Highlights
-
•
Adolescents self-reported substance use yearly from ages 10 to 16.
-
•
Nucleus accumbens seed-based functional connectivity measured at age 16.
-
•
Earlier use predicts stronger connectivity with right frontoparietal network.
Keywords: Adolescence, fMRI, Striatum, Cognitive regulation, Resting state
Abstract
Background
Early adolescent onset of substance use is a robust predictor of future substance use disorders. We examined the relation between age of substance use initiation and resting state functional connectivity (RSFC) of the core reward processing (nucleus accumbens; NAcc) to cognitive control (prefrontal cortex; PFC) brain networks.
Method
Adolescents in a longitudinal study of Mexican-origin youth reported their substance use annually from ages 10 to 16 years. At age 16, 69 adolescents participated in a resting state functional magnetic resonance imaging scan. Seed-based correlational analyses were conducted using regions of interest in bilateral NAcc.
Results
The earlier that adolescents initiated substance use, the stronger the connectivity between bilateral NAcc and right dorsolateral PFC, right dorsomedial PFC, right pre-supplementary motor area, right inferior parietal lobule, and left medial temporal gyrus.
Discussion
The regions that demonstrated significant positive linear relationships between the number of adolescent years using substances and connectivity with NAcc are nodes in the right frontoparietal network, which is central to cognitive control. The coupling of reward and cognitive control networks may be a mechanism through which earlier onset of substance use is related to brain function over time, a trajectory that may be implicated in subsequent substance use disorders.
1. Introduction
Adolescence is a period of increased health-risk behavior, including substance use. Adolescent substance use is common and increases in prevalence as adolescents move through high school. National estimates indicate that 20–30% of adolescents have used alcohol or other drugs by 9th grade, and this usage increases to 50–70% by 12th grade (Johnson et al., 2014). Although some substance use in adolescence is considered within the range of normative behavior, adolescents who experiment with substance use at subclinical levels have been shown to exhibit more externalizing behaviors (Ernst et al., 2006a) and have poorer mental health and academic outcomes than their abstaining peers (Tucker et al., 2006). Substance use early in adolescence confers heightened risk for not only substance use disorders but also other mental illnesses in later adolescence and adulthood (Brook et al., 2002). Every year older that adolescents get before they initiate substance use, is thought to decrease the odds of substance dependence later in life by 5–15% (DuRant et al., 1999, Grant and Dawson, 1997, Grant and Dawson, 1998). Early substance use additionally predicts other types of health risk behaviors during adolescence, such as reckless driving and participation in violent or criminal activities (Grant et al., 2001, Hingson and Zha, 2009), rendering it a critical health behavior, whose underlying mechanisms warrant further investigation.
Identification of the neurobiological components associated with substance use behaviors allows for a richer understanding of the neural underpinnings of developing substance use problems that can be used to inform treatment targets, whether behavioral and/or pharmacological. Resting state functional connectivity (RSFC) analysis is used to quantify the intrinsic connections between brain regions, independent of task-specific demands, context, and participant engagement (Biswal et al., 1995). The connections between brain regions measured by RSFC may be a function of direct communication via structural connections, association with a third region, and/or long-term patterns of coordinated activity engaged during certain behaviors or psychological processes. Functional connectivity can be positive or negative, reflecting the direction of correlation in regional activity patterns (Hulvershorn et al., 2014). The type of connectivity patterns observed indicates the nature of the functional relationship between these regions. Activity of functionally related and structurally connected networks of brain regions are strongly and positively correlated at rest (Damoiseaux et al., 2006, Yeo et al., 2011), whereas networks with opposing functions demonstrate strong negative correlations (Fox et al., 2005). From childhood to adulthood, negative connectivity increases overall as neural regions become more specialized and as the regulatory influence of regions such as the prefrontal cortex (PFC) gains more control (Fox et al., 2005, Stevens et al., 2009). The present study investigates how variations in connectivity patterns in the midst of these developmental shifts relate to adolescent substance use behavior.
Recent work has documented age-related shifts in nucleus accumbens (NAcc) connectivity, suggesting that positive connectivity with anterior cingulate cortex (ACC) and anterior insula peaks during adolescence, then declines into adulthood (Porter et al., 2015). These patterns relate to conceptual frameworks regarding the role of neurodevelopment in shaping adolescents’ motivated behavior. The triadic model (Ernst et al., 2006b, Ernst and Fudge, 2009) hypothesizes that motivated behavior results from the balance between three influences: (1) reward drive, mediated by the NAcc, a region within the striatum, (2) harm avoidance, mediated by the amygdala, and (3) regulation of these systems by the PFC. Research also points to the contribution of salience, emotional or cognitive, mediated by the insula and ACC, in shaping motivated behavior (Seeley et al., 2007). Different patterns of functional connectivity between and functional specialization among regions implicated in reward drive (e.g., NAcc), avoidance (e.g. amygdala), salience (e.g. insula and ACC), and the cognitive control of the above (e.g., PFC) may therefore differentially help or hinder adolescents’ ability to regulate their substance use behaviors.
Individual differences in NAcc functional connectivity have previously been associated with risk for substance use problems. In a RSFC study examining family history as a risk factor for later substance abuse, adolescents with no family history of alcoholism were found to have significant negative correlations between NAcc and ventrolateral PFC (vlPFC), suggesting more segregation and functional calibration between these reward-processing and regulatory regions, respectively, whereas adolescents with a family history of alcoholism demonstrated this relationship to a much lesser extent (Cservenka et al., 2014). Because a family history of substance dependence is a known risk factor for substance use problems (Merikangas et al., 1998), these findings suggest that a lack of negative connectivity (i.e., a positive or zero correlation) between NAcc and regions of PFC during adolescence may signal risk for later addiction.
Adolescent substance use occurs during a period in which the brain undergoes dramatic maturation and refinement of key neural pathways, particularly in the structural connections between the PFC and subcortical circuits (Asato et al., 2010). These changes are widely thought to contribute to the onset of substance use behavior in adolescence (Spear, 2000, Steinberg, 2007, Casey and Jones, 2010), and may make the development of the adolescent brain all the more vulnerable to the negative effects of substance use (Lubman et al., 2007). A number of neuroimaging studies have documented differences in activation in both striatal, including NAcc, and PFC regions both preceding and following onset of heavy substance use (Clark et al., 2013, Norman et al., 2011, Tapert et al., 2007; Schweinsburg et al., 2005, Squeglia et al., 2009).
Behavioral tendencies previously found to confer risk for substance use behavior, such as high levels of sensation seeking (SS) (Zuckerman and Link, 1968) may shed further light on how neural connectivity patterns relate to substance use behavior. Increased SS has been shown to mediate the relation between earlier pubertal development and increased substance use behavior across adolescence (Martin et al., 2002). In adolescents with a family history of alcoholism, task-related functional connectivity between NAcc and both supplementary sensorimotor area and precuneus was found to mediate the positive relationship between sensation seeking and alcohol consumption (Weiland et al., 2013). This suggests that increasing substance use rates in adolescence may result from increased exploratory tendencies, driven by an increase in the optimal level of arousal that adolescents require to feel motivated and balanced (Ernst et al., 2006b, Ernst and Fudge, 2009).
While the NAcc is of particular interest in understanding the mechanisms underlying adolescent substance use, other regions, particularly the amygdala and PFC have also been demonstrated to undergo changes in functional connectivity during adolescence. Across adolescence, negative connectivity has been found to increase between dorsolateral PFC (dlPFC), a region within networks especially active during focused attention and medial PFC, a region within the default mode network, which is especially active at rest (Chai et al., 2014). Connectivity between amygdala and medial PFC has also been shown to switch from positive to negative during adolescence (Gee et al., 2013).
The goal of the present study was to examine the interrelations among adolescent substance use, NAcc-based RSFC, and sensation seeking. We conducted the current study with a sub-sample of Mexican-origin adolescent participants drawn from a multigenerational longitudinal study of the emergence of substance use and subsequent addiction problems. Latinos are a rapidly growing segment of the population, but are largely underrepresented in psychiatric and neuroscience research (Arnett, 2008, Chiao, 2009), despite evidence for higher rates of substance use among Latino adolescents (Johnson et al., 2014). Nonetheless, the racial and cultural homogeneity of the current Mexican-origin sample provides an inherent control for the influence of race and ethnicity and provides data on an understudied ethnic group while producing novel results that should be generalizable to and replicable within other populations. Three aims guided the current study: (1) to investigate the relation between age of substance use onset and NAcc-based RSFC, (2) to examine whether the relation between SS and age of substance use onset relates to and potentially mediates any relation between NAcc-based RSFC and age of substance use onset (3) to examine the specificity of the role of NAcc in the above analyses by also exploring amygdala-, and dlPFC-based RSFC.
2. Materials and method
2.1. Participants
Participants were 73 Mexican-origin adolescents (40 female, MAge = 16.26 years, SD = 0.50) enrolled in a functional neuroimaging sub-study of the California Families Project (CFP), a 10-year, prospective, longitudinal study of risk for and resilience to substance use problems. Participants in the main CFP study included 674 single and two-parent families of Mexican origin with a fifth grade child (MAge = 10.85, 50.2% female) who were drawn at random from school rosters of students during the 2006–2007 and 2007–2008 school years. Participants in the parent study were ranked based on the number of items endorsed on the two substance use scales at age 15. Participants for the neuroimaging study were randomly drawn from the top (N = 37) and bottom (N = 36) third of this distribution, excluding any adolescents taking psychotropic medications. Four adolescents were excluded from analyses due to excessive movement in the scanner, resulting in a sample of 69 youths for all reported analyses.
2.2. Measures
2.2.1. Substance use
Participants reported their history of substance use using the Alcohol, Tobacco, and Other Drugs (ATOD) survey (Elliott et al., 1982) at each annual CFP assessment from ages 10–16, as well as on the day of the MRI visit prior to turning 17. This measure, chosen for its sensitivity to low to moderate levels of use, consists of 26 items about participants’ respective use of cigarettes, beer, wine or wine coolers, hard liquor, marijuana, inhalants, and other drugs as well as their experience with binge drinking, being drunk on alcohol, or being high on drugs. For each of those substances or experiences, they were asked, “have you ever…,” and if yes, then asked “at what age did you first…,” and “in the last 3 months, how many times have you…” Finally, on the day of the MRI visit, an Oralert oral saliva drug test was used to assess the presence of substances within the preceding few days (Transmetron, Salt Lake City, Utah). Participants who tested positive were asked if they were currently under the influence of the drug. If they reported no, they continued with the scan (100%). Participants’ age of substance use onset, as shown in Table 1, was determined based on when they first reported having used any substance, or presence of substance was detected in saliva. This number was subtracted from 17, participants’ maximum age in years by the end of MRI data collection, to calculate years since substance use onset. Years since substance use onset ranged from 0 = never reported having used substances, no substances present at MRI visit to 7 = reported having used substances for the first time at age 10. Frequency of use in the 3 months preceding the scan was scored on a 0–4 scale (0 = no use, 1 = less than once per week, 2 = once per week, 3 = two or three times per week, 4 = almost every day). Participants also completed the substance use disorder section of the Diagnostic Interview Schedule for Children-IV (DISC, Shaffer et al., 2000) at each of the seven annual visits to determine lifetime diagnosis for substance abuse and dependence. Four adolescents met criteria for a diagnosis of alcohol abuse. Two met criteria for a diagnosis of alcohol dependence. One met criteria for marijuana abuse, and one met criteria for marijuana dependence. Finally, one adolescent met criteria for both marijuana and alcohol abuse.
Table 1.
Substance use type and frequency by years since substance use onset and gender (N = 69, MAge = 16.2 years).
| Years since substance use onset (0 = No substance use; 1 = Onset at age 16; 7 = Onset at age 10) | Any Substance |
Alcohol |
Marijuana |
Cigarettes |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | F | T | Freq | M | F | T | Freq | M | F | T | Freq | M | F | T | Freq | |
| 0 | 6 | 13 | 19 | 0 | 11 | 16 | 27 | 0 | 13 | 21 | 34 | 0 | 20 | 27 | 47 | 0 |
| 1 | 8 | 3 | 11 | .55 | 6 | 2 | 8 | .62 | 6 | 4 | 10 | .50 | 4 | 2 | 6 | 0 |
| 2 | 1 | 2 | 3 | .33 | 1 | 2 | 3 | .33 | 1 | 1 | 2 | .50 | 2 | 2 | 4 | 0 |
| 3 | 4 | 7 | 11 | .63 | 4 | 7 | 11 | .70 | 4 | 9 | 13 | 1.15 | 0 | 3 | 3 | 0 |
| 4 | 3 | 6 | 9 | .89 | 3 | 5 | 8 | .75 | 5 | 1 | 6 | 1.33 | 3 | 1 | 4 | 1.00 |
| 5 | 3 | 3 | 6 | 1.8 | 2 | 3 | 5 | .60 | 3 | 0 | 3 | .33 | 2 | 1 | 3 | 1.33 |
| 6 | 5 | 2 | 7 | .57 | 4 | 2 | 6 | .25 | 1 | 0 | 1 | 4.00 | 0 | 0 | 0 | .0 |
| 7 | 3 | 0 | 3 | 3 | 3 | 0 | 3 | 2.67 | 0 | 0 | 0 | 0 | 2 | 0 | 2 | .50 |
| Total | 33 | 36 | 69 | .67 | 22 | 26 | 69 | .46 | 33 | 36 | 69 | .49 | 33 | 36 | 69 | .13 |
M = male, F = female, T = total (male and female), Freq = mean age 16 frequency of use within last 3 months for males and females.
2.2.2. Demographic characteristics and psychiatric symptoms
All adolescents in the CFP completed the Woodcock-Johnson III IQ test (Woodcock et al., 2001) on the first visit in fifth grade. Parents of adolescents reported education, income and household roster at the first visit, which was then used to calculate socioeconomic status (SES, See Supplementary Materials). Participants self-reported on their pubertal development using the Pubertal Development Scale (Petersen et al., 1983). The scale consisted of 6 items total (4 items for both boys and girls, then 2 items per gender that are specific to their own gender development). Tanner stage at age 16 was calculated based on the average of these 6 items from the visit in 10th grade. Descriptive statistics are summarized in Table 2. Participants self-reported symptoms of attention deficit hyperactivity disorder (ADHD), anxiety, depression, conduct disorder, and oppositional defiance, based on the DISC, at each of visit. Two participants in the sample met criteria for conduct disorder in 10th grade. No other participant met criteria for any other psychiatric disorder (see Supplementary Materials for descriptive statistics of each psychiatric disorder).
Table 2.
Descriptive statistics by gender.
| Male |
Female |
|||
|---|---|---|---|---|
| M | SD | M | SD | |
| Age | 16.27 | 0.51 | 16.21 | 0.42 |
| Pubertal status | 2.76 | 0.30 | 3.14 | 0.39 |
| Verbal IQ | 95.00 | 10.57 | 90.23 | 10.87 |
| Fluid IQ | 95.87 | 14.58 | 101.00 | 11.68 |
| Disinhibition | 14.17 | 2.77 | 13.51 | 2.73 |
| Boredom susceptibility | 12.84 | 1.73 | 12.89 | 1.79 |
| Thrill/adventure seeking | 17.80 | 1.49 | 17.03 | 2.37 |
| Experience seeking | 14.68 | 2.01 | 15.13 | 1.84 |
2.2.3. Sensation seeking
The Sensation Seeking Scale (Zuckerman and Link, 1968), completed on the day of the fMRI scan, consists of four subscales with 10 items each. For each item, participants chose between two statements that reflected high or low sensation seeking. The four subscales were: Thrill and Adventure Seeking (e.g., “I often wish I could be a mountain climber” versus “I can’t understand people who risk their necks climbing mountains.”; α = 0.61); Disinhibition (e.g., “I like wild, uninhibited parties” versus “I prefer quiet parties with good conversation.”; α = 0.77); Experience Seeking (e.g., “people should dress in individual ways, even if the effects are sometimes strange” versus “people should dress according to some standard of taste, neatness, and style”; α = 0.47); and Boredom Susceptibility (e.g., “I can’t stand watching a movie I’ve seen before” versus “there are some movies I enjoy seeing a second or even a third time”; α = 0.56). The Disinhibition subscale contains 3 items that specifically refer to substance use (e.g., “I have tried marijuana or would like to” versus “I would never smoke marijuana”; α = 0.69). All analyses involving the total SS and Disinhibition scales were conducted both with (α = 0.77) and without (α = 0.67) these substance-related items.
2.3. Resting state fMRI
2.3.1. Resting state scan
Participants were instructed to lie still and relax in the scanner for several minutes with their eyes opened and focused on a white fixation cross presented on a black screen through a mirror attached to the head coil. Resting state fMRI data were collected for 7 min 24 s.
2.3.2. Image acquisition and data preprocessing
The scan was conducted on a Siemens 3T TIM Trio MRI scanner with a 32-channel head coil. Parameters for image acquisition were voxel size = 3.5 × 3.5 × 3.5 mm, slices = 35, slice thickness = 3.5 mm, repetition time = 2000 ms, echo time = 27 ms, flip angle = 80 degrees, interleaved slice geometry, field of view = 224 mm. Images were T2 weighted. The first three volumes were discarded to ensure magnet stabilization. Preprocessing was conducted using the FMRIB Software Library (FSL) (Smith et al., 2004) and Analysis of Functional NeuroImaging (AFNI) software (Cox, 1996). Preprocessing consisted of slice timing correction, rigid body motion correction with six degrees of freedom, and spatial smoothing with a 6 mm half-maximum Gaussian kernel. “Denoising” of the data was accomplished through independent component analysis using FSL's MELODIC, with components rated as either signal or noise using criteria for visual inspection described by Kelly et al. (2010). The noise components were then filtered out of the functional data. Each participant's functional data were then co-registered with their brain-extracted structural images and normalized to Montreal Neurological Institute (MNI) stereotaxic space using FSL's two-stage registration method via FLIRT. Alignment was visually confirmed for all participants. AFNI was then used for de-spiking, band-pass filtering above 0.1 Hz and below 0.01 Hz, and censoring of volumes with head motion greater than 0.3 mm from the previous volume, resulting in the aforementioned exclusion of four participants for whom censoring resulted in the removal of more than 44/220 volumes (i.e., 20% of the data). As such, 69 participants were included in the analyses.
2.3.3. Data analysis
A region of interest (ROI) based on bilateral spheres with a radius of 3.5 mm each was created at the locations of the left and right NAcc (MNI coordinates: x = ±9, y = 9, z = −8) based on prior work (Di Martino et al., 2008). An ROI was created combining the left and right amygdala using the probabilistic coordinates defined within AFNI's MNI stereotaxic atlas. Individual spherical ROIs with a radius of 6 mm were created at the location of left and right dlPFC (MNI coordinates: x = ±40, y = 40, z = 24). Correct alignment of ROIs was confirmed for each participant by visual inspection. The average time course of the voxels in each ROI was extracted using AFNI's 3dmaskave and correlated with every voxel in the brain for each participant using AFNI's 3dfim+. These correlation coefficients were transformed using Fisher's z transformation. More positive values in the resulting z-score for each voxel indicate more similarity between the BOLD time course of the ROI and that voxel, while more negative values indicate anticorrelation between the BOLD time courses.
Based on AFNI's 3dClustSim program, which uses Monte Carlo simulations to determine appropriate cluster sizes, accounting for the number of voxels and a 6 mm smoothing kernel, a voxel-wise threshold of t = 3.433, p = 0.001, and a minimum cluster size of 45 voxels produced an overall alpha <0.01. Regression analysis was conducted using AFNI's 3dttest++ to determine the effect of years since substance use onset on the z-transformed correlation maps representing connectivity for each ROI for each participant.
3. Results
3.1. Associations of substance use onset age with other variables of interest
Pearson correlations were calculated between age of substance use onset, age at scan, gender, pubertal status, IQ, SES, self-reported substance use frequency concurrent to the scan, lifetime symptoms of psychiatric disorders, and sensation seeking subscales. As shown in Table 3, years since substance use onset was not significantly correlated with age, gender, pubertal status at age 16, IQ, or SES. Therefore, these variables were not included as covariates in the resting state analysis. Years since substance use onset was significantly correlated with conduct disorder symptoms, oppositional defiance symptoms, ADHD symptoms, and depression symptoms (see Supplementary Materials). Years since substance use onset significantly correlated with the Thrill and Adventure seeking subscale of SS and to a greater extent with the Disinhibition subscale score.
Table 3.
Pearson correlations among demographic characteristics, substance use, and sensation seeking.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Years of use | 1 | |||||||||||
| 2. Female | −.18 | 1 | ||||||||||
| 3. Age | .15 | −.06 | 1 | |||||||||
| 4. Pubertal status | .01 | .49** | .09 | 1 | ||||||||
| 5. Verbal IQ | −.11 | −.22 | −.26* | −.03 | 1 | |||||||
| 6. Fluid IQ | −.06 | .19 | −.31* | .13 | .27* | 1 | ||||||
| 7. SES | .06 | .04 | −.07 | .10 | .05 | .12 | 1 | |||||
| 8. SU frequency | .59** | −.22 | .19 | .06 | −.08 | −.12 | −.07 | 1 | ||||
| 9. Disinhibition | .48** | −.12 | −.03 | .24 | −.06 | −.05 | .02 | .61** | 1 | |||
| 10. Boredom susceptibility | .14 | .01 | −.13 | .20 | .13 | .07 | .04 | .14 | .48** | 1 | ||
| 11. Thrill/adventure seeking | .26* | −.19 | .13 | .08 | .01 | .03 | .20 | .05 | .13 | .10 | 1 | |
| 12. Experience seeking | .14 | .12 | .02 | .35** | .12 | .11 | -.12 | .13 | .32** | .24 | .09 | 1 |
Correlation is significant at the 0.05 level (2-tailed).
Correlation is significant at the 0.01 level (2-tailed).
3.2. Years since substance use onset and RSFC
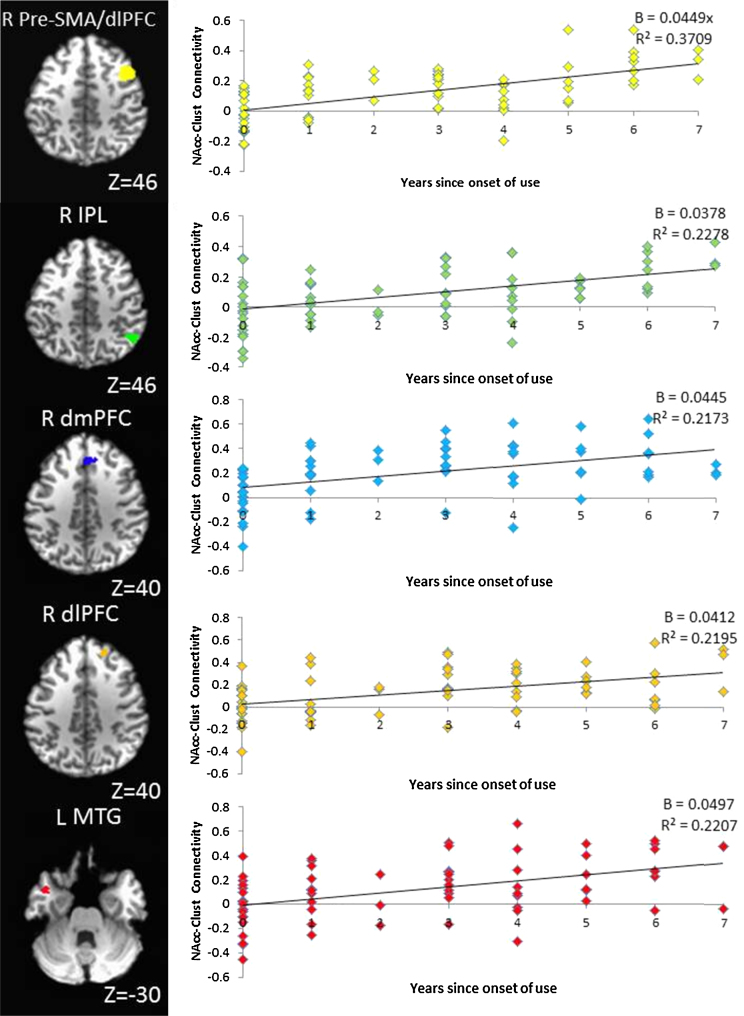
Five regions demonstrated a significant positive relation between the number of years since age 10 that adolescents had engaged in substance use and the strength of connectivity with the NAcc. As shown in Table 4 and Fig. 1, the earlier that adolescents initiated substance use, the more positive the connectivity between bilateral NAcc and right pre-supplementary motor area (pre-SMA), right dorsolateral PFC (dlPFC), right dorsomedial PFC (dmPFC), right inferior parietal lobule (IPL), and left medial temporal gyrus (MTG). These clusters remained significant when conducting separate analyses excluding participants who had tested positive for marijuana in saliva, and excluding participants who met subclinical criteria for conduct disorder.
Table 4.
Clusters with a significant positive relation between bilateral NAcc RSFC and years since age 10 of substance use onset (N = 69, MAge = 16.2 years).
| Voxels | Peak (x, y, z) | Region | BA |
|---|---|---|---|
| 792 | 44, 12, 54 | Right pre-supplementary motor area, dorsolateral prefrontal cortex | 6, 8, 9 |
| 153 | 50, −52, 46 | Right inferior parietal lobule | 40 |
| 99 | 4, 32, 40 | Right dorsomedial prefrontal cortex | 6 |
| 66 | 18, 38, 46 | Right dorsolateral prefrontal cortex | 9 |
| 45 | −44, 6, −28 | Left middle temporal gyrus | 21 |
NAcc = nucleus accumbens; RSFC = resting state functional connectivity; Peak (x, y, z) = MNI coordinates for the voxel with the highest coefficient within each cluster; BA = Brodmann's area; Voxel-wise threshold: t = 3.433, p = 0.001 for minimum cluster size of 45 voxels, alpha <0.01.
Fig. 1.
Points on the scatter plots depict the Z-transformed correlations of the voxel time courses within each significant cluster with the NAcc time course, averaged across the voxels in each cluster, and plotted against years since age 10 of substance use onset for each participant. B = Regression coefficient for the relation between years since onset of use and NAcc-Cluster connectivity, R Pre-SMA/dlPFC = Right pre-supplementary motor area, dorsolateral prefrontal cortex; R IPL = Right inferior parietal lobule; R dmPFC = Right dorsomedial prefrontal cortex; R dlPFC = Right dorsolateral prefrontal cortex, L MTG = Left middle temporal gyrus.
As shown in Table 5, six regions demonstrated a significant relation between the number of years since age 10 that adolescents had engaged in substance use and the strength of connectivity with the right dlPFC. Examining the overall connectivity of these regions with right dlPFC, independent of substance use, revealed that three clusters had overall negative connectivity with right dlPFC, and three clusters had overall positive connectivity with right dlPFC. Right ACC, ventromedial prefrontal cortex, and right dorsomedial prefrontal cortex had overall negative connectivity with right dlPFC, but the connectivity was less negative the earlier that adolescents initiated substance use. Two clusters in right and left dlPFC and another in the right lingual gyrus demonstrated overall positive connectivity with right dlPFC, and the connectivity was more positive the earlier that adolescents initiated substance use. In addition, a single cluster in right ACC demonstrated significantly less negative connectivity with left dlPFC the younger adolescents were when they first engaged in substance use.
Table 5.
Clusters with a significant positive relation between right and left dlPFC RSFC and years since age 10 of substance use onset.
| Voxels | Peak (x, y, z) | Region | BA |
|---|---|---|---|
| Right dlPFC | |||
| 172 | 16, 42, 28 | Right medial prefrontal cortex, anterior cingulate | 9 |
| 157 | 0, 58, −16 | Ventromedial prefrontal cortex | 11 |
| 111 | 36, 14, 50 | Right dorsolateral prefrontal cortex | 8 |
| 81 | 8, 54, 34 | Right dorsomedial prefrontal cortex | 9 |
| 69 | 20, −104, −5 | Right lingual gyrus | 18 |
| 57 | −44, 48, −12 | Left ventrolateral prefrontal cortex | 11 |
| Left dlPFC | |||
| 160 | 16, 40, 28 | Right anterior cingulate | 9 |
dlPFC = dorsolateral prefrontal cortex; PFC = prefrontal cortex; ACC = anterior cingrulate cortex; RSFC = resting state functional connectivity; Peak (x, y, z) = MNI coordinates for the voxel with the highest coefficient within each cluster; BA = Brodmann's area; Voxel-wise threshold: t = 3.433, p = 0.001 for minimum cluster size of 45 voxels, alpha <0.01.
In contrast to results for the NAcc and dlPFC seeds, no regions demonstrated significant relations between age of substance use onset and connectivity with the amygdala seed.
There was no significant relation between substance use frequency concurrent with the fMRI scan and NAcc connectivity. Thus, there was no justification for investigating whether RSFC differences related to substance use frequency could explain the relation between RSFC and number of years using substances.
Neither overall SS nor Disinhibition demonstrated a significant relation to NAcc RSFC. Thus, there was no justification for investigating whether RSFC differences related to SS could explain the relation between number of years using substances and RSFC. Due to the lack of a relation between amygdala RSFC and substance use, amygdala RSFC was not investigated with regard to SS.
4. Discussion
This study was designed to determine the relation between the age of substance use onset across early to middle adolescence and the intrinsic connections of the brain's reward circuitry. A robust and consistent relation was observed, such that the earlier adolescents initiated substance use, the greater the coordinated activity of bilateral NAcc and five neural regions: right pre-SMA, right dlPFC, right dmPFC, right IPL, and left MTG. This pattern is striking in both expected and novel ways. First, the relations between NAcc connectivity with each of the five other regions and years since substance use onset were consistent, whereby a linear increase in NAcc connectivity with these regions corresponded with every year earlier that the adolescents initiated substance use. Second, these right hemisphere regions have been shown in previous research to be strongly connected to one another both functionally and structurally, and to be primary nodes in what has commonly been called the right fronto-parietal control network (Fassbender et al., 2006, Seeley et al., 2007; Nindam et al., 2012). Co-activation of the regions in this network has been observed in resting state studies (Damoiseaux et al., 2006, Spreng et al., 2013) as well as during performance of a variety of tasks requiring cognitive control (Fassbender et al., 2006, Seeley et al., 2007). Right dlPFC and right IPL in particular have been implicated as the common brain regions involved in a wide range of executive functions requiring top-down attentional control (Fassbender et al., 2006). They are therefore considered to constitute a network of intrinsically connected regions that generally subserve cognitive control.
Our results suggest that earlier onset of substance use relates to greater integration of striatal and right frontoparietal networks, and thereby a stronger coupling of the brain's reward and cognitive control mechanisms. This increased between-network coupling may not be specific to reward and cognitive control networks, however. More years of substance use was also associated with increased connectivity between dlPFC and several regions within prefrontal cortex, a pattern that runs counter to the developmental trajectory typically seen across adolescence toward more segregation of neural networks that underpin opposing or complementary functions (Chai et al., 2014, Fox et al., 2005, Stevens et al., 2009). The patterns of increased interconnectivity between dlPFC and other prefrontal regions, particularly ACC, are strikingly similar to those found in cocaine-dependent adults (Camchong et al., 2011, Cisler et al., 2013). This suggests that a lack of segregation between cognitive control regions and other brain networks may be related to the duration of substance exposure and risk for future substance use problems. Finally, amygdala did not show any significant connectivity with any other regions, lending support to the specificity of reward processing and cognitive control circuitry connections to the adolescent substance use phenotype.
Increased coupling between reward and cognitive control regions is consistent with research demonstrating that monetary incentives improved performance and increased activation in frontoparietal control networks during cognitive control tasks for adolescents with substance use disorder, relative to healthy controls (Chung et al., 2011). A stronger coupling of reward and cognitive control networks may suggest a strong influence of external appetitive motivators on goal-directed neural activity. Alternatively, increased connectivity between NAcc and the frontoparietal network may represent a compensatory mechanism by which those that have longer lifetime use of substances recruit normative levels of cognitive control behavior when required.
Greater connectivity between NAcc and the right frontoparietal network does not appear to be a neural signature of sensation seeking. Although strongly correlated with the number of years since substance use onset, neither Sensation Seeking nor Disinhibition was associated with NAcc-based RSFC; thus, NAcc-based RSFC could not mediate the link between SS and earlier adolescent substance use. SS is by no means an exhaustive measure of the psychosocial tendencies that predispose adolescents to early substance use onset, and previous studies have failed to find a connection between SS and neurodevelopmental trajectories in adolescence thought to underlie risk-taking (Mills et al., 2014). Nonetheless, these findings suggest that NAcc functional connectivity with the right frontoparietal control network is related to adolescent substance use behavior, irrespective of its association with a pre-existing tendency to pursue rewards. A more likely explanation may therefore be that substance use during adolescence engages NAcc and the right frontoparietal network in tandem, perhaps especially as behavior is regulated toward the pursuit of rewards such as substances, and the earlier that substance use takes place, the stronger the coactivations become. This connectivity pattern may therefore be one mechanism by which early adolescent substance use alters brain function and contributes to the development of substance use disorders.
Some important limitations must be considered in interpreting the current findings. First, the single time-point of fMRI data collection leaves the direction of causality undetermined. Structural and functional differences in the brains of adolescent substance users have been identified both before and after the initiation of substance use (Squeglia et al., 2009). It is unknown from the present study's results whether differences in connectivity preceded and contributed to earlier substance use, or vice versa. To address this issue, longitudinal study of adolescents’ neurodevelopment and substance use into adulthood is essential. These efforts should also assess other psychosocial tendencies and behaviors related to substance use and risk-taking as potential mediators of brain-behavior relationships in order to more rigorously characterize the nature of their causal relationship. In fact, the participants in this study will continue to be followed annually, and data on their substance use behavior and psychosocial tendencies will be collected into adulthood.
Second, although the use of MNI standard space, based on adult brains, is common practice in studies of adolescent brain function (Stevens et al., 2009, Cservenka et al., 2014, Chung et al., 2011), and we visually confirmed localization of all ROI seeds, the appropriateness of using the adult brain to normalize and locate ROIs on adolescent brains remains open to debate (Muzik et al., 2000). Future research might create and employ the use of a template based on the adolescent brain to study substance use in adolescent samples.
Third, despite there being a wide range of substances, substances were treated as a general category in connectivity analyses. Given the high degree of overlap in substance usage and the small number of adolescents engaging in the use of substances other than alcohol and marijuana, the present study was underpowered to test for NAcc-based RSFC differences related to specific drugs, which should be addressed in future work. Moreover, future researchers should note the comorbidity of alcohol and marijuana use when investigating the specific effects of either substance.
Furthermore, our measure of age of onset suggests different time windows over which participants had engaged in substance use. This was found to more strongly relate to NAcc-based RSFC than concurrent frequency of use. However, other approaches to operationalizing substance use across all these years could contribute to variations in the consequences for neural functioning. Further investigations could attempt to identify particular developmental windows in which onset, duration, and frequency of use may be differentially influential on neural functioning.
The current study suggests other new avenues for future work. First, the right lateralization of these functional connectivity patterns for the NAcc seed as well as the more profuse prefrontal interconnectivity differences for the right versus the left dlPFC seed raise questions for future investigation. Although left and right frontoparietal control networks frequently demonstrate coactivity during cognitive control (Seeley et al., 2007, Niendam et al., 2012), the right and left frontoparietal control networks have also been found to be dissociable functional networks (Damoiseaux et al., 2006, Yeo et al., 2011, Spreng et al., 2013). It is unclear what it is about adolescent substance use that would specifically promote greater connectivity with the right, and not the left, frontoparietal control network. Further research is necessary to investigate the functional specificity of these regions, their connectivity with striatum, and their relation to substance use.
A growing body of research connects aberrant RSFC with adolescent psychopathology, including anxiety symptoms and disorders (Burghy et al., 2012, Roy et al., 2013), depression (Cullen et al., 2009), attention-deficit/hyperactive disorder (Sripada et al., 2014), and now, with the present study, substance use. With continued investigation and replication, RSFC shows promise as a method for identifying the mechanisms by which early adolescent substance use may lead to later substance abuse and dependence, and as a unique indicator of risk, with the potential to reflect underlying genetic and developmental variability in neurotransmission. Indeed, NAcc-PFC RSFC has been linked with genotypic differences impacting dopamine neurotransmission (Gordon et al., 2015, Gordon et al., 2012), an increase in which is thought to lead to increased incentive motivation in adolescence (Luciana et al., 2012). As such, examining RSFC as an endophenotype may be applied to future work focused on understanding risk from genotype to phenotype. Incorporating a multi-level perspective on the genetic and neurobiological contributors to the development of substance use disorders into translational efforts has the potential to lead to transformative prevention and intervention efforts.
5. Conclusion
The earlier substance use was initiated in adolescence, the stronger the coupling between NAcc and brain regions that make up the right frontoparietal network, a network known to be involved in cognitive control, as well as increased interconnectivity between dlPFC and other PFC regions. These patterns occurred independently of the equally strong relations between the age of substance use onset and disinhibition, and age of substance use onset and substance use frequency. Because of the strong connection between age of substance use onset and later substance abuse and dependence, these findings suggest that connectivity between reward and cognitive control networks may serve as a mechanism influencing the transition to substance abuse and dependence. This study demonstrates the utility of seed-based resting state functional connectivity analyses in improving our understanding of variations in neural functioning that underlie psychiatric illness, indicating its burgeoning potential in the identification of risk and targeted intervention.
Financial disclosures
None.
Acknowledgments
This research was supported by a William T. Grant Foundation Scholars Award 180021 (AEG), a William T. Grant Foundation Mentoring Award 182606 (AEG, RAS), National Institutes of Health grant R01 DA017902 (RWR), National Science Foundation grant 1327768 (AEG; PDH), the University of California, Davis Interdisciplinary Frontiers of Humanities and the Arts initiative (AEG; PDH), and the University of California, Davis – Imaging Research Center Pilot Program (AEG). The author was supported by a University of California, Davis First Year Provost Fellowship.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.dcn.2015.07.002.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- Arnett J.J. The neglected 95%: why American psychology needs to become less American. Am. Psychol. 2008;63(7):602. doi: 10.1037/0003-066X.63.7.602. [DOI] [PubMed] [Google Scholar]
- Asato M.R., Terwilliger R., Woo J., Luna B. White matter development in adolescence: a DTI study. Cereb. Cortex. 2010;20(9):2122–2131. doi: 10.1093/cercor/bhp282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B., Zerrin Yetkin F., Haughton V.M., Hyde J.S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Brook D.W., Brook J.S., Zhang C., Cohen P., Whiteman M. Drug use and the risk of major depressive disorder, alcohol dependence, and substance use disorders. Arch. Gen. Psychiatry. 2002;59(11):1039–1044. doi: 10.1001/archpsyc.59.11.1039. [DOI] [PubMed] [Google Scholar]
- Burghy C.A., Stodola D.E., Ruttle P.L., Molloy E.K., Armstrong J.M., Oler J.A., Birn R.M. Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nat. Neurosci. 2012;15(12):1736–1741. doi: 10.1038/nn.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camchong J., MacDonald A.W., Nelson B., Bell C., Mueller B.A., Specker S., Lim K.O. Frontal hyperconnectivity related to discounting and reversal learning in cocaine subjects. Biol. Psychiatry. 2011;69(11):1117–1123. doi: 10.1016/j.biopsych.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Jones R.M. Neurobiology of the adolescent brain and behavior: implications for substance use disorders. J. Am. Acad. Child Adolesc. Psychiatry. 2010;49(12):1189–1201. doi: 10.1016/j.jaac.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai X.J., Ofen N., Gabrieli J.D., Whitfield-Gabrieli S. Selective development of anticorrelated networks in the intrinsic functional organization of the human brain. J. Cogn. Neurosci. 2014;26(3):501–513. doi: 10.1162/jocn_a_00517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao J.Y. Cultural neuroscience: a once and future discipline. Prog. Brain Res. 2009;178:287–304. doi: 10.1016/S0079-6123(09)17821-4. [DOI] [PubMed] [Google Scholar]
- Chung T., Geier C., Luna B., Pajtek S., Terwilliger R., Thatcher D., Clark D.B. Enhancing response inhibition by incentive: comparison of adolescents with and without substance use disorder. Drug Alcohol Depend. 2011;115(1):43–50. doi: 10.1016/j.drugalcdep.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler J.M., Elton A., Kennedy A.P., Young J., Smitherman S., James G.A., Kilts C.D. Altered functional connectivity of the insular cortex across prefrontal networks in cocaine addiction. Psychiatry Res.: Neuroimaging. 2013;213(1):39–46. doi: 10.1016/j.pscychresns.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D.B., Chung T., Pajtek S., Zhai Z., Long E., Hasler B. Neuroimaging methods for adolescent substance use disorder prevention science. Prev. Sci. 2013;14(3):300–309. doi: 10.1007/s11121-012-0323-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cservenka A., Casimo K., Fair D.A., Nagel B.J. Resting state functional connectivity of the nucleus accumbens in youth with a family history of alcoholism. Psychiatry Res.: Neuroimaging. 2014;221(3):210–219. doi: 10.1016/j.pscychresns.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen K.R., Gee D.G., Klimes-Dougan B., Gabbay V., Hulvershorn L., Mueller B.A., Milham M.P. A preliminary study of functional connectivity in comorbid adolescent depression. Neurosci. Lett. 2009;460(3):227–231. doi: 10.1016/j.neulet.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux J.S., Rombouts S.A.R.B., Barkhof F., Scheltens P., Stam C.J., Smith S.M., Beckmann C.F. Consistent resting-state networks across healthy subjects. Proc. Natl. Acad. Sci. 2006;103(37):13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A., Scheres A., Margulies D.S., Kelly A.M.C., Uddin L.Q., Shehzad Z., Milham M.P. Functional connectivity of human striatum: a resting state FMRI study. Cereb. Cortex. 2008;18(12):2735–2747. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- DuRant R.H., Smith J.A., Kreiter S.R., Krowchuk D.P. The relationship between early age of onset of initial substance use and engaging in multiple health risk behaviors among young adolescents. Arch. Pediatr. Adolesc. Med. 1999;153(3):286–291. doi: 10.1001/archpedi.153.3.286. [DOI] [PubMed] [Google Scholar]
- Elliott D.S., Ageton S.S., Huizinga D. Behavioral Research Inst; Boulder, CO: 1982. Explaining Delinquency and Drug Use. [Google Scholar]
- Ernst M., Fudge J.L. A developmental neurobiological model of motivated behavior: anatomy, connectivity and ontogeny of the triadic nodes. Neurosci. Biobehav. Rev. 2009;33(3):367–382. doi: 10.1016/j.neubiorev.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M., Luckenbaugh D.A., Moolchan E.T., Leff M.K., Allen R., Eshel N., Kimes A. Behavioral predictors of substance-use initiation in adolescents with and without attention-deficit/hyperactivity disorder. Pediatrics. 2006;117(6):2030–2039. doi: 10.1542/peds.2005-0704. [DOI] [PubMed] [Google Scholar]
- Ernst M., Pine D.S., Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychol. Med. 2006;36(03):299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassbender C., Simoes-Franklin C., Murphy K., Hester R., Meaney J., Robertson I.H., Garavan H. The role of a right fronto-parietal network in cognitive control. J. Psychophysiol. 2006;20(4):286–296. [Google Scholar]
- Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Van Essen D.C., Raichle M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. U. S. A. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee D.G., Humphreys K.L., Flannery J., Goff B., Telzer E.H., Shapiro M., Tottenham N. A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. J. Neurosci. 2013;33(10):4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon E.M., Devaney J.M., Bean S., Vaidya C.J. Resting-state striato-frontal functional connectivity is sensitive to DAT1 genotype and predicts executive function. Cereb. Cortex. 2015;25(2):336–345. doi: 10.1093/cercor/bht229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon E.M., Stollstorff M., Devaney J.M., Bean S., Vaidya C.J. Effect of dopamine transporter genotype on intrinsic functional connectivity depends on cognitive state. Cereb. Cortex. 2012;22(9):2182–2196. doi: 10.1093/cercor/bhr305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant B.F., Dawson D.A. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J. Subst. Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Grant B.F., Dawson D.A. Age of onset of drug use and its association with DSM-IV drug abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J. Subst. Abuse. 1998;10(2):163–173. doi: 10.1016/s0899-3289(99)80131-x. [DOI] [PubMed] [Google Scholar]
- Grant B.F., Stinson F.S., Harford T.C. Age at onset of alcohol use and DSM-IV alcohol abuse and dependence: a 12-year follow-up. J. Subst. Abuse. 2001;13(4):493–504. doi: 10.1016/s0899-3289(01)00096-7. [DOI] [PubMed] [Google Scholar]
- Hingson R.W., Zha W. Age of drinking onset, alcohol use disorders, frequent heavy drinking, and unintentionally injuring oneself and others after drinking. Pediatrics. 2009;123(6):1477–1484. doi: 10.1542/peds.2008-2176. [DOI] [PubMed] [Google Scholar]
- Hulvershorn L.A., Cullen K.R., Francis M.M., Westlund M.K. Developmental resting state functional connectivity for clinicians. Curr. Behav. Neurosci. Rep. 2014;1(3):161–169. doi: 10.1007/s40473-014-0020-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L.D., O’Malley P.M., Miech R.A., Bachman J.G., Schulenberg J.E. Institute for Social Research, The University of Michigan; Ann Arbor: 2014. Monitoring the Future National Survey Results on Drug Use: 1975–2013: Overview, Key Findings on Adolescent Drug Use. [Google Scholar]
- Kelly R.E., Alexopoulos G.S., Wang Z., Gunning F.M., Murphy C.F., Morimoto S.S., Hoptman M.J. Visual inspection of independent components: defining a procedure for artifact removal from fMRI data. J. Neurosci. Methods. 2010;189(2):233–245. doi: 10.1016/j.jneumeth.2010.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubman D.I., Yücel M., Hall W.D. Substance use and the adolescent brain: a toxic combination? J. Psychopharmacol. 2007;21(8):792–794. doi: 10.1177/0269881107078309. [DOI] [PubMed] [Google Scholar]
- Luciana M., Wahlstrom D., Porter J.N., Collins P.F. Dopaminergic modulation of incentive motivation in adolescence: age-related changes in signaling, individual differences, and implications for the development of self-regulation. Dev. Psychol. 2012;48(3):844. doi: 10.1037/a0027432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C.A., Kelly T.H., Rayens M.K., Brogli B.R., Brenzel A., Smith W.J., Omar H.A. Sensation seeking, puberty, and nicotine, alcohol, and marijuana use in adolescence. J. Am. Acad. Child Adolesc. Psychiatry. 2002;41(12):1495–1502. doi: 10.1097/00004583-200212000-00022. [DOI] [PubMed] [Google Scholar]
- Merikangas K.R., Stolar M., Stevens D.E., Goulet J., Preisig M.A., Fenton B., Rounsaville B.J. Familial transmission of substance use disorders. Arch. Gen. Psychiatry. 1998;55(11):973–979. doi: 10.1001/archpsyc.55.11.973. [DOI] [PubMed] [Google Scholar]
- Mills K.L., Goddings A.L., Clasen L.S., Giedd J.N., Blakemore S.J. The developmental mismatch in structural brain maturation during adolescence. Dev. Neurosci. 2014;36(3–4):147–160. doi: 10.1159/000362328. [DOI] [PubMed] [Google Scholar]
- Muzik O., Chugani D.C., Juhász C., Shen C., Chugani H.T. Statistical parametric mapping: assessment of application in children. Neuroimage. 2000;12(5):538–549. doi: 10.1006/nimg.2000.0651. [DOI] [PubMed] [Google Scholar]
- Niendam T.A., Laird A.R., Ray K.L., Dean Y.M., Glahn D.C., Carter C.S. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn. Affect. Behav. Neurosci. 2012;12(2):241–268. doi: 10.3758/s13415-011-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman A.L., Pulido C., Squeglia L.M., Spadoni A.D., Paulus M.P., Tapert S.F. Neural activation during inhibition predicts initiation of substance use in adolescence. Drug Alcohol Depend. 2011;119(3):216–223. doi: 10.1016/j.drugalcdep.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen A.C., Tobin-Richards M., Boxer A. Puberty: its measurement and its meaning. J. Early Adolesc. 1983;3:47–62. [Google Scholar]
- Porter J.N., Roy A.K., Benson B., Carlisi C., Collins P.F., Leibenluft E., Pine D.S., Luciana M., Ernst M. Age-related changes in the intrinsic functional connectivity of the human ventral vs. dorsal striatum from childhood to middle age. Dev Cogn Neurosci. 2015;11:83–95. doi: 10.1016/j.dcn.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A.K., Fudge J.L., Kelly C., Perry J.S., Daniele T., Carlisi C., Ernst M. Intrinsic functional connectivity of amygdala-based networks in adolescent generalized anxiety disorder. J. Am. Acad. Child Adoles. Psychiatry. 2013;52(3):290–299. doi: 10.1016/j.jaac.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg A.D., Schweinsburg B.C., Cheung E.H., Brown G.G., Brown S.A., Tapert S.F. fMRI response to spatial working memory in adolescents with comorbid marijuana and alcohol use disorders. Drug Alcohol Depend. 2005;79(2):201–210. doi: 10.1016/j.drugalcdep.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Greicius M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer D., Fisher P., Lucas C.P., Dulcan M.K., Schwab-Stone M.E. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J. Am. Acad. Child Adolesc. Psychiatry. 2000;39(1):28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E., Johansen-Berg H., Matthews P.M. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Spear L.P. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spreng R.N., Sepulcre J., Turner G.R., Stevens W.D., Schacter D.L. Intrinsic architecture underlying the relations among the default, dorsal attention, and frontoparietal control networks of the human brain. J. Cogn. Neurosci. 2013;25(1):74–86. doi: 10.1162/jocn_a_00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia L.M., Jacobus J., Tapert S.F. The influence of substance use on adolescent brain development. Clin. EEG Neurosci. 2009;40(1):31–38. doi: 10.1177/155005940904000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada C., Kessler D., Fang Y., Welsh R.C., Prem Kumar K., Angstadt M. Disrupted network architecture of the resting brain in attention-deficit/hyperactivity disorder. Hum. Brain Mapp. 2014;35(9):4693–4705. doi: 10.1002/hbm.22504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. Risk taking in adolescence new perspectives from brain and behavioral science. Curr. Dir. Psychol. Sci. 2007;16(2):55–59. [Google Scholar]
- Stevens M.C., Pearlson G.D., Calhoun V.D. Changes in the interaction of resting-state neural networks from adolescence to adulthood. Hum. Brain Mapp. 2009;30(8):2356–2366. doi: 10.1002/hbm.20673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapert S.F., Schweinsburg A.D., Drummond S.P., Paulus M.P., Brown S.A., Yang T.T., Frank L.R. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology. 2007;194(2):173–183. doi: 10.1007/s00213-007-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker J.S., Ellickson P.L., Collins R.L., Klein D.J. Are drug experimenters better adjusted than abstainers and users? A longitudinal study of adolescent marijuana use. J. Adolesc. Health. 2006;39(4):488–494. doi: 10.1016/j.jadohealth.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Weiland B.J., Welsh R.C., Yau W.Y.W., Zucker R.A., Zubieta J.K., Heitzeg M.M. Accumbens functional connectivity during reward mediates sensation-seeking and alcohol use in high-risk youth. Drug Alcohol Depend. 2013;128(1):130–139. doi: 10.1016/j.drugalcdep.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock R.W., McGrew K.S., Mather N. Riverside Publishing; Itasca, IL: 2001. Woodcock-Johnson III Tests of Cognitive Abilities. [Google Scholar]
- Yeo B.T., Krienen F.M., Sepulcre J., Sabuncu M.R., Lashkari D., Hollinshead M., Buckner R.L. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011;106(3):1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M., Link K. Construct validity for the sensation-seeking scale. J. Consult. Clin. Psychol. 1968;32(4):420. doi: 10.1037/h0026047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.