Highlights
-
•
Error-related brain activity increases during adolescence.
-
•
Weaker response to errors predicts future initiation of tobacco use.
-
•
Faster development of error monitoring function can be a protective factor.
Keywords: Error monitoring, ERN, Adolescence, Substance use, Tobacco, Longitudinal
Abstract
Deficits in self-regulation of behavior can play an important role in the initiation of substance use and progression to regular use and dependence. One of the distinct component processes of self-regulation is error monitoring, i.e. detection of a conflict between the intended and actually executed action. Here we examined whether a neural marker of error monitoring, Error-Related Negativity (ERN), predicts future initiation of tobacco use. ERN was assessed in a prospective longitudinal sample at ages 12, 14, and 16 using a flanker task. ERN amplitude showed a significant increase with age during adolescence. Reduced ERN amplitude at ages 14 and 16, as well as slower rate of its developmental changes significantly predicted initiation of tobacco use by age 18 but not transition to regular tobacco use or initiation of marijuana and alcohol use. The present results suggest that attenuated development of the neural mechanisms of error monitoring during adolescence can increase the risk for initiation of tobacco use. The present results also suggest that the role of distinct neurocognitive component processes involved in behavioral regulation may be limited to specific stages of addiction.
1. Introduction
Adolescence is the period of the highest risk for initiation of substance use, and tobacco remains one of the most frequently used substance in this period. Despite some progress in the reduction of the rates of tobacco use, about 38.1% adolescents initiate cigarette smoking by the 12th grade (Johnston et al., 2014). As the leading cause of preventable disease and mortality in the United States, tobacco use and cigarette smoking in particular represents a major public health problem. Furthermore, emerging evidence suggests that the exposure of the developing brain to nicotine may potentially lead to long-term adverse consequences for brain function and cognition (Naude et al., 2014, van Ewijk et al., 2014). Finally, some evidence suggests that nicotine can facilitate heavier alcohol use due to its ability to counter alcohol's sedative effects when the substances are used together (Funk et al., 2006) and can serve as a “gateway” drug by paving the way to the use of harder drugs (Kandel and Kandel, 2015). A better knowledge of factors increasing risk for tobacco use is essential for the development of more efficient prevention and intervention methods.
The etiology of nicotine addiction involves a complex interplay between genetic predisposition and environmental factors (reviewed in Ray et al., 2009). It is reasonable to expect that individual differences in the liability to nicotine addiction are mediated by relatively distinct neurocognitive processes involved in reward learning and self-regulation of behavior, all of which contribute to the risk for addiction in both additive and interactive way. Identification and characterization of the unique role of each of these “component processes” in addictive behaviors and elucidation of their genetic basis is needed for building an integrative model of addiction. It is important to note that the etiology of addictions is a dynamic process that involves distinct stages such as initiation of drug use, progression to regular use, and the development of dependence on the drug. In particular, genetic factors influencing the risk for initiation of tobacco use appear to be distinct from those affecting progression to regular smoking and nicotine dependence (Heath et al., 2002, Munafo et al., 2004), suggesting distinct underlying biological liability. However, little is known about specific neurocognitive mechanisms operating at different stages of substance involvement.
One of the neurocognitive component processes contributing to addiction risk may be error monitoring, a fundamental mechanism of self-regulation of behavior that involves automatic, largely pre-conscious detection of the mismatch between the intended and actually executed action and subsequent cognitive and emotional appraisal of the detected conflict prompting the recruitment of cognitive control for adjustments of ongoing behavior (Segalowitz and Dywan, 2009, van Noordt and Segalowitz, 2012). These stages of error processing are reflected in ERP components associated with commission of errors, the error-related negativity (ERN) and error positivity (Pe) depicted in Fig. 1. Converging evidence from studies using ERP source localization analyses, multimodal imaging (EEG and fMRI), single unit recording, and studies of patients with brain lesions indicates that the main anatomical source of ERN is the anterior cingulate cortex (Debener et al., 2005, Herrmann et al., 2004, Mathalon et al., 2003, Miltner et al., 2003, Ridderinkhof et al., 2004). A previous study in our laboratory has demonstrated significant heritability of individual differences in ERN and Pe components, suggesting that ERN can serve as an endophenotype for disorders characterized by self-regulation deficits (Anokhin et al., 2008). Over the past decade, ERN has been increasingly used in the investigation of neurocognitive mechanisms mediating the risk for psychopathology, including addictive disorders. A thorough review of these studies is beyond the scope of this introduction and we refer the reader to comprehensive reviews on this topic (Moser et al., 2013, Olvet and Hajcak, 2008, van Noordt and Segalowitz, 2012). Briefly, this evidence suggests that increased ERN, presumably indicating abnormally over-active error monitoring system, is associated with obsessive–compulsive, depressive and anxiety-spectrum symptomatology (Aarts et al., 2013b), whereas reduced ERN is associated with personality traits indicating impulsivity, poor socialization, and externalizing symptoms in children and adults (Dikman and Allen, 2000, Hall et al., 2007, Santesso et al., 2005, Stieben et al., 2007). These correlations with psychopathology are broadly consistent with the notion that ERN reflects not only cognitive but also emotional processing of errors (Aarts et al., 2013a, Koban and Pourtois, 2014).
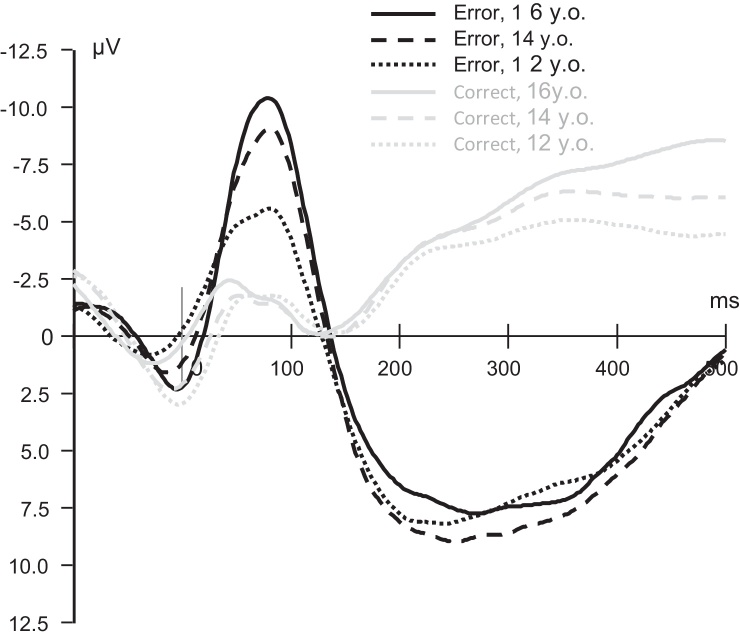
Fig. 1.
ERPs elicited by in the flanker task. Response-locked neural activity at the midline frontocentral (FCz) sensor is shown. Motor response is marked by a vertical line. Each waveform represents the signal averaged across trials and participants (grand average) separately for each condition (erroneous and correct responses) and assessment wave (ages 12, 14, and 16).
Given this pattern of findings, it is reasonable to hypothesize that deficits in the neural mechanisms of error monitoring may contribute to poor self-regulation of behavior and thus increase the risk for initiation of substance use in adolescents. In particular, a large portion of adolescents (>40%) initiate tobacco use by age 18, despite increasing public awareness of substantial health risks associated with smoking and overall decline in smoking rates. One potential mechanism mediating the hypothesized link between poor action monitoring and tobacco use is impulsivity. This hypothesis is supported by three lines of evidence. First, adolescent smokers tend to score higher on laboratory and self-repot measures of impulsivity (Reynolds et al., 2007), and impulsivity is one of important prospective predictors of smoking initiation in adolescence (O’Loughlin et al., 2014). Second, studies reported associations between reduced ERN and higher impulsivity (Potts et al., 2006), broader externalizing and impulse-control problems (Hall et al., 2007), ADHD (Shiels and Hawk, 2010), and risk-taking (Santesso and Segalowitz, 2009). Third, developmental neuroscience has demonstrated that the brain continues to develop during adolescence. Areas of the prefrontal cortex supporting behavioral regulation are characterized by the longest development lasting into the young adulthood, and their relative immaturity may be responsible for poorer self-regulation of behavior in adolescents compared with adults (Casey et al., 2008, Richards et al., 2012, Spear, 2013). These lines of evidence converge to suggest that the neural mechanisms of action monitoring may be immature and continue to develop during adolescence, and individuals with slower or attenuated development may be more prone to impulsive and risky actions such as experimenting with tobacco and other drugs.
The few studies that investigated ERN in relation to tobacco use yielded mixed results. Luijten et al. (2011) reported reduced ERN in smokers after smoking cues exposure, however, other studies using ERN paradigms without smoking cues found no significant differences between smokers and non-smokers with respect to ERN amplitude (Franken et al., 2010, Rass et al., 2014). In a study using reward and punishment contingencies (Potts et al., 2006), smokers showed ERN reduction but only on punishment-motivated trials. Thus, the question of whether and how error monitoring is related to tobacco use and dependence warrants further investigation. In part, these disparate findings can be attributed to small sample sizes of most studies cited above. Across these studies, results for Pe were also disparate: while two studies (Franken et al., 2010, Luijten et al., 2011) found reduced Pe components in smokers, a recent study (Rass et al., 2014) reported increased Pe in intermittent smokers relative to both non-smokers and regular dependent smokers, with the latter two groups showing no significant differences.
However, the main limitation of previous research has been its correlational nature, i.e. reliance on cross-sectional comparisons between tobacco users and non-users. Due to the well- known fact that correlation does not imply causation, cross-sectional designs preclude strong causal inferences because differences between users and non-users with respect to measures of brain activity can be interpreted both as a marker of predisposition to substance use and as a consequence of substance exposure (Anokhin et al., 1999, Anokhin et al., 2000). The most powerful approach to delineating determinants and consequences of substance use is a prospective longitudinal design, in which potential biological markers of risk are assessed before the onset of substance use, preferably at multiple time points across development.
The present study addressed the above limitation by using a prospective longitudinal design to examine whether individual differences in the neurophysiological indicator of error monitoring (ERN) assessed during early and middle adolescence can predict tobacco use in emerging adults. Specifically, we hypothesized that adolescents showing smaller ERN components (presumably indicating poorly developed error monitoring mechanisms) will be at greater risk for initiating tobacco use by age 18. We also expected that ERN would increase with age, consistent with previous developmental studies (Tamnes et al., 2013), and hypothesized that individual differences in the rate of development would be associated with risk for tobacco use, such that individuals showing steeper developmental increase in ERN and thus faster maturation of the error-monitoring system will be less likely to use tobacco compared with individuals showing weaker developmental changes.
2. Method
2.1. Participants and the study overview
The subjects (n = 602, 306 female) were adolescent participants in the study of Brain, Genetics, Development and Addiction (BGDA) at Washington University School of Medicine, a longitudinal study of three consecutive birth-year cohorts of adolescent twins ascertained from the general population through birth records at age 12. Exclusion criteria were minimal and included a history of head trauma with loss of consciousness for more than 5 min, known history of epilepsy, currently taking a psychoactive medication, as well as hearing, visual and other physical and mental impairments that could prevent the subjects from understanding and following task instructions. The study was approved by Washington University's Institutional Review Board, and after a complete description of the study to the subjects and their parents, a written informed assent and consent was obtained from adolescent participants and their parents, respectively. Participants completed the first (baseline) laboratory assessment at the age of 12 and returned to the laboratory for follow-up assessments every two years. During the ERP recording sessions at age 12, 14, and 16, participants were administered the Eriksen flanker task (described below in detail). A total of 602 participants, including 306 females, were included in the present analyses based on the availability of relevant data. For those participants who completed the interview at age 18, ERN data were available for 598, 528, and 242 participants at ages 12, 14, and 16, respectively (only a random subset of subjects was administered this task at age 16). Full longitudinal data spanning ages 12, 14, and 16 were available for 236 participants. Subjects were assessed every two years, about the same time of year, with minimal variation of time intervals between assessments was minimal (within 2–3 months). Follow-up assessments of this sample at age 20 are currently in progress.
2.2. Experimental procedure
To elicit ERN, we used Eriksen's Flanker Task, a commonly used experimental paradigm in the ERN research. The flanker task has been shown to produce more reliable ERN components compared to other tasks (Riesel et al., 2013). On each trial, a string of 5 white letters was presented on black background in the center of the screen (letter dimensions: 10 mm high, 7 mm wide). The letter in the center of the string was the target, and the remaining letters were flankers. Two types of strings were presented in a fixed pseudorandom order with equal frequency (50%); in compatible trials, flankers were identical to the target (SSSSS and HHHHH), and in incompatible trials flankers and targets were different (SSHSS and HHSHH). Stimulus duration was 150 ms, and inter-stimulus interval was 1500 ms. A total of 480 trials were administered in 4 blocks of 120 trials with 2 min breaks between the blocks. The test trials were preceded by a block of 12 training trials that was repeated if needed to achieve a full understanding of the task. Participants were seated in a comfortable recliner chair in a semi-darkened booth in front of the monitor and were instructed to avoid major body movements and muscle tension and keep their gaze on the fixation cross. Participants were instructed to keep their thumbs on the left and right buttons of the response pad and to make a left-hand button-press if the target letter was S, and a right-hand button-press if the target letter was H. Response speed and accuracy were equally emphasized by the instruction.
2.3. Electrophysiological data acquisition and analysis
The EEG was recorded from 30 scalp locations according to the 10–20 system using an elastic cap with Ag/AgCl electrodes and a ground electrode on the forehead, with high- and low-pass filters set at .05 and 70 Hz, respectively. The left mastoid served as reference, and an averaged mastoid reference was digitally computed off-line using the right mastoid recording as a separate channel. Vertical electro-oculogram recording was used for eyeblink artifact correction using a regression-based procedure (Semlitsch et al., 1986). After screening for artifacts, EEG signals were subjected to 30 Hz low-pass filtering, and 1-s epochs time-locked to the response were extracted (from −.1 to .9 s) and averaged separately for correct and incorrect responses. The ERN component was detected as the most negative value within 0–150 ms window following incorrect responses and scored as the difference between the peak and the preceding positive trough (determined in −50 to 60 ms window). The Pe peak was detected as the most positive value within 200 to 500 ms window after the incorrect response. Performance variables were computed including average response latency and the percentage of error and correct responses. Subjects with less than 6 error trials available after artifact screening were excluded from analysis because previous studies indicate that a minimum of 6 trials are needed for ERN measurement (Foti et al., 2013, Olvet and Hajcak, 2009, Pontifex et al., 2010). Individuals with random or near-random performance (<60% correct responses) were also excluded. The mean number of trials (±SD) included in the ERN averaging was 56.5 ± 36.4, 43.0 ± 29.3, and 36.6 ± 26.4 at ages 12, 14, and 16, respectively.
2.4. Assessment of tobacco use
At each biannual laboratory visit (ages 12–18), participants were administered a semi-structured interview about their current and lifetime tobacco use. For the present analyses, we used two outcome variables: smoking initiation (any tobacco use during lifetime) and history of regular tobacco use defined according to Centers for Disease Control criteria as having smoked 100 or more cigarettes in lifetime. We did not include nicotine dependence diagnosis because the currently available longitudinal data are limited to the age of 18. Since nicotine dependence typically develops after a prolonged period of regular use, it may be premature to assess it at age 18, given that most users in this sample started using tobacco within the past 1–2 years of this age. Prevalence of any tobacco use at ages 12, 14, 16, 1nd 18 was 2.0, 5.1, 15.9, and 44.4%, respectively, indicating that most onsets occurred between ages 16 and 18, which is comparable with national U.S.A. data (Johnston et al., 2012).
2.5. Statistical analysis
To examine age-related changes in ERN across adolescence, we used linear mixed-effect modeling (MIXED procedure in SPSS) with ERN amplitudes at ages 12, 14, and 16 treated as 3 levels of within-subject, repeated-measures factor Age. To test the hypothesis that ERN amplitude measured at earlier age (12, 14, and 16) were associated with tobacco use at age 18, we extended the above repeated-measures model to a mixed-design model by adding lifetime tobacco use status at the age of 18 as a between-subject (grouping) factor with two levels (0 = never used; 1 = used) and tested for the main effect of Group and Age by Group interaction effect. A significant effect of Group would indicate that future smokers and non-smokers differ with respect to ERN amplitude during adolescence, whereas a significant Age by Group interaction effect would indicate that predictive value of ERN varies as a function of age. Next, to examine whether individual differences in the rate of age-related changes in ERN amplitude could predict future tobacco use status, we modeled individual change (individual-level regression slope) as a random effect and tested for group differences in the slope (group by time interaction). To rule out the possibility that early tobacco use might have influenced ERN amplitude, the above analyses were repeated after restricting the sample to only those participants who initiated tobacco use after age 16. Since demographic factors such as gender and ethnicity can potentially be associated with both tobacco use and ERPs, we included gender and ethnicity in the model. Ethnicity was coded as two groups, Caucasians and ethnic minorities, predominantly African-Americans (83.1 and 16.9%, respectively). Next, because our preliminary analyses indicated that ERN amplitude is correlated with performance accuracy, we also included the number of errors as a covariate in the model to control for possible confounding of the ERN and tobacco use association. Finally, to adjust for the non-independence (clustering) of observations within twin pairs, we explicitly modeled the hierarchical structure of the data by specifying family as the unit of analysis using the “|SUBJECT” option in the MIXED procedure. To examine whether ERN amplitude and the rate of its developmental increase affects transition to regular smoking in those adolescents who already initiated tobacco use, we have conducted the same analyses as described above using transition to regular smoking assessed at age 18 as a grouping variable (0 = used tobacco but not regularly; 1 = used regularly, i.e. 100 or more cigarettes in lifetime). It is important to note that Satterthwaite's approximation used to estimate denominator degrees of freedom of the F statistic in SPSS MIXED procedure may result in decimal numbers.
3. Results
3.1. Main effects of task conditions and demographic covariates
Response-locked ERPs elicited by error and correct responses at ages 12, 14 and 16 are presented in Fig. 1. As expected, error trials elicited a prominent ERN component with the largest (i.e. most negative) amplitude at the midline fronto-central location (FCz), followed by a slower positive deflection (Pe). The difference between error and correct responses was highly significant (p < .001) for both ERP components at each of the three time points.
3.2. Effects of demographic covariates on ERN
The linear mixed-effect model showed no significant effect of gender on the ERN amplitude (p > .1), however, the effect of ethnicity was significant (F(1,408.1) = 9.07, p < .01), indicating larger ERN amplitude in Caucasians relative to minorities. Ethnicity was also significantly associated with tobacco use initiation by age 18 (χ2 = 5.89, df = 1, p < .05), with Caucasians showing higher prevalence of tobacco use than minorities (44.8% and 31.1%, respectively). Since ethnicity could be a potential confounder, it was retained in the model, while gender was dropped.
3.3. Age-related changes in the ERN during adolescence
Age-related changes in ERN amplitude are shown in Fig. 2. The linear effect of age on ERN amplitude was highly significant after controlling for all covariates in the model (F(2,454.7) = 39.93, p < .001), however, a quadratic effect was non-significant (p > .05). Pairwise tests indicated that significant age-related changes occurred in both time intervals, 12 to 14 years and 14 to 16 years (p < .001 and p < .05, respectively, after Sidak correction for multiple comparisons).
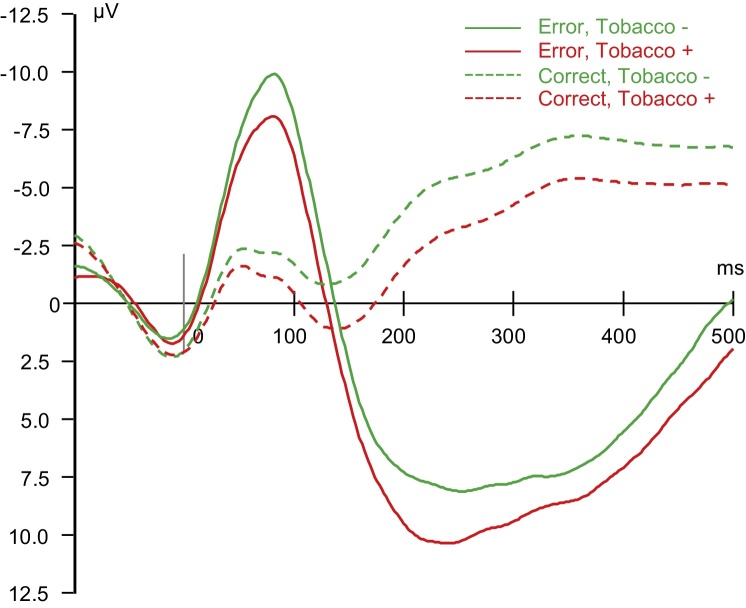
Fig. 2.
ERP waveforms at age 14 for two groups of participants: those who initiated tobacco use by age 18 (Tobacco+) and those who remained abstinent (Tobacco−).
3.4. Does ERN in early and mid-adolescence predict future tobacco use?
Analyses of fixed effects in the linear mixed model revealed a significant main effect of Group (F(1,423.8) = 8.08, p < .01) indicating that participants who initiated tobacco use by age 18 (43.1% of the sample) had smaller, i.e. less negative, ERN components across adolescence (Fig. 2).
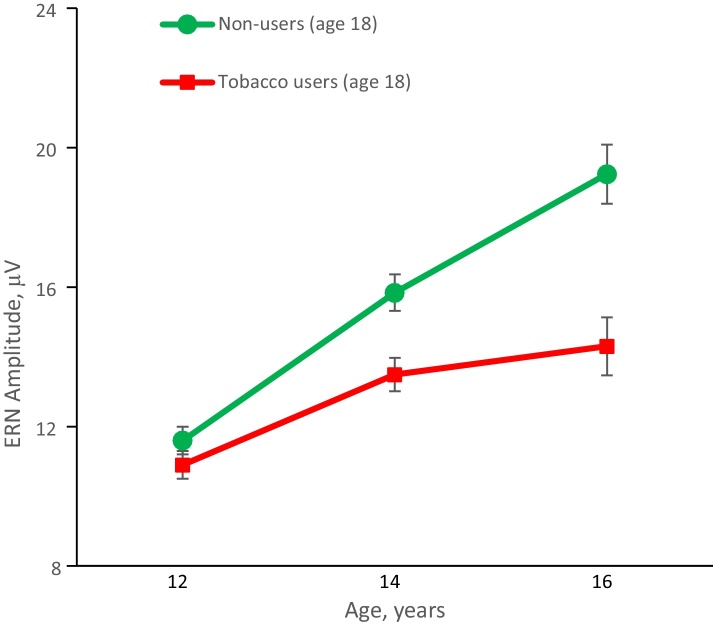
The analysis also showed a significant Age by Group interaction effect (F(2,339.2) = 8.73, p < .001) indicating that ERN measured at different time points during adolescence differentially predicts future tobacco use (Fig. 3). Pairwise group comparisons at each age of ERN assessment within the mixed-effect model (i.e. after controlling for all covariates) showed a lack of significant association between ERN measured at age 12 and future tobacco use (p = .66, n.s.), whereas ERN at ages 14 and 16 showed a highly significant association with tobacco use status assessed at age 18 (p = .015 and p = .001, respectively, after Sidak correction for multiple comparisons). The magnitude of observed ERN differences between the groups increases as a function of age: .7 μV at age 12; 2.4 μV at age 14, and 4.9 μV at age 16 (Cohen's d: .10, .29, and .53, respectively).
Fig. 3.
Age-related changes in ERN amplitude (Mean ± S.E.) during adolescence in future tobacco users and non-users. Data for the FCz electrode are shown.
Importantly, the association between ERN amplitude and subsequent tobacco use initiation remained significant after the exclusion of individuals who have already initiated tobacco use by the age of 16, despite a substantial reduction in the sample size (main effect of Group: F(1,197.9) = 4.35, p < .05; Group X Age interaction: F(2,243.6) = 5.416, p < .01).
The analysis of fixed effects described above indicates that ERN measured at different age shows differential association with future tobacco use status, however, these analyses characterize group-level effects and do not take into account individual variability in developmental trajectories. To examine whether the amount of age-related increase in ERN amplitude (i.e. the rate of developmental changes of the ERN) could predict future tobacco use, we included both intercept and slope of age-related changes in ERN as random effects in the mixed model. These analyses revealed a highly significant Group X Age interaction (F(1,415.4) = 12.54, p < .001) indicating that the rate of change (slope) differs significantly between the tobacco use groups. Specifically, this effect reflects the fact that future tobacco users show attenuated growth of ERN amplitude from age 12 to 16 compared with those adolescents who remained abstinent from tobacco by age 18.
To examine whether the above findings generalize to other aspects of tobacco use such as progression to regular smoking once tobacco use has been initiated (“smoking persistence” phenotype), we examined the relationship between ERN and regular tobacco use at age 18 operationalized as having smoked 100 or more cigarettes during lifetime. In this analysis, we contrasted regular smokers with those who initiated tobacco use but never progressed to regular smoking. However, there was no significant main effect of regular smoking or regular smoking by age interaction effects, (all p > .2) indicating that individual differences in the ERN and the rate of its developmental changes selectively predicted the onset of tobacco use but not the progression to regular use.
Finally, unlike ERN, error positivity (Pe) amplitude did not show significant associations future tobacco use.
3.5. Task performance measures
The two groups did not differ significantly with respect to task performance, although the abstinent group tended to show higher response accuracy at age 12, but the group difference did not survive the multiple comparison correction (F(1,396.2) = 2.78, p = .096, Sidak-corrected). Response latency did not show any significant differences between the groups. There was a significant inverse correlation between the number of errors committed and absolute ERN amplitude (r = −.35, −.30, and −.32 at ages 12, 14, and 16, respectively, all p < .001), indicating that individuals showing better performers (fewer errors) had larger ERN components. To account for the possibility that the present results could be influenced by the number of errors committed and hence the number of trials used for ERN computation, we included the number of errors as a covariate in the model. Although the number of errors had a strong effect on the ERN amplitude (F(1, 960.2) = 83.0, p < .001), both the main effect of Group (F(1, 522.1) = 6.86, p < .01) and Group X Age interaction (F(1, 436.6) = 15.55, p < .001) remained significant after controlling for the number of errors and other covariates in the model.
3.6. ERN and other substance use
To determine whether reduced ERN is a substance-specific marker of risk for tobacco use or, rather, it reflects a broader non-specific liability for substance use, we examined prospective association between ERN and the alcohol and marijuana use at age 18 operationalized as any marijuana use, any alcohol use, and binge drinking (5 or more drinks for boys, 4 or more drinks for girls within 2 h). Although there were trends in the expected direction, namely, lower ERN amplitude in substance users, none of the effects reached significance after controlling for tobacco use and other variables in the model. However, the effect of tobacco (F(1,494.4) = 6.61, p < .05) and tobacco by age interaction(F(2,339.0) = 8.54, p < .001) remained significant after controlling for marijuana and alcohol use.
4. Discussion
4.1. The development of error monitoring system during adolescence
To the best of our knowledge, the present study provides the first longitudinal evidence for developmental changes in the brain mechanisms of error monitoring during adolescence. This finding is in good agreement with previous cross-sectional studies (Davies et al., 2004, Santesso and Segalowitz, 2008, van Meel et al., 2012) as well as neuroanatomical evidence for brain development, particularly in the anterior cingulated cortex (ACC) and other regions of the brain supporting cognitive control of behavior (Mills and Tamnes, 2014, Vijayakumar et al., 2014). The ACC serves as an interface between subcortical regions of the brain and the prefrontal cortex and plays a key role in the integrative functions of the brain such as linking cognition and emotion, evaluation of action outcome, and learning from experience (Etkin et al., 2011, Posner et al., 2007, Rushworth et al., 2007). Given the convergent evidence for the predominantly ACC origin of ERN (Debener et al., 2005, Mathalon et al., 2003, Ridderinkhof et al., 2004), the present findings suggest that ERN amplitude can serve as a marker of ACC functional maturation during adolescence. This interpretation is supported by a recent report that higher ERN amplitudes are associated with increased activation in a large coherent cluster comprising the ACC, the rostral cingulate zone, and pre-SMA, as revealed using simultaneous EEG-fMRI analysis (Iannaccone et al., 2015).
Furthermore, the present analyses using random-coefficient models have shown substantial heterogeneity in individual developmental trajectories during adolescence, such that some individuals exhibit substantial gains in ERN amplitude while others showed little change from 12 to 16 years of age. This finding of large individual differences in ACC functional maturation during a critical period in adolescent development may have important implication for both normal and abnormal behavior, including susceptibility to substance use and other risky behavior. In particular, rapidly changing social environment such as decreasing parental supervision and increasing peer pressure presents a challenge for adolescents’ self-regulation abilities. Adolescents with relatively immature action-regulation system for their chronological age may not respond effectively to this challenge and thus be at higher risk for potentially harmful behaviors compared with their peers who may be better prepared to meet these challenges thanks to faster development of brain regions that are critical for self-regulation of behavior.
4.2. Error monitoring and tobacco use
The present findings suggest that adolescents with poorly developed neurophysiological substrates of error monitoring are at higher risk for initiating tobacco use by age 18. Conversely, accelerated development of error monitoring system in the period from 12 to 16 years of age predicts continued abstinence from tobacco at age 18 and therefore can be viewed as a protective factor. It is important to note that smoking was predicted by ERN measured at ages 14 and 16, but not 12, suggesting that those aspects of ACC function that develop during adolescence, rather than childhood (i.e. before the age of 12) play a particularly important role in continued abstinence from tobacco. One important finding is that ERN prospectively predicts the initiation of tobacco use but not the progression to regular use, suggesting that these two stages of substance involvement may be mediated by distinct neurocognitive processes. This finding is consistent with genetic studies indicating separable genetic influences on smoking initiation and progression to regular use and nicotine dependence (Heath et al., 2002, Munafo et al., 2004). Furthermore, this finding underscores the dynamic complexity of etiological pathways to addiction, where different neurocognitive processes may come into play at distinct stages of the addiction process.
An important issue is whether reduced error-related brain activity represents a substance-specific risk factor for initiation of tobacco use or it reflects a broader risk that generalizes to other substances. The lack of significant associations of reduced ERN with marijuana and alcohol use (despite a non-significant trend in the expected direction) suggests relative substance specificity of the observed relationships. Although the use of different substances is highly comorbid in adolescents, different neurocognitive processes may play differential role in the liability to different substances, which is consistent with genetic studies showing the existence of both common and specific liability (Agrawal et al., 2012).
4.3. Possible cognitive and behavioral mechanisms linking reduced ERN and tobacco use
What neurobehavioral mechanisms and psychological constructs could mediate the link between deficient error monitoring mechanism and tobacco use? One possible mechanism is that the underdeveloped error monitoring mechanism contributes to increased impulsivity, which is a known risk factor for smoking initiation in adolescents. This pathway is suggested by the evidence for associations of reduced ERN with impulsivity and related constructs (Hall et al., 2007, Potts et al., 2006, Santesso and Segalowitz, 2009). However, this interpretation is not quite supported by performance data in the present study: there was no significant association between tobacco use and performance (comission errors) after controlling for covariates.
Another possibility is that the relationship between ERN and tobacco use is mediated by individual differences in anxiety proneness. Anxiety has been shown to be associated with enhanced ERN response (Hajcak et al., 2003). A recent meta-analysis suggested ERN amplitude is specifically associated with anxious apprehension that includes worry associated with ambiguous future threats (Moser et al., 2013). It is reasonable to expect that individuals scoring high on trait worry would be more likely to maintain abstinence in the face of known threats associated with tobacco use. This hypothesis is also supported by evidence that anxiety predicted later onset of smoking in adolescents (Costello et al., 1999). In this context, high activity of the error monitoring system presumably leading to elevated trait anxiety can be viewed as a protective factor that reduces the risk of engaging in potentially harmful behaviors such as smoking. This hypothesis should be tested in future studies.
A third possibility is that ERN is a non-specific marker of the ACC development. As such, reduced ERN may not be related to tobacco use through error monitoring mechanisms specifically; rather, it may indicate a general developmental deficit affecting broader ACC functions including conflict monitoring, response inhibition, etc. A large body of evidence suggests that ACC, due to its rich connections to the dorsolateral prefrontal cortex (higher cognitive processing), the limbic system (emotion and motivation), thalamus and brainstem (arousal), and the motor output systems, plays an integrative role in the organization of adaptive, goal-directed behavior and its willful regulation (Paus, 2001, Shackman et al., 2011). Since the ACC plays a key role in reinforcement learning (Rushworth and Behrens, 2008), delayed ACC development may affect reward processing and reward-based learning. A recent study suggested that attenuated response in the ventral striatum during reward anticipation may represent a risk factor for smoking onset in adolescents (Peters et al., 2011).
Finally, another possible mechanism linking ERN and the likelihood of smoking experimentation is conflict monitoring. According to the conflict monitoring account of the ERN, ACC generates a conflict signal that engages brain regions responsible for conflict resolution and implementation of behavioral adjustments, most notably, the lateral prefrontal cortex (Carter et al., 2000, Huster et al., 2011, Ridderinkhof et al., 2004, van Veen and Carter, 2002). Importantly, both the conflict between competing action representations and actually committed errors activate overlapping regions of the ACC (Ridderinkhof et al., 2004, van Veen and Carter, 2002). This broader account of the ACC function has important implications for its possible role in impulsive actions and substance use. Suppressing an impulsive act requires the engagement of active inhibition process, which is only possible if the conflict between the representations of impending erroneous action and the “right” action is detected. By engaging the broader cognitive control network, the conflict detection in the ACC can switch the control of behavior from the automatic, impulsive mode to the deliberate, reflexive mode. In a real-life situation, when e.g. an adolescent is offered a cigarette by a friend, the ACC could detect a conflict between competing action representations, namely, the representation of the impending act of smoking and the appropriate action in a given context (i.e. refusal) that had been formed previously by social influences and knowledge of harm associated with smoking. If this “early warning” signal generated in the ACC is sufficiently strong, it can trigger the engagement of cognitive control including the response inhibition network. Conversely, a weak or absent conflict signal may fail to trigger the recruitment of cognitive control leading to unimpeded execution of the impulsive act, i.e. smoking a cigarette. This interpretation is also in line with the notion that the action monitoring mechanism involves the attribution of affective and motivational salience to specific action, or “affective tagging” which is strongly influenced by social factors (Koban and Pourtois, 2014). Of course, the above interpretations remain speculative and further research is needed to elucidate the role of conflict monitoring and detection in the inhibition of impulsive and maladaptive behaviors.
4.4. Limitations and future directions
Several limitations of the present study need to be acknowledged. First, the effect sizes reported here are modest, which suggests that reduced error-monitoring brain activity is only one of many factors contributing to the risk of future tobacco use. Second, currently available longitudinal data are limited to age 18 only. Although most onsets of tobacco use occur by this age, the data are nevertheless censored, i.e. some those who never tried tobacco by age 18 may still do so in the future. Therefore, the interpretation of the findings should be limited to age 18. The present results warrant a systematic study of the role of error monitoring mechanisms in the etiology of addictive disorders. Most important, exactly how developmental deficits in the mechanism of error monitoring confers increased risk for substance involvement remains to be established. In the above discussion (Section 4.3) we have hypothesized possible pathways that might link individual differences in the maturation of the error monitoring system and self-regulation competencies that are crucial for substance involvement versus abstinence, however, at present these proposed mechanisms remain speculative. In particular, an important goal for future studies would be to elucidate in greater detail the relationships between ERN, dimensional variation in impulsivity, and anxiety traits, and substance use. Another important direction for future research would be to establish the relative role of deficits in error and conflict monitoring in liability to substance use using tasks that allow for delineation of these aspects of cognitive control.
4.5. Conclusions
To the best of our knowledge, this study is the first attempt to clarify causal relationship between developmental differences in the brain mechanisms of error monitoring and subsequent substance use behavior using a prospective longitudinal design. The results strongly suggest that developmental deficits in the error-related brain activity as indicated by ERN predate the onset of tobacco use by age 18. The findings are also consistent with the notion that ERN differences between future substance users and non-users emerge as a result of differential rate of maturation in the neural substrates of error monitoring during adolescence. We conclude that developmental deficits in the neural mechanisms of error-monitoring may represent a risk factor for substance involvement during adolescence.
Conflict of interest statement
There are no known conflicts of interest associated with this publication.
Acknowledgements
This work was supported by grants DA018899, DA027096, R01 AA016812 and P60 AA011998 from the National Institutes of Health (NIH). The authors acknowledge organizational and technical assistance by Tara Tinnin, MSW, Olga Novak, and other project staff. The authors also acknowledge the generous giving of time and effort by the study participants.
References
- Aarts K., De Houwer J., Pourtois G. Erroneous and correct actions have a different affective valence: evidence from ERPs. Emotion. 2013;13:960–973. doi: 10.1037/a0032808. [DOI] [PubMed] [Google Scholar]
- Aarts K., Vanderhasselt M.A., Otte G., Baeken C., Pourtois G. Electrical brain imaging reveals the expression and timing of altered error monitoring functions in major depression. J. Abnorm. Psychol. 2013;122:939–950. doi: 10.1037/a0034616. [DOI] [PubMed] [Google Scholar]
- Agrawal A., Verweij K.J., Gillespie N.A., Heath A.C., Lessov-Schlaggar C.N., Martin N.G., Nelson E.C., Slutske W.S., Whitfield J.B., Lynskey M.T. The genetics of addiction—a translational perspective. Transl. Psychiatry. 2012;2:e140. doi: 10.1038/tp.2012.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anokhin A.P., Golosheykin S., Heath A.C. Heritability of frontal brain function related to action monitoring. Psychophysiology. 2008;45:524–534. doi: 10.1111/j.1469-8986.2008.00664.x. [DOI] [PubMed] [Google Scholar]
- Anokhin A.P., Todorov A.A., Madden P.A., Grant J.D., Heath A.C. Brain event-related potentials, dopamine D2 receptor gene polymorphism, and smoking. Genet. Epidemiol. 1999;17(Suppl. 1):S37–S42. doi: 10.1002/gepi.1370170707. [DOI] [PubMed] [Google Scholar]
- Anokhin A.P., Vedeniapin A.B., Sirevaag E.J., Bauer L.O., O’Connor S.J., Kuperman S., Porjesz B., Reich T., Begleiter H., Polich J., Rohrbaugh J.W. The P300 brain potential is reduced in smokers. Psychopharmacology (Berl) 2000;149:409–413. doi: 10.1007/s002130000387. [DOI] [PubMed] [Google Scholar]
- Carter C.S., Macdonald A.M., Botvinick M., Ross L.L., Stenger V.A., Noll D., Cohen J.D. Parsing executive processes: strategic vs. evaluative functions of the anterior cingulate cortex. Proc. Natl. Acad. Sci. U.S.A. 2000;97:1944–1948. doi: 10.1073/pnas.97.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Getz S., Galvan A. The adolescent brain. Dev. Rev. 2008;28:62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello E.J., Erkanli A., Federman E., Angold A. Development of psychiatric comorbidity with substance abuse in adolescents: effects of timing and sex. J. Clin. Child Psychol. 1999;28:298–311. doi: 10.1207/S15374424jccp280302. [DOI] [PubMed] [Google Scholar]
- Davies P.L., Segalowitz S.J., Gavin W.J. Development of error-monitoring event-related potentials in adolescents. Ann. N.Y. Acad. Sci. 2004;1021:324–328. doi: 10.1196/annals.1308.039. [DOI] [PubMed] [Google Scholar]
- Debener S., Ullsperger M., Siegel M., Fiehler K., von Cramon D.Y., Engel A.K. Trial-by-trial coupling of concurrent electroencephalogram and functional magnetic resonance imaging identifies the dynamics of performance monitoring. J. Neurosci. 2005;25:11730–11737. doi: 10.1523/JNEUROSCI.3286-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikman Z.V., Allen J.J. Error monitoring during reward and avoidance learning in high- and low-socialized individuals. Psychophysiology. 2000;37:43–54. [PubMed] [Google Scholar]
- Etkin A., Egner T., Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn. Sci. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D., Kotov R., Hajcak G. Psychometric considerations in using error-related brain activity as a biomarker in psychotic disorders. J. Abnorm. Psychol. 2013;122:520–531. doi: 10.1037/a0032618. [DOI] [PubMed] [Google Scholar]
- Franken I.H., van Strien J.W., Kuijpers I. Evidence for a deficit in the salience attribution to errors in smokers. Drug Alcohol Depend. 2010;106:181–185. doi: 10.1016/j.drugalcdep.2009.08.014. [DOI] [PubMed] [Google Scholar]
- Funk D., Marinelli P.W., Le A.D. Biological processes underlying co-use of alcohol and nicotine: neuronal mechanisms, cross-tolerance, and genetic factors. Alcohol Res. Health. 2006;29:186–192. [PMC free article] [PubMed] [Google Scholar]
- Hajcak G., McDonald N., Simons R.F. Anxiety and error-related brain activity. Biol. Psychol. 2003;64:77–90. doi: 10.1016/s0301-0511(03)00103-0. [DOI] [PubMed] [Google Scholar]
- Hall J.R., Bernat E.M., Patrick C.J. Externalizing psychopathology and the error-related negativity. Psychol. Sci. 2007;18:326–333. doi: 10.1111/j.1467-9280.2007.01899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath A.C., Martin N.G., Lynskey M.T., Todorov A.A., Madden P.A. Estimating two-stage models for genetic influences on alcohol, tobacco or drug use initiation and dependence vulnerability in twin and family data. Twin Res. 2002;5:113–124. doi: 10.1375/1369052022983. [DOI] [PubMed] [Google Scholar]
- Herrmann M.J., Rommler J., Ehlis A.C., Heidrich A., Fallgatter A.J. Source localization (LORETA) of the error-related-negativity (ERN/Ne) and positivity (Pe) Brain Res. Cogn. Brain Res. 2004;20:294–299. doi: 10.1016/j.cogbrainres.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Huster R.J., Eichele T., Enriquez-Geppert S., Wollbrink A., Kugel H., Konrad C., Pantev C. Multimodal imaging of functional networks and event-related potentials in performance monitoring. NeuroImage. 2011;56:1588–1597. doi: 10.1016/j.neuroimage.2011.03.039. [DOI] [PubMed] [Google Scholar]
- Iannaccone R., Hauser T.U., Staempfli P., Walitza S., Brandeis D., Brem S. Conflict monitoring and error processing: new insights from simultaneous EEG–fMRI. NeuroImage. 2015;105:395–407. doi: 10.1016/j.neuroimage.2014.10.028. [DOI] [PubMed] [Google Scholar]
- Johnston L.D., O’Malley P.M., Bachman J.G., Schulenberg J.E. Institute for Social Research, The University of Michigan; Ann Arbor, MI: 2012. Monitoring the Future National Results on Adolescent Drug Use: Overview of Key Findings. [Google Scholar]
- Johnston L.D., O’Malley P.M., Miech R.A., Bachman J.G., Schulenberg J.E. Institute for Social Research, The University of Michigan; Ann Arbor, MI: 2014. Monitoring the Future National Results on Drug Use: 1975–2013: Overview, Key Findings on Adolescent Drug Use. [Google Scholar]
- Kandel D., Kandel E. The gateway hypothesis of substance abuse: developmental, biological and societal perspectives. Acta Paediatr. 2015;104:130–137. doi: 10.1111/apa.12851. [DOI] [PubMed] [Google Scholar]
- Koban L., Pourtois G. Brain systems underlying the affective and social monitoring of actions: an integrative review. Neurosci. Biobehav. Rev. 2014;46(PART 1):71–84. doi: 10.1016/j.neubiorev.2014.02.014. [DOI] [PubMed] [Google Scholar]
- Luijten M., van Meel C.S., Franken I.H. Diminished error processing in smokers during smoking cue exposure. Pharmacol. Biochem. Behav. 2011;97:514–520. doi: 10.1016/j.pbb.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Mathalon D.H., Whitfield S.L., Ford J.M. Anatomy of an error: ERP and fMRI. Biol. Psychol. 2003;64:119–141. doi: 10.1016/s0301-0511(03)00105-4. [DOI] [PubMed] [Google Scholar]
- Mills K.L., Tamnes C.K. Methods and considerations for longitudinal structural brain imaging analysis across development. Dev. Cogn. Neurosci. 2014;9:172–190. doi: 10.1016/j.dcn.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miltner W.H., Lemke U., Weiss T., Holroyd C., Scheffers M.K., Coles M.G. Implementation of error-processing in the human anterior cingulate cortex: a source analysis of the magnetic equivalent of the error-related negativity. Biol. Psychol. 2003;64:157–166. doi: 10.1016/s0301-0511(03)00107-8. [DOI] [PubMed] [Google Scholar]
- Moser J.S., Moran T.P., Schroder H.S., Donnellan M.B., Yeung N. On the relationship between anxiety and error monitoring: a meta-analysis and conceptual framework. Front. Hum. Neurosci. 2013;7:466. doi: 10.3389/fnhum.2013.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafo M., Clark T., Johnstone E., Murphy M., Walton R. The genetic basis for smoking behavior: a systematic review and meta-analysis. Nicotin Tob. Res. 2004;6:583–597. doi: 10.1080/14622200410001734030. (Official journal of the Society for Research on Nicotine and Tobacco) [DOI] [PubMed] [Google Scholar]
- Naude J., Dongelmans M., Faure P. Nicotinic alteration of decision-making. Neuropharmacology. 2014;96(Pt B):244–254. doi: 10.1016/j.neuropharm.2014.11.021. [DOI] [PubMed] [Google Scholar]
- O’Loughlin J.L., Dugas E.N., O’Loughlin E.K., Karp I., Sylvestre M.P. Incidence and determinants of cigarette smoking initiation in young adults. J. Adolesc. Health. 2014;54 doi: 10.1016/j.jadohealth.2013.07.009. 26.e24-32.e24. [DOI] [PubMed] [Google Scholar]
- Olvet D.M., Hajcak G. The error-related negativity (ERN) and psychopathology: toward an endophenotype. Clin. Psychol. Rev. 2008;28:1343–1354. doi: 10.1016/j.cpr.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olvet D.M., Hajcak G. The stability of error-related brain activity with increasing trials. Psychophysiology. 2009;46:957–961. doi: 10.1111/j.1469-8986.2009.00848.x. [DOI] [PubMed] [Google Scholar]
- Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat. Rev. Neurosci. 2001;2:417–424. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- Peters J., Bromberg U., Schneider S., Brassen S., Menz M., Banaschewski T., Conrod P.J., Flor H., Gallinat J., Garavan H., Heinz A., Itterman B., Lathrop M., Martinot J.L., Paus T., Poline J.B., Robbins T.W., Rietschel M., Smolka M., Strohle A., Struve M., Loth E., Schumann G., Buchel C., Consortium I. Lower ventral striatal activation during reward anticipation in adolescent smokers. Am. J. Psychiatry. 2011;168:540–549. doi: 10.1176/appi.ajp.2010.10071024. [DOI] [PubMed] [Google Scholar]
- Pontifex M.B., Scudder M.R., Brown M.L., O’Leary K.C., Wu C.T., Themanson J.R., Hillman C.H. On the number of trials necessary for stabilization of error-related brain activity across the life span. Psychophysiology. 2010;47:767–773. doi: 10.1111/j.1469-8986.2010.00974.x. [DOI] [PubMed] [Google Scholar]
- Posner M.I., Rothbart M.K., Sheese B.E., Tang Y. The anterior cingulate gyrus and the mechanism of self-regulation. Cogn., Affect. Behav. Neurosci. 2007;7:391–395. doi: 10.3758/cabn.7.4.391. [DOI] [PubMed] [Google Scholar]
- Potts G.F., George M.R., Martin L.E., Barratt E.S. Reduced punishment sensitivity in neural systems of behavior monitoring in impulsive individuals. Neurosci. Lett. 2006;397:130–134. doi: 10.1016/j.neulet.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Rass O., Fridberg D.J., O’Donnell B.F. Neural correlates of performance monitoring in daily and intermittent smokers. Clin. Neurophysiol. 2014;125:1417–1426. doi: 10.1016/j.clinph.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray R., Schnoll R.A., Lerman C. Nicotine dependence: biology, behavior, and treatment. Annu. Rev. Med. 2009;60:247–260. doi: 10.1146/annurev.med.60.041707.160511. [DOI] [PubMed] [Google Scholar]
- Reynolds B., Patak M., Shroff P., Penfold R.B., Melanko S., Duhig A.M. Laboratory and self-report assessments of impulsive behavior in adolescent daily smokers and nonsmokers. Exp. Clin. Psychopharmacol. 2007;15:264–271. doi: 10.1037/1064-1297.15.3.264. [DOI] [PubMed] [Google Scholar]
- Richards J.M., Plate R.C., Ernst M. Neural systems underlying motivated behavior in adolescence: implications for preventive medicine. Prev. Med. 2012;55(Suppl.):S7–S16. doi: 10.1016/j.ypmed.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof K.R., Ullsperger M., Crone E.A., Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Riesel A., Weinberg A., Endrass T., Meyer A., Hajcak G. The ERN is the ERN is the ERN?. Convergent validity of error-related brain activity across different tasks. Biol. Psychol. 2013;93:377–385. doi: 10.1016/j.biopsycho.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Rushworth M.F., Behrens T.E. Choice, uncertainty and value in prefrontal and cingulate cortex. Nat. Neurosci. 2008;11:389–397. doi: 10.1038/nn2066. [DOI] [PubMed] [Google Scholar]
- Rushworth M.F., Buckley M.J., Behrens T.E., Walton M.E., Bannerman D.M. Functional organization of the medial frontal cortex. Curr. Opin. Neurobiol. 2007;17:220–227. doi: 10.1016/j.conb.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Santesso D.L., Segalowitz S.J. Developmental differences in error-related ERPs in middle- to late-adolescent males. Dev. Psychol. 2008;44:205–217. doi: 10.1037/0012-1649.44.1.205. [DOI] [PubMed] [Google Scholar]
- Santesso D.L., Segalowitz S.J. The error-related negativity is related to risk taking and empathy in young men. Psychophysiology. 2009;46:143–152. doi: 10.1111/j.1469-8986.2008.00714.x. [DOI] [PubMed] [Google Scholar]
- Santesso D.L., Segalowitz S.J., Schmidt L.A. ERP correlates of error monitoring in 10-year olds are related to socialization. Biol. Psychol. 2005;70:79–87. doi: 10.1016/j.biopsycho.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Segalowitz S.J., Dywan J. Individual differences and developmental change in the ERN response: implications for models of ACC function. Psychol. Res. 2009;73:857–870. doi: 10.1007/s00426-008-0193-z. [DOI] [PubMed] [Google Scholar]
- Semlitsch H.V., Anderer P., Schuster P., Presslich O. A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology. 1986;23:695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Shackman A.J., Salomons T.V., Slagter H.A., Fox A.S., Winter J.J., Davidson R.J. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat. Rev. Neurosci. 2011;12:154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels K., Hawk L.W., Jr. Self-regulation in ADHD: the role of error processing. Clin. Psychol. Rev. 2010;30:951–961. doi: 10.1016/j.cpr.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear L.P. Adolescent neurodevelopment. J. Adolesc. Health. 2013;52:S7–S13. doi: 10.1016/j.jadohealth.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieben J., Lewis M.D., Granic I., Zelazo P.D., Segalowitz S., Pepler D. Neurophysiological mechanisms of emotion regulation for subtypes of externalizing children. Dev. Psychopathol. 2007;19:455–480. doi: 10.1017/S0954579407070228. [DOI] [PubMed] [Google Scholar]
- Tamnes C.K., Walhovd K.B., Torstveit M., Sells V.T., Fjell A.M. Performance monitoring in children and adolescents: a review of developmental changes in the error-related negativity and brain maturation. Dev. Cogn. Neurosci. 2013;6C:1–13. doi: 10.1016/j.dcn.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ewijk H., Groenman A.P., Zwiers M.P., Heslenfeld D.J., Faraone S.V., Hartman C.A., Luman M., Greven C.U., Hoekstra P.J., Franke B., Buitelaar J., Oosterlaan J. Smoking and the developing brain: altered white matter microstructure in attention-deficit/hyperactivity disorder and healthy controls. Hum. Brain Mapp. 2014;36(3):1180–1189. doi: 10.1002/hbm.22695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meel C.S., Heslenfeld D.J., Rommelse N.N., Oosterlaan J., Sergeant J.A. Developmental trajectories of neural mechanisms supporting conflict and error processing in middle childhood. Dev. Neuropsychol. 2012;37:358–378. doi: 10.1080/87565641.2011.653062. [DOI] [PubMed] [Google Scholar]
- van Noordt S.J., Segalowitz S.J. Performance monitoring and the medial prefrontal cortex: a review of individual differences and context effects as a window on self-regulation. Front. Hum. Neurosci. 2012;6:197. doi: 10.3389/fnhum.2012.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veen V., Carter C.S. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiol. Behav. 2002;77:477–482. doi: 10.1016/s0031-9384(02)00930-7. [DOI] [PubMed] [Google Scholar]
- Vijayakumar N., Whittle S., Dennison M., Yucel M., Simmons J., Allen N.B. Development of temperamental effortful control mediates the relationship between maturation of the prefrontal cortex and psychopathology during adolescence: a 4-year longitudinal study. Dev. Cogn. Neurosci. 2014;9:30–43. doi: 10.1016/j.dcn.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]