Abstract
Acetaminophen (APAP) hepatotoxicity is a serious public health problem in western countries. Current treatment options for APAP poisoning are limited and novel therapeutic intervention strategies are needed. A recent publication suggested that benzyl alcohol (BA) protects against APAP hepatotoxicity and could serve as a promising antidote for APAP poisoning. To assess the protective mechanisms of BA, C56Bl/6J mice were treated with 400mg/kg APAP and/or 270mg/kg BA. APAP alone caused extensive liver injury at 6h and 24h post-APAP. This injury was attenuated by BA co-treatment. Assessment of protein adduct formation demonstrated that BA inhibits APAP metabolic activation. In support of this, in vitro experiments also showed that BA dose-dependently inhibits cytochrome P450 activities. Correlating with the hepatoprotection of BA, APAP-induced oxidant stress and mitochondrial dysfunction were reduced. Similar results were obtained in primary mouse hepatocytes. Interestingly, BA alone caused mitochondrial membrane potential loss and cell toxicity at high doses, and its protective effect could not be reproduced in primary human hepatocytes (PHH). We conclude that BA protects against APAP hepatotoxicity mainly by inhibiting cytochrome P450 enzymes in mice. Considering its toxic effect and the loss of protection in PHH, BA is not a clinically useful treatment option for APAP overdose patient.
Keywords: acetaminophen hepatotoxicity, benzyl alcohol, acute liver failure, mitochondria, sterile inflammation, human hepatocytes
1. INTRODUCTION
Acetaminophen (APAP) is a commonly used analgesic and antipyretic drug in many western countries. However, an overdose can cause severe liver injury and even liver failure, resulting in almost 70,000 hospitalizations and 500 deaths annually in the US (Budnitz et al., 2011; Manthripragada et al., 2011). Studies in the last few decades have greatly enhanced our understanding of the mechanisms of APAP hepatotoxicity in the mouse model (Jaeschke et al., 2012a) and also in humans (McGill and Jaeschke, 2014; Jaeschke, 2015). Formation of the reactive metabolite N-acetyl-p-benzoquinone imine (NAPQI), which is catalyzed by P-450 enzymes, has been recognized as the initiating event in the toxicity (Nelson, 1990). Excessive NAPQI formation after APAP overdose depletes hepatic glutathione (GSH) and binds to cellular proteins (Cohen et al., 1997). Protein binding in mitochondria disturbs the mitochondria electron transport chain and enhances the formation of reactive oxygen species (ROS) and peroxynitrite (Meyers et al., 1988; Jaeschke, 1990; Cover et al., 2005). The initial oxidant stress is amplified through c-jun-N-terminal kinase (JNK) signaling, leading to extensive mitochondrial dysfunction (Du et al., 2015; Hanawa et al., 2008), membrane permeability transition pore opening and collapse of the mitochondrial membrane potential (Kon et al., 2004). In addition, mitochondrial dysfunction causes release of mitochondrial nucleases, which cleave nuclear DNA (Bajt et al., 2006). These signaling events induce cellular necrosis (Gujral et al., 2002). Damage-associated molecular patterns (DAMPs) released from the injured hepatocytes mediate the activation of toll-like receptors (TLRs) on Kupffer cells and lead to an inflammatory response including cytokine/chemokine formation and liver-specific immune cell recruitment (Jaeschke et al., 2012b).
N-acetylcysteine (NAC) was introduced as the clinical antidote against APAP poisoning in the 1970s (Prescott et al., 1977). Multiple protective mechanisms have been reported for NAC, including replenishment of the GSH pool, which enhances the detoxification of NAPQI during the metabolism phase (Corcoran et al, 1985; Corcoran and Wong, 1986) and later scavenges ROS and peroxynitrite in the mitochondria (Knight et al., 2002; Cover et al., 2005). In addition, excess NAC can be degraded to form Krebs cycle intermediates, which support mitochondrial energy production (Saito et al., 2010). However, although NAC is very effective during the early phase of the injury, many patients seek medical attention relatively late (Larson, 2007). Therefore, a drug that is effective after the metabolism phase is needed.
A recent study identified benzyl alcohol (BA), which has already been approved by the FDA for treatment of head lice, as a promising intervention in APAP hepatotoxicity (Cai et al. 2014). Remarkably, the authors demonstrated that BA not only offers almost complete protection over a dose range of 270–540 mg/kg when given as a co-treatment, but can also reduce the injury when administered as late as 2h post-APAP (Cai et al. 2014). This study was highlighted by an editorial commentary, which suggested developing BA as a therapy for APAP overdose and acute liver failure (Patman, 2014). However, there are several concerns regarding the interpretation of the data. Many studies from our laboratory and others have shown that the innate inflammatory response after APAP overdose does not exaggerate the liver injury but rather is beneficial by promoting tissue repair (Williams et al., 2010a; 2011; 2014; Holt et al., 2008; You et al., 2013; Dambach et al., 2002), while BA was suggested to mainly protect by interfering with pro-inflammatory signaling (Cai et al., 2014). In addition, the effect of BA on APAP metabolic activation was not addressed. The nearly complete protection observed when BA was given around the time of APAP treatment raises the possibility that BA might have an effect on APAP metabolic activation. Furthermore, despite the close similarities between the mouse model and the human pathophysiology (Jaeschke, 2015), the effectiveness of BA against APAP hepatotoxicity should be tested using primary human hepatocytes before clinical testing is contemplated. Given the potential benefits of BA and the concerns listed above, we investigated the protective mechanisms of BA in an in vivo mouse model and also in primary mouse and human hepatocytes.
2. MATERIALS AND METHODS
2.1 Animals
Male C57Bl/6J mice (8–12 weeks old) used in our experiments were purchased from Jackson Laboratories (Bar Harbor, ME) and kept in an environmentally controlled room with light/dark cycle of periods of 12 hr. All the animals were accustomed at least 3 days before experiments with free access to food and water. All experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of Kansas Medical Center.
2.2 Experimental design
After overnight fasting, mice were treated i.p. with 400 mg/kg APAP (Sigma-Aldrich, St. Louis, MO) dissolved in warm saline. Benzyl alcohol (270 mg/kg) (Sigma-Aldrich) dissolved in saline or saline alone (20 ml/kg) were co-administered (i.p.) or 2 h after APAP. At 0.5 h, 2 h, 6 h or 24 h post-APAP mice were euthanized and blood and livers were harvested. Blood was drawn into a heparinized syringe and centrifuged to obtain plasma. The liver was sliced into pieces, and then fixed in phosphate-buffered formalin for histology, or used for mitochondrial isolation, or was snap-frozen in liquid nitrogen and subsequently stored at −80°C.
2.3 Isolation of subcellular fractions
The right and caudate lobes of the liver were minced and homogenized in ice cold isolation buffer (pH 7.4, containing 22 mM mannitol, 70 mM sucrose, 2.5 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 10 mM EDTA, 1 mM ethylene glycol tetraacetic acid, and 0.1% bovine serum albumin) with 15–20 strokes using a tight-fitting motorized Teflon pestle. Cell debris was removed by spinning the homogenate at 2,500 g for 10 min, and the resulting supernatant was then centrifuged at 20,000 g for 10 min to pellet mitochondria. The supernatant was preserved as cytosolic fraction. The mitochondria were washed with isolation buffer and flash frozen in liquid nitrogen. Both the cytosolic and the mitochondrial fractions were stored long-term at −80°C (Du et al., 2014).
2.4 Cell culture experiments
Primary mouse hepatocytes were isolated with a 2-step collagenase perfusion technique as described previously (Bajt et al., 2004). All isolations had cell viability > 90% and hepatocyte purity > 95%. The cells were plated in a density of 6 ×105 cells/well in six-well plates (BioCoat collagen I cellware plates; Becton Dickinson, Franklin Lakes, NJ), and grown in Williams E medium (Life Technologies, Grand Island, NY) containing 100 U/mL penicillin/streptomycin, 1×10−7 M insulin, and 10% fetal bovine serum. All studies with human material were approved by the University of Kansas Medical Center Institutional Review Board and followed the ethical and institutional guidelines. Primary human hepatocytes were isolated as described previously (Xie et al., 2014). Primary mouse hepatocytes or human hepatocytes were co-treated with 5 or 10 mM APAP and 0–46 mM BA, or APAP or BA alone. Cells were harvested for the JC-1 assay, APAP-protein adducts determination, ALT or lactate dehydrogenase (LDH) activity measurement.
2.5 Biochemical assays
ALT activity was determined using an ALT kit (Pointe Scientific, MI). LDH activity was performed as described in detail (Bajt et al., 2004). GSH and GSSG levels were measured using a modified method of the Tietze assay (Jaeschke and Mitchell, 1990). JC-1 Mitochondrial Membrane Potential Kit (Cell Technology, Mountain View, CA) was used to detect the mitochondrial membrane potential as described in detail (Bajt et al., 2004).
2.6 APAP protein adducts and cytochrome P450 activity
APAP-protein adducts in liver tissues and mitochondrial pellets were measured as described (Ni et al., 2012; McGill et al., 2012). 7-ethoxy-4-trifluoromethylcoumarin (7EFC) deethylase assay was performed to determine the cytochrome P450 activity in liver homogenates, as described in detail (Buters et al., 1993; (Ramachandran et al., 2011). The substrate 7EFC is known to be metabolized by Cyp1A2 and 2E1 (Buters et al., 1993).
2.7 Histology and western blotting
Formalin-fixed tissue samples embedded in paraffin were cut in 5 μm thickness and stained with hematoxylin and eosin (H&E) for necrosis assessment (Gujral et al., 2002). Western blotting was performed as described (Bajt et al., 2000). The primary antibodies were rabbit anti-Bax polyclonal antibody, rabbit anti-AIF antibody (Cell Signaling Technology, Danvers, MA) and mouse anti-Smac/DIABLO (BD Biosciences, San Diego, CA). A horseradish peroxidase-coupled anti-rabbit or anti-mouse IgG (Santa Cruz) was used as secondary antibody.
2.8 Statistics
All results were expressed as mean ± SEM. Statistical significance was assessed by one-way analysis of variance (ANOVA), followed by Student-Newman-Keul’s test for multiple comparisons. For non-normally distributed data, ANOVA was performed on ranks, followed by Dunn’s multiple comparisons. P < 0.05 was considered significant.
3. RESULTS
3.1 BA reduced APAP-induced liver injury
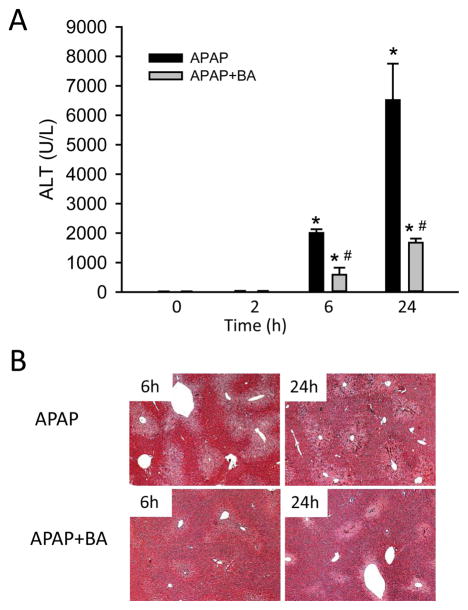
Mice treated with 400 mg/kg APAP had severe liver injury at 6 h and 24 h post-APAP, as indicated by increased plasma ALT activities (Fig. 1A) and centrilobular necrosis (Fig. 1B). Co-treatment with BA (270 mg/kg) significantly attenuated the increase in ALT activities and reduced areas of necrosis at both 6 h and 24 h (Fig. 1A,B), indicating that BA reduced APAP-induced liver injury. However, when mice were treated with BA 2 h after APAP, no significant protection was observed at 6 h as indicated by the similar increase in plasma ALT activities (APAP: 2573±162 U/L; APAP+BA: 2358±335 U/L; n=4 per group) and a similar oxidant stress as indicated by hepatic GSSG levels (data not shown). Together, our data confirmed the significant beneficial effect of BA against APAP hepatotoxicity when BA was co-treated with APAP, but not when it was given 2 h post-APAP as described by Cai et al. (2014).
Figure 1.
BA reduced APAP-induced liver injury. Mice were co-treated with 400 mg/kg APAP and 270 mg/kg BA, and sacrificed at 0–24 h post-APAP. (A) Time course of plasma ALT values. (B) Representative H&E-stained liver sections (×50 magnification). Data are expressed as means ± SE, n=3–6 mice per group. *P<0.05 (compared to 0h). #P<0.05 (compared to APAP).
3.2 BA reduced APAP metabolic activation and parameters of oxidant stress
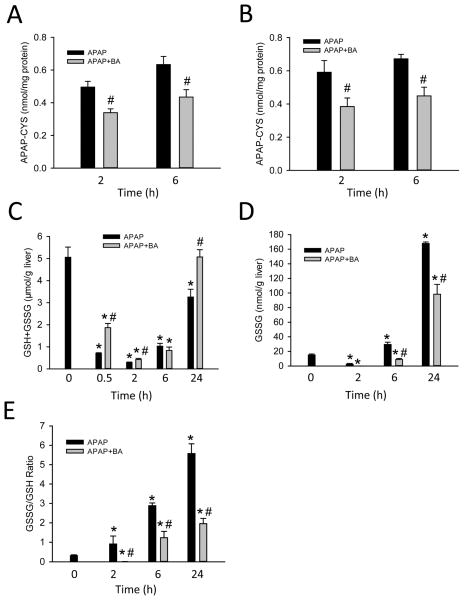
To evaluate the protective mechanisms of BA, APAP-protein adducts in both the total liver and mitochondria were measured at 2 h, the peak of adduct formation in the liver (McGill et al., 2013), and 6 h after APAP (Fig. 2A, B). Compared to APAP alone, co-treatment with BA resulted in a reduction of protein adducts in total liver (Fig. 2A) and mitochondria (Fig. 2B) at both time points. These results suggested significant inhibition of APAP metabolic activation by BA. In support of this, there was also a significant delay in hepatic GSH depletion at both 0.5 h and 2 h in BA-treated mice (Fig. 2C). Consistent with the protection by BA, the recovery of hepatic GSH in BA-treated mice was significantly higher at 24 h (Fig. 2C). Formation of reactive oxygen species was assessed by GSSG levels and the GSSG-to-GSH ratio (Fig. 2D, E). APAP dramatically increased both absolute GSSG levels and the GSSG-to-GSH at 6 and 24 h, and co-treatment with BA significantly reduced both (Fig. 2D, E), indicating reduced oxidant stress after BA treatment. However, as demonstrated previously, inhibition of metabolic activation of APAP will effectively reduce the down-stream oxidant stress (Jaeschke et al., 2006; Knight et al., 2002). Thus, the lower oxidant stress in livers of BA-treated animals is most likely a consequence of the reduced upstream events.
Figure 2.
BA reduced APAP-protein adduct formation and parameters of oxidant stress. Mice were co-treated with 400 mg/kg APAP and 270 mg/kg BA, and sacrificed at 0–24 h post-APAP. (A) Total liver and (B) mitochondrial APAP–cysteine adducts quantified by HPLC-ECD method. (C) Hepatic GSH levels, (D) GSSG levels and (E) GSSG-to-GSH ratio. Data are expressed as means ± SE, n=3–6 mice per group. *P<0.05 (compared to 0h). #P<0.05 (compared to APAP).
3.3 BA reduced APAP-induced mitochondrial dysfunction
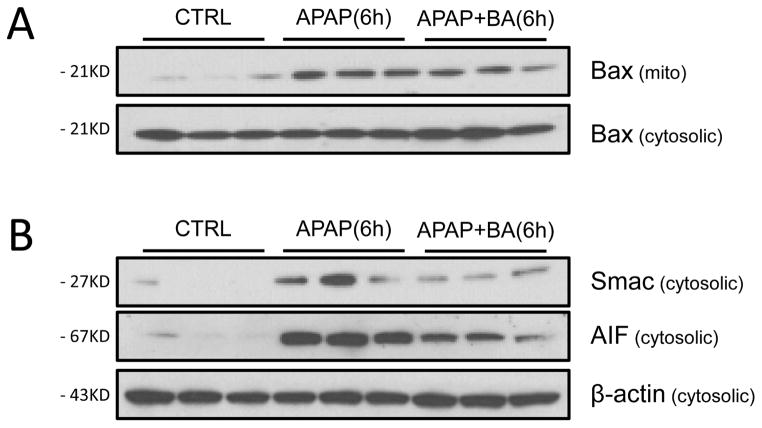
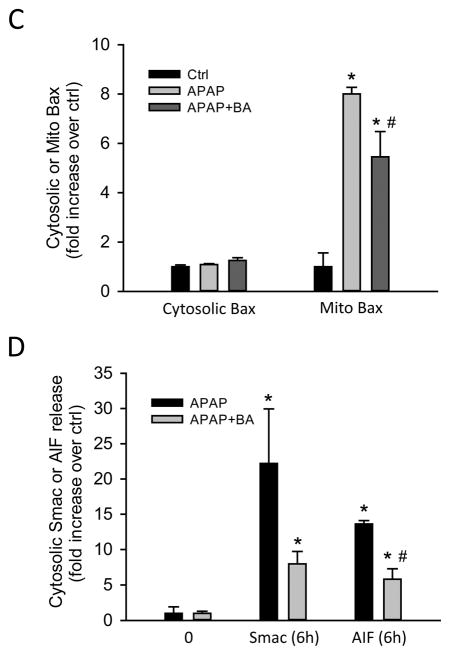
Mitochondrial Bax translocation causes the formation of mitochondrial outer membrane pores, contributing to the release of intermembrane proteins including Smac and AIF after APAP overdose (Bajt et al., 2008). We assessed mitochondrial translocation of Bax and the cytosolic release of Smac and AIF at 6 h after APAP (Fig. 3A,B). APAP treatment induced Bax translocation from the cytosol to the mitochondria (Fig. 3A), as indicated by a dramatic increase in the mitochondrial fraction (Fig 3C). BA caused a significant reduction in mitochondrial Bax translocation (Fig. 3A, C). The mitochondrial Bax pore formation triggered the release of Smac and AIF into the cytosol (Fig. 3B, D). BA treatment significantly reduced AIF release, which was supported by densitometric analysis (Fig. 3B, D).
Figure 3.
Effect of BA on mitochondrial translocation of Bax and mitochondrial release of Smac and AIF. Mice were co-treated with 400 mg/kg APAP and 270 mg/kg BA, and sacrificed at 0–6 h post-APAP. (A) Western blots for Bax in subcellular fractions and (C) densitometric analysis mitochondrial/cytosolic Bax ratio. (B) Western blots for cytosolic Smac and AIF and (D) densitometric analysis. Data are expressed as means ± SE, n=3–6 mice per group. *P<0.05 (compared to vehicle control).
3.4 BA directly inhibited P450 activities
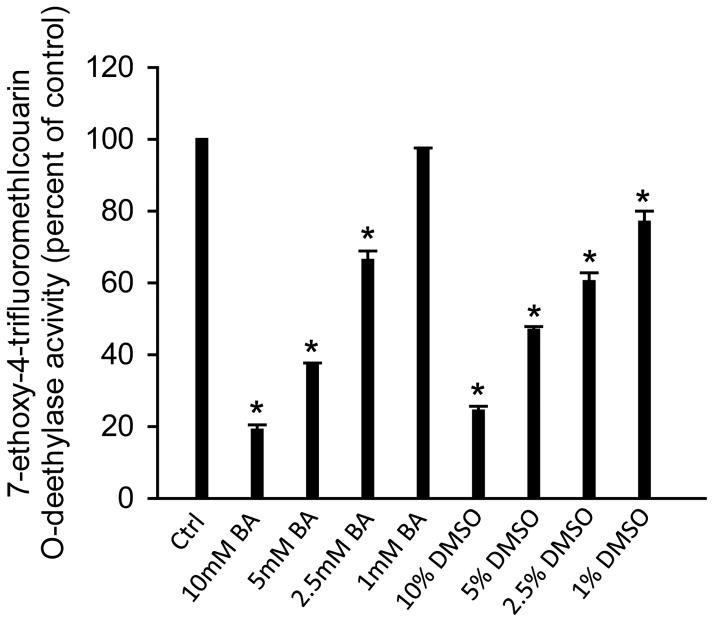
APAP toxicity is initiated by its metabolic activation, which is catalyzed by cytochrome P450 enzymes such as Cyp2E1 and Cyp1A2 (Zaher et al., 1998). To test whether BA can directly inhibit these cytochrome P450 enzymes, the 7EFC deethylase assay was used. The general P450 enzyme inhibitor DMSO was included as a positive control (Buters et al., 1993; Du et al., 2013). As indicated in Figure 4, both DMSO (1–10%) and BA dose-dependently inhibited cytochrome P450 activities. BA caused >30% inhibition at 2.5 mM and >80% inhibition at 10 mM (Figure 4). These data clearly demonstrated that BA can directly inhibit P450 enzyme activities, which are relevant for metabolic activation of APAP.
Figure 4.
BA and DMSO inhibited P450 activities. Cytochrome P450 activities affected by 1–10 mM BA or 1–10 % DMSO was measured by the 7EFC deethylase assay using the 14,000 ×g supernatant of mouse liver homogenate. n=3 mice per group. *P<0.05 (compared to control).
3.5 Effect of BA on APAP toxicity in primary mouse hepatocytes
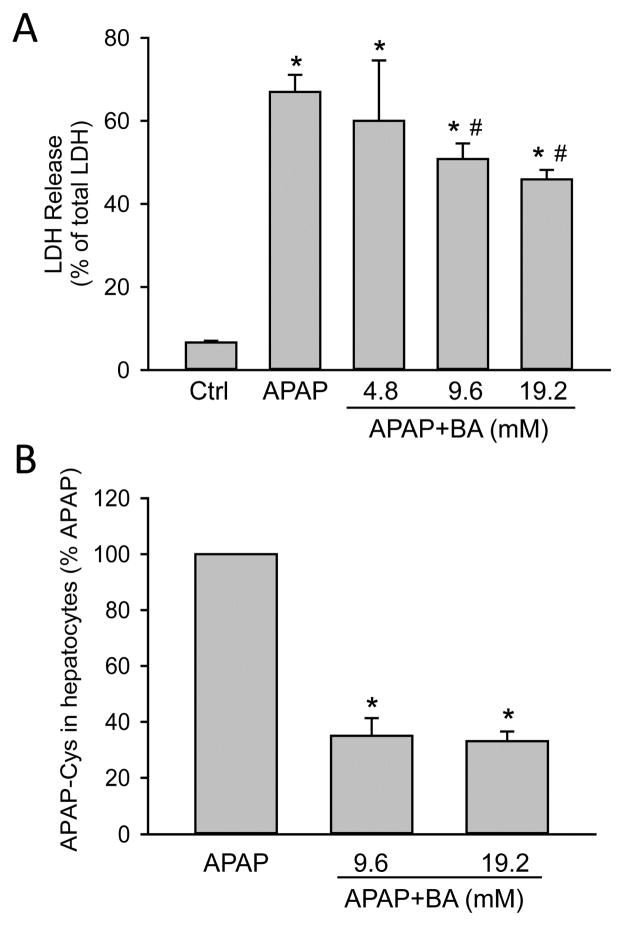
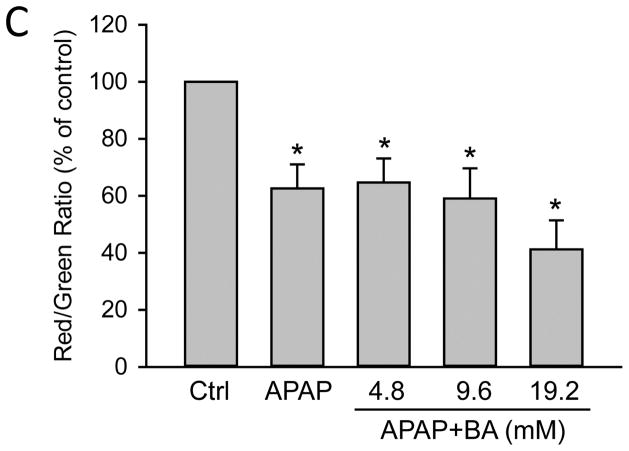
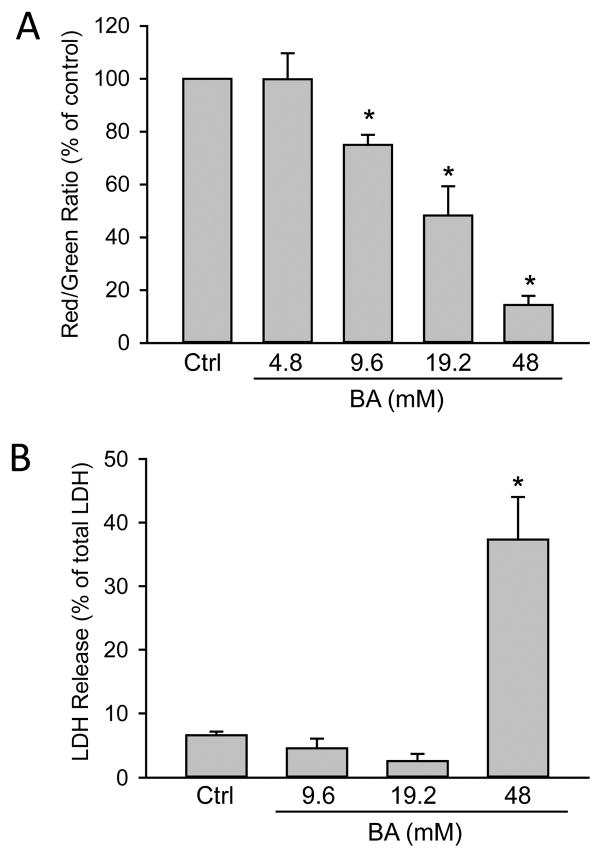
In order to assess the direct effect of BA on hepatocytes, freshly isolated primary mouse hepatocytes were treated with 5mM APAP, 0–5 μl/mL BA (equivalent to 0–46 mM BA) or both. APAP alone caused extensive necrotic cell death (70 ± 4%) as indicated by the release of LDH at 16 h post-APAP. Co-treatment with BA dose-dependently protected against APAP-induced cell injury (Fig. 5A). Importantly, this experiment was performed using pure hepatocytes in the absence of inflammatory cells. Both concentrations of BA that protected also showed reduced APAP-protein adduct formation compared to APAP alone (Fig. 5B), which supports our in vivo finding that the protection with BA correlates with inhibition of metabolic activation of APAP. Interestingly, BA did not prevent loss of mitochondrial membrane potential, as indicated by JC-1 assay (Fig. 5C). Since a previous study suggested that BA could inhibit the mitochondrial electron transport (Chazotte and Vanderkooi, 1981), we measured the direct effect of BA on mitochondria in primary mouse hepatocytes. BA treatment dose-dependently resulted in mitochondrial membrane potential loss at 4.5 h (Fig. 6A), and also caused significant cell death at the highest concentration of 46 mM at 16 h (Fig. 6B). Therefore, although BA protects against APAP-induced toxicity by inhibiting APAP bioactivation, its own toxicity needs to be considered at higher concentrations.
Figure 5.
Effects of BA on APAP-induced toxicity and BA toxicity in primary mouse hepatocytes. Cells were treated with APAP (5 mM), or BA (0–46 mM), or both. (A) LDH release 16 h-post APAP+BA treatment. (B) APAP protein adducts 4.5 h post-APAP. (C) Mitochondrial membrane potential 4.5 h-post APAP+BA, based on JC-1 red/green fluorescence ratio. *P<0.05 (compared to control). #P<0.05 (compared to APAP).
Figure 6.
BA toxicity in primary mouse hepatocytes. Cells were treated with 0–46 mM BA. (A) Mitochondrial membrane potential 4.5 h-post BA treatment. (B) LDH release 16 h-post BA treatment. Data represent means ± SE of n=3–4 separate experiments. *P<0.05 (compared to control).
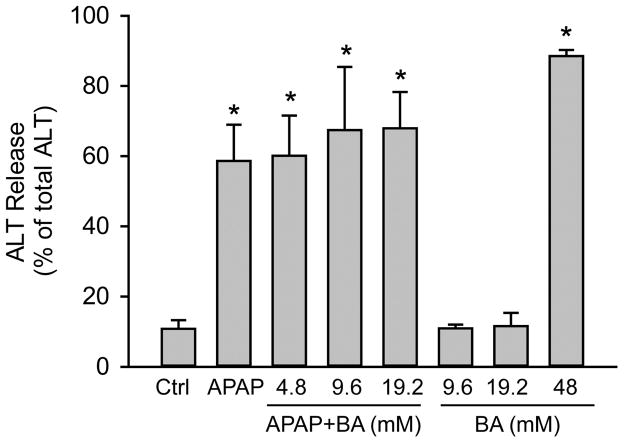
3.6 Effect of BA on APAP toxicity in primary human hepatocytes
Because it was suggested that BA could be an effective therapy for APAP poisoning in humans (Cai et al., 2014; Patman, 2014), we tested its effect on APAP toxicity in primary human hepatocytes. Surprisingly, BA did not prevent ALT release at 48 h post-APAP (Fig. 7), suggesting that BA does not protect in human hepatocytes. Moreover, BA itself was toxic at high concentrations, which is consistent with our findings in mouse hepatocytes (Fig. 7).
Figure 7.
BA did not inhibit APAP-induced toxicity in primary human hepatocytes. Cells were treated with APAP (10 mM), or BA (0–46 mM), or both, and ALT release was measured at 48 h after treatment. Data represent means ± SE of n=3 separate experiments. *P<0.05 (compared to control).
4. DISCUSSION
The main objective of this study was to investigate the hepatoprotective mechanism of BA in APAP-induced liver injury in vivo and in isolated hepatocytes. Our data clearly demonstrated that BA reduces liver injury and oxidant stress mainly by inhibiting cytochrome P450 enzymes. In addition, we found that BA itself is cytotoxic at high concentrations and is not effective in primary human hepatocytes.
4.1 BA inhibits the metabolic activation of APAP
It is well established that APAP hepatotoxicity is initiated by the formation of the reactive metabolite NAPQI, which depletes GSH and binds to cysteine residues on cellular proteins (Mitchell et al., 1973; Cohen et al., 1997; McGill and Jaeschke, 2013). Although protein adduct formation is insufficient to cause cell death directly, it is thought that these protein adducts, especially in mitochondria, are the first steps in a cascade of events that eventually leads to cell necrosis (Jaeschke et al., 2012a). This is supported by numerous publications showing that inhibition of P450 enzymes or their deficiency eliminates APAP toxicity (Jollow et al., 1973; Mitchell et al., 1973; Jaeschke, 1990; Jaeschke et al., 2006; Zaher et al., 1998). Therefore, any effect on the cytochrome P450-dependent metabolic activation of APAP would have a profound effect on the downstream mitochondrial dysfunction, oxidant stress, liver injury and sterile inflammatory response. For this reason, assessing the metabolic activation of APAP is a prerequisite for the proper interpretation of the mechanisms of any intervention. Inattention to this has led to misinterpretation of the mechanisms of protection in numerous studies in recent years, especially those with natural products (Jaeschke et al., 2013). Our data first demonstrated that BA delayed the early GSH depletion, especially at 30 min after APAP. Because there is an exponential decline of hepatic GSH levels after an APAP overdose during the first 30 min, this time point most accurately reflects the initial reactive metabolite formation in vivo (Jaeschke, 1990). As has been discussed (Jaeschke et al., 2011), later time points may be less informative about a potential difference in reactive metabolite formation as the slower declining GSH levels in the drug-treated group have time to catch up. This is illustrated by the results shown in Figure 2C, where there is a much more pronounced difference in GSH levels between APAP-treated animals with or without BA co-treatment at 30 min compared to 2 h.
Further evidence for the reduced metabolic activation of APAP after BA is the significantly attenuated protein adduct formation. This directly indicates the inhibitory effect of BA on the metabolic activation of APAP in vivo under the experimental conditions that showed protection against liver injury. This is corroborated by results from the 7EFC deethylase assay, which directly demonstrated that BA can dose-dependently inhibit P450 enzyme activities at concentrations ≥2.5mM. In addition, the inhibitory concentrations of BA used in this assay correlated very well with the reduced protein adduct formation and protection with BA treatment in isolated hepatocytes, where 9 mM of BA resulted in a 60% reduction in protein adduct formation and subsequently a significant reduction in cell death. As a consequence of the reduced metabolic activation of APAP, we observed less oxidant stress and mitochondrial dysfunction in BA co-treated mice, which was indicated by the reduced hepatic GSSG levels reflecting mainly GSSG formation in mitochondria (Jaeschke, 1990). A reduced APAP-induced oxidant stress due to inhibition of metabolic activation by the P450 inhibitor DMSO has been previously reported (Jaeschke et al., 2006). Likewise, the reduced release of intermembrane proteins such as Smac and AIF from the mitochondria can also be considered a down-stream effect. There is no known function for Smac in APAP hepatotoxicity; the elevated levels of Smac in the cytosol likely simply reflect the permeabilization of the outer membrane due to the initial formation of a Bax pore at early time points or due to matrix swelling and outer membrane rupture caused by the MPT at later time points (Bajt et al., 2008). In contrast, AIF release has pathophysiological consequences. AIF can translocate to the nucleus and is involved in nuclear degradation (Bajt et al., 2006, 2011). Thus, the reduced mitochondrial release of AIF and Smac indicates reduced mitochondrial dysfunction, likely as a consequence of reduced oxidant stress and the inhibited upstream metabolic activation.
4.2 APAP hepatotoxicity and sterile inflammatory response
In APAP hepatotoxicity, endogenous damage-associated molecular patterns (DAMPs) are released from the necrotic cells, act on Kupffer cells through toll-like receptors (TLRs), and subsequently lead to a sterile inflammatory response (Jaeschke et al., 2012b). It was previously reported that TLR4 was involved in APAP hepatotoxicity and mice deficient in TLR4 signaling were protected (Yohe et al., 2006; Shah et al., 2013). However, Cai et al. (2014) failed to reproduce these observations. Nevertheless, the authors still concluded that BA protects against APAP hepatotoxicity through TLR4 signaling. This is mainly based on the findings that BA is equally effective in a number of knockout mice deficient for genes related to innate immunity (TLR9, TLR2, RAGE, etc) but was not protective in TLR4-deficient mice, suggesting that TLR4 signaling was required for the protective effect of BA (Cai et al., 2014). The authors also implicated the involvement of the Nalp3 inflammasome (Cai et al., 2014). Although TLR4 signaling can induce IL-1β mRNA transcription and trigger pro-IL-1β protein formation, a second signal, e.g. ATP, through the purinergic receptor is necessary to activate the inflammasome and caspase-1 (Gross et al., 2011). There are reports suggesting a role of the Nalp3 inflammasome and purinergic receptors in APAP hepatotoxicity (Imaeda et al., 2009; Hoque et al., 2012), but the receptor antagonist used in this study appears to protect because it inhibits the metabolic activation of APAP (Xie et al., 2013). Importantly, the relatively minor increase of IL-1β after APAP hepatotoxicity actually has no impact on the liver injury (Williams et al., 2010b). Moreover, even large amounts of IL-1β generated by endotoxin or added exogenously do not affect the pathophysiology of APAP hepatotoxicity (Williams et al., 2010b). In addition, mice deficient in IL-1R gene expression or treated with the pan-caspase inhibitor Z-VD-fmk to block the inflammasome-mediated maturation of IL-1β were not protected from the injury, clearly indicating that IL-1β signaling might not be relevant in the liver injury process (Lawson et al., 1999; Williams et al., 2010b). Furthermore, it is impossible for IL-1R to directly induce cell death due to the lack of a cytoplasmic death domain in rodents (Sims and Smith, 2010). IL-1β, if generated in sufficient quantities, can increase the recruitment of immune cells, especially neutrophils into the liver (Williams et al., 2010b). However, there is extensive evidence against a role for neutrophils in the injury process after APAP overdose in mice (Lawson et al., 2000; Cover et al., 2006; James et al., 2003; Williams et al., 2010a) or humans (Williams et al., 2014). Taken together, the reduced inflammatory response reported by Cai et al. (2014) is likely the consequence of the reduced cell necrosis and DAMP release due to the reduced metabolic activation of APAP and attenuated mitochondrial dysfunction. However, it cannot be excluded that some direct effect of inflammatory mediators on intracellular signaling may have affected the injury process. The extensive inhibition of protein adduct formation in cultured mouse hepatocytes with moderately reduced cell death in vitro compared to the in vivo efficacy of BA supports this conclusion. The increasing number of studies implying a role of various DAMPs in the pathophysiology of APAP-induced liver injury also suggests that inflammatory mediators can have an impact on the intracellular mechanism of cell death (Cai et al., 2014; Chen et al., 2009; Imaeda et al., 2009; Singhal et al., 2007). An example is the role of IL-10 in suppressing this pro-inflammatory response and reducing APAP-induced injury by attenuating inducible nitric oxide synthase induction and peroxynitrite formation (Bourdi et al., 2002).
4.3 BA mitochondrial toxicity and its potential as a therapy for APAP hepatotoxicity
Consistent with a previous study in isolated mitochondria (Chazotte and Vanderkooi, 1981), our findings showed that BA itself can cause loss of mitochondrial membrane potential and induce cell death at high concentrations in both primary mouse and human hepatocytes. These findings indicate that BA may have a limited therapeutic window. Given that NAPQI and BA both target the mitochondrial electron transport chain, a negative effect of BA on APAP toxicity may even occur at doses lower than those causing BA toxicity. Thus, use of BA as drug against APAP-induced liver injury and acute liver failure may pose too much of a risk of adverse effects. In a recent study with human liver microsomes, it was reported that BA can inhibit Cyp2E1, Cyp3A4 and Cyp1A2 by 20–45% using model substrates for each P450 enzyme (Barnes et al., 2014). In contrast to these observations using microsomes from 5 different donors (Barnes et al., 2014), our findings indicate that at concentrations up to 19 mM, there was no protection in primary human hepatocytes. The exact reason for the lack of protection in PHH is unknown, but a species difference between human and mice may contribute. This could be an issue of BA uptake into hepatocytes or that in PHH the adverse effects may overcome the potential beneficial effect of BA. Although the mechanism of the lack of BA protection in PHH was not investigated in detail, our observations suggest that the adverse effects of BA dominate in human hepatocytes. This supports the overall conclusion that BA will not be a useful therapeutic option for acute liver failure in patients.
4.4 Summary and Conclusions
In summary, we demonstrated that the protection of BA in the murine model of APAP hepatotoxicity is mainly caused by inhibition of metabolic activation as indicated by a delay in GSH depletion and reduced protein adduct formation. This conclusion is also supported by the findings that BA is a direct inhibitor of P450 enzymes, and that there was protection in pure primary mouse hepatocyte cultures in which inflammatory cells are absent. The reduced inflammatory response after APAP-induced cell death is most likely a secondary effect of the reduced cell necrosis and DAMP release. Nevertheless, some impact of inflammatory mediator formation on the pathophysiology cannot be excluded. Because BA can trigger mitochondrial dysfunction at higher doses in both murine and human hepatocytes but did not show a significant protection against APAP-induced cell death in human hepatocytes, it is unlikely that BA is a realistic therapeutic option for treatment of APAP overdose or acute liver failure in patients.
Supplementary Material
RESEARCH HIGHLIGHTS.
Benzyl alcohol protects against APAP hepatotoxicity in mice and mouse hepatocytes
BA protects by inhibiting metabolic activation of APAP rather than inflammation
BA is toxic and does not protect against APAP-induced cell death in human hepatocytes
BA is not likely a realistic therapeutic option for treatment of APAP overdose
Acknowledgments
The authors thank Ken Dorko and the Cell Isolation Core for the supply of human hepatocytes and Drs. Sean Kumer and Timothy Schmitt for providing the liver samples. This investigation was supported in part by the National Institutes of Health grants R01 DK070195 and R01 AA12916 to H.J., and by grants from the National Center for Research Resources (5P20RR021940-07) and the National Institute of General Medical Sciences (8 P20 GM103549-07) from the National Institutes of Health. Additional support came from the “Training Program in Environmental Toxicology” T32 ES007079-26A2 (to M.R.M.) from the National Institute of Environmental Health Sciences and an award from the Biomedical Research Training Program (BRTP) from the University of Kansas Medical Center (to K.D.).
List of Abbreviations
- AIF
apoptosis-inducing factor
- ALT
alanine aminotransferase
- APAP
acetaminophen
- BA
benzyl alcohol
- CYP
cytochrome P450
- DAMPs
damage associated molecular patterns
- 7-EFC
7-ethoxy-4-trifluoromethylcoumarin
- GSH
glutathione
- GSSG
glutathione disulfide
- HPLC-ECD
high-pressure liquid chromatography with electrochemical detection
- LDH
lactate dehydrogenase
- MPT
mitochondrial permeability transition
- NAC
N-acetylcysteine
- NAPQI
N-acetyl-p-benzoquinone imine
- PHH
primary human hepatocytes
- ROS
reactive oxygen species
- Smac
second mitochondria-derived activator of caspases
- TLR
toll like receptor
Footnotes
CONFLICT OF INTEREST DISCLOSURE
The authors declare no competing financial interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kuo Du, Email: kdu@kumc.edu.
Mitchell R. McGill, Email: mmcgill@path.wustl.edu.
Yuchao Xie, Email: yxie@kumc.edu.
Hartmut Jaeschke, Email: hjaeschke@kumc.edu.
References
- Bajt ML, Cover C, Lemasters JJ, Jaeschke H. Nuclear translocation of endonuclease G and apoptosis-inducing factor during acetaminophen-induced liver injury. Toxicol Sci. 2006;94:217–225. doi: 10.1093/toxsci/kfl077. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Farhood A, Lemasters JJ, Jaeschke H. Mitochondrial bax translocation accelerates DNA fragmentation and cell necrosis in a murine model of acetaminophen hepatotoxicity. J Pharmacol Exp Ther. 2008;324:8–14. doi: 10.1124/jpet.107.129445. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Knight TR, Lemasters JJ, Jaeschke H. Acetaminophen-induced oxidant stress and cell injury in cultured mouse hepatocytes: protection by N-acetyl cysteine. Toxicol Sci. 2004;80:343–349. doi: 10.1093/toxsci/kfh151. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Lawson JA, Vonderfecht SL, Gujral JS, Jaeschke H. Protection against Fas receptor-mediated apoptosis in hepatocytes and nonparenchymal cells by a caspase-8 inhibitor in vivo: evidence for a postmitochondrial processing of caspase-8. Toxicol Sci. 2000;58:109–117. doi: 10.1093/toxsci/58.1.109. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Ramachandran A, Yan HM, Lebosfky M, Farhood A, Lemasters JJ, Jaeschke H. Apoptosis-inducing factor modulates mitochondrial oxidant stress in acetaminophen hepatotoxicity. Toxicol Sci. 2011;122:598–605. doi: 10.1093/toxsci/kfr116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes KJ, Rowland A, Polasek TM, Miners JO. Inhibition of human drug-metabolising cytochrome P450 and UDP-glucuronosyltransferase enzyme activities in vitro by uremic toxins. Eur J Clin Pharmacol. 2014;70:1097–1106. doi: 10.1007/s00228-014-1709-7. [DOI] [PubMed] [Google Scholar]
- Bourdi M, Masubuchi Y, Reilly TP, Amouzadeh HR, Martin JL, George JW, Shah AG, Pohl LR. Protection against acetaminophen-induced liver injury and lethality by interleukin 10: role of inducible nitric oxide synthase. Hepatology. 2002;35:289–298. doi: 10.1053/jhep.2002.30956. [DOI] [PubMed] [Google Scholar]
- Budnitz DS, Lovegrove MC, Crosby AE. Emergency department visits for overdoses of acetaminophen-containing products. Am J Prev Med. 2011;40:585–592. doi: 10.1016/j.amepre.2011.02.026. [DOI] [PubMed] [Google Scholar]
- Buters JT, Schiller CD, Chou RC. A highly sensitive tool for the assay of cytochrome P450 enzyme activity in rat, dog and man. Direct fluorescence monitoring of the deethylation of 7-ethoxy-4-trifluoromethyl-coumarin. Biochem Pharmacol. 1993;46:1577–1584. doi: 10.1016/0006-2952(93)90326-r. [DOI] [PubMed] [Google Scholar]
- Cai C, Huang H, Whelan S, Liu L, Kautza B, Luciano J, Wang G, Chen G, Stratimirovic S, Tsung A, Billiar TR, Zuckerbraun BS. Benzyl alcohol attenuates acetaminophen-induced acute liver injury in a Toll-like receptor-4-dependent pattern in mice. Hepatology. 2014;60:990–1002. doi: 10.1002/hep.27201. [DOI] [PubMed] [Google Scholar]
- Chazotte B, Vanderkooi G. Multiple sites of inhibition of mitochondrial electron transport by local anesthetics. Biochim Biophys Acta. 1981;636:153–161. doi: 10.1016/0005-2728(81)90088-8. [DOI] [PubMed] [Google Scholar]
- Chen GY, Tang J, Zheng P, Liu Y. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science. 2009;323:1722–1725. doi: 10.1126/science.1168988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SD, Pumford NR, Khairallah EA, Boekelheide K, Pohl LR, Amouzadeh HR, Hinson JA. Selective protein covalent binding and target organ toxicity. Toxicol Appl Pharmacol. 1997;143:1–12. doi: 10.1006/taap.1996.8074. [DOI] [PubMed] [Google Scholar]
- Corcoran GB, Racz WJ, Smith CV, Mitchell JR. Effects of N-acetylcysteine on acetaminophen covalent binding and hepatic necrosis in mice. J Pharmacol Exp Ther. 1985;232:864–872. [PubMed] [Google Scholar]
- Corcoran GB, Wong BK. Role of glutathione in prevention of acetaminophen-induced hepatotoxicity by N-acetyl-L-cysteine in vivo: studies with N-acetyl-D-cysteine in mice. J Pharmacol Exp Ther. 1986;238:54–61. [PubMed] [Google Scholar]
- Cover C, Liu J, Farhood A, Malle E, Waalkes MP, Bajt ML, Jaeschke H. Pathophysiological role of the acute inflammatory response during acetaminophen hepatotoxicity. Toxicol Appl Pharmacol. 2006;216:98–107. doi: 10.1016/j.taap.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Cover C, Mansouri A, Knight TR, Bajt ML, Lemasters JJ, Pessayre D, Jaeschke H. Peroxynitrite-induced mitochondrial and endonuclease-mediated nuclear DNA damage in acetaminophen hepatotoxicity. J Pharmacol Exp Ther. 2005;315:879–887. doi: 10.1124/jpet.105.088898. [DOI] [PubMed] [Google Scholar]
- Dambach DM, Watson LM, Gray KR, Durham SK, Laskin DL. Role of CCR2 in macrophage migration into the liver during acetaminophen-induced hepatotoxicity in the mouse. Hepatology. 2002;35:1093–1103. doi: 10.1053/jhep.2002.33162. [DOI] [PubMed] [Google Scholar]
- Du K, Williams CD, McGill MR, Jaeschke H. Lower susceptibility of female mice to acetaminophen hepatotoxicity: Role of mitochondrial glutathione, oxidant stress and c-jun N-terminal kinase. Toxicol Appl Pharmacol. 2014;281:58–66. doi: 10.1016/j.taap.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du K, Williams CD, McGill MR, Xie Y, Farhood A, Vinken M, Jaeschke H. The gap junction inhibitor 2-aminoethoxy-diphenyl-borate protects against acetaminophen hepatotoxicity by inhibiting cytochrome P450 enzymes and c-jun N-terminal kinase activation. Toxicol Appl Pharmacol. 2013;273:484–491. doi: 10.1016/j.taap.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du K, Xie Y, McGill MR, Jaeschke H. Pathophysiological significance of c-jun N-terminal kinase in acetaminophen hepatotoxicity. Expert Opin Drug Metab Toxicol. 2015 Jul;20:1–11. doi: 10.1517/17425255.2015.1071353. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross O, Thomas CJ, Guarda G, Tschopp J. The inflammasome: an integrated view. Immunol Rev. 2011;243:136–151. doi: 10.1111/j.1600-065X.2011.01046.x. [DOI] [PubMed] [Google Scholar]
- Gujral JS, Knight TR, Farhood A, Bajt ML, Jaeschke H. Mode of cell death after acetaminophen overdose in mice: apoptosis or oncotic necrosis? Toxicol Sci. 2002;67:322–328. doi: 10.1093/toxsci/67.2.322. [DOI] [PubMed] [Google Scholar]
- Hanawa N, Shinohara M, Saberi B, Gaarde WA, Han D, Kaplowitz N. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J Biol Chem. 2008;283:13565–13577. doi: 10.1074/jbc.M708916200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt MP, Cheng L, Ju C. Identification and characterization of infiltrating macrophages in acetaminophen-induced liver injury. J Leukoc Biol. 2008;84:1410–1421. doi: 10.1189/jlb.0308173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoque R, Sohail MA, Salhanick S, Malik AF, Ghani A, Robson SC, Mehal WZ. P2X7 receptor-mediated purinergic signaling promotes liver injury in acetaminophen hepatotoxicity in mice. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1171–G1179. doi: 10.1152/ajpgi.00352.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaeda AB, Watanabe A, Sohail MA, Mahmood S, Mohamadnejad M, Sutterwala FS, Flavell RA, Mehal WZ. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J Clin Invest. 2009;119:305–314. doi: 10.1172/JCI35958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H. Glutathione disulfide formation and oxidant stress during acetaminophen-induced hepatotoxicity in mice in vivo: the protective effect of allopurinol. J Pharmacol Exp Ther. 1990;255:935–941. [PubMed] [Google Scholar]
- Jaeschke H. Acetaminophen: Dose-dependent drug hepatotoxicity and acute liver failure in patients. Dig Dis. 2015;33:464–471. doi: 10.1159/000374090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H, Cover C, Bajt ML. Role of caspases in acetaminophen-induced liver injury. Life Sci. 2006;78:1670–1676. doi: 10.1016/j.lfs.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, McGill MR, Ramachandran A. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: lessons learned from acetaminophen hepatotoxicity. Drug Metab Rev. 2012a;44:88–106. doi: 10.3109/03602532.2011.602688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H, McGill MR, Williams CD, Ramachandran A. Current issues with acetaminophen hepatotoxicity--a clinically relevant model to test the efficacy of natural products. Life Sci. 2011;88:737–745. doi: 10.1016/j.lfs.2011.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H, Mitchell JR. Use of isolated perfused organs in hypoxia and ischemia/reperfusion oxidant stress. Methods Enzymol. 1990;186:752–759. doi: 10.1016/0076-6879(90)86175-u. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Williams CD, McGill MR, Xie Y, Ramachandran A. Models of drug-induced liver injury for evaluation of phytotherapeutics and other natural products. Food Chem Toxicol. 2013;55:279–289. doi: 10.1016/j.fct.2012.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H, Williams CD, Ramachandran A, Bajt ML. Acetaminophen hepatotoxicity and repair: the role of sterile inflammation and innate immunity. Liver Int. 2012b;32:8–20. doi: 10.1111/j.1478-3231.2011.02501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James LP, McCullough SS, Knight TR, Jaeschke H, Hinson JA. Acetaminophen toxicity in mice lacking NADPH oxidase activity: role of peroxynitrite formation and mitochondrial oxidant stress. Free Radic Res. 2003;37:1289–1297. doi: 10.1080/10715760310001617776. [DOI] [PubMed] [Google Scholar]
- Jollow DJ, Mitchell JR, Potter WZ, Davis DC, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. II Role of covalent binding in vivo. J Pharmacol Exp Ther. 1973;187:195–202. [PubMed] [Google Scholar]
- Knight TR, Ho YS, Farhood A, Jaeschke H. Peroxynitrite is a critical mediator of acetaminophen hepatotoxicity in murine livers: protection by glutathione. J Pharmacol Exp Ther. 2002;303:468–475. doi: 10.1124/jpet.102.038968. [DOI] [PubMed] [Google Scholar]
- Kon K, Kim JS, Jaeschke H, Lemasters JJ. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology. 2004;40:1170–1179. doi: 10.1002/hep.20437. [DOI] [PubMed] [Google Scholar]
- Larson AM. Acetaminophen hepatotoxicity. Clin Liver Dis. 2007;11:525–548. doi: 10.1016/j.cld.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Lawson JA, Farhood A, Hopper RD, Bajt ML, Jaeschke H. The hepatic inflammatory response after acetaminophen overdose: Role of neutrophils. Toxicol Sci. 2000;54:509–516. doi: 10.1093/toxsci/54.2.509. [DOI] [PubMed] [Google Scholar]
- Lawson JA, Fisher MA, Simmons CA, Farhood A, Jaeschke H. Inhibition of Fas receptor (CD95)-induced hepatic caspase activation and apoptosis by acetaminophen in mice. Toxicol Appl Pharmacol. 1999;156:179–186. doi: 10.1006/taap.1999.8635. [DOI] [PubMed] [Google Scholar]
- Manthripragada AD, Zhou EH, Budnitz DS, Lovegrove MC, Willy ME. Characterization of acetaminophen overdose-related emergency department visits and hospitalizations in the United States. Pharmacoepidemiol Drug Saf. 2011;20:819–826. doi: 10.1002/pds.2090. [DOI] [PubMed] [Google Scholar]
- McGill MR, Jaeschke H. Metabolism and disposition of acetaminophen: recent advances in relation to hepatotoxicity and diagnosis. Pharm Res. 2013;30:2174–2187. doi: 10.1007/s11095-013-1007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Jaeschke H. Mechanistic biomarkers in acetaminophen-induced hepatotoxicity and acute liver failure: from preclinical models to patients. Expert Opin Drug Metab Toxicol. 2014;10:1005–1017. doi: 10.1517/17425255.2014.920823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Lebofsky M, Norris HR, Slawson MH, Bajt ML, Xie Y, Williams CD, Wilkins DG, Rollins DE, Jaeschke H. Plasma and liver acetaminophen-protein adduct levels in mice after acetaminophen treatment: dose-response, mechanisms, and clinical implications. Toxicol Appl Pharmacol. 2013;269:240–249. doi: 10.1016/j.taap.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Williams CD, Xie Y, Ramachandran A, Jaeschke H. Acetaminophen-induced liver injury in rats and mice: comparison of protein adducts, mitochondrial dysfunction, and oxidative stress in the mechanism of toxicity. Toxicol Appl Pharmacol. 2012;264:387–394. doi: 10.1016/j.taap.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers LL, Beierschmitt WP, Khairallah EA, Cohen SD. Acetaminophen-induced inhibition of hepatic mitochondrial respiration in mice. Toxicol Appl Pharmacol. 1988;93:378–387. doi: 10.1016/0041-008x(88)90040-3. [DOI] [PubMed] [Google Scholar]
- Mitchell JR, Jollow DJ, Potter WZ, Davis DC, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. I Role of drug metabolism. J Pharmacol Exp Ther. 1973;187:185–194. [PubMed] [Google Scholar]
- Nelson SD. Molecular mechanisms of the hepatotoxicity caused by acetaminophen. Semin Liver Dis. 1990;10:267–278. doi: 10.1055/s-2008-1040482. [DOI] [PubMed] [Google Scholar]
- Ni HM, Boggess N, McGill MR, Lebofsky M, Borude P, Apte U, Jaeschke H, Ding WX. Liver-specific loss of Atg5 causes persistent activation of Nrf2 and protects against acetaminophen-induced liver injury. Toxicol Sci. 2012;127:438–450. doi: 10.1093/toxsci/kfs133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patman G. Benzyl alcohol limits acute liver injury. Nat Rev Gastroenterol Hepatol. 2014;11:396. doi: 10.1038/nrgastro.2014.77. [DOI] [PubMed] [Google Scholar]
- Prescott LF, Park J, Ballantyne A, Adriaenssens P, Proudfoot AT. Treatment of paracetamol (acetaminophen) poisoning with N-acetylcysteine. Lancet. 1977;27:432–434. doi: 10.1016/s0140-6736(77)90612-2. [DOI] [PubMed] [Google Scholar]
- Ramachandran A, Lebofsky M, Baines CP, Lemasters JJ, Jaeschke H. Cyclophilin D deficiency protects against acetaminophen-induced oxidant stress and liver injury. Free Radic Res. 2011;45:156–164. doi: 10.3109/10715762.2010.520319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito C, Zwingmann C, Jaeschke H. Novel mechanisms of protection against acetaminophen hepatotoxicity in mice by glutathione and N-acetylcysteine. Hepatology. 2010;51:246–254. doi: 10.1002/hep.23267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah N, Montes de Oca M, Jover-Cobos M, Tanamoto K, Muroi M, Sugiyama K, Davies NA, Mookerjee RP, Dhar DK, Jalan R. Role of toll-like receptor 4 in mediating multiorgan dysfunction in mice with acetaminophen induced acute liver failure. Liver Transpl. 2013;19:751–761. doi: 10.1002/lt.23655. [DOI] [PubMed] [Google Scholar]
- Sims JE, Smith DE. The IL-1 family: regulators of immunity. Nat Rev Immunol. 2010;10:89–102. doi: 10.1038/nri2691. [DOI] [PubMed] [Google Scholar]
- Singhal R, Ganey PE, Roth RA. Complement activation in acetaminophen-induced liver injury in mice. J Pharmacol Exp Ther. 2012;341:377–385. doi: 10.1124/jpet.111.189837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CD, Antoine DJ, Shaw PJ, Benson C, Farhood A, Williams DP, Kanneganti TD, Park BK, Jaeschke H. Role of the Nalp3 inflammasome in acetaminophen-induced sterile inflammation and liver injury. Toxicol Appl Pharmacol. 2011;252:289–297. doi: 10.1016/j.taap.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CD, Bajt ML, Farhood A, Jaeschke H. Acetaminophen-induced hepatic neutrophil accumulation and inflammatory liver injury in CD18-deficient mice. Liver Int. 2010a;30:1280–1292. doi: 10.1111/j.1478-3231.2010.02284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CD, Bajt ML, Sharpe MR, McGill MR, Farhood A, Jaeschke H. Neutrophil activation during acetaminophen hepatotoxicity and repair in mice and humans. Toxicol Appl Pharmacol. 2014;275:122–133. doi: 10.1016/j.taap.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CD, Farhood A, Jaeschke H. Role of caspase-1 and interleukin-1beta in acetaminophen-induced hepatic inflammation and liver injury. Toxicol Appl Pharmacol. 2010b;247:169–178. doi: 10.1016/j.taap.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, McGill MR, Dorko K, Kumer SC, Schmitt TM, Forster J, Jaeschke H. Mechanisms of acetaminophen-induced cell death in primary human hepatocytes. Toxicol Appl Pharmacol. 2014;279:266–274. doi: 10.1016/j.taap.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Williams CD, McGill MR, Lebofsky M, Ramachandran A, Jaeschke H. Purinergic receptor antagonist A438079 protects against acetaminophen-induced liver injury by inhibiting P450 isoenzymes, not by inflammation activation. Toxicol Sci. 2013;131:325–35. doi: 10.1093/toxsci/kfs283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yohe HC, O’Hara KA, Hunt JA, Kitzmiller TJ, Wood SG, Bement JL, Bement WJ, Szakacs JG, Wrighton SA, Jacobs JM, Kostrubsky V, Sinclair PR, Sinclair JF. Involvement of Toll-like receptor 4 in acetaminophen hepatotoxicity. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1269–G1279. doi: 10.1152/ajpgi.00239.2005. [DOI] [PubMed] [Google Scholar]
- You Q, Holt M, Yin H, Li G, Hu CJ, Ju C. Role of hepatic resident and infiltrating macrophages in liver repair after acute injury. Biochem Pharmacol. 2013;86:836–843. doi: 10.1016/j.bcp.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaher H, Buters JT, Ward JM, Bruno MK, Lucas AM, Stern ST, Cohen SD, Gonzalez FJ. Protection against acetaminophen toxicity in CYP1A2 and CYP2E1 double-null mice. Toxicol Appl Pharmacol. 1998;152:193–199. doi: 10.1006/taap.1998.8501. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.