Highlights
-
•
Increased OFC functional connectivity was observed in heavy MJ users compared to HC.
-
•
Greater MJ use correlated with increased OFC functional connectivity in MJ users.
-
•
Motor impulsivity correlated with increased OFC functional connectivity in MJ users.
Keywords: Marijuana, Adolescents, Resting-state, fMRI
Abstract
Introduction
Orbitofrontal (OFC) circuits have been implicated in the pathophysiology of substance use disorders. The current study examined OFC functional connectivity differences in marijuana-using adolescents (MJ) and non-using healthy controls (HC).
Methods
Functional magnetic resonance imaging (fMRI) resting-state data were obtained on a 3 T MRI scanner on 31 HC and 43 heavy MJ smokers. Image analyses were performed between groups (MJ, HC) for the left and right OFC separately. Regression analyses between OFC functional connectivity and lifetime MJ use, age of first MJ use and impulsivity also were performed.
Results
Increased OFC functional connectivity to frontal and motor regions was observed in heavy MJ users compared to HC. Earlier age of first MJ use was associated with increased functional connectivity of the right OFC to motor regions. High lifetime MJ use was associated with increased OFC functional connectivity to posterior brain regions in MJ youth.
Discussion
Findings indicate atypical OFC functional connectivity patterns in attentional/executive, motor and reward networks in adolescents with heavy MJ use. These anomalies may be related to suboptimal decision making capacities and increased impulsivity. Results also suggest different OFC connectivity patterns may be present in adolescents with early onset of MJ use and high lifetime exposure to MJ.
1. Introduction
There has been a sharp resurgence in adolescent substance abuse since the early 1990s (Weinberg et al., 1998, Bachman et al., 1998) with a recent report from the United Nations Office on Drugs (UNODC, 2010), indicating that marijuana remains the most widely abused substance among adolescents. Moreover, marijuana (MJ) is the most commonly used illicit drug in the United States by adolescents (SAMHSA, 2009). Dysfunction or imbalance between cognitive control (fronto-parietal) networks involved in attention and executive function, as well as reward (fronto-striatal) networks involved in impulsivity and decision-making have been implicated in substance use disorders (SUD). Within the prefrontal region, the orbitofrontal cortex (OFC) has been linked to impulsivity, poor decision-making, and increased risk for substance misuse (Whelan et al., 2012, Filbey et al., 2014). The current study examined OFC functional connectivity in marijuana using adolescents (MJ) as compared to healthy controls (HC) to determine if difference in connectivity patterns could be seen in the adolescent MJ cohort.
Impaired decision-making and impulsivity are characteristic of damage to OFC regions (Bechara et al., 2000, Berlin et al., 2004) and studies applying morphometric imaging techniques have demonstrated that alterations in OFC gray matter volume are correlated with observed and self-reported impulsivity in healthy adults and adolescents (Matsuo et al., 2009, Boes et al., 2009). For example, a negative correlation between right OFC gray matter volume and the BIS nonplanning subscale was observed in a large community sample including males and females (Matsuo et al., 2009) and decreased right OFC cortical volume was also shown to be associated with increased impulsivity in a sample of adolescent males (Boes et al., 2009). As compared to healthy controls, MJ using adolescents have also been found to have decreased right OFC volumes (Churchwell et al., 2010) suggesting a potential relationship between OFC volume and onset of substance use. Interestingly, in a recent study by Cheetham and associates (Cheetham et al., 2012), smaller OFC volumes at age 12 were found to predict initiation of marijuana use by age 16. Furthermore, two other studies of adult participants reported reduced OFC volumes in MJ users (Filbey et al., 2014, Battistella et al., 2014).
While most investigations on OFC changes in subjects with substance misuse have focused on morphometric imaging techniques, a number of studies have also used functional MRI (fMRI) approaches to assess either resting-state or task-dependent activity in adult and adolescent MJ users. For example, functional imaging studies of marijuana craving cues have found greater activity or increased functional connectivity in OFC regions in adults with marijuana misuse (Filbey and Dunlop, 2014, Filbey et al., 2009, Cousijn et al., 2014). Furthermore, differential OFC activation and connectivity patterns have also been found in adults with marijuana misuse during response inhibition (Filbey and Yezhuvath, 2013) and decision-making (Wesley et al., 2011, Bolla et al., 2005) tasks. In one of these studies, earlier age of onset of regular use was associated with greater activation in the OFC during the response inhibition task (Filbey and Yezhuvath, 2013). More recently, Filbey and colleagues also reported increased functional connectivity within the OFC network, and higher white matter connectivity as measured by fractional anisotropy in a major white matter tract connecting bilateral OFC regions (forceps minor) in adult MJ users compared to HC (Filbey et al., 2014). In adolescents, a study done by Tapert and colleagues also reported OFC activation abnormalities in MJ using participants as compared to HC (Tapert et al., 2007). In a large study of 1593 adolescents, abnormal orbitofrontal cortical network activity on an inhibitory task was associated with greater likelihood of drug use initiation (Whelan et al., 2012).
Unlike structural magnetic resonance (MR) measures of MR imaging such as voxel based morphometry and diffusion tensor imaging, resting state fMRI (rs-fMRI) is thought to index connectivity between brain regions which may be associated with specific brain functionality (Van Den Heuvel and Pol, 2010). A number of investigations have shown that rs-fMRI measures are significantly associated with clinically relevant behaviors, such as craving, impulsivity, and decision-making. For example, studies by Liu and colleagues have reported functional connectivity changes in heroin users that were associated with the amount of craving for the drug (Liu et al., 2009, Liu et al., 2011). Additionally, Davis and colleagues applied resting state techniques to examine and predict impulsivity on decision-making tasks (Davis et al., 2013) and Li and colleagues found that resting state networks reflected performance on delay discounting tasks thought to be impacted by impulsive processing (Davis et al., 2013, Li et al., 2013). More recently, Gordon and colleagues reported that striato-frontal functional connectivity reflected both behavioral impulsivity and an association with the DAT 1 genotype (Gordon et al., 2015). Given previous reports of associations between rs-fMRI and behavioral traits related to substance abuse and impulsivity as well as orbitofrontal morphometry, the current investigation was aimed at improving our understanding of these symptoms in relation to OFC connectivity with MJ users.
Thus far, only a limited number of resting-state functional connectivity studies have been performed in either adult or adolescents with marijuana misuse. Furthermore, of the few studies (Filbey et al., 2014, Filbey and Dunlop, 2014, Orr et al., 2013, Harding et al., 2012, Houck et al., 2013) that have been reported, only one recent study specifically examined the OFC (Filbey et al., 2014). In this study, conducted on adult MJ users, Filbey and colleagues found increased functional connectivity within the OFC with higher OFC connectivity associated with earlier onset of MJ use (Filbey et al., 2014). In addition, little is know about how OFC functional connectivity is related to symptoms of craving and impulsivity or decision-making. Given the paucity of resting-state studies in adolescents with marijuana misuse and the important role that OFC circuits play in craving, impulsivity and decision-making, the current study examined OFC functional connectivity in a group of adolescents with heavy MJ use as compared to HC. Based on previous studies in adult and adolescents with marijuana misuse, we hypothesized that we would find increased OFC functional connectivity to other prefrontal brain regions in adolescent marijuana users as compared to HC. In addition to examining between group differences, the relationship between age of first MJ use and lifetime MJ use on OFC circuits was evaluated to determine if different patterns of functional connectivity are associated with risk (age of onset) or exposure (lifetime MJ use) during adolescent development. Furthermore associations between the measures of trait impulsivity, including motor, attention and non-planning as indexed by the Barratt Impulsivity Scale (Barratt, 1994, Moeller et al., 2001) and functional connectivity patterns of the OFC were examined.
2. Materials and methods
2.1. Subjects
The Institutional Review Board at the University of Utah approved this study. All subjects were recruited from the community via local advertisements and by word of mouth. Inclusion criteria for all subjects in this analysis were: age 14–20 years old. Inclusion criteria for MJ users included a self-report of heavy MJ use with at least 100 minimum lifetime smoking events in the previous year. Healthy controls had no Diagnostic and Statistical Manual of Mental Disorders IV (DSM-IV) Axis I diagnosis based on structured and clinical interviews and had no first-degree family history of bipolar disorder (BPD), psychosis or any other psychiatric family history. Family history was obtained by clinical interview with participants and/or parents. Exclusion criteria for all MJ users and HC were: major sensorimotor handicaps (e.g., deafness, blindness, paralysis); full scale IQ <70 or learning disabilities; lifetime history of claustrophobia, autism, schizophrenia, BPD, conduct disorder, anorexia nervosa or bulimia; other drug or alcohol dependence (during 2 months prior to scan or total past history ≥12 months), or the use of any illicit drug (except MJ) greater than 15× in their lifetime; presence of active medical or neurological disease; history of electroconvulsive therapy; history of head injury with loss of consciousness greater than 30 min; metal fragments or implants; and current pregnancy or lactation.
All subjects provided written assent and their parents or legal guardians provided written informed consent for their adolescent's participation. Both groups underwent a clinical and diagnostic semi-structured interview by either a board-certified child psychiatrist (MLL) or a licensed clinical psychologist. Adolescents under the age of 18 were administered the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Episode (K-SADS-PL) (Kaufman et al., 1997a) with additional mood onset and offset items derived from the WASH-U K-SADS (K-SADS-PL-W) (Geller et al., 2001). The K-SADS-PL is a semi-structured interview used to assess psychiatric disorders in children and adolescents. Since this instrument consists of the K-SADS-PL with supplemental items based on the WASH-U-KADS, we will refer to this instrument as the K-SADS-PL-W. For participants 18 and older, the Structured Clinical Interview for DSM-IV Patient Version (SCID-P) was used with the K-SADS-PL-W (Kaufman et al., 1997b).
The DSM-IV-TR Global Assessment of Functioning (GAF) (Endicott et al., 1976) was used to assess global functioning with a scale from 1 (worst) to 100 (best). Impulsivity was assessed using the Barratt Impulsiveness Scale version 11 (BIS-11), which is a 30 item self-report questionnaire given to the adolescents. The BIS-11 is designed to measure a total composite score for impulsivity, which includes the subscale scores for motor impulsivity (acting without thinking), the Barratt Impulsiveness Scale Motor (BIS M), non-planning, the Barratt Impulsiveness Scale Nonplanning (BIS NP), and inattention (or inability to concentrate), the Barratt Impulsiveness Scale Attention (BIS A) (Patton et al., 1995).
In addition to the detailed substance use histories, a urine drug test was performed prior to MRI scanning and positive results were quantified to give current drug levels for the participant. The urine test was done to confirm alcohol and drug histories obtained from the participant. Any participant with a urine screen that tested positive for anything other than MJ was excluded from the study. Also, if the urine drug screen was negative for MJ for the MJ group, participants were excluded from the study. In addition, information regarding age of first MJ use, age of regular use, and frequency of MJ use was obtained on all participants. Total lifetime MJ use was calculated after taking an extensive MJ use history over the lifetime of a participant. It was calculated by taking into account the changes in use over a lifetime and did not assume a constant rate of MJ use. Total lifetime MJ use was calculated by averaging the number of smoking events/occasions per day and then multiplying that number per week and then again by duration of use in months. For instance, if a participant used 3× a day, 5× a week for 3 months and then 1 time a day once a week for 1 month, the episodes of use (or each smoking event per day) would be calculated separately for each time period and then added together to get total a total lifetime MJ use. Equality of groups on demographic and clinical variables was evaluated by ANOVAs and t-tests for continuous variables and chi-square tests for categorical variables.
2.2. Data acquisition
Structural and functional imaging was performed at the Utah Center for Advanced Imaging Research (UCAIR) using a 3T Siemens Trio scanner with a 12-channel head coil. Structural acquisitions included a T1-weighted 3D MPRAGE grappa sequence acquired sagittally, with TE/TR/TI = 3.38 ms/2.0 s/1.1 s, 8° flip, 256 × 256 acquisition matrix, 256 mm2 FOV, 160 slices, and 1.0 mm slice thickness. The scanning protocol consisted of an initial 1 mm isotropic MPRAGE acquisition acquired in the axial plane for an anatomic template. BOLD echo planar images (TR = 2.0 s, TE = 28 ms, GRAPPA parallel acquisition with acceleration factor = 2, 40 slices at 3 mm slice thickness, 64 × 64 matrix) were obtained during the resting-state, where subjects were given the following instructions: “Keep your eyes open and remain awake and try to let thoughts pass through your mind without focusing on any particular mental activity.” An 8-min resting-state scan (providing a total of 240 image volumes) was performed for each subject. An additional field map scan was obtained for each subject for the purposes of distortion correction. The stimulus computer was synchronized to the onset of the first BOLD image via fiber optic pulse emitted by the scanner.
2.3. Functional MRI post-processing
FMRI images were analyzed using SPM8 (Wellcome Department of Imaging Neuroscience, University College, London, UK) and Data Processing Assistant for Resting-State fMRI (DPARSF) software (Chao-Gan and Yu-Feng, 2010) running in Matlab (MathWorks, Natick, MA, USA). The first 10 volumes of functional images were discarded for the signal equilibrium and to allow for the participants’ adaptation to scanning noise. Initially, the data underwent rigid body realignment to correct for head movement using rigid body motion correction procedure. We calculated 6 motion parameters – three for displacement (in x, y and z) and three for rotation (pitch, roll or yaw). The exclusion criteria were 2 degrees or 2 mm in either the rotational or translational plane. No participants were excluded on this basis. There was no statistically significant difference in average motion being observed between the study groups. The realigned images were then normalized to an EPI template in Montreal Neurological Institute (MNI) stereotactic space. Normalized images were re-sampled into 3 mm cubic voxels and then spatially smoothed using an isotropic Gaussian kernel with 6 mm full width at half maximum (FWHM). Next, linear regression was used to reduce the effect of nuisance signals (motion parameters, global signal, and signals derived from cerebrospinal fluid and white matter) and temporal band-pass filtering (0.01–0.08 Hz) was applied to reduce the effect of both very low and high frequency physiological noise. We included all 43 MJ users in our comparison and 31 controls in the initial image analysis. These results are seen in Fig. 1 and Table 2. To isolate the effects of MJ without the potential confound of comorbid nicotine and alcohol use we also carried out a second analysis including only adolescents with only a history of MJ use.
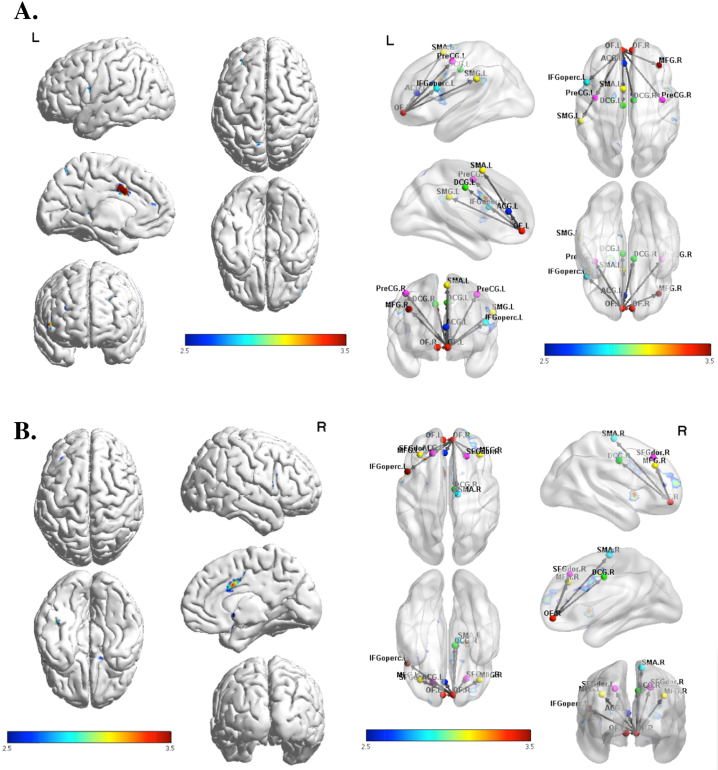
Fig. 1.
(A) Functional connectivity using the left OFC as a seed region, thresholded at p < .005 uncorrected, demonstrating greater activation in MJ relative to HC in cingulate and prefrontal brain regions. (B) Functional connectivity using the right OFC as a seed region, thresholded at p < .005 uncorrected, demonstrating greater activation in MJ relative to HC in cingulate and prefrontal brain regions.
Table 2.
Functional connectivity analysis between groups controlling for age and sex.
| Hemisphere | Regions | # Voxels | T | Z | X | y | z | PFDR-corr |
|---|---|---|---|---|---|---|---|---|
| HC>MJ | Seed: Right OFC | |||||||
| R | Superior Parietal Lobe | 121 | 3.74 | 3.59 | 31 | −61 | 53 | .08 |
| MJ>HC | Seed: Right OFC | |||||||
| L | Anterior & Middle Cingulate | 180 | 4.63 | 4.35 | −2 | 8 | 28 | .01 |
| R | Medial, Middle, & Superior Frontal | 145 | 3.65 | 3.50 | 29 | 41 | 20 | .02 |
| L | Precentral | 101 | 3.99 | 3.81 | −51 | 0 | 11 | .05 |
| Seed: Left OFC | ||||||||
| R | Middle frontal | 260 | 3.78 | 3.62 | 32 | 37 | 13 | .002 |
| R | Anterior & middle cingulate | 108 | 5.28 | 4.88 | 3 | 6 | 31 | .08 |
The left and right OFC regions were derived from the Automated Anatomical Labeling (AAL) atlas (Tzourio-Mazoyer et al., 2002) and the functional maps were computed by using a standard seed-based whole brain correlation method (Wang et al., 2007, Tang et al., 2011). For each seed region, the time series of the voxels within the seed region were averaged to generate the reference time series. For each subject and each seed region, the correlation coefficient was computed between the reference time series and the time course of each voxel of the brain. Correlation coefficients were converted to z-values using Fisher's r-to-z transform (Mayer et al., 2011). The individual z-values were entered into a one-sample t-test in SPM8 to determine brain regions showing significant functional connectivity to the left and right OFC within each group (p < .05, FDR corrected; k > 100 voxels). Similarly, two-sample t-tests were performed for the two groups, controlling for both age and gender, for the left and right OFC separately. The statistical threshold for between group analysis was set at p < .005 and k was set at >20 voxels. Finally, in order to determine the relationship between MJ use on resting-state functional connectivity measures, regression analyses were completed between BOLD signal data and age of onset of MJ use, total lifetime MJ use and on BIS measures of motor, attention and nonplanning impulsivity (p < .005, and k > 20 voxels). Given the study hypotheses the OFC connectivity correlations with BIS measure were conducted for the MJ users only. All regression analyses were controlled for both age and gender.
3. Results
3.1. Subject characteristics
This study included 31 HC (77% male), ranging in age from 14 to 20 (mean 17.2 ± 1.4) and 43 adolescents (93% male) with heavy MJ use, ranging in age from 14 to 20 (mean 18.0 ± 1.2) (see Table 1). As can be seen, MJ smoking adolescents were slightly older than HC participants and had significantly lower GAF scores when compared to HC. On BIS measures of impulsivity, higher impulsivity scores were observed for BIS total, BIS NP and BIS A subscale scores in MJ users as compared to HC; however, no significant difference was observed in BIS M scores (see Table 1). Although our groups were similar in age, we also attempted to standardize our raw impulsivity measures by performing univariate analyses for each BIS measure covarying for age. We found BIS NP was still significantly different between groups with adjusted means for MJ and HC, 25.1 ± 0.63 and 22.56 ± 0.78, respectively (F = 6.03, p = 0.02). The other impulsivity measures were not significantly different between groups after covarying for age (BIS-M: F = 0.09; p = 0.77, BIS-A: F = 2.94, p = 0.09; BIS-Total: F = 3.41, p = 0.07). In our MJ smoking adolescents, age of first use was 14.7 ± 1.4 year of age and frequency of use and total lifetime MJ use was 14.8 ± 15.0 and 1993.2 ± 2157.9, respectively. For the MJ group, 14% (6/43) were currently smoking cigarettes at least 7× a week with an average nicotine use of 49 ± 50.8 cigarettes over 32.3 ± 30.9 months. Furthermore, 11.6% (5/43) of the MJ participants used alcohol regularly (more than once a week). These 5 individuals used alcohol an average of 1.5 ± 1.3× per week over the course of 19.3 ± 19.5 months.
Table 1.
Demographic and clinical measures.
| HC (n = 31) |
MJ (n = 43) |
HC vs. MJ | |||
|---|---|---|---|---|---|
| N | % | N | % | χ2 | |
| Sex (male) | 24 | 77.42 | 40 | 93.00 | .05 |
| Mean | SD | Mean | SD | p-Value | |
|---|---|---|---|---|---|
| Age | 17.20 | 1.38 | 18.00 | 1.22 | .01 |
| Age of first MJ use | – | – | 14.66 | 1.41 | – |
| Frequency of MJ use (#/week) | – | – | 14.78 | 15.02 | – |
| Total lifetime MJ use (# events) | – | – | 1993.15 | 2157.92 | – |
| GAF | 90.56 | 4.14 | 79.49 | 10.01 | <.001 |
| BIS non planning | 22.18 | 3.79 | 25.19 | 4.13 | <.01 |
| BIS motor | 19.04 | 3.91 | 19.81 | 4.54 | ns |
| BIS attention | 16.11 | 3.75 | 17.74 | 3.07 | .05 |
| BIS total | 57.32 | 9.33 | 62.74 | 8.58 | .01 |
HC = healthy control; MJ = marijuana; SD = standard deviation; GAF = Global Assessment of Functioning; BIS = Barratt Impulsiveness Scale.
3.2. Functional connectivity findings
Healthy controls had greater functional connectivity between the right OFC seed and the right superior parietal lobe compared to MJ using adolescents at a trend level. Marijuana using adolescents had greater functional connectivity than HC between the right and left OFC seeds and the cingulate and right middle frontal gyrus. The right OFC also had increased functional connectivity in MJ using adolescents to the right superior frontal gyrus and left precentral gyrus as compared to HC (see Fig. 1 and Table 2). The addition of lifetime MJ use as a covariate in the analyses did not significantly change the functional connectivity results. To isolate the effects of MJ, we also carried out analyses excluding MJ smokers with comorbid nicotine and alcohol use. The connectivity projections from the left and right OFC remained essentially the same as those reported in the larger sample.
3.3. OFC regressions with marijuana use metrics and Barratt Impulsivity measures
To examine the impact of lifetime MJ use and age of first MJ use, we carried out regression analysis using the functional connectivity data in our MJ group only. Because we found increased connectivity between OFC and motor regions on our group comparisons, only behavioral regressions associated with increased OFC connectivity will be reported.
3.3.1. Lifetime marijuana use
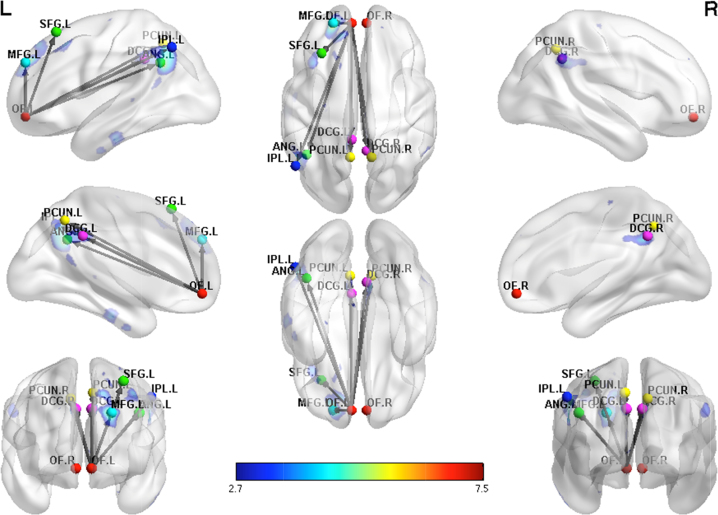
In the MJ group, a positive association for functional connectivity was found for lifetime MJ use and left and right OFC functional connectivity between primarily frontal, parietal, middle and posterior cingulate/precuneus and cerebellar regions (see Fig. 2 and Table 3).
Fig. 2.
Positive regression in marijuana using adolescents showing increased functional connectivity of the left OFC with increasing lifetime use in primarily posterior brain regions. Functional connectivity maps shown at a threshold of p < .005 uncorrected.
Table 3.
Positive regression for MJ with lifetime use.
| Hemisphere | Regions | # voxels | T | Z | x | y | z | PFDR-corr |
|---|---|---|---|---|---|---|---|---|
| Seed: Left OFC | ||||||||
| R, L | Cerebellum | 1359 | 5.62 | 4.80 | −18 | −30 | −39 | <.001 |
| L | Parahippocampal | |||||||
| L | Fusiform | |||||||
| L | Middle, & superior frontal | 377 | 6.37 | 5.26 | −21 | 51 | 33 | <.001 |
| L | Parietal | 333 | 7.50 | 5.89 | −42 | −54 | 33 | <.001 |
| R, L | Middle & posterior cingulate/precuneus | 244 | 4.57 | 4.08 | 6 | −51 | 36 | <.001 |
| Seed: Right OFC | ||||||||
| R, L | Cerebellum | 772 | 5.74 | 4.88 | −9 | −27 | −33 | <.001 |
| L | Hippocampus | |||||||
| L | Fusiform | |||||||
| R, L | Middle & posterior cingulate | |||||||
| L | Superior frontal | 129 | 5.02 | 4.39 | −15 | −9 | 72 | .03 |
| L | Precentral | |||||||
| L | Paracentral lobule | |||||||
| L | Supplementary motor | |||||||
| L | Angular & inferior parietal | 124 | 4.27 | 3.85 | −48 | −66 | 48 | .03 |
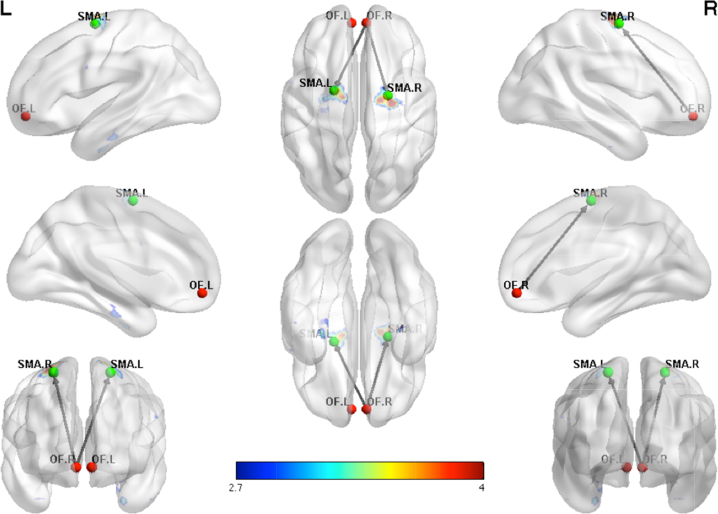
3.3.2. Age of first MJ use
In the MJ only group, a positive association was found between age of first MJ use and right OFC connections to the left occipital and left cerebellar regions. A negative association was also found for age of first MJ use and right OFC functional connectivity for frontal and motor regions including the superior frontal, precentral and supplementary motor regions (see Fig. 3 and Table 4).
Fig. 3.
Negative regression in marijuana using adolescents showing increased functional connectivity with earlier age of first use between the right OFC and motor regions. Functional connectivity maps shown at a threshold of p < .005 uncorrected.
Table 4.
Negative regression with age of first use.
| Hemisphere | Regions | # voxels | T | Z | x | y | z | PFDR-corr |
|---|---|---|---|---|---|---|---|---|
| Seed: Right OFC | ||||||||
| R | Superior frontal | 145 | 4.88 | 4.30 | 27 | −12 | 66 | .04 |
| R | Precentral | |||||||
| R | Supplementary motor | |||||||
| L | Superior frontal | 110 | 4.51 | 4.03 | −12 | −9 | 72 | .06 |
| L | Precentral | |||||||
| L | Supplementary motor | |||||||
| L | Paracentral lobule | |||||||
3.3.3. Barratt Impulsivity measures
There were no significant associations found for left or right OFC connectivity and BIS A or BIS NP However, a positive association was found for BIS M and left OFC for parietal, precunus, post-central gyrus, middle and inferior frontal, superior temporal, supplementary motor area, mid-cingulum, precentral gyrus and occipital regions in the MJ group. For the right OFC regions, a positive association was found with BIS M for occipital, cuneus and lingual regions (see Table 5).
Table 5.
Positive regression for BIS-M.
| Hemisphere | Regions | # voxels | T | x | y | z | PFDR-corr |
|---|---|---|---|---|---|---|---|
| Seed: Left OFC | |||||||
| L | Superior & inferior parietal | 646 | 6.12 | −21 | −66 | 51 | <0.0001 |
| L | Precuneus | ||||||
| L | Postcentral gyrus | ||||||
| R | Middle & inferior frontal | 353 | 5.95 | 36 | 33 | 30 | <0.0001 |
| R, L | Calcarine, middle occipital | 735 | 4.38 | −21 | −75 | 12 | <0.0001 |
| R, L | Lingual | ||||||
| R, L | Middle occipital | ||||||
| R, L | Cuneus | ||||||
| R | Superior occipital | ||||||
| R | Rolandic operculum | 152 | 5.83 | 51 | −3 | 0 | 0.003 |
| R | Superior temporal | ||||||
| R | Insula | ||||||
| R, L | Supplementary motor area | 162 | 4.38 | 0 | 12 | 45 | 0.003 |
| R, L | Middle cingulum | ||||||
| L | Precentral | 144 | 4.21 | −57 | 9 | 21 | 0.003 |
| L | Inferior frontal operculum | ||||||
| Seed: Right OFC | |||||||
| R, L | Calcarine | 588 | 4.24 | 15 | −75 | 3 | <0.0001 |
| R | Middle & superior occipital | ||||||
| R | Cuneus | ||||||
| R | Lingual | ||||||
4. Discussion
In summary, we found differences in functional connectivity between the OFC and frontal and motor regions in adolescents with MJ use as compared to HC. In adolescents with MJ, increasing lifetime MJ use was associated with increased functional connectivity of the left and right OFC to frontal, parietal, mid and posterior cingulate/precuneus and cerebellar regions. An earlier age of onset of MJ use was associated with greater right OFC functional connectivity, particularly between prefrontal and motor regions. Furthermore, higher BIS M impulsivity symptoms were associated with increased left OFC connectivity to frontal, parietal, and motor regions (precentral gyrus, supplementary motor area, mid-cingulum, precentral gyrus). We did not find significant associations for left or right OFC connectivity and BIS A or BIS NP.
Marijuana using adolescents had greater functional connectivity than HC between the right and left OFC seeds and the cingulate and right middle frontal gyrus. The right OFC also had increased functional connectivity in MJ using adolescents to the left precentral gyrus as compared to HC. These findings suggest the OFC is hyperconnected to anterior frontal-motor brain regions that support attention/executive function (ACC and superior/middle frontal gyrus) and motor planning (superior frontal gyrus, precentral gyrus and cingulate) in MJ users. These findings are consistent with several other studies in adults that have also reported increased activation or functional connectivity in OFC regions in adult MJ users (Filbey and Dunlop, 2014, Filbey et al., 2009, Cousijn et al., 2013).
Interestingly, a study of cortical thickness in adolescent MJ users found decreased cortical thickness in right middle frontal and bilateral superior frontal gyri (Lopez-Larson et al., 2011). Furthermore, Mata and colleagues (Mata et al., 2010) described decreased concavity of the sulci and thinner sulci in the right frontal lobe in adolescent MJ users. These authors reported that HC had age-related reductions in gyrification and cortical thickness, which was not noted in MJ users suggesting an atypical neurodevelopment pattern was present in their MJ group (Mata et al., 2010). Therefore, the increased OFC functional connectivity to frontal and motor brain regions may reflect predisposing neuromaturational abnormalities in OFC-mediated brain networks that are associated with MJ misuse. Alternatively, the hyperconnectivity of the OFC to other frontal and motor regions may be a neuroadaptive mechanism related specifically to MJ exposure. For example, Abbas et al. has suggested that use of alternate pathways may result in hyperconnectivity between brain regions (Abbas et al., 2014). Lastly the observed hyperconnectivity may be the result of an interaction between predisposing brain maturational abnormalities and MJ exposure.
In the current investigation, greater lifetime MJ use was associated with increased functional connectivity of the OFC in multiple frontal (middle and inferior frontal, OFC and insula) and posterior brain regions including middle cingulate and cerebellum (motor), posterior cingulate/precuneus (default mode network) and hippocampus/parahippocampus (limbic) regions in the MJ user group. These findings suggest that exposure to MJ may affect multiple brain networks involved in widespread cognitive, emotional, and motor functions. In a separate study, our group also found negative correlations between prefrontal cortical thickness and current MJ exposure which suggests that severity and duration of marijuana use may preferentially affect frontal brain network development (Lopez-Larson et al., 2011). Given the potential impact of MJ exposure on brain tissue, it is possible that the increased connectivity of the OFC may be an adaptive mechanism associated with tissue loss.
Notably, in our MJ group, a younger age of onset was associated with increased functional connectivity for the right OFC and motor network brain regions including the bilateral superior frontal, bilateral precentral, bilateral supplementary motor area and left paracentral lobule and reduced functional connectivity for the right OFC to left occipital-cerebellar regions. Interestingly, motor impulsivity, and not attentional or nonplanning impulsivity, was significantly associated with both left and right OFC regions. Our findings are consistent with Filbey and colleagues who also reported increased functional connectivity within the OFC network in adult MJ users that was associated with earlier age of onset (Filbey et al., 2014). Similarly, in a large study of 1593 adolescents, abnormal orbitofrontal cortical network activity on an inhibitory task was also associated with greater likelihood of drug use initiation (Whelan et al., 2012). In previous studies of brain morphology, a younger age of onset of MJ use has been linked to smaller right OFC volumes (Churchwell et al., 2010), and increased right superior frontal cortical thickness (Lopez-Larson et al., 2011) in adolescents MJ users. Furthermore, in a longitudinal study, Cheetham and associates (Cheetham et al., 2012) reported that smaller OFC volumes at age 12 predicted initiation of marijuana use by age 16. Orbitofrontal gray matter volumes have also been found to correlate with observed and self-reported impulsivity measures in both healthy adults and adolescents (Matsuo et al., 2009, Boes et al., 2009). Taken together, the recent published literature and current examination of OFC functional connectivity highlights the role of the right OFC connectivity to motor brain regions as a key region associated with onset of MJ use. Further examination of these key fronto-motor brain regions may improve our understanding of the neurobiological risk for early initiation of substance use.
5. Conclusion
The current study adds to the growing literature on the importance of OFC networks in MJ use disorders in adolescents. Limitations of the study include modest sample sizes and the cross-sectional nature of the study. In addition there were a few participants included in the study with current alcohol and/or nicotine use. Interestingly, analyses completed on adolescents with only MJ use showed resting state connectivity patterns that were essentially the same as those including comorbid MJ, alcohol, and nicotine use. However, the low frequency of nicotine and alcohol use in the current study did not allow additional analyses to explore the impact of these substances on functional connectivity measures of the OFC. Nonetheless, the interaction of MJ and other drugs of misuse is an important area of future study. Furthermore, the GAF was used to assess daily functioning across a broad range of psychological, social, and occupational/educational functioning. The lower GAF scores in the MJ users was likely a result of the negative consequences of marijuana misuse such as educational, social, legal problems and the time spent obtaining or using MJ. However, although we screened all participants for comorbid axis I psychiatric disorders, we cannot rule out that lower GAF scores could also be the result of sub-clinical psychopathology, which may have also impacted our findings.
Despite these limitations, this was a novel study designed to assess the differences in OFC functional connectivity in adolescents with heavy MJ use and HC. The findings of atypical OFC functional connectivity patterns in adolescents with MJ use in key attentional/executive, motor and reward networks, maybe related to the suboptimal decision making capacities and increased impulsivity leading to substance use initiation, abuse and dependence. Additional studies examining the distinct biological risk factors for initiation of SUD and chronic use of substances on brain developmental trajectories of key OFC networks is needed in individuals with SUD as these biological differences may impact prognosis and treatment.
Acknowledgements
This work was supported by NIMH Award K23 MH087831 (Lopez-Larson, MP) and NIDA grant 1R01 DA020269 (Yurgelun-Todd, DA).
References
- Abbas K. Alteration of default mode network in high school football athletes due to repetitive subconcussive mild traumatic brain injury: a Resting-State Functional Magnetic Resonance Imaging Study. Brain Connect. 2014 doi: 10.1089/brain.2014.0279. [DOI] [PubMed] [Google Scholar]
- Bachman J.G., Johnson L.D., O’Malley P.M. Explaining recent increases in students’ marijuana use: impacts of perceived risks and disapproval, 1976 through 1996. Am. J. Public Health. 1998;88(6):887–892. doi: 10.2105/ajph.88.6.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barratt E.S. Impulsiveness and aggression. In: Monahan J., Steadman H.J., editors. Violence and Mental Disorder: Developments in Risk Assessment. University of Chicago Press; Chicago, IL: 1994. pp. 61–79. [Google Scholar]
- Battistella G. Long-term effects of cannabis on brain structure. Neuropsychopharmacology. 2014;39(9):2041–2048. doi: 10.1038/npp.2014.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A., Tranel D., Damasio H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain. 2000;123(Pt 11):2189–2202. doi: 10.1093/brain/123.11.2189. [DOI] [PubMed] [Google Scholar]
- Berlin H.A., Rolls E.T., Kischka U. Impulsivity, time perception, emotion and reinforcement sensitivity in patients with orbitofrontal cortex lesions. Brain. 2004;127(5):1108–1126. doi: 10.1093/brain/awh135. [DOI] [PubMed] [Google Scholar]
- Boes A.D. Right ventromedial prefrontal cortex: a neuroanatomical correlate of impulse control in boys. Soc. Cogn. Affect. Neurosci. 2009;4(1):1–9. doi: 10.1093/scan/nsn035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla K.I. Neural substrates of faulty decision-making in abstinent marijuana users. Neuroimage. 2005;26(2):480–492. doi: 10.1016/j.neuroimage.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Chao-Gan Y., Yu-Feng Z. DPARSF: a MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Front. Syst. Neurosci. 2010;4:13. doi: 10.3389/fnsys.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheetham A. Orbitofrontal volumes in early adolescence predict initiation of cannabis use: a 4-year longitudinal and prospective study. Biol. Psychiatry. 2012;71(8):684–692. doi: 10.1016/j.biopsych.2011.10.029. [DOI] [PubMed] [Google Scholar]
- Churchwell J.C., Lopez-Larson M., Yurgelun-Todd D.A. Altered frontal cortical volume and decision making in adolescent cannabis users. Front. Psychol. 2010;1:225. doi: 10.3389/fpsyg.2010.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousijn J. Neural responses associated with cue-reactivity in frequent cannabis users. Addict. Biol. 2013;18(3):570–580. doi: 10.1111/j.1369-1600.2011.00417.x. [DOI] [PubMed] [Google Scholar]
- Cousijn J. Relationship between working-memory network function and substance use: a 3-year longitudinal fMRI study in heavy cannabis users and controls. Addict. Biol. 2014;19(2):282–293. doi: 10.1111/adb.12111. [DOI] [PubMed] [Google Scholar]
- Davis F.C. Impulsivity and the modular organization of resting-state neural networks. Cereb. Cortex. 2013;23(6):1444–1452. doi: 10.1093/cercor/bhs126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endicott J. The global assessment scale. A procedure for measuring overall severity of psychiatric disturbance. Arch. Gen. Psychiatry. 1976;33(6):766–771. doi: 10.1001/archpsyc.1976.01770060086012. [DOI] [PubMed] [Google Scholar]
- Filbey F.M., Dunlop J. Differential reward network functional connectivity in cannabis dependent and non-dependent users. Drug Alcohol Depend. 2014;140:101–111. doi: 10.1016/j.drugalcdep.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey F., Yezhuvath U. Functional connectivity in inhibitory control networks and severity of cannabis use disorder. Am. J. Drug Alcohol Abuse. 2013;39(6):382–391. doi: 10.3109/00952990.2013.841710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey F.M. Marijuana craving in the brain. Proc. Natl. Acad. Sci. U. S. A. 2009;106(31):13016–13021. doi: 10.1073/pnas.0903863106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey F.M. Long-term effects of marijuana use on the brain. Proc. Natl. Acad. Sci. U. S. A. 2014 doi: 10.1073/pnas.1415297111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller B. Reliability of the Washington University in St Louis Kiddie Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS) mania and rapid cycling sections. J. Am. Acad. Child Adolesc. Psychiatry. 2001;40(4):450–455. doi: 10.1097/00004583-200104000-00014. [DOI] [PubMed] [Google Scholar]
- Gordon E.M. Resting-state striato-frontal functional connectivity is sensitive to DAT1 genotype and predicts executive function. Cereb. Cortex. 2015;25(2):336–345. doi: 10.1093/cercor/bht229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding I.H. Functional connectivity in brain networks underlying cognitive control in chronic cannabis users. Neuropsychopharmacology. 2012;37(8):1923–1933. doi: 10.1038/npp.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houck J.M., Bryan A.D., Feldstein Ewing S.W. Functional connectivity and cannabis use in high-risk adolescents. Am. J. Drug Alcohol Abuse. 2013;39(6):414–423. doi: 10.3109/00952990.2013.837914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kaufman J. Kiddie-Schedule for Affective Disorders and Schizophrenia-Present and Lifetime version (K-SADS-PL): initial reliability and validity data. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Li N. Resting-state functional connectivity predicts impulsivity in economic decision-making. J. Neurosci. 2013;33(11):4886–4895. doi: 10.1523/JNEUROSCI.1342-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. Dysfunctional connectivity patterns in chronic heroin users: an fMRI study. Neurosci. Lett. 2009;460(1):72–77. doi: 10.1016/j.neulet.2009.05.038. [DOI] [PubMed] [Google Scholar]
- Liu J. Interaction between dysfunctional connectivity at rest and heroin cues-induced brain responses in male abstinent heroin-dependent individuals. PLoS ONE. 2011;6(10):e23098. doi: 10.1371/journal.pone.0023098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Larson M. Altered prefrontal and insular cortical thickness in adolescent marijuana users. Behav. Brain Res. 2011;220(1):164–172. doi: 10.1016/j.bbr.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata I. Gyrification brain abnormalities associated with adolescence and early-adulthood cannabis use. Brain Res. 2010;1317:297–304. doi: 10.1016/j.brainres.2009.12.069. [DOI] [PubMed] [Google Scholar]
- Matsuo K. A voxel-based morphometry study of frontal gray matter correlates of impulsivity. Hum. Brain Mapp. 2009;30(4):1188–1195. doi: 10.1002/hbm.20588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A.R. Functional connectivity in mild traumatic brain injury. Hum. Brain Mapp. 2011;32(11):1825–1835. doi: 10.1002/hbm.21151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller F.G. Psychiatric aspects of impulsivity. Am. J. Psychiatry. 2001;158(11):1783–1793. doi: 10.1176/appi.ajp.158.11.1783. [DOI] [PubMed] [Google Scholar]
- Orr C. Altered resting-state connectivity in adolescent cannabis users. Am. J. Drug Alcohol Abuse. 2013;39(6):372–381. doi: 10.3109/00952990.2013.848213. [DOI] [PubMed] [Google Scholar]
- Patton J.H., Stanford M.S., Barratt E.S. Factor structure of the Barratt impulsiveness scale. J. Clin. Psychol. 1995;51(6):768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- SAMHSA . Office of Applied Studies, NSDUH Series H-36, HHS Publication No. SMA 09-4434; Rockville, MD: 2009. Results from the 2008 National Survey on Drug Use and Health: National Findings. [Google Scholar]
- Tang L. Thalamic resting-state functional networks: disruption in patients with mild traumatic brain injury. Radiology. 2011;260(3):831–840. doi: 10.1148/radiol.11110014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapert S.F. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology (Berlin) 2007;194(2):173–183. doi: 10.1007/s00213-007-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- U.N.O.o.D.a.C. (UNDOC) 2010. World Drug Report 2010. New York. [Google Scholar]
- Van Den Heuvel M.P., Pol H.E.H. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur. Neuropsychopharmacol. 2010;20(8):519–534. doi: 10.1016/j.euroneuro.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Wang K. Altered functional connectivity in early Alzheimer's disease: a resting-state fMRI study. Hum. Brain Mapp. 2007;28(10):967–978. doi: 10.1002/hbm.20324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg N.Z. Adolescent substance abuse: a review of the past 10 years. J. Am. Acad. Child Adolesc. Psychiatry. 1998;37(3):252–261. doi: 10.1097/00004583-199803000-00009. [DOI] [PubMed] [Google Scholar]
- Wesley M.J., Hanlon C.A., Porrino L.J. Poor decision-making by chronic marijuana users is associated with decreased functional responsiveness to negative consequences. Psychiatry Res. 2011;191(1):51–59. doi: 10.1016/j.pscychresns.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan R. Adolescent impulsivity phenotypes characterized by distinct brain networks. Nat. Neurosci. 2012;15(6):920–925. doi: 10.1038/nn.3092. [DOI] [PubMed] [Google Scholar]