerr T, Marshall BDL, Li K, Zhang R, Hogg RS, et al. Longitudinal community plasma HIV-1 RNA concentrations and incidence of HIV-1 among injecting drug users: prospective cohort study. Brit Med J. 2009 Apr;30:338. doi: 10.1136/bmj.b1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen MS, Chen YQ, Fleming TR, Team HS. Prevention of HIV-1 Infection with Antiretroviral Therapy REPLY. New Engl J Med. 2011 Nov 17;365(20):1935. doi: 10.1056/NEJMc1110588. [DOI] [PubMed] [Google Scholar]
- 20.Tanser F, Barnighausen T, Grapsa E, Zaidi J, Newell ML. High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science. 2013 Feb 22;339(6122):966–71. doi: 10.1126/science.1228160. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stover J, Hallett TB, Wu Z, Warren M, Gopalappa C, Pretorius C, et al. How can we get close to zero? The potential contribution of biomedical prevention and the investment framework towards an effective response to HIV. PLoS One. 2014;9(11):e111956. doi: 10.1371/journal.pone.0111956. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skarbinski JRE, Paz-Bailey G, Hall I, Rose CE, Viall A, Fagan JL, Lansky A, Mermin JH. Human Immunodeficiency Virus Transmission at Each Step of the Care Continuum in the United States. JAMA Internal Medicine [Original Investigation] 2015 Feb 23;175(4):588–96. doi: 10.1001/jamainternmed.2014.8180. [DOI] [PubMed] [Google Scholar]
- 23.Nosyk B, Montaner JS, Colley G, Lima VD, Chan K, Heath K, et al. The cascade of HIV care in British Columbia, Canada, 1996–2011: a population-based retrospective cohort study. Lancet Infect Dis. 2013 Sep 26; doi: 10.1016/S1473-3099(13)70254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montaner JS, Lima VD, Harrigan PR, Lourenco L, Yip B, Nosyk B, et al. Expansion of HAART coverage is associated with sustained decreases in HIV/AIDS morbidity, mortality and HIV transmission: the “HIV Treatment as Prevention” experience in a Canadian setting. PLoS One. 2014;9(2):e87872. doi: 10.1371/journal.pone.0087872. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heath K, Samji H, Nosyk B, Colley G, Gilbert M, Hogg RS, et al. Cohort Profile: Seek and Treat for the Optimal Prevention of HIV/AIDS in British Columbia (STOP HIV/AIDS BC) Int J Epidemiol. 2014 Apr 2; doi: 10.1093/ije/dyu070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nosyk B, Colley G, Yip B, Chan K, Heath K, Lima VD, et al. Application and validation of case-finding algorithms for identifying individuals with human immunodeficiency virus from administrative data in British Columbia, Canada. PLoS One. 2013;8(1):e54416. doi: 10.1371/journal.pone.0054416. [Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yeni PG, Hammer SM, Carpenter CCJ, Cooper DA, Fischl MA, Gatell JM, et al. Antiretroviral treatment for adult HIV infection in 2002 - Updated recommendations of the international AIDS Society-USA panel. Jama-J Am Med Assoc. 2002 Jul 10;288(2):222–35. doi: 10.1001/jama.288.2.222. [DOI] [PubMed] [Google Scholar]
- 28.Thompson MA, Aberg JA, Cahn P, Montaner JSG, Rizzardini G, Telenti A, et al. Antiretroviral Treatment of Adult HIV Infection 2010 Recommendations of the International AIDS Society-USA Panel. Jama-J Am Med Assoc. 2010 Jul 21;304(3):321–33. doi: 10.1001/jama.2010.1004. [DOI] [PubMed] [Google Scholar]
- 29.Hammer SM, Eron JJ, Reiss P, Schooley RT, Thompson MA, Walmsley S, et al. Antiretroviral treatment of adult HIV infection - 2008 recommendations of the International AIDS Society USA panel. Jama-J Am Med Assoc. 2008 Aug 6;300(5):555–70. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]
- 30.Thompson MA, Aberg JA, Hoy JF, Telenti A, Benson C, Cahn P, et al. Antiretroviral Treatment of Adult HIV Infection 2012 Recommendations of the International Antiviral Society-USA Panel. Jama-J Am Med Assoc. 2012 Jul 25;308(4):387–402. doi: 10.1001/jama.2012.7961. [DOI] [PubMed] [Google Scholar]
- 31.Carroll KJ. On the use and utility of the Weibull model in the analysis of survival data. Control Clin Trials. 2003 Dec;24(6):682–701. doi: 10.1016/s0197-2456(03)00072-2. [DOI] [PubMed] [Google Scholar]
- 32.Lima VD, Geller J, Bangsberg DR, Patterson TL, Daniel M, Kerr T, et al. The effect of adherence on the association between depressive symptoms and mortality among HIV-infected individuals first initiating HAART. Aids. 2007 May 31;21(9):1175–83. doi: 10.1097/QAD.0b013e32811ebf57. [Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- 33.Andersen M, Hockman E, Smereck G, Tinsley J, Milfort D, Wilcox R, et al. Retaining women in HIV medical care. J Assoc Nurse Aids C. 2007 May-Jun;18(3):33–41. doi: 10.1016/j.jana.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 34.Bradford JB, Coleman S, Cunningham W. HIV system navigation: An emerging model to improve HIV care access. Aids Patient Care St. 2007 Jun;21:S49–S58. doi: 10.1089/apc.2007.9987. [DOI] [PubMed] [Google Scholar]
- 35.Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008 Jul 26;372(9635):293–9. doi: 10.1016/S0140-6736(08)61113-7. [Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Higa DH, Marks G, Crepaz N, Liau A, Lyles CM. Interventions to improve retention in HIV primary care: a systematic review of U.S. studies. Curr HIV/AIDS Rep [Review] 2012 Dec;9(4):313–25. doi: 10.1007/s11904-012-0136-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gardner LI, Marks G, Craw JA, Wilson TE, Drainoni ML, Moore RD, et al. A low-effort, clinic-wide intervention improves attendance for HIV primary care. Clin Infect Dis. 2012 Oct;55(8):1124–34. doi: 10.1093/cid/cis623. [Multicenter Study Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S. Research Support, U.S. Gov’t, P.H.S.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herwehe J, Wilbright W, Abrams A, Bergson S, Foxhood J, Kaiser M, et al. Implementation of an innovative, integrated electronic medical record (EMR) and public health information exchange for HIV/AIDS. J Am Med Inform Assn. 2012 May;19(3):448–52. doi: 10.1136/amiajnl-2011-000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Israelski D, Gore-Felton C, Power R, Wood MJ, Koopman C. Sociodemographic characteristics associated with medical appointment adherence among HIV-seropositive patients seeking treatment in a county outpatient facility. Prev Med. 2001 Nov;33(5):470–5. doi: 10.1006/pmed.2001.0917. [DOI] [PubMed] [Google Scholar]
- 40.Poole WK, Perritt R, Shah KB, Lou Y, Turner J, Kvale P, et al. A characterisation of patient drop outs in a cohort of HIV positive homosexual/bisexual men and intravenous drug users. J Epidemiol Community Health. 2001 Jan;55(1):66–7. doi: 10.1136/jech.55.1.66. [Research Support, U.S. Gov’t, P.H.S.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arici C, Ripamonti D, Maggiolo F, Rizzi M, Finazzi MG, Pezzotti P, et al. Factors associated with the failure of HIV-positive persons to return for scheduled medical visits. HIV Clin Trials. 2002 Jan-Feb;3(1):52–7. doi: 10.1310/2XAK-VBT8-9NU9-6VAK. [DOI] [PubMed] [Google Scholar]
- 42.Rumptz MH, Tobias C, Rajabiun S, Bradford J, Cabral H, Young R, et al. Factors associated with engaging socially marginalized HIV-positive persons in primary care. Aids Patient Care St. 2007 Jun;21:S30–S9. doi: 10.1089/apc.2007.9989. [DOI] [PubMed] [Google Scholar]
- 43.Giordano TP, Visnegarwala F, White AC, Jr, Troisi CL, Frankowski RF, Hartman CM, et al. Patients referred to an urban HIV clinic frequently fail to establish care: factors predicting failure. AIDS Care. 2005 Aug;17(6):773–83. doi: 10.1080/09540120412331336652. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 44.Waldrop-Valverde D, Guo Y, Ownby RL, Rodriguez A, Jones DL. Risk and Protective Factors for Retention in HIV Care. AIDS Behav. 2013 Oct 2; doi: 10.1007/s10461-013-0633-7. [DOI] [PMC free article] [PubMed] [Google Scholar]