Highlights
-
•
Adolescent binge drinkers have reduced cerebellar activity during reward outcome.
-
•
Average drinks consumed/drinking day was negatively related to brain activity.
-
•
Salience of rewards may be blunted because of alcohol-induced neurotoxicity.
Keywords: Adolescence, Alcohol, Binge, Reward, Cerebellum
Abstract
Due to ongoing development, adolescence may be a period of heightened vulnerability to the neurotoxic effects of alcohol. Binge drinking may alter reward-driven behavior and neurocircuitry, thereby increasing risk for escalating alcohol use. Therefore, we compared reward processing in adolescents with and without a history of recent binge drinking. At their baseline study visit, all participants (age = 14.86 ± 0.88) were free of heavy alcohol use and completed a modified version of the Wheel of Fortune (WOF) functional magnetic resonance imaging task. Following this visit, 17 youth reported binge drinking on ≥3 occasions within a 90 day period and were matched to 17 youth who remained alcohol and substance-naïve. All participants repeated the WOF task during a second visit (age = 16.83 ± 1.22). No significant effects were found in a region of interest analysis of the ventral striatum, but whole-brain analyses showed significant group differences in reward response at the second study visit in the left cerebellum, controlling for baseline visit brain activity (p/α < 0.05), which was negatively correlated with mean number of drinks consumed/drinking day in the last 90 days. These findings suggest that binge drinking during adolescence may alter brain activity during reward processing in a dose-dependent manner.
1. Introduction
Adolescence is a period marked by continued structural and functional development in the brain (for review, see Blakemore, 2012), as well as many associated behavioral and cognitive changes (for review, see Paus, 2005). Adolescence is also a time of increased risk-taking behavior, including experimentation with drugs and alcohol (Eaton et al., 2012). One possible explanation for increased risk-taking observed during adolescence involves the continued development of reward-related neurocircuitry (Galvan, 2010). Brain regions, such as the orbitofrontal cortex (OFC), medial prefrontal cortex (mPFC), and striatum have been implicated as key components in the brain's reward system (McClure et al., 2004, Schultz, 2000) and are regions that undergo development during adolescence (Goddings et al., 2014, Gogtay et al., 2004). For example, dopamine receptor levels and binding in the striatum are higher during adolescence than adulthood (Seeman et al., 1987) and are accompanied by an increase in density of dopaminergic projections to the prefrontal cortex (Kalsbeek et al., 1988, Rosenberg and Lewis, 1994, Tunbridge et al., 2007). These developmental changes in the reward system during adolescence may impact how the brain responds to reward. Specifically, some neuroimaging studies have found greater reward-related activation during adolescence, compared to that seen in adults and children, in regions such as the ventral striatum and mPFC (Van Leijenhorst et al., 2010), which may be associated with an increase in ventral striatal dopamine release observed during rewarding events (Jonasson et al., 2014, Koepp et al., 1998). This heightened plasticity of the reward system during adolescence may render the adolescent brain more vulnerable to the neurotoxic effects of drugs of abuse.
Currently, the most common form of substance use among adolescents is the use of alcohol. In the United States, by the end of the 12th grade, 68% of adolescents have reported drinking alcohol, 52% report being drunk in their lifetime, and 26% report being drunk within the last 30 days (Johnston et al., 2014). Furthermore, binge drinking, the most common form of alcohol misuse among adolescents (Deas, 2006), is reported by over 22% of adolescents (Johnston et al., 2014). In line with findings of continued development of the reward system during adolescence, pre-clinical models have found that the reward system in adolescence responds differently to alcohol than during adulthood. Increased dopamine release within the ventral striatum during acute alcohol exposure is prominent in adolescents (Pascual et al., 2009, Philpot et al., 2009) and appears to be associated with greater rewarding effects of alcohol during this developmental stage (Pautassi et al., 2008, Ristuccia and Spear, 2008), which may promote future drinking. In support of this notion, rodent models indicate that alcohol exposure during adolescence, compared to adulthood, increases reward driven behavior (McMurray et al., 2014, Schindler et al., 2014).
A growing body of literature has documented numerous abnormalities in brain structure and functioning among adolescent alcohol users. Binge drinking during adolescence has been associated with differences in frontal lobe cortical thickness (Squeglia et al., 2012) and reductions in cerebellar volume (Lisdahl et al., 2013), as well as widespread reductions in white matter integrity with and without comorbid marijuana use (Jacobus et al., 2013). Additionally, binge drinking has been shown to have an effect on brain activity during affective decision making (Xiao et al., 2013) and spatial working memory (Schweinsburg et al., 2008, Squeglia et al., 2011). Cross-sectional neuroimaging studies have begun to explore the effects of alcohol on reward processing in the brain, but this work has been limited to adults. Alcohol-dependent adults have shown increased activation in the ventral striatum and mesial frontal cortex during reward notification compared to controls (Bjork et al., 2008b), as well as reduced reward outcome-related positivity in the brain, measured with event-related potentials, suggestive of deficient reward processing (Kamarajan et al., 2010). This latter “reward deficiency” finding is further supported by studies in adult alcoholics which found hyporesponsive activity in the ventral striatum during reward anticipation (Beck et al., 2009, Wrase et al., 2007). While both hyper- and hyporesponsive activity in the ventral striatum have been seen in human adults, preclinical models in adolescents primarily support the idea of reward deficiency, such that repeated alcohol exposure leads to a hypodopaminergic state (Philpot et al., 2009), and alcohol-induced changes to the reward system correlate with increased alcohol seeking behavior and voluntary consumption in adult rats that experienced adolescent alcohol exposure (Pascual et al., 2009). No longitudinal study, to our knowledge, has investigated the effects of binge-level alcohol use on reward-related brain activation in an adolescent population.
The current study sought to expand on this body of literature by examining neural activation to reward in a group of binge-drinking adolescents compared to alcohol/drug-naïve controls using a prospective, longitudinal approach. Adolescents performed a modified version of the Wheel of Fortune (WOF) task (Ernst et al., 2004), a reward-based decision-making task, during functional magnetic resonance imaging (fMRI). Based on previous findings in adult alcoholics of decreased reward-related activation (Beck et al., 2009, Wrase et al., 2007), as well as alcohol's propensity to affect the reward system during adolescence (Pascual et al., 2009, Philpot et al., 2009), we hypothesized that binge-drinking adolescents would show reduced brain activation to reward outcome, compared to their non-using peers, in reward processing regions, such as the mPFC and ventral striatum.
2. Materials and methods
2.1. Participant recruitment and screening
Over 200 participants, ages 12–16, were recruited as part of a longitudinal study on risk factors for and consequences of alcohol use on brain and behavior during adolescence (Cservenka et al., 2012, Ernst et al., 2004, Cservenka et al., 2014a, Cservenka et al., 2014b, Cservenka and Nagel, 2012, Mackiewicz Seghete et al., 2013). Recruitment was conducted through community mailings and local advertising. The participating youth and one of their parents or guardians were interviewed by phone during a pre-screen to determine initial eligibility, following which study consent and assent were obtained from parents and youth, respectively. Next, a longer telephone screening interview was conducted with the youth and parent, separately. During this screen, the Structured Clinical Interview (Brown et al., 1994), the Diagnostic Interview Schedule for Children Predictive Scales (Lucas et al., 2001), and the Family History Assessment Module (Rice et al., 1995) were administered to assess the presence of psychiatric conditions in the youth, and determine family history of psychopathology. Youth who met DSM-IV criteria for an Axis I disorder were not included in the study. Other exclusionary criteria for study participation were (1) lack of family history information, (2) presence of psychotic disorders in first-degree biological parents (i.e. schizophrenia or bipolar I), (3) parent report of prenatal alcohol exposure, (4) MRI contraindications (including pregnancy), (4) head injury with loss of consciousness, (5) use of psychotropic medication, (6) left-handedness (Oldfield, 1971), and (7) serious medical/neurological conditions. Furthermore, since this study was aimed at understanding the neural consequences of initiating heavy drinking during adolescence, youth were excluded from the initial study visit if they reported >10 lifetime alcoholic beverages, >2 drinks/occasion, >5 lifetime uses of marijuana, or >4 cigarettes/day (Brief Lifetime version of the Customary Drinking and Drug Use Record (CDDR, Brown et al. (1998)). Finally, the Hollingshead Index of Social Position (Hollingshead, 1957) was administered to the participating parent or guardian to estimate socioeconomic status (SES) of the youth, based on the parents’ educational and occupational attainment. All procedures were in accordance with the ethical guidelines of the Oregon Health & Science University (OHSU) Institutional Review Board.
2.2. Study procedures and follow-up assessments
Eligible male youth were scheduled for study visits at any time, while female youth were scheduled during the first 10 days of their menstrual cycle (follicular phase) to account for cycle-related variations in hormone levels. At the initial baseline study visit (age range: 13.15–16.34), participants underwent neuroimaging, during which they completed a modified version of the WOF fMRI task (Cservenka et al., 2013, Cservenka and Nagel, 2012, Ernst et al., 2004) and a structural MRI scan for co-registration of functional data (other components of the MRI scan are not reported in this study). Furthermore, youth completed a neuropsychological battery within one week of the imaging session, which included estimates of intellectual functioning using the Wechsler Abbreviated Scale of Intelligence (WASI, Wechsler (1999)). Pubertal status was assessed with gender-specific line drawings representing pubertal development with Tanner's Sexual Maturation Scale (Taylor et al., 2001). The Children's Depression Inventory (Kovacs, 1985) and the State-Trait Anxiety Inventory (Spielberger et al., 1973) were administered at each study visit to assess depressive symptoms and state anxiety, respectively.
Following completion of the initial study visits, participants could elect to participate in the longitudinal portion of the study. If youth assented to participating, they were contacted by phone approximately every 3 months. During these phone interviews, youth were asked to provide information on drinking behavior in the past 90 days with the CDDR (Brown et al., 1998), following which the 90-day Timeline Followback (TLFB) (Sobell and Sobell, 1992, Sobell et al., 1986) was used to collect detailed information on alcohol, marijuana, nicotine, and other drug use, if any use was reported. Based on these interviews, youth who reported binge drinking (≥5 drinks/occasion for males and ≥4 drinks/occasion for females) on at least one occasion in the past 90 days, and had at least two other occasions of ≥4 drinks/occasion in that 3-month period, were invited back for a second study visit1 with identical neuroimaging procedures and neuropsychological measures as administered during their baseline visits. Youth were asked to abstain from alcohol and/or other drug use for 72 h prior to the second study visit. At the time of the second study visit (age range: 14.51–18.68), absence of acute alcohol intoxication prior to the neuroimaging session was confirmed with a breathalyzer, which was negative for all youth. A urine test was also collected at this time to assess for other drugs youth may have taken. Nine youth from the binge-drinking group tested positive for tetrahydrocannabinol, while one of these nine also tested positive for tricyclic antidepressants, although use of this medication was not reported. 17 youth met binge-drinking criteria to be invited back for a revisit (except one male youth whose largest binge was 4 drinks, but also completed the revisit study procedures), and these youth were matched to 17 controls who had not initiated any alcohol or substance use. Alcohol-using youth were matched on age, puberty, and sex to controls and these adolescents also completed the identical revisit study procedures. The interval between visits was variable across subjects as some youth emerged into binge drinking more quickly than others. Youth completed revisits between 0.60 and 4.45 years of being in the study. Binge drinkers and controls were matched on time between visits (mean for bingers = 2.05 ± 1.18 years; mean for controls: 1.90 ± 1.01 years; t32 = −0.40, p = 0.69).
2.3. Wheel of Fortune fMRI task
A modified version (Cservenka et al., 2013, Cservenka and Nagel, 2012) of the WOF fMRI task (Ernst et al., 2004) was administered to all youth during both their baseline and second study visits. This task has been described in detail elsewhere (Cservenka et al., 2013, Cservenka and Nagel, 2012), but briefly, procedures were as follows. The task consisted of two runs of 36 trials each, divided into three phases per trial, with 1–11 second fixation periods jittered between trials. Wheels divided into two portions, were presented in the first phase of each trial. Each portion of the wheel was associated with the probability of winning a certain monetary reward. In the first phase of each trial, participants made a decision about which portion of a wheel they wanted to pick, in the second phase, they anticipated whether or not they would win, and in the third phase, they received feedback on whether or not they won. Winning was based on whether the choice in the decision-making phase of the task matched the pre-defined probabilities that were programmed in the task. The portions of each wheel were categorized as either risky (10% chance of winning $7 or 30% chance of winning $2), safe (90% chance of winning $1 or 70% chance of winning $1), or chance (50% chance of winning $2) (Cservenka et al., 2013).
To assess reward processing differences between adolescent binge drinkers and controls, the third phase of the task was analyzed. The contrast of interest in the third phase was behavior and brain response to Wins vs. No Wins. Wins were classified as those trials in which participants won the portion of the wheel they selected after a risky, chance, or safe choice. No Wins were those trials in which participants did not win the portion of the wheel they selected after a risky or chance choice. Since not winning after making a safe choice was rare, but could represent the greatest expectancy violation during the task based on expected values (0.90 for 10/90 wheels and 0.70 for 30/70 wheels), these trials were not included in the regressor representing No Win brain response. Furthermore, to ensure youth were engaged in the task during reward notification, they were asked to make a button press with their index finger if they won, or a button press with their middle finger, if they did not win during each trial. Accuracy and reaction time on these trials was recorded. All participants practiced the WOF task prior to entering the scanner.
2.4. Image acquisition
Participants were scanned with a Siemens 3T Tim Trio system at OHSU's Advanced Imaging Research Center. A mirror was mounted on a 12-channel head coil, so that participants could view the task projected on a screen at the end of the scanner bore. All youth were given earplugs and headphones to reduce noise from the MRI scan and allow communication with the scan operator through an intercom system. Pillows were placed around each participant's head to limit head motion while lying in the supine position. A four button optical button box, placed in the participant's right hand, was used to make responses during the WOF task. A T1-weighted structural MPRAGE scan (time repetitions (TR) = 2300 ms, time to echo (TE) = 3.58 ms, inversion time (TI) = 900 ms, flip angle = 10°, field of view (FOV) = 240 mm × 256 mm, voxel size = 1 mm × 1 mm × 1.1 mm, 160 slices, acquisition time = 9:14) was acquired sagittally for co-registration of the functional data to the participant's anatomical image. T2*-weighted echo planar imaging, acquired in the axial plane, was used to measure the blood oxygen level-dependent (BOLD) response during the WOF task (TR = 2000 ms, TE = 30 ms, flip angle = 90°, FOV = 240 mm2, voxel size = 3.75 mm × 3.75 mm × 3.8 mm, 33 slices, acquisition time = 2 runs of 300 TRs, lasting 10:00 min each).
2.5. Image preprocessing
Image preprocessing followed conventional procedures previously described in other reports (Cservenka et al., 2013, Cservenka and Nagel, 2012). Analysis of Functional NeuroImages (AFNI) was used for all image preprocessing (Cox, 1996). Briefly, following image reconstruction, anatomical masks were skull-stripped to remove non-brain skull and tissue. First, functional data were subjected to slice timing correction, identification of movement artifact, and realignment of TRs to the volume requiring the least amount of adjustment of rigid body head motion following a least squares algorithm (Cox and Jesmanowicz, 1999). Then, TRs that required more than 2.5 mm or 2.5 degrees of adjustment were censored prior to further analyses to limit artifact induced by head motion or other noise. Functional data were blurred with a 6 mm full width half maximum Gaussian kernel to increase signal-to-noise ratio, fractionized to the anatomical image, and normalized to convert values to relative percent signal change. The two runs of the WOF task were then concatenated as were the six motion regressors from each run of the task. The hemodynamic response function (HRF) was modeled with duration of the event defined as the length of each phase of the trial, while modeling delays in the HRF. Regressors of interest were represented by Wins and No Wins (as defined above), while other task-related regressors, including risky, safe, and chance decisions, and risky, safe, and chance anticipations were also modeled (as defined above), but were not examined for the current analyses. AFNI's baseline model included the six motion regressors of non-interest, unmodeled fixation, linear drift, and the average BOLD signal from the entire timecourse of the task. Data were re-sampled into 3 mm3 voxels and transformed to standardized Talairach space (Talairach and Tournoux, 1988).
2.6. Group-level analyses
2.6.1. Demographics and behavior
Demographic variables and dependent variables from task performance were examined for normal distribution using IBM SPSS Version 20.0 (Corp. Released, 2011). Mixed model analysis of variance (ANOVA) examined effects of group, time, and group-by-time interactions for demographic variables. Non-normal variables, including pubertal stage and accuracy identifying a Win or No Win during the feedback phase of the WOF task, were compared between groups for each study visit with Mann–Whitney U tests, while other variables were compared between groups using independent samples t-tests. Outliers on drinking measures or brain activity greater than 2.5 standard deviations from the mean were excluded from correlational analyses relating brain activity with drinking behavior. Cigarette and marijuana use characteristics were severely positively skewed, so they were log-transformed prior to correlational analyses.
2.6.2. Imaging
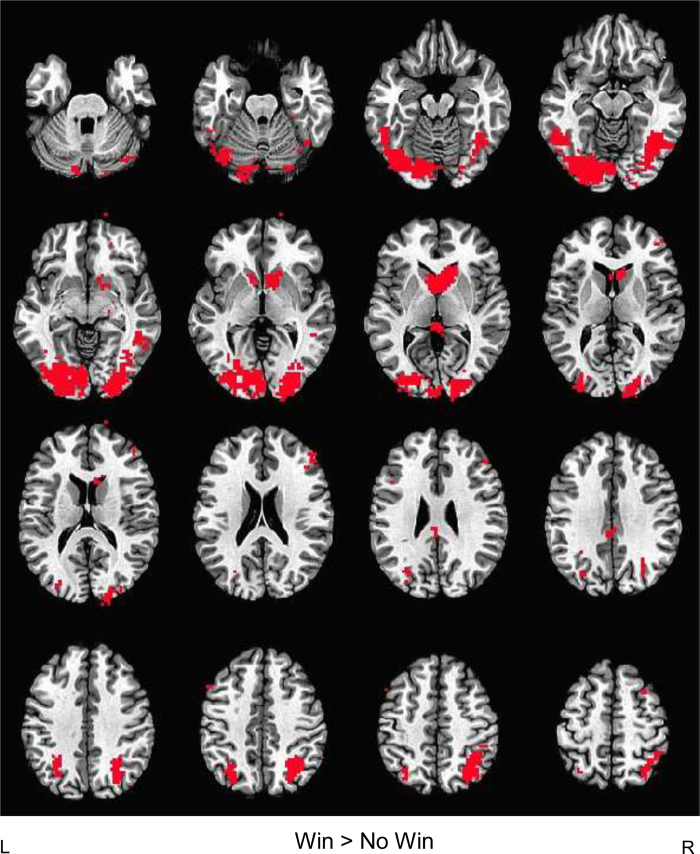
To illustrate significant WOF task-related activity, regardless of group status and study visit, we conducted a conjunction analysis, voxel thresholded at p < 0.05 (Fig. 1), which confirmed expected reward-related and task-positive brain regions activated by the task in the Win vs. No Win contrast, including the ventral striatum, occipital cortex, and fronto-parietal regions. The Win vs. No Win contrast appears to be a valid measure of reward processing, as opposed to more general incentive salience processing, as areas activated in the current study closely resemble those in previous adolescent studies of reward vs. no reward brain activity, which show distinct patterns from loss vs. no loss BOLD response (Bjork et al., 2008a, Bjork et al., 2010).
Fig. 1.
Conjunction map of task-related brain activity during reward processing. Task-related activity maps for each group at each study visit (baseline and revisit) were voxel-thresholded at p < 0.05 and a conjunction map was created for Win > No Win brain response to determine areas commonly activated across participants regardless of group status or time of study visit. Occipital, superior parietal, dorsolateral prefrontal, caudate, and ventral striatal brain activity were seen in the Win > No Win contrast overlaid on AFNI's Talairach template brain in the axial view.
For group-level analyses, an a priori region of interest (ROI) analysis of the ventral striatum was conducted by using the Talairach Daemon atlas in AFNI, and applying 4-mm radius masks (10 voxels each) of the left and right ventral striatum with peak coordinates at 12, −8, −8 (right ventral striatum) and −12, −8, −8 (left ventral striatum). The ventral striatal masks were resampled to 3 mm3 to match the functional data. Percent signal change for the Win vs. No Win contrast was extracted from left and right ventral striatal masks for each participant and mixed model ANOVAs examined the effect of group, time, and group-by-time interactions for the Win vs. No Win contrast in SPSS.
Next, for the whole-brain analyses examining differences in reward processing between binge drinkers and controls, one sample t-tests were voxel thresholded at p < 0.05 for each group and added together to form a map of task-related brain activity at the second study visit. AFNI's 3dttest++ was used to compare groups on differences in brain activity during Wins vs. No Wins, restricted to the pre-defined task-related activity mask. Results of this analysis were multiple comparison corrected using Monte Carlo simulation with both a voxel and cluster threshold (p/α < 0.05) (Forman et al., 1995), yielding a minimum cluster size of 102 voxels. Percent signal change from the cluster in which significant group differences emerged at the second study visit were extracted with 3dROIstats, and signal from the baseline study visit was also extracted from this region. Hierarchical regressions in IBM SPSS Version 20.0 (Corp. Released, 2011) tested whether group differences remained significant after accounting for brain activity in this cluster at the first study visit, age at revisit, and time between scans. Significant findings from this analysis were overlaid on a Talairach brain template in AFNI, while bar graphs created in GraphPad Prism version 5.00 for Windows, GraphPad Software, San Diego California, USA, www.graphpad.com, were used to illustrate percent signal change in each group at first and second study visits in both the significant contrast of interest (Win vs. No Win) and for Win vs. baseline, and No Win vs. baseline brain activity.
In order to examine whether group differences in brain activity were present between youth who emerged into binge drinking and controls, an identical whole-brain analysis to that described above was conducted at baseline (controlling for baseline differences in head movement; p/α < 0.05, ≥94 voxels). Furthermore, two-way ANOVAs examined effects of group, sex, and group-by-sex interactions in clusters where significant differences in BOLD response were present between binge drinkers and controls at revisit.
3. Results
3.1. Demographic characteristics and WOF task behavior
Participant characteristics at each study visit are illustrated in Table 1 (baseline study visit and revisit). Seventeen youth met criteria for binge drinking in the study and completed the second study visit. These youth were matched to 17 controls who had abstained from alcohol and drugs. Valid WOF task data were available from all youth at each study visit. Mixed model ANOVAs examined group-by-time interactions and main effects for all demographic variables in Table 1, except for SES, which was missing for eight controls and 12 binge drinkers at revisit. No significant group-by-time interactions were present for any of the variables. Main effects of time were present for age (F1,32 = 109.92, MSE = 0.60, p < 0.0001, partial η2 = 0.78), pubertal status (F1,32 = 16.48, MSE = 0.27, p < 0.0005, partial η2 = 0.36), and state anxiety (F1,32 = 6.86, MSE = 23.72, p = 0.01, partial η2 = 0.18). Youth were older, more pubertally mature, and less anxious at revisit compared with baseline visit. No main effects of group were present. When examining groups separately at each study visit, significant differences in pubertal stage and head movement were seen at the baseline study visit, such that youth who later emerged into binge drinking were more pubertally mature at this time, while youth who abstained from alcohol use had more head movement during the WOF task (Table 1). No differences in any of the demographic variables were present between the groups at the second study visit. No significant differences in task performance (mean reaction time and accurate identification of winning or not winning) were present at either study visit (all p's >0.10, Table 2).
Table 1.
Demographic characteristics at the baseline visit and revisit.
| Baseline visit |
Revisit |
T2–T1e Binge drinkers |
T2–T1 Controls |
|||||
|---|---|---|---|---|---|---|---|---|
| Binge drinkers (n = 17) | Controls (n = 17) | p | Binge drinkers (n = 17) | Controls (n = 17) | p | p | p | |
| Female (n) | n = 7 | n = 7 | n = 7 | n = 7 | ||||
| Age | 14.90 (1.01) | 14.82 (0.75) | 0.81 | 16.94 (1.28) | 16.72 (1.19) | 0.60 | <0.00001 | <0.00001 |
| Pubertal Statusa | 4.50 (0.63) | 4.00 (0.82) | 0.06 | 4.81 (0.40) | 4.75 (0.45) | 0.78 | 0.06 | 0.005 |
| IQb | 111.59 (9.25) | 112.00 (7.23) | 0.89 | 114.18 (11.08) | 110.82 (9.90) | 0.36 | 0.18 | 0.68 |
| Socioeconomic statusc | 31.41 (15.57) | 30.59 (8.54) | 0.85 | 35.80 (14.86) | 30.33 (12.51) | 0.48 | 0.23 | 0.14 |
| % Risky decisions | 69.68 (24.13) | 59.46 (22.56) | 0.21 | 74.26 (19.78) | 66.02 (24.70) | 0.29 | 0.44 | 0.29 |
| RMSd (Head movement) | 0.17 (0.09) | 0.32 (0.26) | 0.03* | 0.22 (0.16) | 0.24 (0.18) | 0.71 | 0.31 | 0.12 |
| CDIf | 40.76 (4.12) | 41.29 (5.88) | 0.76 | 43.50 (7.93) | 38.93 (4.77) | 0.07 | 0.20 | 0.19 |
| STAIg | 40.89 (5.19) | 40.35 (7.30) | 0.81 | 37.10 (6.04) | 37.95 (7.39) | 0.72 | 0.053 | 0.13 |
Mean (standard deviation).
Tanner's Sexual Maturation Scale (Taylor et al., 2001); missing for one binge-drinking and one control youth at revisit.
Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999).
Hollingshead Index of Social Position; higher scores indicate lower SES (Hollingshead, 1957); missing for twelve binge-drinking and eight control youth at revisit.
Root mean square.
Revisit vs. Baseline Visit.
Children's Depression Inventory (Kovacs, 1985); missing for three binge-drinking and two control youth at revisit.
State-Trait Anxiety Inventory (Spielberger et al., 1973).
Table 2.
WOF task performance at each study visit.
| Binge drinkers (n = 17) |
Controls (n = 17) |
p-value | |
|---|---|---|---|
| Female (n) | n = 7 | n = 7 | |
| Baseline visit | |||
| Win accuracy (%)a | 98.63 (4.26) | 99.78 (0.63) | 0.95 |
| No win accuracy (%)b | 92.25 (23.88) | 97.58 (3.75) | 0.52 |
| Win RT (ms)c | 1237.53 (261.62) | 1318.11 (335.87) | 0.44 |
| No win RT (ms)d | 1200.51 (243.37) | 1317.39 (342.97) | 0.26 |
| Revisit | |||
| Win accuracy (%)a | 96.59 (13.10) | 99.43 (1.17) | 0.79 |
| No win accuracy (%)b | 98.11 (2.56) | 98.91 (2.59) | 0.15 |
| Win RT (ms)c | 1167.99 (238.91) | 1083.50 (253.78) | 0.33 |
| No win RT (ms)d | 1119.80 (229.03) | 1057.47 (251.77) | 0.46 |
Mean (standard deviation), RT = reaction time.
Win accuracy following a 10%, 30%, 50%, 70%, or 90% selection.
No win accuracy following a 10%, 30%, or 50% selection (70% and 90% were not included in behavioral or imaging analyses due to expectancy violations).
Win reaction time following a 10%, 30%, 50%, 70%, or 90% selection.
No Win reaction time following a 10%, 30%, or 50% selection (70% and 90% were not included in behavioral or imaging analyses due to expectancy violations).
3.2. Alcohol and drug use
Drinking characteristics in the binge drinkers at the time of the second study visit are listed in Table 3. The CDDR (Brown et al., 1998) and TLFB (Sobell and Sobell, 1992) were administered to assess previous 90-day alcohol and substance use. Lifetime measures of alcohol, marijuana, and cigarette use were also calculated based on summing the amount of drinks consumed, amount of times marijuana was used, and number of cigarettes smoked from time of study entry through the time of the second study visit. Other substance use characteristics, including age of onset of cigarette and marijuana use are also listed in Table 3. All but two binge-drinking youth, had used marijuana at least once in their lifetime by the time of the second study visit. None of the controls had ever reported consuming alcohol or using any other substances in this study.
Table 3.
Drinking and substance use characteristics of binge drinkers at revisit.
| CDDRa | Binge drinkers (n = 17) |
|---|---|
| Age first drank | 14.65 (1.66) |
| # of days drank/month in past 90 days | 2.57 (2.63) |
| # of drinks/drinking day (past 90 days) | 4.72 (1.66) |
| # of drinks/drinking occasion (past 90 days) | 4.52 (2.35) |
| Largest amount drank on one occasion (past 90 days) | 6.38 (1.84) |
| Average time to consume largest binge (hours) | 1.87 (1.26) Range: 0.01–4.4 |
| Lifetime drinks at revisit | 70.29 (69.71) Range: 16–311.5 |
| Age first used Marijuanab | 14.40 (1.84) |
| Lifetime Marijuana use occasionsb | 81.87 (129.63) Range: 2–476 |
| Age first smoked cigarettesc | 14.67 (1.94) |
| Lifetime cigarette use occasionsc | 213.89 (326.60) Range: 4–800 |
| Other drug used | n = 6 |
Mean (standard deviation).
Customary Drinking and Drug Use Record (Brown et al., 1998).
n = 15.
n = 9.
One participant reported using psychedelic mushrooms and 3,4-methylenedioxy-methamphetamine (once each/lifetime), a second participant reported using psychedelic mushrooms, 3,4-methylenedioxy-methamphetamine, and lysergic acid diethylamide (three times, six times, and one time respectively/lifetime), a third participant reported using psychedelic mushrooms, 3,4-methylenedioxy-methamphetamine, and lysergic acid diethylamide (three times, six times, and one time, respectively/lifetime), a fourth participant reported using 3,4-methylenedioxy-methamphetamine once/lifetime, a fifth participant reported using nitrous oxide once/lifetime, and a sixth participant reported using psychedelic mushrooms once/lifetime.
3.3. Neuroimaging
The a priori ROI analysis of Win vs. No Win brain response in the ventral striatum did not show any significant group differences in brain response at revisit controlling for baseline brain response in these regions (left ventral striatum: F2,31 = 1.31, ΔR2 = 0.003, p = 0.28, β = −0.05, t = −0.30, p = 0.76; right ventral striatum: F2,31 = 0.21, ΔR2 = 0.003, p = 0.81, β = −0.06, t = −0.32, p = 0.75). To follow-up on these negative findings, intra-class correlation coefficients (ICC) using baseline and revisit brain response were calculated in IBM SPSS Version 20.0 (Corp. Released, 2011) for the control group only to limit confounds related to alcohol's effects on the BOLD signal in this region. For the control group, the ICCs were 0.62 and 0.50 for left and right ventral striatum, respectively. These ICCs correspond to fair to good ranges for reliability (Fleiss, 1986), and fall within accepted ranges for neuroimaging analyses (Aron et al., 2006).
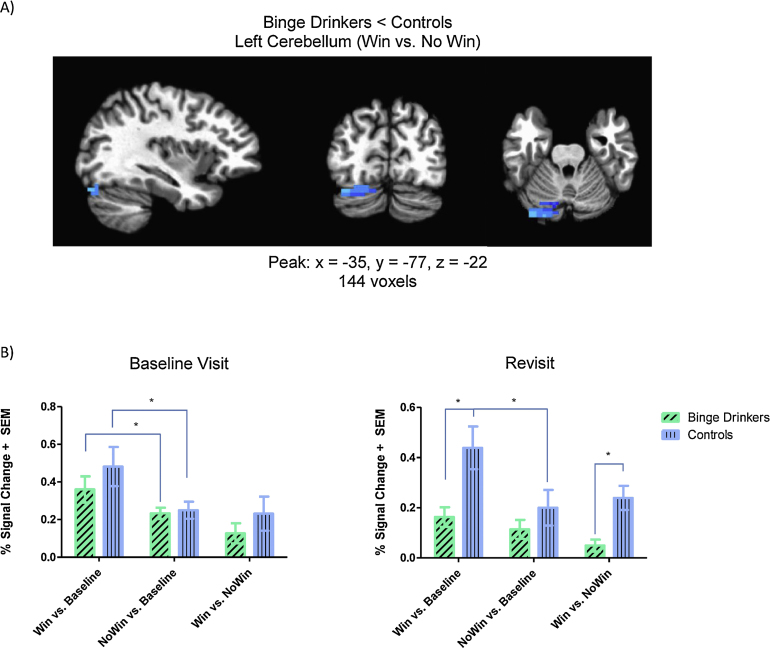
For the whole-brain analysis, a significant group difference in brain activity to Wins vs. No Wins was seen in the left cerebellum (lobule VIIa/crus I) at revisit, while no significant group differences in Win vs. No Win BOLD response were present at the baseline visit. In this cluster, binge drinkers showed reduced Win vs. No Win brain response at the second study visit compared with controls (Fig. 2). Examination of the simple contrasts of Win vs. baseline and No Win vs. baseline brain activity to interpret the results indicated that this effect was driven by greater brain response during Winning in controls compared with binge drinkers (t32 = 2.95, p = 0.007). Binge drinkers showed no significant dissociation between BOLD response to Wins and No Wins at the second study visit (t16 = 2.07, p > 0.05), but distinct brain activity was seen between these two conditions in the control group (t16 = 4.95, p = 0.0001). At the baseline study visit, however, there were no differences in Win vs. baseline (t32 = 0.97, p = 0.34) or No Win vs. baseline (t32 = 0.31, p = 0.76) brain activity in this region between binge drinkers and controls. In fact, both groups showed elevated BOLD response to Wins compared with No Wins (Bingers: (t16 = 2.38, p = 0.03); Controls: (t16 = 2.56, p = 0.02)) in the absence of any heavy drinking.
Fig. 2.
Binge drinkers had reduced cerebellar brain activity during reward processing compared with controls. (A) Significantly reduced posterior cerebellar (lobule VIIa/crus I) activity during Win vs. No Win was seen in binge drinkers at the time of revisit, compared with controls, while accounting for baseline brain activity in this region. Results are displayed in the sagittal, coronal, and axial view overlaid on AFNI's Talairach template brain. (B) Bar graphs of percent signal change illustrating significant differences in brain activity in the contrast of interest (Win vs. No Win) and in simple effects are displayed for each visit. At revisit, binge drinkers had significantly reduced brain response during Win vs. No Win. Simple effects show this was attributed to reduced activity during Win vs. baseline in binge drinkers, compared with controls. While controls showed elevated brain response during Win vs. baseline compared with No Win vs. baseline, brain activity was not distinct in binge drinkers during reward receipt or absence. This is in contrast to brain response in this region at the baseline visit, when both groups showed more activity in response to Wins than No Wins. *p < 0.05.
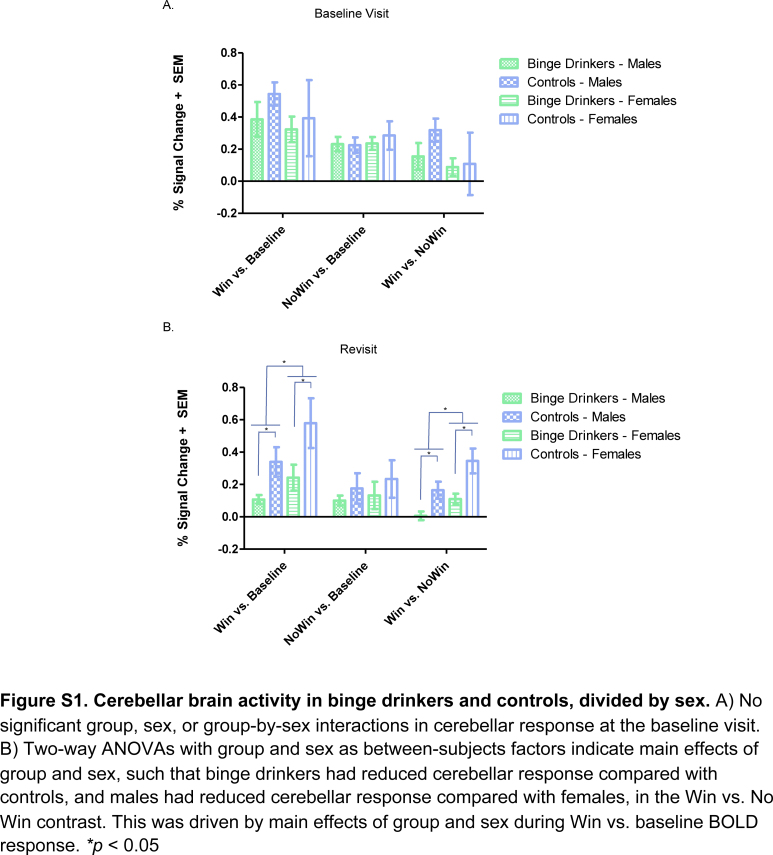
Next, main effects of group, sex, and a group-by-sex interaction were examined using a two-way ANOVA at both revisit and baseline visit. Main effects of group (F1,30 = 15.72, MSE = 0.02, p < 0.0005, partial η2 = 0.34) and sex (F1,30 = 8.23, MSE = 0.02, p = 0.007, partial η2 = 0.22) were present for Win vs. No Win BOLD response at revisit (Fig. S1B). Post hoc t-tests indicated significantly lower Win vs. No Win brain response in binge drinkers compared with controls (t32 = 3.53, p = 0.001) and significantly lower Win vs. No Win brain response for males compared with females (t32 = 2.40, p = 0.02). A follow-up ANOVA on the simple effect contrasts suggested this was driven by Win vs. baseline BOLD response, as main effects of group (F1,30 = 9.68, MSE = 0.07, p = 0.004, partial η2 = 0.24) and sex (F1,30 = 4.12, MSE = 0.07, p = 0.049, partial η2 = 0.12) were also present for this contrast, such that binge drinkers had lower brain response than controls, and males had lower brain response than females. No group-by-sex interaction in cerebellar response was present at revisit. No main effects of group or sex, and no group-by-sex interaction were present for BOLD response in the cerebellar cluster at the baseline visit (Fig. S1A, all p's > 0.10).
To determine whether group differences in brain activity at the second study visit could have been related to preexisting baseline group differences in brain response, age at revisit, or time between visits, we conducted a hierarchical regression, controlling for these variables. Group differences in pubertal stage and head movement (root mean square) were not significantly related to baseline cerebellar activity, so were not covaried for in the analyses. Controlling for baseline activity in this region, age at revisit, and time between visits, differences in left cerebellar activity between binge drinkers and controls remained significant at the second study visit (F4,29 = 5.0, ΔR2 = 0.28, p = 0.003, β = −0.54, t = −3.68, p = 0.001).
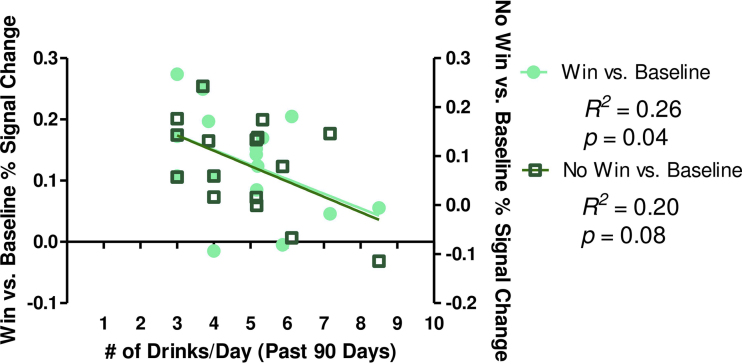
Drinking characteristics were examined in correlation with Win vs. No Win, Win vs. baseline, and No Win vs. baseline BOLD response in the left cerebellar cluster. One youth with percent signal change greater than 2.5 standard deviations from the mean was excluded from correlations. One significant correlation was present when relating past 90 day drinks/drinking day with reward outcome-related brain activity (Fig. 3). Average drinks consumed in the past 90 days was negatively related to No Win vs. baseline BOLD response in the left cerebellum, indicating greater number of drinks/drinking day consumed in the last 90 days was associated with reduced BOLD activity in this region. Marijuana and cigarette use characteristics were also examined for relationships with brain activity, since 15 of the 17 binge drinkers had used marijuana at least once by the time of the second visit, while 9 of the 17 binge drinkers had smoked cigarettes by this time. Age of first marijuana use, lifetime marijuana use, age of first cigarette use, and lifetime cigarette use, were not significantly correlated with Win vs. No Win, Win vs. baseline, or No Win vs. baseline brain response at time of revisit (all p's > 0.10).
Fig. 3.
Drinks consumed/drinking day in the past 3 months was negatively related to cerebellar brain response in binge drinkers. Average drinks/drinking day was negatively associated with reward-related activity in binge drinkers. Win vs. baseline activity is plotted on the left y-axis, while No Win vs. baseline activity is plotted on the right y-axis.
4. Discussion
To our knowledge, the current findings are the first to report alterations in brain functioning during reward processing in adolescent binge drinkers, using a prospective longitudinal design. Since binge drinking is a commonly observed alcohol use pattern during adolescence, reported by nearly a quarter of youth in 12th grade (Johnston et al., 2014), it is important to understand whether this pattern of alcohol use may alter adolescent reward processing. Alcohol-induced neurotoxicity during adolescence (Jacobus and Tapert, 2013) may have long-term effects that extend well into adulthood, thus signaling the need to identify brain areas affected by heavy alcohol consumption during neuromaturation.
4.1. Cerebellum and alcohol-induced neurotoxicity
In contrast to our hypothesis of reduced ventral striatal or mPFC activity, we report a significant difference between binge drinkers and controls in left cerebellar (lobule VIIa/crus I) activity, driven by reduced BOLD response in this region in heavy alcohol users compared with controls during reward receipt. A previous study found that ventral striatal activity was similar in alcohol-dependent adults vs. controls, and that personality characteristics accounted for more variance in nucleus accumbens activity than alcohol-drinking status (Bjork et al., 2012), which may help explain the negative findings in this region. Furthermore, it is possible that effects in mesolimbic circuitry may only be present after prolonged, heavy alcohol use, as seen in adults with alcohol use disorders (AUDs) (Beck et al., 2009, van Holst et al., 2014, Wrase et al., 2007). It is currently unknown whether long-term heavy alcohol use that is initiated during adolescence may alter reward-related ventral striatal response. In the current study, ICC values of percent signal change in the ventral striatum were fair to good (but not excellent), making it difficult to determine if the negative results of binge drinking-related effects in this region were due to lack of power or lack of effect. Importantly, these modest values may also be impacted by developmental change in this region, rendering reliability over time in this age range difficult to assess. However, the negative findings in the ventral striatal ROIs had small effect sizes (Cohen's ƒ2 = 0.003–0.004), suggesting that even with a larger sample, we may not have observed significant group differences in this region.
Nevertheless, the directionality of the findings does support hyporesponsive cerebellar activity during reward receipt in binge drinkers. We found that compared to their baseline study visit, binge drinkers showed significant reductions in cerebellar brain response during reward processing, while control youth did not. The absence of significant differences in brain activity in these youth prior to the initiation of heavy alcohol use provides greater confidence that the current findings could be associated with alcohol consumption, rather than preexisting neural alterations in binge drinkers prior to heavy alcohol use. A significant relationship with mean number of alcoholic beverages consumed/drinking day in the past 3 months was negatively related to reward outcome processing in lobule VIIA/crus I, providing further support for dose-dependent alcohol-related changes in brain functioning. Additionally, cerebellar brain response has been seen in other reward processing studies during both reward anticipation and feedback (Dichter et al., 2012, Koeneke et al., 2008, Nees et al., 2012). For example, cerebellar crus I activity was found during the outcome phase of a wheel-of-fortune game with chocolate bar rewards where individual preference for rewards was correlated with cerebellar crus I activity during reward receipt (Koeneke et al., 2008). Thus, cerebellar crus I activity may be an important region for examining reward outcome processing.
The current study employed the use of a decision-making task to determine alcohol's effects on adolescent reward processing, because animal and human studies suggest that reward-driven behavior (McMurray et al., 2014, Schindler et al., 2014) is altered after heavy alcohol consumption during adolescence. However, it is unclear whether reward-related response is hyper- or hypoactive in human AUDs (Beck et al., 2009, van Holst et al., 2014, Wrase et al., 2007). The present findings support reduced reward response in binge-drinking youth in the cerebellum, a brain region that has consistently been associated with volumetric (Anderson et al., 2010, Chanraud et al., 2007, Chanraud et al., 2010, De Bellis et al., 2005, Sullivan et al., 2000, Sullivan et al., 2010) and functional (Desmond et al., 2003, Jung et al., 2014, Pitel et al., 2013, Rogers et al., 2012, Sullivan et al., 2003) abnormalities in adults and youth with heavy alcohol consumption. Rodent models indicate that chronic ethanol intake induces apoptosis of both Purkinje and granule cells in the cerebellum (Oliveira et al., 2014), and reduces synaptic plasticity (Valenzuela et al., 2010). Human neuroimaging studies have found that cerebellar volume and connectivity in alcoholics are associated with motor (Sullivan et al., 2000) and cognitive (Chanraud et al., 2007, Chanraud et al., 2010, Jung et al., 2014) impairments. These relationships are explained by the cerebellum's role in classic motor functioning (Ito, 2006), but also its functional connections with executive control regions, such as the prefrontal cortex (Diamond, 2000, Kelly and Strick, 2003, Krienen and Buckner, 2009, Schmahmann and Pandya, 1997). Additionally, cerebellar damage results in cerebellar cognitive affective syndrome (Schmahmann and Sherman, 1998), characterized by emotional regulation deficits, implying connectivity with the limbic system (Heath et al., 1978, Schutter and van Honk, 2005). It is believed that cerebellar cognitive affective syndrome may, in part, account for cognitive and emotional deficits observed in alcoholics with cerebellar damage (Fitzpatrick et al., 2008). Thus, prefrontal and limbic connectivity with the cerebellum could explain why brain activity may be altered in heavy alcohol users in this region during tasks with high emotional salience, such as a reward-based decision-making task.
In terms of adolescent-specific drinking behavior, the current findings support previous reports – that a pattern of binge drinking during adolescence may be neurotoxic to the cerebellum. Not only has reduced volume been reported in alcoholics (Sullivan et al., 2000, Sullivan et al., 2010) and youth with AUDs (De Bellis et al., 2005), but a recent study also found reduced cerebellar volume in binge-drinking adolescents, related to the participants’ recent alcohol consumption (Lisdahl et al., 2013). The current study complements this finding by providing evidence for binge drinking-related effects on cerebellar reward response, also related to recent alcohol consumption. While past studies have reported binge drinking effects during adolescence on brain response during working memory (Squeglia et al., 2011), verbal encoding (Schweinsburg et al., 2010, Schweinsburg et al., 2011), and affective decision-making (Xiao et al., 2013), this is the first study to report effects on reward processing. It is possible that emotional salience of trials with reward receipt is reduced in value for binge drinkers, which could explain reduced cerebellar activity to reward and comparable response in this region regardless of whether feedback indicated reward presence or absence. This interpretation is supported by the finding that reduced response to reward in binge drinkers was localized to lobule VIIa/crus I, a posterior part of the cerebellum implicated more in cognitive and emotional functions based on its connectivity patterns, as opposed to motor functions that are localized to anterior portions of the cerebellum (Stoodley, 2012). It is damage to this posterior portion that has been reported to result in cognitive and emotional deficits (Stoodley and Schmahmann, 2010). These findings suggest an emotional component to cerebellar response during reward processing that is supported by the specific cerebellar region in which blunted reward response was observed in binge drinkers. Future studies will need to examine regional specificity of cerebellar alterations in activity and volume in heavy alcohol users to determine whether motor and/or cognitive/affective deficits would be expected.
Since hypoactive reward response has been reported in the ventral striatum of alcoholics in previous studies (Beck et al., 2009, Wrase et al., 2007), the current findings could also be explained by models that suggest changes in the cerebellum with its functional relationships to reward-related brain regions in addiction (Moulton et al., 2014). Reciprocal dopaminergic projections between the basal ganglia and the pontine nuclei provide connectivity to the cerebellar cortex (Bostan and Strick, 2010). This connectivity profile could explain a hypoactive pattern of cerebellar response to reward receipt in binge drinkers if this pathway is altered by heavy alcohol use. Additionally, crus I has been shown to be functionally connected to brain regions involved in emotional salience, such as the anterior insula and anterior cingulate cortex (Buckner et al., 2011), which provides further evidence for posterior cerebellar involvement with addiction (Moulton et al., 2014). The current findings may suggest that heavy alcohol use has reduced the salience or value of rewards in binge drinkers, which could lead to a path of increased reward-seeking behavior that may ultimately heighten vulnerability for the development of AUDs.
4.2. Strengths and limitations
The strengths and limitations of the present study merit discussion. First, by collecting data on participants at both a baseline and second study visit, we were able to examine group differences in alcohol-related effects on brain functioning by accounting for preexisting differences in patterns of brain activity in youth who have not yet engaged in heavy alcohol use. The present results can confirm with greater confidence that the current findings are more likely due to differences in alcohol consumption between the groups, rather than a premorbid difference in vulnerability toward alcohol use in these youth. In fact, the relationship observed between number of drinks consumed/drinking day in the 3 months leading up to the second study visit with cerebellar brain activity, suggests that, in fact, there may be a dose-response relationship between alcohol use and cerebellar reward processing in binge drinkers. Additionally, to our knowledge this is the first longitudinal study to report on the effects of adolescent binge drinking during reward processing. Given that multiple studies report aberrant reward processing in adult alcoholics (Beck et al., 2009, van Holst et al., 2014, Wrase et al., 2007), the current findings suggest that alcohol-induced reward-related alterations during neurodevelopment may already be taking place prior to the onset of an AUD.
Despite prospective methodological rigor, the size of the current sample was too small to examine sex-by-alcohol use interactions on brain functioning at the whole-brain level. However, we expect additional youth to emerge into binge drinking during the longitudinal study, and thus anticipate a larger sample will allow us to answer important questions related to sex interactions that have been reported in the adult alcoholism literature. Additionally, the current sample of adolescent binge drinkers reported use of other substances that may have limited detection of purely alcohol-related effects. A majority of the binge drinkers had used marijuana by the time of the second study visit, and half had used cigarettes. This is not surprising, as cigarette and marijuana use are highly co-morbid with alcohol use during adolescence (Moss et al., 2014, Palmer et al., 2009). Thus, it is difficult to examine binge drinkers who have not used any other substance but alcohol. However, given that none of the cigarette or marijuana use variables related to brain activity in the current study, it is more likely that the current effects are alcohol-related, especially considering the correlations found with drinking variables and BOLD response. Finally, while a relationship between alcohol use and cerebellar brain response was found at revisit, the possibility of other confounding variables that were not assessed (i.e. early life trauma, cumulative stressful life events), which could have increased between the baseline visit and revisit, or differed by group or sex, may have influenced the current findings, and could be assessed in future studies.
5. Conclusions
To our knowledge, this is the first study to report on adolescent binge drinkers and brain activity during reward processing, using a longitudinal design. The cerebellum has consistently been implicated in alcohol-related brain damage across studies of adults and adolescents. We contribute to this literature by showing that binge drinkers have reduced reward-related response in the left posterior cerebellum (lobule VIIa/crus I), a pattern that was not present prior to heavy alcohol use. Lobule VIIa/crus I of the posterior cerebellum is a region implicated in affective processing, cognitive functioning, as well as reward salience (Moulton et al., 2014, Stoodley, 2012). This suggests that response to reward presence may be blunted in binge drinkers due to alterations of pathways to limbic and/or reward systems, which could potentially drive further risky drinking behavior. Importantly, these findings may contribute to targeted early intervention strategies in adolescent binge drinkers, as it suggests that reward functioning may be altered in youth who have just begun to engage in heavy alcohol use.
Conflict of interest
All authors have made significant contributions to this work, and the manuscript has been read and approved by all authors. This manuscript contains original material, and has not been submitted, nor is it in press or published elsewhere in any form. The authors of this manuscript ensure that all work was completed with care to ensure scientific integrity of the research. No conflicts of interest are present.
Acknowledgements
This study was supported by the National Institute on Alcohol Abuse and Alcoholism (R01 AA017664 – Nagel; U01 – AA021691 – Nagel, and P60 AA010760 – PI: Crabbe). We would like to thank the research assistants and volunteers of the Developmental Brain Imaging Lab for their assistance with data collection.
Footnotes
Except for one male youth who completed the second visit after consuming four drinks on four occasions in a 90 day period before his revisit.
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.dcn.2015.06.004.
Appendix A. Supplementary data
The following are the supplementary data to this article:
Fig. S1.
Cerebellar brain activity in binge drinkers and controls, divided by sex. A) No significant group, sex, or group-by-sex interactions in cerebellar response at the baseline visit. B) Two-way ANOVAs with group and sex as between-subjects factors indicate main effects of group and sex, such that binge drinkers had reduced cerebellar response compared with controls, and males had reduced cerebellar response compared with females, in the Win vs. No Win contrast. This was driven by main effects of group and sex during Win vs. baseline BOLD response. *p > 0.05
References
- Anderson C.M., Rabi K., Lukas S.E., Teicher M.H. Cerebellar lingula size and experiential risk factors associated with high levels of alcohol and drug use in young adults. Cerebellum. 2010;9:198–209. doi: 10.1007/s12311-009-0141-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron A.R., Gluck M.A., Poldrack R.A. Long-term test–retest reliability of functional MRI in a classification learning task. Neuroimage. 2006;29:1000–1006. doi: 10.1016/j.neuroimage.2005.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A., Schlagenhauf F., Wustenberg T., Hein J., Kienast T., Kahnt T., Schmack K., Hagele C., Knutson B., Heinz A., Wrase J. Ventral striatal activation during reward anticipation correlates with impulsivity in alcoholics. Biol. Psychiatry. 2009;66:734–742. doi: 10.1016/j.biopsych.2009.04.035. [DOI] [PubMed] [Google Scholar]
- Bjork J.M., Knutson B., Hommer D.W. Incentive-elicited striatal activation in adolescent children of alcoholics. Addiction. 2008;103:1308–1319. doi: 10.1111/j.1360-0443.2008.02250.x. [DOI] [PubMed] [Google Scholar]
- Bjork J.M., Smith A.R., Hommer D.W. Striatal sensitivity to reward deliveries and omissions in substance dependent patients. Neuroimage. 2008;42:1609–1621. doi: 10.1016/j.neuroimage.2008.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork J.M., Smith A.R., Chen G., Hommer D.W. Adolescents, adults and rewards: comparing motivational neurocircuitry recruitment using fMRI. PLoS ONE. 2010;5:e11440. doi: 10.1371/journal.pone.0011440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork J.M., Smith A.R., Chen G., Hommer D.W. Mesolimbic recruitment by nondrug rewards in detoxified alcoholics: effort anticipation, reward anticipation, and reward delivery. Hum. Brain Mapp. 2012;33:2174–2188. doi: 10.1002/hbm.21351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore S.J. Imaging brain development: the adolescent brain. Neuroimage. 2012;61:397–406. doi: 10.1016/j.neuroimage.2011.11.080. [DOI] [PubMed] [Google Scholar]
- Bostan A.C., Strick P.L. The cerebellum and basal ganglia are interconnected. Neuropsychol. Rev. 2010;20:261–270. doi: 10.1007/s11065-010-9143-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S.A., Myers M.G., Mott M.A., Vik P.W. Correlates of success following treatment for adolescent substance abuse. Appl. Prev. Psychol. 1994;3:61–73. [Google Scholar]
- Brown S.A., Myers M.G., Lippke L., Tapert S.F., Stewart D.G., Vik P.W. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): a measure of adolescent alcohol and drug involvement. J. Stud. Alcohol Drugs. 1998;59:427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Krienen F.M., Castellanos A., Diaz J.C., Yeo B.T. The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:2322–2345. doi: 10.1152/jn.00339.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanraud S., Martelli C., Delain F., Kostogianni N., Douaud G., Aubin H.J., Reynaud M., Martinot J.L. Brain morphometry and cognitive performance in detoxified alcohol-dependents with preserved psychosocial functioning. Neuropsychopharmacology. 2007;32:429–438. doi: 10.1038/sj.npp.1301219. [DOI] [PubMed] [Google Scholar]
- Chanraud S., Pitel A.L., Rohlfing T., Pfefferbaum A., Sullivan E.V. Dual tasking and working memory in alcoholism: relation to frontocerebellar circuitry. Neuropsychopharmacology. 2010;35:1868–1878. doi: 10.1038/npp.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corp., I., Released . IBM Corp.; Armonk, NY: 2011. IBM SPSS Statistics for Windows. Version 20.0. [Google Scholar]
- Cox R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cox R.W., Jesmanowicz A. Real-time 3D image registration for functional MRI. Magn. Reson. Med. 1999;42:1014–1018. doi: 10.1002/(sici)1522-2594(199912)42:6<1014::aid-mrm4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Cservenka A., Herting M.M., Nagel B.J. Atypical frontal lobe activity during verbal working memory in youth with a family history of alcoholism. Drug Alcohol Depend. 2012;123:98–104. doi: 10.1016/j.drugalcdep.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A., Herting M.M., Seghete K.L., Hudson K.A., Nagel B.J. High and low sensation seeking adolescents show distinct patterns of brain activity during reward processing. Neuroimage. 2013;66C:184–193. doi: 10.1016/j.neuroimage.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A., Nagel B.J. Risky decision-making: an fMRI study of youth at high risk for alcoholism. Alcohol. Clin. Exp. Res. 2012;36:604–615. doi: 10.1111/j.1530-0277.2011.01650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A., Casimo K., Fair D.A., Nagel B.J. Resting state functional connectivity of the nucleus accumbens in youth with a family history of alcoholism. Psychiatry Res. 2014;221:210–219. doi: 10.1016/j.pscychresns.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A., Fair D.A., Nagel B.J. Emotional processing and brain activity in youth at high risk for alcoholism. Alcohol. Clin. Exp. Res. 2014;38:1912–1923. doi: 10.1111/acer.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis M.D., Narasimhan A., Thatcher D.L., Keshavan M.S., Soloff P., Clark D.B. Prefrontal cortex, thalamus, and cerebellar volumes in adolescents and young adults with adolescent-onset alcohol use disorders and comorbid mental disorders. Alcohol. Clin. Exp. Res. 2005;29:1590–1600. doi: 10.1097/01.alc.0000179368.87886.76. [DOI] [PubMed] [Google Scholar]
- Deas D. Adolescent substance abuse and psychiatric comorbidities. J. Clin. Psychiatry. 2006;67(Suppl. 7):18–23. [PubMed] [Google Scholar]
- Desmond J.E., Chen S.H., DeRosa E., Pryor M.R., Pfefferbaum A., Sullivan E.V. Increased frontocerebellar activation in alcoholics during verbal working memory: an fMRI study. Neuroimage. 2003;19:1510–1520. doi: 10.1016/s1053-8119(03)00102-2. [DOI] [PubMed] [Google Scholar]
- Diamond A. Close interrelation of motor development and cognitive development and of the cerebellum and prefrontal cortex. Child Dev. 2000;71:44–56. doi: 10.1111/1467-8624.00117. [DOI] [PubMed] [Google Scholar]
- Dichter G.S., Kozink R.V., McClernon F.J., Smoski M.J. Remitted major depression is characterized by reward network hyperactivation during reward anticipation and hypoactivation during reward outcomes. J. Affect Disord. 2012;136:1126–1134. doi: 10.1016/j.jad.2011.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton D.K., Kann L., Kinchen S., Shanklin S., Flint K.H., Hawkins J., Harris W.A., Lowry R., McManus T., Chyen D., Whittle L., Lim C., Wechsler H., Centers for Disease, C., Prevention Youth risk behavior surveillance – United States 2011. MMWR Surveill Summ. 2012;61:1–162. [PubMed] [Google Scholar]
- Ernst M., Nelson E.E., McClure E.B., Monk C.S., Munson S., Eshel N., Zarahn E., Leibenluft E., Zametkin A., Towbin K., Blair J., Charney D., Pine D.S. Choice selection and reward anticipation: an fMRI study. Neuropsychologia. 2004;42:1585–1597. doi: 10.1016/j.neuropsychologia.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick L.E., Jackson M., Crowe S.F. The relationship between alcoholic cerebellar degeneration and cognitive and emotional functioning. Neurosci. Biobehav. Rev. 2008;32:466–485. doi: 10.1016/j.neubiorev.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Fleiss J.L. Vol. XXX. John Wiley & Sons; New York: 1986. (The Design and Analysis of Clinical Experiments). [Google Scholar]
- Forman S.D., Cohen J.D., Fitzgerald M., Eddy W.F., Mintun M.A., Noll D.C. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn. Reson. Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Galvan A. Adolescent development of the reward system. Front Hum. Neurosci. 2010;4:6. doi: 10.3389/neuro.09.006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddings A.L., Mills K.L., Clasen L.S., Giedd J.N., Viner R.M., Blakemore S.J. The influence of puberty on subcortical brain development. Neuroimage. 2014;88:242–251. doi: 10.1016/j.neuroimage.2013.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N., Giedd J.N., Lusk L., Hayashi K.M., Greenstein D., Vaituzis A.C., Nugent T.F., 3rd, Herman D.H., Clasen L.S., Toga A.W., Rapoport J.L., Thompson P.M. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. U. S. A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath R.G., Dempesy C.W., Fontana C.J., Myers W.A. Cerebellar stimulation: effects on septal region, hippocampus, and amygdala of cats and rats. Biol. Psychiatry. 1978;13:501–529. [PubMed] [Google Scholar]
- Hollingshead A.B. vol. XXX. New Haven, CT; 1957. (Two Factor Index of Social Position). [Google Scholar]
- Ito M. Cerebellar circuitry as a neuronal machine. Prog. Neurobiol. 2006;78:272–303. doi: 10.1016/j.pneurobio.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Jacobus J., Squeglia L.M., Bava S., Tapert S.F. White matter characterization of adolescent binge drinking with and without co-occurring marijuana use: a 3-year investigation. Psychiatry Res. 2013;214:374–381. doi: 10.1016/j.pscychresns.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J., Tapert S.F. Neurotoxic effects of alcohol in adolescence. Annu. Rev. Clin. Psychol. 2013;9:703–721. doi: 10.1146/annurev-clinpsy-050212-185610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston L.D., O’Malley P.M., Miech R.A., Bachman J.G., Schulenberg J.E. Institute for Social Research, The University of Michigan; Ann Arbor: 2014. Monitoring the Future: National Results on Adolescent Drug Use: 1975–2013: Overview, Key Findings on Adolescent Drug Use. [Google Scholar]
- Jonasson L.S., Axelsson J., Riklund K., Braver T.S., Ogren M., Backman L., Nyberg L. Dopamine release in nucleus accumbens during rewarded task switching measured by [(1)(1)C]raclopride. Neuroimage. 2014;99:357–364. doi: 10.1016/j.neuroimage.2014.05.047. [DOI] [PubMed] [Google Scholar]
- Jung Y.C., Schulte T., Muller-Oehring E.M., Namkoong K., Pfefferbaum A., Sullivan E.V. Compromised frontocerebellar circuitry contributes to nonplanning impulsivity in recovering alcoholics. Psychopharmacology (Berl) 2014;231:4443–4453. doi: 10.1007/s00213-014-3594-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalsbeek A., Voorn P., Buijs R.M., Pool C.W., Uylings H.B. Development of the dopaminergic innervation in the prefrontal cortex of the rat. J. Comp. Neurol. 1988;269:58–72. doi: 10.1002/cne.902690105. [DOI] [PubMed] [Google Scholar]
- Kamarajan C., Rangaswamy M., Tang Y., Chorlian D.B., Pandey A.K., Roopesh B.N., Manz N., Saunders R., Stimus A.T., Porjesz B. Dysfunctional reward processing in male alcoholics: an ERP study during a gambling task. J. Psychiatry Res. 2010;44:576–590. doi: 10.1016/j.jpsychires.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R.M., Strick P.L. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J. Neurosci. 2003;23:8432–8444. doi: 10.1523/JNEUROSCI.23-23-08432.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeneke S., Pedroni A.F., Dieckmann A., Bosch V., Jancke L. Individual preferences modulate incentive values: evidence from functional MRI. Behav. Brain Funct. 2008;4:55. doi: 10.1186/1744-9081-4-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koepp M.J., Gunn R.N., Lawrence A.D., Cunningham V.J., Dagher A., Jones T., Brooks D.J., Bench C.J., Grasby P.M. Evidence for striatal dopamine release during a video game. Nature. 1998;393:266–268. doi: 10.1038/30498. [DOI] [PubMed] [Google Scholar]
- Kovacs M. The Children's Depression Inventory (CDI) Psychopharmacol. Bull. 1985;21:995–998. [PubMed] [Google Scholar]
- Krienen F.M., Buckner R.L. Segregated fronto-cerebellar circuits revealed by intrinsic functional connectivity. Cereb. Cortex. 2009;19:2485–2497. doi: 10.1093/cercor/bhp135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisdahl K.M., Thayer R., Squeglia L.M., McQueeny T.M., Tapert S.F. Recent binge drinking predicts smaller cerebellar volumes in adolescents. Psychiatry Res. 2013;211:17–23. doi: 10.1016/j.pscychresns.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas C.P., Zhang H., Fisher P.W., Shaffer D., Regier D.A., Narrow W.E., Bourdon K., Dulcan M.K., Canino G., Rubio-Stipec M., Lahey B.B., Friman P. The DISC Predictive Scales (DPS): efficiently screening for diagnoses. J. Am. Acad. Child Adolesc. Psychiatry. 2001;40:443–449. doi: 10.1097/00004583-200104000-00013. [DOI] [PubMed] [Google Scholar]
- Mackiewicz Seghete K.L., Cservenka A., Herting M.M., Nagel B.J. Atypical spatial working memory and task-general brain activity in adolescents with a family history of alcoholism. Alcohol. Clin. Exp. Res. 2013;37:390–398. doi: 10.1111/j.1530-0277.2012.01948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure S.M., York M.K., Montague P.R. The neural substrates of reward processing in humans: the modern role of FMRI. Neuroscientist. 2004;10:260–268. doi: 10.1177/1073858404263526. [DOI] [PubMed] [Google Scholar]
- McMurray M.S., Amodeo L.R., Roitman J.D. Effects of voluntary alcohol intake on risk preference and behavioral flexibility during rat adolescence. PLOS ONE. 2014;9:e100697. doi: 10.1371/journal.pone.0100697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss H.B., Chen C.M., Yi H.Y. Early adolescent patterns of alcohol, cigarettes, and marijuana polysubstance use and young adult substance use outcomes in a nationally representative sample. Drug Alcohol Depend. 2014;136:51–62. doi: 10.1016/j.drugalcdep.2013.12.011. [DOI] [PubMed] [Google Scholar]
- Moulton E.A., Elman I., Becerra L.R., Goldstein R.Z., Borsook D. The cerebellum and addiction: insights gained from neuroimaging research. Addict. Biol. 2014;19:317–331. doi: 10.1111/adb.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nees F., Vollstadt-Klein S., Fauth-Buhler M., Steiner S., Mann K., Poustka L., Banaschewski T., Buchel C., Conrod P.J., Garavan H., Heinz A., Ittermann B., Artiges E., Paus T., Pausova Z., Rietschel M., Smolka M.N., Struve M., Loth E., Schumann G., Flor H. A target sample of adolescents and reward processing: same neural and behavioral correlates engaged in common paradigms? Exp. Brain Res. 2012;223:429–439. doi: 10.1007/s00221-012-3272-8. [DOI] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Oliveira S.A., Chuffa L.G., Fioruci-Fontanelli B.A., Neto F.S., Novais P.C., Tirapelli L.F., Oishi J.C., Takase L.F., Stefanini M.A., Martinez M., Martinez F.E. Apoptosis of purkinje and granular cells of the cerebellum following chronic ethanol intake. Cerebellum. 2014;13:728–738. doi: 10.1007/s12311-014-0591-2. [DOI] [PubMed] [Google Scholar]
- Palmer R.H., Young S.E., Hopfer C.J., Corley R.P., Stallings M.C., Crowley T.J., Hewitt J.K. Developmental epidemiology of drug use and abuse in adolescence and young adulthood: Evidence of generalized risk. Drug Alcohol Depend. 2009;102:78–87. doi: 10.1016/j.drugalcdep.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M., Boix J., Felipo V., Guerri C. Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. J. Neurochem. 2009;108:920–931. doi: 10.1111/j.1471-4159.2008.05835.x. [DOI] [PubMed] [Google Scholar]
- Paus T. Mapping brain maturation and cognitive development during adolescence. Trends Cogn. Sci. 2005;9:60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Pautassi R.M., Myers M., Spear L.P., Molina J.C., Spear N.E. Adolescent but not adult rats exhibit ethanol-mediated appetitive second-order conditioning. Alcohol Clin. Exp. Res. 2008;32:2016–2027. doi: 10.1111/j.1530-0277.2008.00789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpot R.M., Wecker L., Kirstein C.L. Repeated ethanol exposure during adolescence alters the developmental trajectory of dopaminergic output from the nucleus accumbens septi. Int. J. Dev. Neurosci. 2009;27:805–815. doi: 10.1016/j.ijdevneu.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Pitel A.L., Chanraud S., Muller-Oehring E.M., Pfefferbaum A., Sullivan E.V. Modulation of limbic-cerebellar functional connectivity enables alcoholics to recognize who is who. Brain Struct. Funct. 2013;218:683–695. doi: 10.1007/s00429-012-0421-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice J.P., Reich T., Bucholz K.K., Neuman R.J., Fishman R., Rochberg N., Hesselbrock V.M., Nurnberger J.I., Jr., Schuckit M.A., Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcohol. Clin. Exp. Res. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Ristuccia R.C., Spear L.P. Adolescent and adult heart rate responses to self-administered ethanol. Alcohol Clin. Exp. Res. 2008;32:1807–1815. doi: 10.1111/j.1530-0277.2008.00752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers B.P., Parks M.H., Nickel M.K., Katwal S.B., Martin P.R. Reduced fronto-cerebellar functional connectivity in chronic alcoholic patients. Alcohol. Clin. Exp. Res. 2012;36:294–301. doi: 10.1111/j.1530-0277.2011.01614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg D.R., Lewis D.A. Changes in the dopaminergic innervation of monkey prefrontal cortex during late postnatal development: a tyrosine hydroxylase immunohistochemical study. Biol. Psychiatry. 1994;36:272–277. doi: 10.1016/0006-3223(94)90610-6. [DOI] [PubMed] [Google Scholar]
- Schindler A.G., Tsutsui K.T., Clark J.J. Chronic alcohol intake during adolescence, but not adulthood, promotes persistent deficits in risk-based decision making. Alcohol Clin. Exp. Res. 2014;38:1622–1629. doi: 10.1111/acer.12404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann J.D., Pandya D.N. The cerebrocerebellar system. Int. Rev. Neurobiol. 1997;41:31–60. doi: 10.1016/s0074-7742(08)60346-3. [DOI] [PubMed] [Google Scholar]
- Schmahmann J.D., Sherman J.C. The cerebellar cognitive affective syndrome. Brain. 1998;121(Pt 4):561–579. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- Schultz W. Multiple reward signals in the brain. Nat. Rev. Neurosci. 2000;1:199–207. doi: 10.1038/35044563. [DOI] [PubMed] [Google Scholar]
- Schutter D.J., van Honk J. The cerebellum on the rise in human emotion. Cerebellum. 2005;4:290–294. doi: 10.1080/14734220500348584. [DOI] [PubMed] [Google Scholar]
- Schweinsburg A.D., Nagel B.J., Schweinsburg B.C., Park A., Theilmann R.J., Tapert S.F. Abstinent adolescent marijuana users show altered fMRI response during spatial working memory. Psychiatry Res. 2008;163:40–51. doi: 10.1016/j.pscychresns.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg A.D., McQueeny T., Nagel B.J., Eyler L.T., Tapert S.F. A preliminary study of functional magnetic resonance imaging response during verbal encoding among adolescent binge drinkers. Alcohol. 2010;44:111–117. doi: 10.1016/j.alcohol.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg A.D., Schweinsburg B.C., Nagel B.J., Eyler L.T., Tapert S.F. Neural correlates of verbal learning in adolescent alcohol and marijuana users. Addiction. 2011;106:564–573. doi: 10.1111/j.1360-0443.2010.03197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P., Bzowej N.H., Guan H.C., Bergeron C., Becker L.E., Reynolds G.P., Bird E.D., Riederer P., Jellinger K., Watanabe S. Human brain dopamine receptors in children and aging adults. Synapse. 1987;1:399–404. doi: 10.1002/syn.890010503. [DOI] [PubMed] [Google Scholar]
- Sobell L.C., Sobell M.B. Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Litten R.Z., Allen J., editors. vol. XXX. Humana Press; New Jersey: 1992. pp. 41–72. (Measuring alcohol consumption: Psychological and biological methods). [Google Scholar]
- Sobell M.B., Sobell L.C., Klajner F., Pavan D. The reliability of a timeline method for assessing normal drinker college students’ recent drinking history: utility for alcohol research. Addict. Behav. 1986;11:149–161. doi: 10.1016/0306-4603(86)90040-7. [DOI] [PubMed] [Google Scholar]
- Spielberger C.D., Edwards C.D., Lushene R.E., Montuori J., Platzek D. Consulting Psychologists Press; Palo Alto, CA: 1973. Manual for The State-Trait Anxiety Inventory for Children. [Google Scholar]
- Squeglia L.M., Schweinsburg A.D., Pulido C., Tapert S.F. Adolescent binge drinking linked to abnormal spatial working memory brain activation: differential gender effects. Alcohol Clin. Exp. Res. 2011;35:1831–1841. doi: 10.1111/j.1530-0277.2011.01527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia L.M., Sorg S.F., Schweinsburg A.D., Wetherill R.R., Pulido C., Tapert S.F. Binge drinking differentially affects adolescent male and female brain morphometry. Psychopharmacology (Berl) 2012;220:529–539. doi: 10.1007/s00213-011-2500-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley C.J., Schmahmann J.D. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010;46:831–844. doi: 10.1016/j.cortex.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley C.J. The cerebellum and cognition: evidence from functional imaging studies. Cerebellum. 2012;11:352–365. doi: 10.1007/s12311-011-0260-7. [DOI] [PubMed] [Google Scholar]
- Sullivan E.V., Deshmukh A., Desmond J.E., Lim K.O., Pfefferbaum A. Cerebellar volume decline in normal aging, alcoholism, and Korsakoff's syndrome: relation to ataxia. Neuropsychology. 2000;14:341–352. doi: 10.1037//0894-4105.14.3.341. [DOI] [PubMed] [Google Scholar]
- Sullivan E.V., Harding A.J., Pentney R., Dlugos C., Martin P.R., Parks M.H., Desmond J.E., Chen S.H., Pryor M.R., De Rosa E., Pfefferbaum A. Disruption of frontocerebellar circuitry and function in alcoholism. Alcohol Clin. Exp. Res. 2003;27:301–309. doi: 10.1097/01.ALC.0000052584.05305.98. [DOI] [PubMed] [Google Scholar]
- Sullivan E.V., Rohlfing T., Pfefferbaum A. Pontocerebellar volume deficits and ataxia in alcoholic men and women: no evidence for “telescoping”. Psychopharmacology (Berl) 2010;208:279–290. doi: 10.1007/s00213-009-1729-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J., Tournoux P. vol. XXX. Thieme, New York; 1988. (Coplanar stereotaxic atlas of the human brain. Three-dimensional proportional system: an approach to cerebral imaging.). [Google Scholar]
- Taylor S.J., Whincup P.H., Hindmarsh P.C., Lampe F., Odoki K., Cook D.G. Performance of a new pubertal self-assessment questionnaire: a preliminary study. Paediatr. Perinat. Epidemiol. 2001;15:88–94. doi: 10.1046/j.1365-3016.2001.00317.x. [DOI] [PubMed] [Google Scholar]
- Tunbridge E.M., Weickert C.S., Kleinman J.E., Herman M.M., Chen J., Kolachana B.S., Harrison P.J., Weinberger D.R. Catechol-o-methyltransferase enzyme activity and protein expression in human prefrontal cortex across the postnatal lifespan. Cereb Cortex. 2007;17:1206–1212. doi: 10.1093/cercor/bhl032. [DOI] [PubMed] [Google Scholar]
- Valenzuela C.F., Lindquist B., Zamudio-Bulcock P.A. A review of synaptic plasticity at Purkinje neurons with a focus on ethanol-induced cerebellar dysfunction. Int. Rev. Neurobiol. 2010;91:339–372. doi: 10.1016/S0074-7742(10)91011-8. [DOI] [PubMed] [Google Scholar]
- van Holst R.J., Clark L., Veltman D.J., van den Brink W., Goudriaan A.E. Enhanced striatal responses during expectancy coding in alcohol dependence. Drug Alcohol Depend. 2014;142:204–208. doi: 10.1016/j.drugalcdep.2014.06.019. [DOI] [PubMed] [Google Scholar]
- Van Leijenhorst L., Gunther Moor B., Op de Macks Z.A., Rombouts S.A., Westenberg P.M., Crone E.A. Adolescent risky decision-making: neurocognitive development of reward and control regions. Neuroimage. 2010;51:345–355. doi: 10.1016/j.neuroimage.2010.02.038. [DOI] [PubMed] [Google Scholar]
- Wechsler D. vol. XXX. Psychological Corporation; San Antonio, TX: 1999. (Wechsler Abbreviated Scale of Intelligence). [Google Scholar]
- Wrase J., Schlagenhauf F., Kienast T., Wustenberg T., Bermpohl F., Kahnt T., Beck A., Strohle A., Juckel G., Knutson B., Heinz A. Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. Neuroimage. 2007;35:787–794. doi: 10.1016/j.neuroimage.2006.11.043. [DOI] [PubMed] [Google Scholar]
- Xiao L., Bechara A., Gong Q., Huang X., Li X., Xue G., Wong S., Lu Z.L., Palmer P., Wei Y., Jia Y., Johnson C.A. Abnormal Affective decision making revealed in adolescent binge drinkers using a functional magnetic resonance imaging study. Psychol. Addict. Behav. 2013;27:443–454. doi: 10.1037/a0027892. [DOI] [PubMed] [Google Scholar]