Abstract
Amorphous silica nanoparticles (NPs) possess unique material properties that make them ideal for many different applications. However, the impact of these materials on human and environmental health needs to be established. We investigated nonporous silica NPs both bare and modified with amine functional groups (3-aminopropyltriethoxysilane (APTES)) in order to evaluate the effect of surface chemistry on biocompatibility. In vitro data showed there to be little to no cytotoxicity in a human lung cancer epithelial cell line (A549) for neither bare silica NPs nor amine-functionalized NPs using doses based on both mass concentration (below 200 μg/mL) and exposed total surface area (below 14 m2/L). To assess lung inflammation, C57/B16 mice were administered bare and amine-functionalized silica NPs via intra-tracheal instillation. Two doses (0.1 and 0.5 mg NPs/mouse) were tested using the in vivo model. At the higher dose used, bare silica NPs elicited a significantly higher inflammatory response, as evidence by increased neutrophils and total protein in bronchoalveolar (BAL) fluid compared to amine-functionalized NPs. From this study, we conclude that functionalization of nonporous silica NPs with APTES molecules reduces murine lung inflammation and improves the overall biocompatibility of the nanomaterial.
Keywords: amine-functionalized silica, inflammation, nanomaterials, nanotoxicology, A549 cells
1. Introduction
Inorganic nanomaterials (<100 nm in size), including silica NPs, have been increasingly utilized in a wide variety of applications due to their unique material properties such as tunable size and ease of surface functionalization.1,2,3 There are well-established methods for the synthesis of silica NPs in addition to commercial availability that make them prime candidates for applications such as drug delivery4,5,6 and biomedical imaging.7,8 Silica NPs are also commonly used as additives in many products including cosmetics, varnishes and printer toners.9,10
Silica materials are composed of silicon dioxide (SiO2) and are present in crystalline and amorphous forms. Quartz is the most common form of crystalline silica. Amorphous and crystalline silica NPs can enter the body through different routes, one of the most common being inhalation of free silica into the lungs which can potentially lead to pulmonary disorders.11 The toxicity of crystalline silica has been studied for many years, especially in connection with chronic bronchitis, emphysema and silicosis.12,13,14 While toxicity related to inhalation of larger, crystalline silica has been well documented15,16,17, more studies are needed to evaluate amorphous particles in the nanometer size range. Although silica NPs are used in many applications because of their small size and large surface area, these same properties could lead to increased toxicity in biological systems compared to larger silica particles.10, 18 For example, the small size of NPs allows for increased interactions with biological tissues and enhanced cellular uptake.19,20,10 It is recognized that particles less than 1 μm in aerodynamic diameter penetrate more distally into lung tissue whereas larger particles (> 5 μm) deposit primarily in the upper airways.21,22 Previously, amorphous silica NPs were considered to be less toxic than crystalline silica; however recent studies found that amorphous silica particles induce substantial lung inflammation.23 In addition to size, surface charge of the NPs could influence NP-cell interactions.24,25 As the potential for human exposure increases, it is critical to evaluate the safety of nano-sized materials especially in occupational settings where engineered nanomaterials are manufactured or handled in bulk quantity.26 Furthermore, development of methodologies for production of safe silica particles that would produce less toxicity or less inflammation are very important. In the last several years, many efforts have been made in particle surface modification with the aim to decrease their potential toxicity.27,28
Numerous in vitro studies have been published in the literature investigating the cytotoxicity of amorphous silica NPs on cultured cell lines. 9,29,30,31 While in vitro experiments are commonly used to predict the toxicity of engineered silica NPs in vivo, there are limitations to the interpretation of the results because of the over-simplified environment of cell culture experiments versus animal models where there is a more complex, physiological environment.32,33,34 Although the evaluation of the side effects of silica NPs have been investigated previously, there is a lack of data in the literature where both in vitro cytotoxicity and in vivo inflammatory response studies have been performed using the same batch of silica NPs and reported together in a single study.
Many applications such as gene delivery,35,36 DNA binding/transport,37 and biomedical imaging38 have been developed utilizing amorphous silica NPs where the surface is functionalized with amine groups in order to change the chemical properties of the material. It is important to investigate how the surface chemistry of silica NPs affects the interaction with biological systems and if there is an influence on toxicity as a result of amine functionalization. Here we present in vitro cytotoxicity and in vivo inflammatory response data in lung models evaluating 50 nm amorphous silica NPs with and without amine-functionalization. Cell cytotoxicity of bare silica NPs and amine-modified silica NPs was measured in human bronchoalveolar cells at three time points. Mice were treated with two different doses of particles via intra-tracheal instillation and markers of pulmonary inflammation in the lungs were evaluated 24 hours after exposure. Silica NP treatments were compared to a crystalline form of silica (Min-U-Sil®5) which is known to be hazardous to human health.39,40 Our studies provide evidence that surface chemistry plays an important role in silica NP-induced inflammation in murine lungs.
2. Materials and Methods
2.1. Synthesis of Silica NPs
Nonporous silica (or Stober silica) was prepared following a modified procedure from the literature.41 In this synthesis no surfactant was used, and ammonia was used as the basic catalyst. In a glass vessel 120 mL anhydrous ethanol (Decon Labs, King of Prussia, PA) was combined with 6.0 mL aqueous ammonia (Sigma-Aldrich, St. Louis, MO) and stirred for 5 minutes. Tetraethyl orthosilicate (TEOS, 4 mL, Sigma) was used as the silicon source and added to the ammonia/ethanol mixture. The reaction mixture was stirred at room temperature for 24 hours and then centrifuged at 11,000 x g for 30 minutes to obtain the products, which were washed three times with water and dried at 60 °C overnight.
2.2. Surface Functionalization
Functional groups were covalently attached to the NP surface using a post-synthesis grafting method.42 Functionalization with amine groups was carried out by refluxing a mixture of 4 g of aminopropyltriethoxysilane (APTES, Sigma) with 1.00 g of silica nanoparticles in 60 mL of toluene for 48 hours. The reaction mixture was then centrifuged at 11,000 x g for 20 minutes, washed three times with 20 mL of dichloromethane and dried overnight at 80°C.
2.3. Material Characterization
NPs were characterized by nitrogen adsorption isotherms, thermogravimetric analysis, scanning electron microscopy (SEM) and zeta potential. Nitrogen adsorption experiments were conducted using a Nova 1200 Nitrogen Adsorption Instrument (Quantachrome). Approximately 100 mg of powder was dried at 120 °C under vacuum overnight. A seven-point BET isotherm and a 50-point adsorption/desorption isotherm in a liquid nitrogen bath were obtained, using pure nitrogen gas as the adsorbate. Surface area was calculated using the BET (Brunauer-Emmett-Teller) method. Functionalized NPs were evaluated by thermogravimetric analysis using a TA Q5000 TGA instrument with a heating rate of 5 °C/minute. The sample was heated from room temperature to ~800 °C under a flow of nitrogen. Mass loss during the run was used to approximate the loading of the organic functional group. SEM was used to image the particles and determine average particle diameter. Particles were dispersed on to silicon wafers on aluminum stubs and then sputter coated (Emitech K550x) with gold and palladium for 3 minutes at 10 mA. Zeta potential of particles were measured in water using a Zeta Sizer nano ZS (Malvern Instrument Ltd., Southborough, MA). Fine ground silica (Min-U-Sil®5 quartz, Berkeley Springs, West Virginia) was used as a positive control for in vivo experiments.
2.4. In Vitro Cytotoxicity Studies
Adenocarcinoma human alveolar basal epithelial (A549) cells were cultured in RPMI-1640 (Gibco®, Life Technologies Corporations, Grand Island, NY) medium containing 10% fetal bovine serum (Atlanta Biologics, Lawrenceville, GA), 10 mM HEPES (Gibco®), 50 μg/mL gentamycin sulfate (IBI Scientific, Peosta, IA), 1 mM sodium pyruvate (Gibco®) and 1 mM Glutamax (Gibco®). Cells were incubated at 37 °C and 5% CO2. Cells were passaged prior to confluence.
A549 cells were plated into 96-well plates at a density of 1 × 104 cells per well. After 24 hours of incubation, the media was removed and replaced with 200 μL of media containing various silica particles types and concentrations. Cells incubated with medium alone served as a control and was used to calculate percent relative cell viability. The cells were exposed to each treatment for 4, 24 or 48 hours after which, the treatment was re moved and the cell viability was assessed using the MTS assay (CellTiter 96 AQueous One Solution Cell Proliferation Assay, Promega Corporation, Madison, WI). Once the treatment was removed, 100 μL of media was added to the wells along with 20 μL of MTS reagent. The cells were incubated with MTS reagent for 1–4 hours. In order to avoid unwanted scattering of light by the silica nanoparticles during analysis, the 96-well plates were centrifuged at 500 x g for 20 minutes and 70 μL of media was carefully removed for absorbance measurements at 490 nm using a SpectraMax Plus 384 microplate reader (Molecular Devices, Sunnyvale, CA). A background absorbance value was established using media and MTS reagent in the absence of cells and all sample data was corrected accordingly.
2.5. In Vivo Toxicology Studies
For in vivo studies, mice (C57Bl/6, males, 12–16 wks old, The Jackson Laboratory, Bar Harbor, ME) were used. After arrival, animals were acclimatized for 10–14 days before exposure while housed in our vivarium in polypropylene, fiber-covered cages in HEPA-filtered Thoren caging units (Hazelton, PA). Food (sterile Teklad 5% stock diet, Harlan, Madison, WI) and water (via an automated watering system) were provided ad libitum. Light-dark cycle (12 hours) was maintained in the animal room. All protocols were approved by the Institutional Animal Care and Use Committee at the University of Iowa. Mice were weighed twice per week (PR 5002 Delta Range balance, Mettler Toledo Inc., Columbus, OH) and monitored throughout the study for any signs of distress. Mice were weighed before intratracheal instillation and 24 hours after exposure to treatments.
Silica NPs were suspended in PBS by sonication for 30 minutes immediately before use. The mice were dosed with 50 μL of NP suspension via intratracheal instillation one time. Two doses were tested, low 0.1 mg/mouse (4 mg/kg) and high 0.5 mg/mouse (20 mg/kg). Mice were anesthetized by inhalation of 3% isoflurane (Fortec vaporizer, Cyprane, Keighley, U.K.). Mice were euthanized 24 hours after exposure and bronchoalveolar lavage fluid and lungs were collected. The number of mice used in each experimental group were as follows: negative control, n=5; positive control (quartz)-low and high dose, n=6 and n=4, respectively, silica NPs-low dose, n=5, silica NPs + APTES-low dose, n=7, silica NPs-high dose, n=4, and silica NPs + APTES-high dose, n=5.
Lungs were lavaged in situ three times with 1 mL of sterile isotonic saline using a cannula inserted into the trachea as described previously.43 The BAL fluid was centrifuged at 800 x g for 5 minutes at 4 °C to collect all cells after which the cells were resuspended in Hank’s balanced salt solution. The total numbers of white cells were quantified using a hemocytometer. The supernatant from each sample was frozen at -80 °C until analysis for total protein and lactate dehydrogenase (LDH) activity. The cells were cytospun (Thermo Shandon, Thermo Scientific, Waltham, MA) at 800 x g for 3 minutes, then stained with HEMA 3 staining kit (Fisher Scientific Company LLC, Midland, MI). The numbers of neutrophils, macrophages, lymphocytes and eosinophils in each BAL sample were determined. BAL fluid was assayed for total protein (Bradford Assay, Bio-Rad Laboratories, Inc., Hercules, CA) and LDH enzyme activity (LDH) (Roche Diagnostics, Mannheim, Germany) in order to quantify the membrane integrity of cells.
Generation of free radical species was assessed using Oxiselect in vitro reactive oxygen species (ROS)/reactive nitrogen species (RNS) assay kit (Cell Biolabs, Inc. San Diego, CA). Half of the left lobe of lung tissue was resuspended at 25–30 mg/mL in PBS and homogenized on ice. Samples were spun at 10,000 x g for 5 minutes. The supernatant was collected and stored at −80°C until analysis. Then, the samples were incubated for 45 minutes with dichlorodihydrofluorescein DiOxyQ (DCFH-DiOxyQ), which reacts with free radicals to form a fluorescent product. The fluorescence intensity of each sample was measured using 480 nm excitation and 530 nm emission. Levels of total free radicals in each sample were determined by comparison with the predetermined DCF standard curve. The levels were normalized for total protein content in homogenates.
2.6. Statistical Analysis
The data are expressed as the mean ± standard error. The differences between data sets were determined using GraphPad Prism version 6 (Graphpad Software, Inc., San Diego, CA) with Tukey’s test for multiple comparisons along with two-way analysis of variance (ANOVA). A p- value < 0.05 was the threshold considered for statistical significance.
3. Results and Discussion
3.1. Particle Characterization
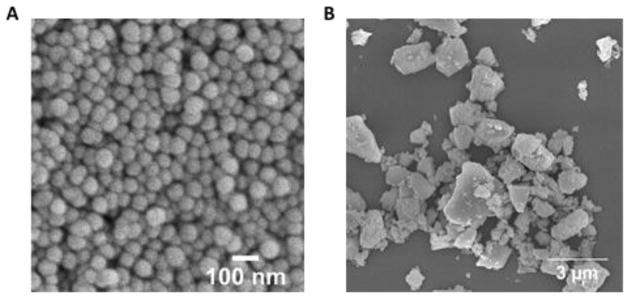
The average diameter of amorphous silica NPs used in this study was approximately 50 nm (figure 1). The diameter of NPs plays an important role in particle-cell interactions. Multiple studies have shown that when comparing variously sized particles of the same composition, the optimal diameter in terms of being taken up by cells is 50 nm or less.19,44,45,46 Using a seven-point nitrogen adsorption isotherm, the surface area of the bare silica NPs and APTES functionalized silica NPs was found to be 66 (±3) m2/g and 42 (±2) m2/g, respectively. Using thermogravimetric analysis, the organic loading was approximated to be 0.582 mmol of APTES per gram of material (table 1). The APTES-functionalization was also confirmed by the increase in zeta potential before and after the covalent attachment of amine molecules to the particle surface (−25 mV and 19 mV, respectively). Min-U-Sil® quartz had an average diameter of ~700 nm although there was a large size distribution (figure 1B) and had a higher negative surface potential (−36 mV) compared to the silica NPs.
Fig. 1.
SEM image of nonporous silica NPs(A) and Min-U-Sil(B).
Table 1.
Physical characterization of the materials used.
| Particle Type | Diameter (nm)a | Surface Area (m2/g) | APTES Loading (mmol/g) | Z-Potential (mV) |
|---|---|---|---|---|
| Bare Silica NPs | 54 ± 9 | 66 ± 3 | - | −25 ± 12 |
| APTES - NPs | 50 ± 8 | 42 ± 2 | 0.582 ± 0.009 | 19 ± 4 |
| Min-U-Sil® Quartz | 700 | 7.6 ± 0.3 | - | −36 ± 6 |
Determined from analysis of SEM images
3.2. In Vitro Toxicity Results
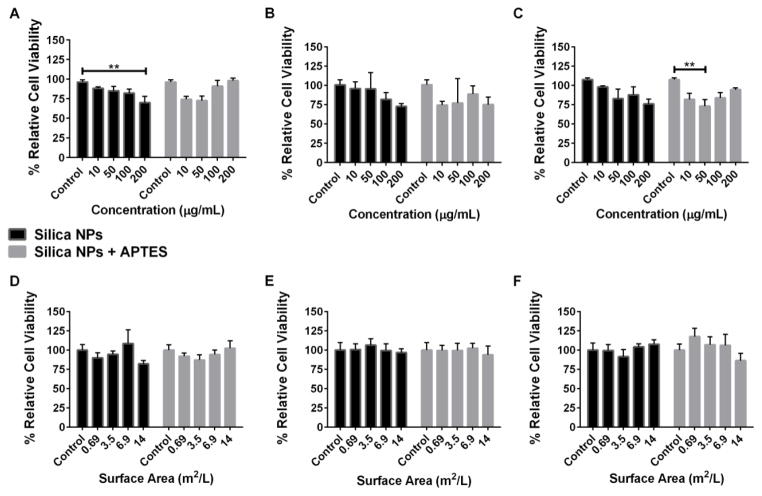
A549 human lung epithelial cells are commonly used as a model system for evaluating lung damage after NP exposures.47 In order to compare the cytotoxicity of bare nonporous silica NPs before and after amine-functionalization on alveolar lung cells, we employed the colorimetric MTS cell viability assay. The NPs were suspended in culture medium and incubated with A549 cells for 4, 24 or 48 hours. Figure 2 (A, B and C) shows the cell viability of A549 cells when using various concentrations of bare and APTES-functionalized silica NP treatments. There was a slight reduction in cell viability for the APTES-modified silica NPs after 4 hours of treatment with 10 and 50 μg/mL (74% and 72% relative cell viability, respectively), while only the highest concentration (200 μg/mL) of bare silica NPs displayed a significant reduction in cell viability to 70%. At longer time points, 24 and 48 hours, there was some apparent cytotoxicity to A549 cells from the bare silica NPs at the highest dose (72% and 75% relative cell viability, respectively); however it was not significant from the control. The APTES-modified silica NPs also induced significant toxicity after 48 hours of treatment but the trend was not dose-dependent.
Fig. 2.
Percent relative cell viability of A549 cells treated with bare silica NPs (black) and APTES-functionalized NPs (grey). The first row shows percent relative cell viability from doses prepared using mass concentration of NPs ranging from 10–200 μg/mL at 4(A), 24(B) and 48(C) hours. The second row shows percent relative cell viability from doses prepared using exposed surface area of NPs ranging from 0.69–14 m2/L at 4(D), 24(E) and 48(F) hours. Data are expressed at mean ± standard error. ** p < 0.01
Using mass concentration when comparing different particle types may not be completely accurate since the number of available surface molecules that could potentially interact with cells increases as the surface area increases.10,48 Previous studies have demonstrated that toxicity outcomes can vary differently with mass concentration than with exposed surface area of NPs.49 Because APTES-functionalized NPs have a lower surface area, there would be less exposed surface area per treatment compared to bare silica NPs when the samples are prepared via mass concentration. Therefore, the MTS assay was repeated with the same time points, however, the particle treatments were calculated according to exposed particle surface area per volume of medium (figure 2 D, E and F). Subsequent analysis by the MTS assay indicated that there was no significant cytotoxicity from the silica NPs before or after amine-modification.
Nowak et al29 reported that when A549 cells were treated with 20 nm silica NPs (bare and APTES functionalized) at concentrations below 250 μg/mL, there was no significant decrease in cell viability after 72 hours of exposure. The same group also saw a greater amount of uptake of amine functionalized silica NPs compared to bare silica NPs over the course of 10 hours. The larger 50 nm silica NPs investigated in our studies perhaps affected the cells in a similar manner explaining the low cytotoxicity at the concentrations tested (10–200 μg/mL). Another group, Lin et al, used nonporous silica NPs of a similar size to those used in our study (46 nm) to determine the cytotoxic effect on A549 cells after 24, 48 and 72 hours of silica NP exposure.9 The NPs were administered using mass concentration doses in the range of 10–100 μg/mL. Their study is in agreement with our cell viability data at 48 hours where at the lowest concentration of 10 μg/mL there was negligible cytotoxicity from bare silica NPs. At 100 μg/mL, there was an increase in the cytotoxicity after 48 hours of bare silica NP exposure reported by Lin et al.; however, we saw no significant difference at the same concentration compared to the control cells. Although in vitro experiments are commonly used to screen drugs or treatments for cytotoxic effects, it is difficult to use the results to predict inflammatory responses in animal models.50 In addition, there are many limitations when using cultured cells in a cytotoxicity assay as it cannot represent the complexity of a biological environment such as the lungs where there are numerous cell types or airway surface molecules present and the nature and impact of the inflammatory response cannot be easily predicted. Furthermore, most in vitro studies are performed in submerged culture conditions that may influence the overall cellular response to tested material. Amorphous silica NPs were found to be less toxic to human lung cells when cells were exposed at an air-liquid interface (ALI).51 However, when pulmonary surfactant was added to cell cultures at an ALI, amine-modified silica NPs augmented cytotoxicity but not the release of IL-8.52
3.3. Effects of NP Treatments on Mouse Body Weight
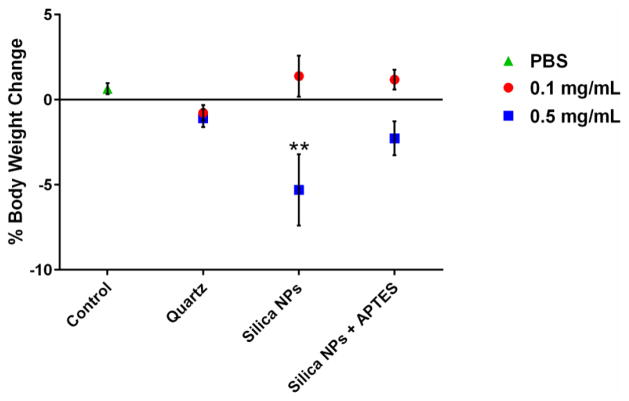
Mice exposed to 0.1 mg of NPs for 24 hours demonstrated, no significant change in body weight associated with any of the particle types compared to the control (figure 3). When the dose was increased to 0.5 mg silica per mouse, a decrease in the body weight in treated mice was evident for each different silica type tested after 24 hours although only the bare silica NP treatment was significantly different from the control (5% decrease). These results indicate that at a dose of 0.5 mg NPs per mouse, unmodified silica NPs have greater adverse side effects compared to APTES-functionalized NPs and quartz.
Fig. 3.
Percent change in body weight of mice after 24 hours of treatment of 0.1 mg/mouse and 0.5 mg/mouse. Control mice were treated with PBS instead of particles. ** p < 0.01
3.4. Total and Differential Cells, Total Protein and LDH Activity in BAL Fluid
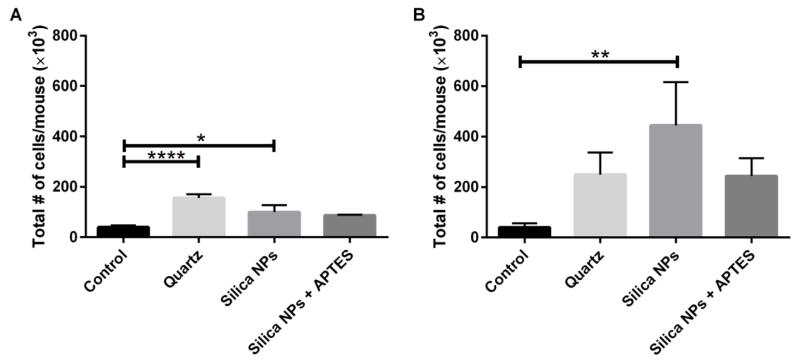
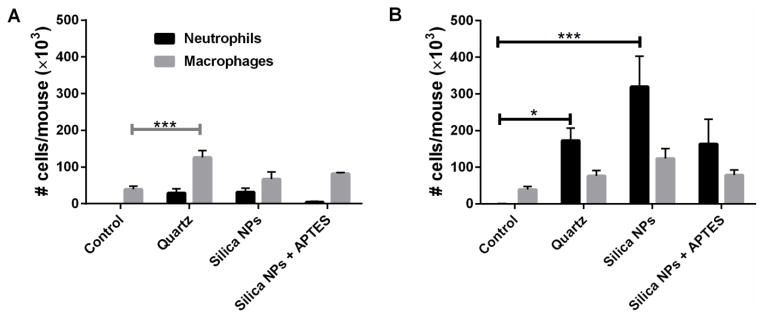
To investigate silica NP-induced inflammation of the lungs, the total numbers of cells present in BAL fluid of silica NP treated mice were counted 24 hours after bronchial instillation. At the dose of 0.1 mg NPs per mouse there was a significantly higher number of total cells in the BAL fluid of mice exposed to unfunctionalized silica NPs (figure 4A) and quartz compared to the control group. When the higher dose (0.5 mg/mouse) was used, all treatments had higher numbers of total cells, compared to control mice. However, the only significant difference observed was the 10-fold increase in total cells in BAL fluid when mice treated with bare silica NPs and control mice were compared (figure 4B). Our results suggest that, when a higher dose was implemented, that 50 nm unmodified silica NPs trended toward being more toxic than the larger crystalline quartz and the APTES-functionalized silica NPs. Thus, surface functionalization of silica NPs with APTES was beneficial in terms of reducing lung inflammation.
Fig. 4.
Total number of cells in BAL fluid after administration of 0.1 mg silica/mouse (A) or 0.5 mg silica/mouse (B). * p < 0.05, ** p < 0.01, **** p < 0.0001
Neutrophils, macrophages, lymphocytes and eosinophils were quantified in BAL fluid to provide insight into lung inflammation caused by silica NPs (figure 5). There was minimal eosinophils recruitment into BAL fluid in any of the experimental groups; thus no eosinophils are listed in the plots. For both doses tested, there was no noticeable difference in the number of lymphocytes between all of the groups, with numbers being very low (also not shown in plot). At a dose of 0.1 mg particles per mouse (figure 5A), both types of silica NPs and quartz induced a greater influx of macrophages into the lungs compared to the control sample but only the quartz treatment yielded values that were significantly different.
Fig. 5.
Neutrophils and macrophages in BAL fluid after administration of 0.1 mg silica/mouse (A) or 0.5 mg silica/mouse (B). * p < 0.05, *** p < 0.001
Once tissue damage has occurred, neutrophils are recruited to the site of injury by cytokine/chemokines and serve as a marker of lung inflammation.53 None of the treatments (at 0.1 mg/mouse) resulted in significant increases in neutrophils compared to control mice. However, it was noted that the APTES-functionalized silica NPs induced very low levels of neutrophil infiltration in the lungs compared to other treatments (figure 5A). At the higher dose of 0.5 mg silica NPs per mouse (figure 5B), there was a 1000-fold increase in neutrophil numbers in the BAL fluid of mice exposed to bare silica NPs and approximately a 500-fold increase in neutrophils resulting from APTES-functionalized silica NP treatment compared to control mice. A 500-fold increase in neutrophils infiltrating into the lungs was also noted for mice treated with quartz. Although all types of silica materials tested induced neutrophil recruitment at 0.5 mg per mouse, there were significantly more neutrophils (about twice as many) in the mice exposed to bare silica NPs compared to the other two treatment groups (figure 5B). It is also likely that the increase in total cell infiltration into the lungs noted in figure 4B for mice treated with bare silica NPs was probably mostly due to the increase in the numbers of neutrophils. A study by Brown et al. indicated that 50 nm bare silica NPs and amine-functionalized NPs both induced neutrophil recruitment in rats to a similar degree (24 hours after instillation) compared to the control. However, they used a dose of 30 μg silica per animal, whereas in our study the doses were higher and could potentially be the reason we saw a larger difference between the bare silica NP and amine-silica NP treatments.54 Our results show that the modification of 50 nm silica NPs with amine groups reduces the amount of acute inflammation, particularly neutrophil infiltration, in the lungs of mice.
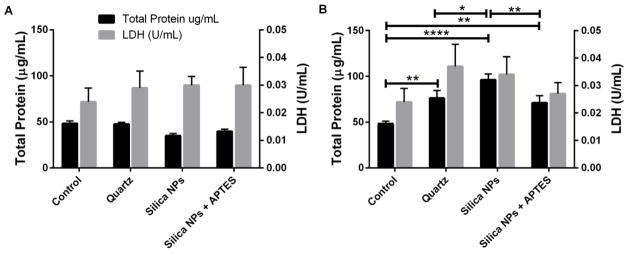
An increase in the total protein concentration in BAL fluid is a sign of lung injury due to increased alveolar-capillary permeability. Another marker of cytotoxicity is a spike in LDH concentration of BAL fluid indicating that the membrane integrity is compromised. Therefore, the total amount of protein in BAL fluid of mice was determined in order to investigate in vivo pulmonary toxicity as well as LDH enzyme activity (figure 6). There was no significant increase in either total protein or LDH activity for the lower dose (0.1 mg/mouse) treatments for any of the sample groups (figure 6B). However, at the higher dose (0.5 mg/mouse), there was a significant difference in the total protein for all of the treatments compared to the control (figure 6B). Again, while there was some indication of inflammation from both types of NPs, there was a significantly larger amount of total protein in the mice treated with bare silica NPs compared to APTES-functionalized silica NPs and quartz treatments. Although the levels of LDH were not significantly different they tended to mirror the results for total protein (figure 6B).
Fig. 6.
Total protein and LDH activity after administration of 0.1 mg silica/mouse (A) or 0.5 mg silica/mouse (B). * p < 0.05, ** p < 0.01, **** p < 0.0001
3.5. Generation of Intracellular ROS/RNS
It is well known that elevated concentrations of intracellular ROS and RNS are indicative of oxidative stress. The results reported herein (figure 7) showed that all experimental treatments induced significantly increased levels of oxidative stress at a dose of 0.5 mg/mouse compared to the controls (figure 7B). Importantly, there was a 60% increase and a 35% increase in the amount of ROS/RNS in mice treated with bare silica NPs and amine-modified silica NPs, respectively. The difference in ROS/RNS production between the two particle types was significant (p-value < 0.01) and we conclude that bare silica NPs induced increased oxidative stress in murine lungs compared to amine-modified silica NPs.
Fig. 7.
Production of intracellular ROS/RNS after administration of 0.1 mg silica/mouse (A) or 0.5 mg silica/mouse (B). * p < 0.05, *** p < 0.001, **** p < 0.0001
There is a large amount of evidence in the literature indicating that silanol groups on the surface of silica materials play a major role in particle-cell interactions and can cause degradation of membrane proteins as well as free radical production. 55,56,57,58 The increase in intracellular ROS/RNS after treatment with bare silica NPs could be related to a higher concentration of exposed surface silanol groups compared to amine-functionalized silica NPs where the silanol moieties are replaced with aminopropyl functional groups. Our work demonstrates that silica NP-induced ROS/RNS can be reduced by functionalizing the surface with amine groups.
4. Conclusions
As nanotechnology is rapidly advancing, it is important to evaluate the potential health effects of silica NP-related exposures. Amine-functionalization of silica NPs had no impact on cell viability of A549 cell cultures for any of the doses tested after 48 hours. Additionally, modification of silica NPs with APTES resulted in significantly reduced inflammatory responses in the lungs of mice treated with these particles versus unmodified silica NPs. This was indicated by decreased accumulation of total cells, especially neutrophils, as well as a marked decrease of total protein in the BAL fluid from lungs of mice. Furthermore, surface modification of silica NPs also significantly reduced ROS production in the alveolar tissue. These findings strongly support the process of surface modification of silica NPs to reduce their potential inflammatory effects.
Highlights.
We investigated nonporous silica NPs both bare and modified with amine functional groups (3-aminopropyltriethoxysilane (APTES)) in order to evaluate the effect of surface chemistry on biocompatibility.
No cytotoxicity was observed in a human lung cancer epithelial cell line (A549) for bare silica NPs or amine-functionalized NPs.
Bare silica NPs elicited a significantly higher inflammatory response compared to amine-functionalized NPs.
Acknowledgments
The authors gratefully acknowledge support from the NIH NIEHS P30 ES005605 grant. AKS acknowledges support from the Lyle and Sharon Bighley Professorship. ASM acknowledges support from an Institutional National Research Service Award (NRSA T90) in Oral Health Research from the National Institute of Dental & Craniofacial Research. We thank Tasneem Al Rashaideh for her help with mouse necropsies. We acknowledge Paul Mueller for the synthesis of silica NPs. We also acknowledge Sean M. Geary for expert reading of the manuscript
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stöber WF, Bohn AE. Controlled growth of monodispere silica spheres in the micron size range. J Colloid Interface Sci. 1968;26:62–69. [Google Scholar]
- 2.He X, Nie H, Wang K, Tan W, Wu X, Zhang P. In vivo study of biodistribution and urinary excretion of surface-modified silica nanoparticles. Anal Chem. 2008;80(24):9597–603. doi: 10.1021/ac801882g. [DOI] [PubMed] [Google Scholar]
- 3.Bharali DJ, Klejbor I, Stachowiak EK, Dutta P, Roy I, Kaur N, Bergey EJ, Prasad PN, Stachowiak MK. Organically modified silica nanoparticles: a nonviral vector for in vivo gene delivery and expression in the brain. Proc Natl Acad Sci U S A. 2005;102(32):11539–44. doi: 10.1073/pnas.0504926102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao A, Ye Z, Cai Z, Dong E, Yang X, Liu G, Deng X, Wang Y, Yang ST, Wang H, Wu M, Liu Y. A facile method to encapsulate proteins in silica nanoparticles: encapsulated green fluorescent protein as a robust fluorescence probe. Angew Chem Int Ed Engl. 2010;49(17):3022–5. doi: 10.1002/anie.200906883. [DOI] [PubMed] [Google Scholar]
- 5.Stevens EV, Carpenter AW, Shin JH, Liu J, Der CJ, Schoenfisch MH. Nitric oxide-releasing silica nanoparticle inhibition of ovarian cancer cell growth. Mol Pharm. 2010;7(3):775–85. doi: 10.1021/mp9002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang L, Fan TM, Borst LB, Cheng J. Synthesis and biological response of size-specific, monodisperse drug-silica nanoconjugates. ACS Nano. 2012;6(5):3954–66. doi: 10.1021/nn300149c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benezra M, Penate-Medina O, Zanzonico PB, Schaer D, Ow H, Burns A, DeStanchina E, Longo V, Herz E, Iyer S, Wolchok J, Larson SM, Wiesner U, Bradbury MS. Multimodal silica nanoparticles are effective cancer-targeted probes in a model of human melanoma. J Clin Invest. 2011;121(7):2768–80. doi: 10.1172/JCI45600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradbury MS, Phillips E, Montero PH, Cheal SM, Stambuk H, Durack JC, Sofocleous CT, Meester RJ, Wiesner U, Patel S. Clinically-translated silica nanoparticles as dual-modality cancer-targeted probes for image-guided surgery and interventions. Integr Biol (Camb) 2013;5(1):74–86. doi: 10.1039/c2ib20174g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin W, Huang YW, Zhou XD, Ma Y. In vitro toxicity of silica nanoparticles in human lung cancer cells. Toxicol Appl Pharmacol. 2006;217(3):252–9. doi: 10.1016/j.taap.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Nel A, Xia T, Madler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311(5761):622–7. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 11.Rimal B, Greenberg AK, Rom WN. Basic pathogenetic mechanisms in silicosis: current understanding. Curr Opin Pulm Med. 2005;11(2):169–73. doi: 10.1097/01.mcp.0000152998.11335.24. [DOI] [PubMed] [Google Scholar]
- 12.Hnizdo E, Sullivan PA, Bang KM, Wagner G. Association between chronic obstructive pulmonary disease and employment by industry and occupation in the US population: a study of data from the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2002;156(8):738–46. doi: 10.1093/aje/kwf105. [DOI] [PubMed] [Google Scholar]
- 13.Hnizdo E, Vallyathan V. Chronic obstructive pulmonary disease due to occupational exposure to silica dust: a review of epidemiological and pathological evidence. Occup Environ Med. 2003;60(4):237–43. doi: 10.1136/oem.60.4.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ross MH, Murray J. Occupational respiratory disease in mining. Occup Med (Lond) 2004;54(5):304–10. doi: 10.1093/occmed/kqh073. [DOI] [PubMed] [Google Scholar]
- 15.Hamilton RF, Jr, Thakur SA, Holian A. Silica binding and toxicity in alveolar macrophages. Free Radic Biol Med. 2008;44(7):1246–58. doi: 10.1016/j.freeradbiomed.2007.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reiser KM, Last JA. Silicosis and fibrogenesis: fact and artifact. Toxicology. 1979;13(1):51–72. [PubMed] [Google Scholar]
- 17.Saffiotti U. Lung cancer induction by crystalline silica. Prog Clin Biol Res. 1992;374:51–69. [PubMed] [Google Scholar]
- 18.Oberdorster G, Oberdorster E, Oberdorster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005;113(7):823–39. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang W, Kim BY, Rutka JT, Chan WC. Nanoparticle-mediated cellular response is size-dependent. Nat Nanotechnol. 2008;3(3):145–50. doi: 10.1038/nnano.2008.30. [DOI] [PubMed] [Google Scholar]
- 20.Verma A, Uzun O, Hu Y, Hu Y, Han HS, Watson N, Chen S, Irvine DJ, Stellacci F. Surface-structure-regulated cell-membrane penetration by monolayer-protected nanoparticles. Nat Mater. 2008;7(7):588–95. doi: 10.1038/nmat2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oberdorster G. Pulmonary effects of inhaled ultrafine particles. Int Arch Occup Environ Health. 2001;74(1):1–8. doi: 10.1007/s004200000185. [DOI] [PubMed] [Google Scholar]
- 22.Sager TM, Kommineni C, Castranova V. Pulmonary response to intratracheal instillation of ultrafine versus fine titanium dioxide: role of particle surface area. Part Fibre Toxicol. 2008;5:17. doi: 10.1186/1743-8977-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park EJ, Park K. Oxidative stress and pro-inflammatory responses induced by silica nanoparticles in vivo and in vitro. Toxicol Lett. 2009;184(1):18–25. doi: 10.1016/j.toxlet.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 24.Nel AE, Madler L, Velegol D, Xia T, Hoek EM, Somasundaran P, Klaessig F, Castranova V, Thompson M. Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater. 2009;8(7):543–57. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- 25.Kairdolf BA, Mancini MC, Smith AM, Nie S. Minimizing nonspecific cellular binding of quantum dots with hydroxyl-derivatized surface coatings. Anal Chem. 2008;80(8):3029–34. doi: 10.1021/ac800068q. [DOI] [PubMed] [Google Scholar]
- 26.Seipenbusch M, Binder A, Kasper G. Temporal evolution of nanoparticle aerosols in workplace exposure. Ann Occup Hyg. 2008;52(8):707–16. doi: 10.1093/annhyg/men067. [DOI] [PubMed] [Google Scholar]
- 27.Shen M, Cai H, Wang X, Cao X, Li K, Wang SH, Guo R, Zheng L, Zhang G, Shi X. Facile one-pot preparation, surface functionalization, and toxicity assay of APTS-coated iron oxide nanoparticles. Nanotechnology. 2012;23(10):105601. doi: 10.1088/0957-4484/23/10/105601. [DOI] [PubMed] [Google Scholar]
- 28.van Schooneveld MM, Vucic E, Koole R, Zhou Y, Stocks J, Cormode DP, Tang CY, Gordon RE, Nicolay K, Meijerink A, Fayad ZA, Mulder WJ. Improved biocompatibility and pharmacokinetics of silica nanoparticles by means of a lipid coating: a multimodality investigation. Nano Lett. 2008;8(8):2517–25. doi: 10.1021/nl801596a. [DOI] [PubMed] [Google Scholar]
- 29.Nowak JS, Mehn D, Nativo P, Garcia CP, Gioria S, Ojea-Jimenez I, Gilliland D, Rossi F. Silica nanoparticle uptake induces survival mechanism in A549 cells by the activation of autophagy but not apoptosis. Toxicol Lett. 2014;224(1):84–92. doi: 10.1016/j.toxlet.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Yang X, Liu J, He H, Zhou L, Gong C, Wang X, Yang L, Yuan J, Huang H, He L, Zhang B, Zhuang Z. SiO2 nanoparticles induce cytotoxicity and protein expression alteration in HaCaT cells. Part Fibre Toxicol. 2010;7:1. doi: 10.1186/1743-8977-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ye Y, Liu J, Chen M, Sun L, Lan M. In vitro toxicity of silica nanoparticles in myocardial cells. Environ Toxicol Pharmacol. 2010;29(2):131–7. doi: 10.1016/j.etap.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 32.Kim YH, Boykin E, Stevens T, Lavrich K, Gilmour MI. Comparative lung toxicity of engineered nanomaterials utilizing in vitro, ex vivo and in vivo approaches. J Nanobiotechnology. 2014;12(1):47. doi: 10.1186/s12951-014-0047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frohlich E, Salar-Behzadi S. Toxicological assessment of inhaled nanoparticles: role of in vivo, ex vivo, in vitro, and in silico studies. Int J Mol Sci. 2014;15(3):4795–822. doi: 10.3390/ijms15034795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yildirimer L, Thanh NT, Loizidou M, Seifalian AM. Toxicology and clinical potential of nanoparticles. Nano Today. 2011;6(6):585–607. doi: 10.1016/j.nantod.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Csogor Z, Nacken M, Sameti M, Lehr CM, Schmidt H. Modified silica particles for gene delivery. Mat Sci Eng C-Bio S. 2003;23(1–2):93–97. [Google Scholar]
- 36.Bharali DJ, Klejbor I, Stachowiak EK, Dutta P, Roy I, Kaur N, Bergey EJ, Prasad PN, Stachowiak MK. Organically modified silica nanoparticles: A nonviral vector for in vivo gene delivery and expression in the brain. P Natl Acad Sci USA. 2005;102(32):11539–11544. doi: 10.1073/pnas.0504926102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kneuer C, Sameti M, Haltner EG, Schiestel T, Schirra H, Schmidt H, Lehr CM. Silica nanoparticles modified with aminosilanes as carriers for plasmid DNA. Int J Pharm. 2000;196(2):257–261. doi: 10.1016/s0378-5173(99)00435-4. [DOI] [PubMed] [Google Scholar]
- 38.Kumar R, Roy I, Hulchanskyy TY, Goswami LN, Bonoiu AC, Bergey EJ, Tramposch KM, Maitra A, Prasad PN. Covalently dye-linked, surface-controlled, and bioconjugated organically modified silica nanoparticles as targeted probes for optical imaging. Acs Nano. 2008;2(3):449–456. doi: 10.1021/nn700370b. [DOI] [PubMed] [Google Scholar]
- 39.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Silica, Some Silicates, Coal Dust and Para-Aramid Fibrils. Lyon, 15–22 October 1996. IARC Monogr Eval Carcinog Risks Hum. 1997;68:1–475. [PMC free article] [PubMed] [Google Scholar]
- 40.Donaldson K, Borm PJ. The quartz hazard: a variable entity. Ann Occup Hyg. 1998;42(5):287–94. doi: 10.1016/s0003-4878(98)00044-1. [DOI] [PubMed] [Google Scholar]
- 41.Stober W, Fink A, Bohn E. Controlled Growth of Monodisperse Silica Spheres in Micron Size Range. J Colloid Interf Sci. 1968;26(1):62. [Google Scholar]
- 42.Lehman SE, Tataurova Y, Larsen SC. NMR studies of functionalized mesoporous silica nanomaterials in aqueous solution. Abstr Pap Am Chem S. 2014;248 [Google Scholar]
- 43.Wongrakpanich A, Adamcakova-Dodd A, Xie W, Joshi VB, Mapuskar KA, Geary SM, Spitz DR, Thorne PS, Salem AK. The absence of CpG in plasmid DNA-chitosan polyplexes enhances transfection efficiencies and reduces inflammatory responses in murine lungs. Mol Pharm. 2014;11(3):1022–31. doi: 10.1021/mp400689r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chithrani BD, Chan WC. Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes. Nano Lett. 2007;7(6):1542–50. doi: 10.1021/nl070363y. [DOI] [PubMed] [Google Scholar]
- 45.Chithrani BD, Ghazani AA, Chan WC. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006;6(4):662–8. doi: 10.1021/nl052396o. [DOI] [PubMed] [Google Scholar]
- 46.Lu F, Wu SH, Hung Y, Mou CY. Size effect on cell uptake in well-suspended, uniform mesoporous silica nanoparticles. Small. 2009;5(12):1408–13. doi: 10.1002/smll.200900005. [DOI] [PubMed] [Google Scholar]
- 47.Verstraelen S, Remy S, Casals E, De Boever P, Witters H, Gatti A, Puntes V, Nelissen I. Gene expression profiles reveal distinct immunological responses of cobalt and cerium dioxide nanoparticles in two in vitro lung epithelial cell models. Toxicology Letters. 2014;228(3):157–169. doi: 10.1016/j.toxlet.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 48.DeLoid G, Cohen JM, Darrah T, Derk R, Rojanasakul L, Pyrgiotakis G, Wohlleben W, Demokritou P. Estimating the effective density of engineered nanomaterials for in vitro dosimetry. Nat Commun. 2014;5 doi: 10.1038/ncomms4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bowman CR, Bailey FC, Elrod-Erickson M, Neigh AM, Otter RR. Effects of silver nanoparticles on zebrafish (Danio rerio) and Escherichia coli (ATCC 25922): a comparison of toxicity based on total surface area versus mass concentration of particles in a model eukaryotic and prokaryotic system. Environ Toxicol Chem. 2012;31(8):1793–800. doi: 10.1002/etc.1881. [DOI] [PubMed] [Google Scholar]
- 50.Sauer UG, Vogel S, Hess A, Kolle SN, Ma-Hock L, van Ravenzwaay B, Landsiedel R. In vivo-in vitro comparison of acute respiratory tract toxicity using human 3D airway epithelial models and human A549 and murine 3T3 monolayer cell systems. Toxicol In Vitro. 2013;27(1):174–90. doi: 10.1016/j.tiv.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 51.Panas A, Comouth A, Saathoff H, Leisner T, Al-Rawi M, Simon M, Seemann G, Dossel O, Mulhopt S, Paur HR, Fritsch-Decker S, Weiss C, Diabate S. Silica nanoparticles are less toxic to human lung cells when deposited at the air-liquid interface compared to conventional submerged exposure. Beilstein J Nanotechnol. 2014;5:1590–602. doi: 10.3762/bjnano.5.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kasper JY, Feiden L, Hermanns MI, Bantz C, Maskos M, Unger RE, Kirkpatrick CJ. Pulmonary surfactant augments cytotoxicity of silica nanoparticles: Studies on an in vitro air-blood barrier model. Beilstein J Nanotechnol. 2015;6:517–28. doi: 10.3762/bjnano.6.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cho WS, Choi M, Han BS, Cho M, Oh J, Park K, Kim SJ, Kim SH, Jeong J. Inflammatory mediators induced by intratracheal instillation of ultrafine amorphous silica particles. Toxicology Letters. 2007;175(1–3):24–33. doi: 10.1016/j.toxlet.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 54.Brown DM, Kanase N, Gaiser B, Johnston H, Stone V. Inflammation and gene expression in the rat lung after instillation of silica nanoparticles: effect of size, dispersion medium and particle surface charge. Toxicol Lett. 2014;224(1):147–56. [PubMed] [Google Scholar]
- 55.Hemenway D, Absher A, Fubini B, Trombley L, Vacek P, Volante M, Cavenago A. Surface Functionalities Are Related to Biological Response and Transport of Crystalline Silica. Inhaled Particles Vii. 1994:447–454. [Google Scholar]
- 56.Slowing II, Wu CW, Vivero-Escoto JL, Lin VSY. Mesoporous Silica Nanoparticles for Reducing Hemolytic Activity Towards Mammalian Red Blood Cells. Small. 2009;5(1):57–62. doi: 10.1002/smll.200800926. [DOI] [PubMed] [Google Scholar]
- 57.Yu T, Malugin A, Ghandehari H. Impact of Silica Nanoparticle Design on Cellular Toxicity and Hemolytic Activity. Acs Nano. 2011;5(7):5717–5728. doi: 10.1021/nn2013904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pavan C, Tomatis M, Ghiazza M, Rabolli V, Bolis V, Lison D, Fubini B. In Search of the Chemical Basis of the Hemolytic Potential of Silicas. Chem Res Toxicol. 2013;26(8):1188–1198. doi: 10.1021/tx400105f. [DOI] [PubMed] [Google Scholar]