Abstract
Sapienic acid, 16:1n-10 is the most abundant unsaturated fatty acid on human skin where its synthesis is mediated by FADS2 in the sebaceous glands. The FADS2 product introduces a double bond at the Δ6, Δ4 and Δ8 positions by acting on at least ten substrates, including 16:0, 18:2n-6, and 18:3n-3. Our aim was to characterize the competition for accessing FADS2 mediated Δ6 desaturation between 16:0 and the most abundant polyunsaturated fatty acids (PUFA) in the human diet, 18:2n-6 and 18:3n-3, to evaluate whether competition may be relevant in other tissues and thus linked to metabolic abnormalities associated with FADS2 or fatty acid levels. MCF7 cells stably transformed with FADS2 biosynthesize 16:1n-10 from exogenous 16:0 in preference to 16:1n-7, the immediate product of SCD highly expressed in cancer cell lines, and 16:1n-9 via partial β-oxidation of 18:1n-9. Increasing availability of 18:2n-6 or 18:3n-3 resulted in decreased bioconversion of 16:0 to 16:1n-10, simultaneously increasing the levels of highly unsaturated products. FADS2 cells accumulate the desaturation-elongation products 20:3n-6 and 20:4n-3 in preference to the immediate desaturation products 18:3n-6 and 18:4n-3 implying prompt/coupled elongation of the nascent desaturation products. MCF7 cells incorporate newly synthesized 16:1n-10 into phospholipids. These data suggest that excess 16:0 due to, for instance, de novo lipogenesis from high carbohydrate or alcohol consumption, inhibits synthesis of highly unsaturated fatty acids, and may in part explain why supplemental preformed EPA and DHA in some studies improves insulin resistance and other factors related to diabetes and metabolic syndrome aggravated by excess calorie consumption.
Keywords: Palmitic acid, Sapienic acid, Δ6 desaturase, fatty acid desaturases, monounsaturated fatty acids, polyunsaturated fatty acids
Introduction
Saturated and unsaturated fatty acids are ubiquitous, essential components of cell membranes with many structural and metabolic functions. In mammals, the products of 3 genes (SCD, FADS1 and FADS2) desaturate specific fatty acids [1]. Among these, FADS2 is most promiscuous, producing a classical transcript that desaturates in three specific carbon positions along the chain of at least ten substrates: eight polyunsaturated fatty acids (PUFA), one monounsaturated fatty acid (MUFA) when PUFA are low, and one saturate, palmitic acid 16:0→16:1n-10 (sapienic acid) in human skin (Table 1).
Table 1.
FADS2 catalyzes reactions of at least 10 substrates at three positions.
| Desaturation Position | Comments | |
|---|---|---|
| Δ 4-desaturation | 22:5n-3→22:6n-3 | Slow |
| 22:4n-6→22:5n-6 | Slow | |
| Δ 6-desaturation | 18:3n-3→18:4n-3 | Fast |
| 18:2n-6→18:3n-6 | Fast | |
| 18:1n-9→18:2n-9 | When PUFA are low | |
| 24:5n-3→24:6n-3 | ||
| 24:4n-6→24:5n-6 | ||
| 16:0→16:1n-10 | When 16:0 is in excess of SCD capacity | |
| Δ 8-desaturation | 20:3n-3→20:4n-3 | Slow and when C18 PUFA are low |
| 20:2n-6→20:3n-6 | Slow and when C18 PUFA are low |
Stearoyl CoA desaturase (SCD) codes for a Δ9-desaturase protein catalyzing the biosynthesis of MUFA from dietary or de novo synthesis, principally stearic acid (18:0→18:1n-9) but also palmitic acid (16:0→16:1n-7) [2]. In humans, pigs, chickens and ruminants, two isoforms of SCD have been reported (SCD1, SCD5), whereas, in mice four SCD isoforms (SCD1-4), sharing Δ9-desaturase function have been identified [3]. Unlike humans, rodent skin lipid secretions are dominated by 9-desaturated monoenes [4]. A targeted disruption of SCD1 (SCD1−/−), the predominant isoform in mice, caused >70% reduction in 16:1n-7 and 28% reduction in 18:1n-9, with >2-fold increase in the Δ6-desaturated product 16:1n-10 in the preputial glands residing on the skin [5]. Inhibition of SCD activity in microsomal preparations from rat liver showed formation of very low levels of 16:1n-10 [6]. Both these observations in rodents indicate that the SCD1 protein operates to catalyze 16:0→16:1n-7, but when SCD is limiting the FADS2 protein catalyzes 16:0→16:1n-10 which then accumulates.
Uniquely among hair bearing mammals, human sebum sapienic acid is the most abundant unsaturated fatty acid, constituting 25% of total fatty acids [7]. SCD reportedly is not active in human sebaceous glands [8]. In the absence of SCD mediated Δ9-desaturation, FADS2 mediated 16:0→16:1n-10 yields sapienic acid as the predominant unsaturate on human skin.
Numerous chronic conditions of widespread concern, for instance diabetes and both alcohol and non-alcohol induced fatty liver diseases, are at least in part caused by intake of excess carbohydrate and alcohol, leading to de novo lipogenesis. Excess carbohydrate and alcohol can both be converted to fat via acetate, which seeds cytosolic fatty acid synthase (FASN) in liver and adipose and produces primarily 16:0-CoA. 16:0-CoA may then translocate into the ER where the protein products of SCD [9], FADS2 and the fatty acid chain elongation system reside, most likely catalyzing 16:0→18:0 prior to action of SCD via 18:0→18:1n-9. Numerous studies investigate the possibility that the n-3 highly unsaturated fatty acids (HUFA) EPA and DHA taken as supplements can partially ameliorate symptoms of chronic disease. Viewed alternatively, supplementary HUFA may be correcting local deficiencies. We speculated that the excess palmitate could interfere with the conversion of FADS2 mediated conversion of PUFA to HUFA, thereby reducing their availability for metabolically essential functions.
MCF7 human breast cancer cells have no detectable Δ6-desaturase activity but like other cancer cell lines have active SCD mediated Δ9-desaturase activity. MCF7 cells do not synthesize LCPUFA from 18:2n-6 or 18:3n-3 unless transfected with FADS2. Our aim was to characterize competition between 16:0 and 18:2n-6 or 18:3n-3 for desaturation mediated by FADS2 using a stably transformed MCF7 human cell system.
Materials and methods
RNA Isolation, cDNA synthesis and vector construction
Total RNA was isolated from a 12-week old baboon neonate liver tissue, cDNA synthesized and open reading frames of baboon FADS1 (GenBank Accession# EF531577) and FADS2 (GenBank Accession# EU780003) were cloned into pcDNA3.1 expression vector as previously described [10]. FADS2 from humans and baboons share 99% sequence homology [11].
Studies on baboons were approved by Cornell University (IACUC, protocol # 02–105) and Texas Biomedical Research Institute (formerly the Southwest Foundation for Biomedical Research) Institutional Animal Care and Use Committees.
Mammalian cell culture and fatty acid supplementation
MCF7 cells were grown in MEM-α with 10% FBS, 10 mM HEPES buffer and 0.5 mg/ml geneticin in a humidified environment at 37°C with 5% CO2. Dose and competition studies were performed using MCF7 cells stably expressing FADS1, FADS2 and control (empty vector). Both dose and competition studies used 1×106 cells. Dosage study was carried out by using albumin bound palmitic acid (16:0) substrate incubated for 24 hour (h) at concentrations of 5-100 μM. After 24 h, the incubated cells were washed twice with 1× PBS and harvested using trypsin for fatty acid analysis. Competition studies were carried out by incubating FADS2 cells for 24 h with 1) constant molar concentration of 16:0 (50 μM) and various concentrations of 18:2n-6 (20 μM, 50 μM and 100 μM) 2) constant molar concentration of 16:0 (50 μM) and various concentrations of 18:3n-3 (20-100 μM) 3) constant molar concentration of 18:2n-6 (50 μM) and various concentrations of 16:0 (20-100 μM) 4) constant molar concentration of 18:3n-3 (50 μM) and various concentrations of 16:0 (20-100 μM) 5) various concentrations of 16:0 (30-90 μM) and constant molar concentrations of 18:2n-6 (20 μM) and 18:3n-3 (20 μM) together. After 24 h incubation, cells were washed twice with 1× PBS and harvested using trypsin for fatty acid analysis.
Fatty acid extraction and analysis
Harvested cell pellets were used for fatty acid extraction and analysis. Fatty acid methyl esters (FAME) were prepared using modified one-step method of Garces and Mancha [12]. Methylated fatty acids were structurally identified by gas chromatography covalent adduct chemical ionization tandem mass spectrometry (GC-CACI-MS/MS) [13, 14]. An equal weight FAME mixture (462A; Nu-Chek Prep, Inc.) was used to calculate response factors on a daily basis, and peak areas were normalized to 18:1n-7. 18:1n-7 was used for normalization because it did not change between FADS2, FADS1 and Control treatments. GC analyses were performed in triplicate.
Thin layer chromatography (TLC) analysis
Silica gel G, 20×20 TLC plates (Analtech Inc, Newark, DE) before performing TLC were activated at 100°C for 1 h. Lipids from the FADS2 cells were extracted according to the modified Bligh and Dyer method [15]. Extracted lipid samples along with the lipid standards and piglet plasma were loaded on the TLC plate and developed first in chloroform : methanol : acetic acid : water (25:15:4:2, v:v:v:v), dried, and then redeveloped in hexane : diethyl-ether : formic acid (80:20:2, v:v:v). Lipid bands were visualized with iodine vapor. Visualized bands were carefully scrapped, methylated and analyzed by GC-FID.
Chemicals
Fatty acids (16:0, 18:2n-6, and 18:3n-3) were purchased from Nu-Chek Prep (Elysian, MN). Solvents for lipid extraction were HPLC grade from Sigma-Aldrich (St. Louis, MO) and Burdick & Jackson (Muskegon, MI). Media, FBS and reagents for cell culture work were obtained from Life Technologies (NY), Corning (MA) and Thermo Fisher Scientific (MA).
Results
The two fatty acid products resulting from FADS2 operation on 16:0 and 18:1n-9 β-oxidation, 16:1n-10 and 16:1n-9, respectively, have nearly identical retention times under most standard GC conditions (e.g. [8]). To enable positive identification and sensitive detection, and avoid long GC times [16], we used CACI-MS/MS to distinguish 16:1n-10 from 16:1n-9. Figure 1A presents the CACI-MS spectrum of 16:1, identical for both isomers, showing the familiar peaks at m/z 322, 269, 237, and 219, corresponding to the [M+54]+, [MH]+, [M+54-32]+, and [MH+54-32-18]+ ions, respectively, characteristic of a 16:1 FAME. Figure 1B is the collisionally induced activation spectrum (MS/MS) of [M+54]+ of 16:1n-9, yielding ions at m/z 224 and 208 corresponding to the α and ω diagnostic ions, respectively [17]. Figure 1C shows the MS/MS spectrum of 16:1n-10 with diagnostic ions at m/z 222 and 210, thus the diagnostic ions establish double bond locations unequivocally. In the present chromatographic overlaps, some common ions from ion series (m/z 14 spacings) appear, however these are minor.
Figure 1.
FADS2 action on 16:0 (A) CACI-MS of 16:1 showing the characteristic ions (m/z 322, 269, 237, 219); (B) CACI-MS/MS of 16:1n-9 showing the diagnostic ions (m/z 224, 208); (C) CACI-MS/MS of 16:1n-10 showing the diagnostic ions (m/z 222, 210).
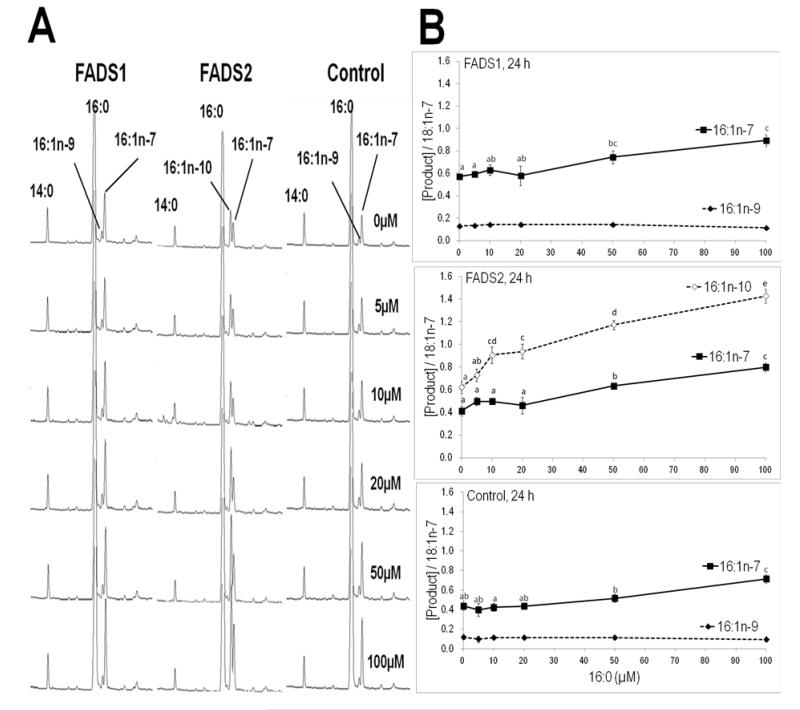
MCF7 cells stably expressing FADS1, FADS2, and control (empty vector) were incubated with various concentrations (5 to 100 uM) of albumin bound palmitic acid (16:0). Control and FADS1 cells do not synthesize 16:1n-10 but rather synthesize 16:1n-9, likely from chain shortening of 18:1n-9 (Figure 2). In contrast, FADS2 cells accumulate 16:1n-10 while 16:1n-9 remains at trace levels. Higher amounts of newly synthesized 16:1n-10 was detected with increasing concentrations of the substrate. Basal 16:1n-10 levels in these cells was greater than 16:1n-7, the SCD product, and rose by about 50% when incubated with 10 μM added 16:0, while 16:1n-7 did not rise significantly. A similar minor rise in 16:1n-7 is observed with all cells. No evidence of FADS2 action on other saturated fatty acids 14:0 or 18:0 was detected. Subsequent experiments were performed with FADS2 cells only.
Figure 2.
Chromatograms of FADS1, FADS2 and Control stable MCF7 cells dosed with 16:0 (left panel, A) and conversion of 16:0 (right panel, B) by FADS1, FADS2 and Control stable MCF7 cells at various concentrations. FADS2 cells accumulate higher amounts of 16:1n-10 with increasing concentrations of the substrate. FADS1 and Control cells show only 16:1n-9, no synthesis of 16:1n-10 was observed. 16:1n-7 synthesis is mediated by stearoyl-CoA desaturase (SCD). The y-axis is presented as product(s) of 16:0 with synthesis mediated by FADS2 or SCD. The 16:1n-9 product is biosynthesized via partial β-oxidation of 18:1n-9.
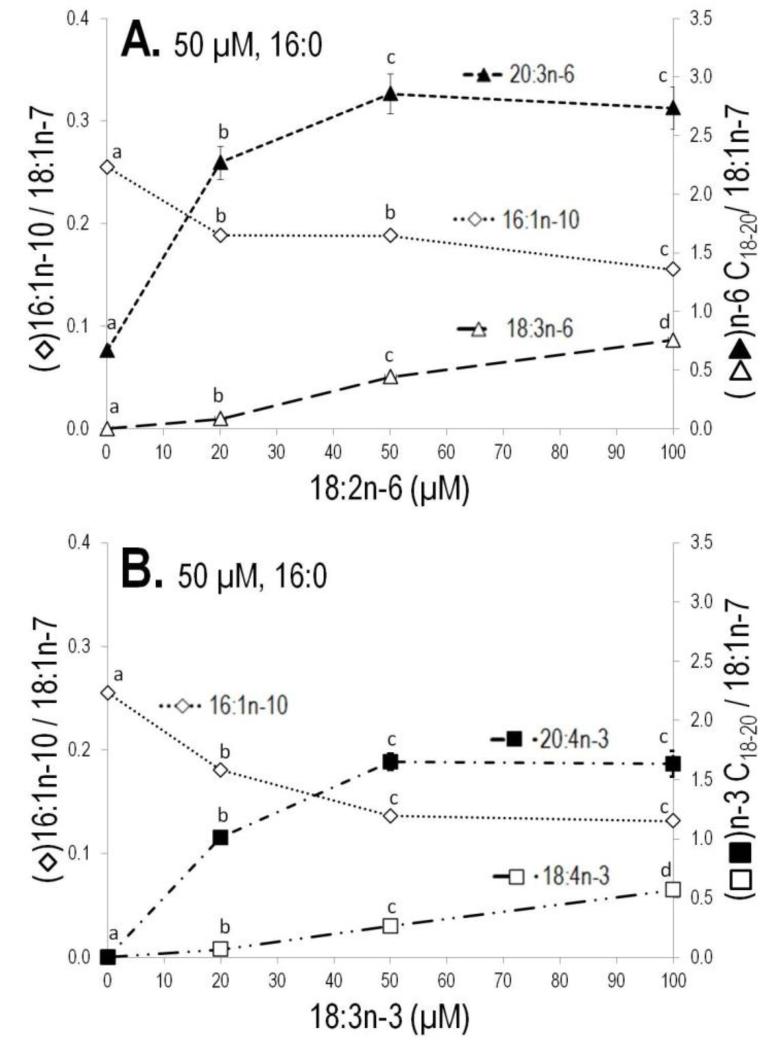
Competition for FADS2 mediated desaturation was investigated by holding 16:0 constant in media and ramping 18:2n-6 or 18:3n-3 either separately or as an equimolar mixture, or holding 18:2n-6 and/or 18:3n-3 constant and ramping 16:0 concentrations. FADS2 cells were incubated for 24 h with 50 μM of 16:0 and various concentrations (20 μM, 50 μM and 100 μM) of 18:2n-6 (Figure 3A). At baseline, no detectable 18:3n-6 is found in FADS2 cells; it rises linearly with increasing concentrations of 18:2n-6. The elongation product (20:3n-6) of 18:3n-6 rises sharply at 20 μM 18:2n-6 and plateaus at higher concentrations, indicating prompt (channeled) elongation of the 18:3n-6 product before it can accumulate. As expected, 16:1n-10 decreased with increasing concentrations of 18:2n-6, most significantly at 20 μM. Analogous trends were observed for incubations of ramped concentrations of 18:3n-3 (Figure 3B).
Figure 3.
Competition study for Δ6-desaturase between 16:0 and polyunsaturated fatty acids (18:2n-6 or 18:3n-3). (A) 24 h Incubation with the constant molar concentration of 16:0 along with the various concentrations of 18:2n-6 and (B) 18:3n-3.
Consistent trends were found when FADS2 cells were incubated with various concentrations of 16:0 and constant molar concentrations of 18:2n-6 or 18:3n-3 (50 μM) or combined 18:2n-6-18:3n-3 at 20 μM each (Figure 4). 16:1n-10 increased linearly with similar slopes for 18:2n-6 and 18:3n-3 separate incubations (Figure 4A and 4B), and similarly increased for the combined incubation (Figure 4C). Again, the C20 product concentrations were more strongly affected by the 16:0 concentrations, reflecting channeled desaturation-elongation. Slopes for 20:3n-6 and 20:4n-3 were similar in both incubations. We also observed even stronger inhibition of the elongated products 20:2n-6 and 20:3n-3 formation (Figures 4A and 4B), respectively, possibly reflecting competition with the nascent 6-desaturated products. In the combined incubation, the 16:0 elongation product 18:0 increased and its SCD desaturation product increased more, again suggesting channeling (Figure 4D).
Figure 4.
Competition study for Δ6-desaturase between polyunsaturated fatty acids (18:2n-6 or/and 18:3n-3) and 16:0. (A) 24 h incubation with constant molar concentration of 18:2n-6 along with various concentrations of 16:0. In a dose dependent manner, 16:1n-10 rises while the desaturation-elongation product 20:3n-6 and the elongation product 20:2n-6 falls. The immediate FADS2 product 18:3n-6 is unaffected except for the highest concentration. The slope of the 20:2n-6 decrease (s20:2n-6) is greater than the slope of the decrease in 20:3n-6 (s20:3n-6) suggesting nascent 18:3n-6 outcompetes the greater concentration 18:2n-6 for elongation. (B) 18:3n-3 constant 16:0 variable shows results analogous to 18:2n-6 incubations in (A). (C and D) Combined 18:2n-6 and 18:3n-3 incubations. The immediate desaturation products are nearly unchanged with dose. The n-3 desaturation-elongation product 20:4n-3 decreases in a dose-dependent manner while the n-6 desaturation-elongation product 20:3n-6 decreases only at the highest concentration, indicating that 16:0 competes more effectively with 18:3n-3 than with 18:2n-6. The 16:0 dose dependent rise in 18:1n-9 is greater than that of 18:0 (s18:1>s18:0) again consistent with coupled desaturation and elongation.
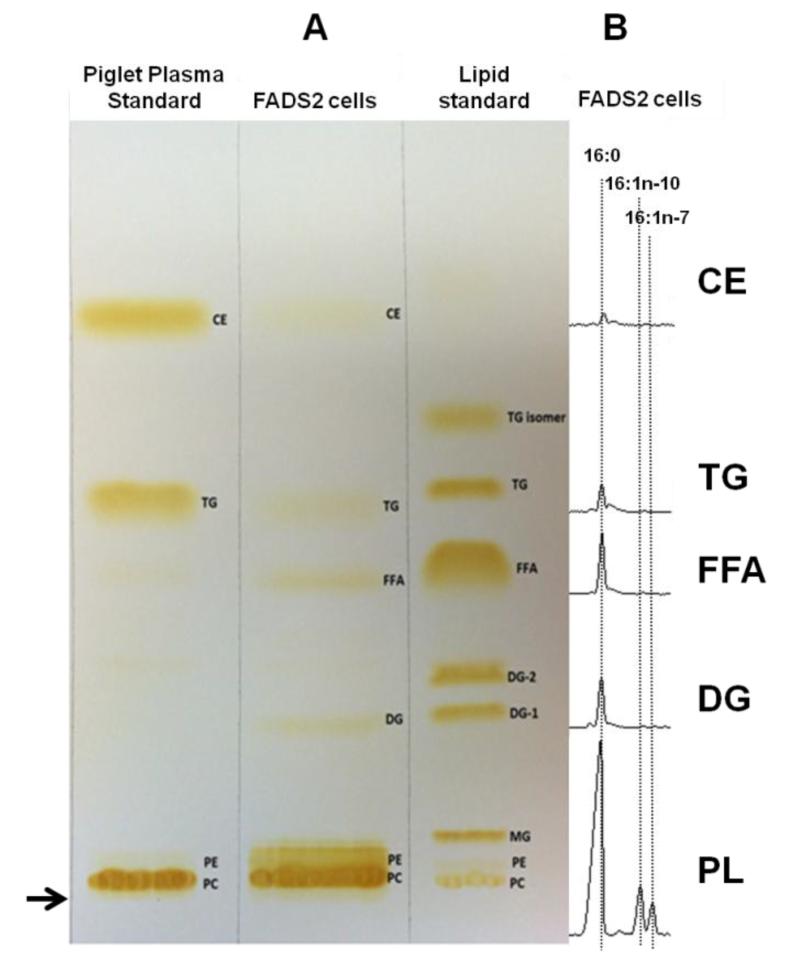
To determine the fate of FADS2 and SCD products 16:1n-10 and 16:1n-7, respectively, TLC plates were used to separate lipid classes of cultured MCF7 cells stably expressing FADS2 (Figure 5A). The particular cells used in these experiments correspond to the cells presented in Figure 2B, center panel, not treated with any exogenous fatty acid (0 μM 16:0 point), made with 16:0 imported from media and from de novo synthesis. All separated bands were identified based on lipid standards run on the same plate. Figure 5B shows the GC peaks in the region of the relevant peaks from various fractions. PL constituted > 90% of lipids. Phosphatidylcholine (PC) and phosphatidylethanolamine (PE) were combined for these analyses (PL). 16:1n-10 and 16:1n-7 were detected only in PL fraction and were, at most, trace in other fractions, despite the presence of 16:0 in all other fractions.
Figure 5.
Thin-layer chromatography of the lipid extracts of the FADS2 cells and the lipid standards. The arrow → denotes the origin. (A) Lipid extracts were loaded onto a 20 × 20 silica gel G plate, developed and visualized by iodine vapor. CE, cholesteryl esters; TG, triglycerides; FFA, free fatty acids; DG, diacylglycerols; PL, phospholipids including phosphatidylethanolamines (PE) and phosphatidylcholines (PC). (B) Each fraction was carefully scrapped and extracted, methylated and analyzed by GC-FID. 16:0 is seen in all fractions. 16:1n-10 and 16:1n-7 are detected only in PL fraction.
Discussion
The fatty acid desaturation activities mediated by the FADS and SCD genes are usually considered separately because their substrate specificities are most important for unsaturated, and saturated fatty acids, respectively. Palmitic acid is the unique exception, serving as a substrate for both desaturases. Further, in human skin palmitate desaturation is unique among mammals being mediated by the action of FADS2 to produce 16:1n-10, the most prominent unsaturate in human sebum. FADS2 principally mediates Δ6-desaturation of 18:2n-6 and 18:3n-3; no activity of SCD products towards 18:2n-6 and 18:3n-3 has been reported.
The 16:1 product of FADS2, 16:1n-10, is not separated from the oxidation product of oleic acid, 16:1n-9, in most GC analyses of the corresponding methyl esters, nor can the two be distinguished from one another or 16:1n-7 by single stage mass spectra, thus special methods are needed for positive identification. Recently using 100 m ionic liquid based capillary column and performing GC-MS with electronic impact (EI) ionization revealed 16:1n-10 and other n-10s in human hair and nail samples [18]. A 60 m column was sufficient to detect 16:1n-10 in human plasma, however conformation of double bond position was accomplished with offline fragmentation methods. Importantly, that work demonstrated the presence of sapienic acid in blood, and reported enrichment of >5% in LDL triacylglycerols and also in cholesteryl esters [19], while it is found in triacylglycerols and wax esters in sebum [20, 21] our data demonstrate that a shift in the cell’s balance between SCD and FADS2 can influence 16:1 product distribution and enrichment in PL. Sapienic acid detection in human plasma and its appearance in physiologically active phospholipid pools in breast cells demonstrate that it may be biosynthesized in internal tissues and thus more prevalent than currently appreciated. The recent interest around palmitoleate (16:1n-7) as a lipokine [22], the possibility for unsuspected overlapping peaks, and the various lipid classes that 16:1n-10 is found warrants caution in assigning double bond position as novel metabolism is discovered [19].
Cancer cell lines typically exhibit high SCD1 expression and Δ9-desaturase activity [23]. MCF7 cells have active SCD1 [24] but no detectable FADS2 mediated Δ6-desaturase activity, thus we developed a MCF7 human cell system stably transformed with FADS2. Here, we evaluated the competition between 16:0 and 18:2n-6 and/or 18:3n-3 for accessing desaturase activity mediated by FADS2. Our data apply directly to substrate competition for catalysis by the FADS2 classical gene product functioning within the MCF7 cell milieu, and as we have reported previously [10, 11]. Increasing concentrations of 18:2n-6 or 18:3n-3 decreased accumulation of 16:1n-10 in a dose dependent manner, directly demonstrating competition between saturated and PUFA precursors for accessing FADS2 mediated Δ6-desaturation in human cells also expressing SCD. Our results show that similar competition is plausible in normal primary cells and in vivo to be investigated in future studies. 16:1n-10 is not normally resolved in broad LC/MS/MS lipidomics scans, and local reductions in LCPUFA may not be readily detected based on quantitative analysis. Sapienic acid has been recently observed in humans by specialized methods [19]. Further research on the relationship of sapienic acid concentrations and metabolic state is required to establish the origin of sapienic acid in internal tissue and its possible value as a biomarker.
Metabolic conditions characterized by relatively high de novo lipogenesis, such as high carbohydrate or alcohol intake, may lead to increased cellular 16:0. Our data show that 16:0 competes with 18:2n-6 and 18:3n-3 for desaturation, but in these human cells this competition is manifested prominently in a decrease in the elongation products 20:3n-6 and 20:4n-3. 20:3n-6 is a direct precursor of eicosanoids generally regarded as anti-inflammatory [25]. Chronic low grade inflammation is now well recognized as a morbidity risk in some but not all obesity, and is also associated with diabetes [26, 27]. A recent prospective cohort study showed that circulating 16:0 and 18:0 were associated with higher diabetes risk, insulin resistance, and inflammatory markers [28]. Considerable evidence supports positive effects of LCPUFA on physiology that reduce diabetes symptoms and severity. In vitro and ex vivo studies indicate EPA/DHA support improved insulin secretion and reduced inflammatory signaling milieu [29]. The American Diabetes Association (ADA) recommends two or more servings of fish per week for diabetes management [30]. Together these observations lead us to suggest the hypothesis that higher 16:0 induced by, for instance high carbohydrate or alcohol intake, may induce LCPUFA reduction leading to metabolic disease that is at least partially corrected by ingestion of preformed LCPUFA.
Desaturase activity is cumbersome and expensive to measure directly; accurate in vivo and non-invasive desaturase measurements are nearly impossible because of reactions competing for the precursors and products. Tissue activity can be measured but requires physical sampling and cumbersome bench work [31]. In contrast, major fatty acid profiles and concentrations are relatively simple measurements, and as a result precursor-product concentration ratios usually referred to a “desaturation index” are widely used as proxies for desaturase activities. For instance the ratio of 18:3n-6/18:2n-6 is commonly used as a proxy for Δ6-desaturase activity in GWAS and clinical studies [32]. Inspection of Figure 4 shows that these ratios are poorly related to activity, which must be identical because FADS2 expression is identical under all conditions shown. For instance, Figure 4A shows that increasing precursor 18:2n-6 does not increase product 18:3n-6 but does increase 20:3n-6, the Δ6-desaturation and elongation product (and active eicosanoid precursor). Similar considerations apply to the analogous n-3 fatty acids in Figure 4B, and the situation is more complex with the more natural condition of combined/competing substrates 18:2n-6 and 18:3n-3 in Figure 4C/D. These results highlight the complexity of fatty acid precursor-product relationships which have straightforward meaning only in complex ways. In our simple experimental system, coupled desaturation-elongation products more accurately reflect desaturation activity than do the immediate desaturation products.
FADS2 is abundantly expressed in a normal human skin cDNA library, comprising 152 out of 7985 (1.9%) expressed sequence tags (ESTs) [8]. Converging evidence from cell culture and localization studies provide evidence of delta-6 desaturase preference for palmitic acid (16:0) in human sebaceous glands. Cell cultures established from human skin biopsy punches show selective incorporation and utilization of 16:0, but in contrast oxidation and degradation of 18:2n-6 predominates [20]. Partial β-oxidation of 18:2n-6 yields acetyl-CoA, and apart from enhancing de novo lipogenesis, also enhances the biosynthetic pathway leading to squalene and cholesterol [20]. Localization studies show high expression of FADS2 and 15-LOX-2 in differentiated sebocytes [8, 33]. 15-LOX-2 reduces the availability of 18:2n-6 to FADS2 by selectively metabolizing 18:2n-6 to oxylipins, and enhancing 16:0 desaturation [8, 34].
Conclusion
The FADS2 gene product is a versatile even numbered desaturase that operates on many substrates and serves as a common pivot point for PUFA. Our data extend that concept to include competition for 16:0. Sapienic acid is well known on the skin, but despite its presence in the circulation is not well studied and in particular it is not thought of as a competitor for PUFA, and vice versa. The high concentration of 16:0 in essentially all tissues contributes to the difficulty in studying conversion of small fraction of 16:0 to 16:1s which may well have important physiological consequences, particularly since they appear in physiologically active phospholipids.
Highlights.
In mammals the desaturation of saturated and polyunsaturated fatty acids (PUFA) is almost exclusively considered separately: the FADS genes apply to PUFA and the SCD apply to saturates.
Palmitic acid is the only exception, being a substrate for both SCD (16:0 → 16:1n-7) and FADS2 (16:0 → 16:1n-10).
Competition experiments shows excess of 16:0 suppresses endogenous eicosanoid and docosanoid precursor synthesis by competing for FADS2 mediated desaturation with essential PUFA.
Metabolic conditions that induce local excess of 16:0 such as excess carbohydrate intake can inhibit synthesis of PUFA precursors for membrane synthesis and alter signaling precursors.
Acknowledgements
This work was supported by NIH grant R01 AT007003 from the National Center for Complementary and Integrative Health (NCCIH) and the Office of Dietary Supplements (ODS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Abbreviations
- PUFA
polyunsaturated fatty acids
- MUFA
monounsaturated fatty acid
- FADS
Fatty acid desaturase
- SCD
Stearoyl CoA desaturase
- FASN
fatty acid synthase
- HUFA
highly unsaturated fatty acids
- FAME
Fatty acid methyl esters
- GC-CACI-MS/MS
gas chromatography covalent adduct chemical ionization tandem mass spectrometry
- PC
Phosphatidylcholine
- PE
phosphatidylethanolamine
- PL
phospholipids
- EI
electronic impact
- ADA
American Diabetes Association
- LCPUFA
long chain PUFA
- EPA
eicosapentaenoic acid
- DHA
docosahexaenoic acid
- ESTs
expressed sequence tags
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
J.T.B., K.S.D.K., H.P. designed research; H.P., W.P., C.D., L.L., A.L. executed the research; J.T.B. and K.S.D.K. contributed new reagents/analytic tools; J.T.B., K.S.D.K., H.P., W.P. and P.L. analyzed and interpreted the data; and J.T.B., K.S.D.K., and H.P. wrote the first draft and all authors approved the final draft.
CONFLICT OF INTEREST
All authors declare no conflict of interest.
References
- [1].Nakamura MT, Nara TY. Structure, function, and dietary regulation of delta6, delta5, and delta9 desaturases. Annu Rev Nutr. 2004;24:345–376. doi: 10.1146/annurev.nutr.24.121803.063211. [DOI] [PubMed] [Google Scholar]
- [2].Miyazaki M, Ntambi JM. Role of stearoyl-coenzyme A desaturase in lipid metabolism. Prostaglandins Leukot Essent Fatty Acids. 2003;68:113–121. doi: 10.1016/s0952-3278(02)00261-2. [DOI] [PubMed] [Google Scholar]
- [3].Bernard L, Leroux C, Chilliard Y. Springer; New York, NY: 2013. Expression and Nutritional Regulation of Stearoyl-CoA Desaturase Genes in the Ruminant Mammary Gland: Relationship with Milk Fatty Acid Composition. [Google Scholar]
- [4].Nicolaides N, Ansari MN. Fatty acids of unusual double-bond positions and chain lengths found in rat skin surface lipids. Lipids. 1968;3:403–410. doi: 10.1007/BF02531278. [DOI] [PubMed] [Google Scholar]
- [5].Miyazaki M, Gomez FE, Ntambi JM. Lack of stearoyl-CoA desaturase-1 function induces a palmitoyl-CoA Delta6 desaturase and represses the stearoyl-CoA desaturase-3 gene in the preputial glands of the mouse. J Lipid Res. 2002;43:2146–2154. doi: 10.1194/jlr.m200271-jlr200. [DOI] [PubMed] [Google Scholar]
- [6].Pollard MR, Gunstone FD, James AT, Morris LJ. Desaturation of positional and geometric isomers of monoenoic fatty acids by microsomal preparations from rat liver. Lipids. 1980;15:306–314. doi: 10.1007/BF02533545. [DOI] [PubMed] [Google Scholar]
- [7].Picardo M, Ottaviani M, Camera E, Mastrofrancesco A. Sebaceous gland lipids. Dermatoendocrinol. 2009;1:68–71. doi: 10.4161/derm.1.2.8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ge L, Gordon JS, Hsuan C, Stenn K, Prouty SM. Identification of the delta-6 desaturase of human sebaceous glands: expression and enzyme activity. J Invest Dermatol. 2003;120:707–714. doi: 10.1046/j.1523-1747.2003.12123.x. [DOI] [PubMed] [Google Scholar]
- [9].Enoch HG, Catala A, Strittmatter P. Mechanism of rat liver microsomal stearyl-CoA desaturase. Studies of the substrate specificity, enzyme-substrate interactions, and the function of lipid. J Biol Chem. 1976;251:5095–5103. [PubMed] [Google Scholar]
- [10].Park WJ, Kothapalli KS, Reardon HT, Lawrence P, Qian SB, Brenna JT. A novel FADS1 isoform potentiates FADS2-mediated production of eicosanoid precursor fatty acids. J Lipid Res. 2012;53:1502–1512. doi: 10.1194/jlr.M025312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Park HG, Park WJ, Kothapalli KS, Brenna JT. The fatty acid desaturase 2 (FADS2) gene product catalyzes Delta4 desaturation to yield n-3 docosahexaenoic acid and n-6 docosapentaenoic acid in human cells. FASEB J. 2015 doi: 10.1096/fj.15-271783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Garces R, Mancha M. One-step lipid extraction and fatty acid methyl esters preparation from fresh plant tissues. Anal Biochem. 1993;211:139–143. doi: 10.1006/abio.1993.1244. [DOI] [PubMed] [Google Scholar]
- [13].Lawrence P, Brenna JT. Acetonitrile covalent adduct chemical ionization mass spectrometry for double bond localization in non-methylene-interrupted polyene fatty acid methyl esters. Anal Chem. 2006;78:1312–1317. doi: 10.1021/ac0516584. [DOI] [PubMed] [Google Scholar]
- [14].Van Pelt CK, Brenna JT. Acetonitrile chemical ionization tandem mass spectrometry to locate double bonds in polyunsaturated fatty acid methyl esters. Anal Chem. 1999;71:1981–1989. doi: 10.1021/ac981387f. [DOI] [PubMed] [Google Scholar]
- [15].Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- [16].Akaza N, Akamatsu H, Numata S, Matsusue M, Mashima Y, Miyawaki M, Yamada S, Yagami A, Nakata S, Matsunaga K. Fatty acid compositions of triglycerides and free fatty acids in sebum depend on amount of triglycerides, and do not differ in presence or absence of acne vulgaris. J Dermatol. 2014;41:1069–1076. doi: 10.1111/1346-8138.12699. [DOI] [PubMed] [Google Scholar]
- [17].Michaud AL, Diau GY, Abril R, Brenna JT. Double bond localization in minor homoallylic fatty acid methyl esters using acetonitrile chemical ionization tandem mass spectrometry. Anal Biochem. 2002;307:348–360. doi: 10.1016/s0003-2697(02)00037-4. [DOI] [PubMed] [Google Scholar]
- [18].Destaillats F, Guitard M, Cruz-Hernandez C. Identification of Delta6-monounsaturated fatty acids in human hair and nail samples by gas-chromatography-mass-spectrometry using ionic-liquid coated capillary column. J Chromatogr A. 2011;1218:9384–9389. doi: 10.1016/j.chroma.2011.10.095. [DOI] [PubMed] [Google Scholar]
- [19].Sansone A, Melchiorre M, Chatgilialoglu C, Ferreri C. Hexadecenoic fatty acid isomers: a chemical biology approach for human plasma biomarker development. Chem Res Toxicol. 2013;26:1703–1709. doi: 10.1021/tx400287u. [DOI] [PubMed] [Google Scholar]
- [20].Pappas A, Anthonavage M, Gordon JS. Metabolic fate and selective utilization of major fatty acids in human sebaceous gland. J Invest Dermatol. 2002;118:164–171. doi: 10.1046/j.0022-202x.2001.01612.x. [DOI] [PubMed] [Google Scholar]
- [21].Pappas A. Epidermal surface lipids. Dermatoendocrinol. 2009;1:72–76. doi: 10.4161/derm.1.2.7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hodson L, Karpe F. Is there something special about palmitoleate? Curr Opin Clin Nutr Metab Care. 2013;16:225–231. doi: 10.1097/MCO.0b013e32835d2edf. [DOI] [PubMed] [Google Scholar]
- [23].Scaglia N, Chisholm JW, Igal RA. Inhibition of stearoylCoA desaturase-1 inactivates acetyl-CoA carboxylase and impairs proliferation in cancer cells: role of AMPK. PLoS One. 2009;4:e6812. doi: 10.1371/journal.pone.0006812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mauvoisin D, Charfi C, Lounis AM, Rassart E, Mounier C. Decreasing stearoyl-CoA desaturase-1 expression inhibits beta-catenin signaling in breast cancer cells. Cancer Sci. 2013;104:36–42. doi: 10.1111/cas.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wang X, Lin H, Gu Y. Multiple roles of dihomo-gamma-linolenic acid against proliferation diseases. Lipids Health Dis. 2012;11:25. doi: 10.1186/1476-511X-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Stehouwer CD, Gall MA, Twisk JW, Knudsen E, Emeis JJ, Parving HH. Increased urinary albumin excretion, endothelial dysfunction, and chronic low-grade inflammation in type 2 diabetes: progressive, interrelated, and independently associated with risk of death. Diabetes. 2002;51:1157–1165. doi: 10.2337/diabetes.51.4.1157. [DOI] [PubMed] [Google Scholar]
- [27].Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ma W, Wu JH, Wang Q, Lemaitre RN, Mukamal KJ, Djousse L, King IB, Song X, Biggs ML, Delaney JA, Kizer JR, Siscovick DS, Mozaffarian D. Prospective association of fatty acids in the de novo lipogenesis pathway with risk of type 2 diabetes: the Cardiovascular Health Study. The American journal of clinical nutrition. 2015;101:153–163. doi: 10.3945/ajcn.114.092601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wang X, Chan CB. n-3 polyunsaturated fatty acids and insulin secretion. The Journal of endocrinology. 2015;224:R97–106. doi: 10.1530/JOE-14-0581. [DOI] [PubMed] [Google Scholar]
- [30].Bantle JP, Wylie-Rosett J, Albright AL, Apovian CM, Clark NG, Franz MJ, Hoogwerf BJ, Lichtenstein AH, Mayer-Davis E, Mooradian AD, Wheeler ML. Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2008;31(Suppl 1):S61–78. doi: 10.2337/dc08-S061. [DOI] [PubMed] [Google Scholar]
- [31].Su HM, Brenna JT. Simultaneous measurement of desaturase activities using stable isotope tracers or a nontracer method. Anal Biochem. 1998;261:43–50. doi: 10.1006/abio.1998.2706. [DOI] [PubMed] [Google Scholar]
- [32].Kroger J, Schulze MB. Recent insights into the relation of Delta5 desaturase and Delta6 desaturase activity to the development of type 2 diabetes. Current opinion in lipidology. 2012;23:4–10. doi: 10.1097/MOL.0b013e32834d2dc5. [DOI] [PubMed] [Google Scholar]
- [33].Shappell SB, Keeney DS, Zhang J, Page R, Olson SJ, Brash AR. 15-Lipoxygenase-2 expression in benign and neoplastic sebaceous glands and other cutaneous adnexa. J Invest Dermatol. 2001;117:36–43. doi: 10.1046/j.1523-1747.2001.01378.x. [DOI] [PubMed] [Google Scholar]
- [34].Prouty SM, Pappas A. Sapienic Acid: Species-Specific Fatty Acid Metabolism of the Human Sebaceous Gland. Springer Science+Business Media; New York: 2015. [Google Scholar]