Abstract
Introduction
Both acute kidney injury (AKI) and chronic kidney disease (CKD) are common yet underappreciated risk factors for adverse perioperative outcomes. We hypothesize that AKI and CKD are associated with similar increases in ninety-day mortality and cost in patients undergoing major vascular surgery.
Methods
We used multivariable regression analyses to evaluate the associations between acute and chronic kidney disease and incremental ninety-day mortality and hospital cost in a single-center cohort of 3,646 adult patients undergoing major vascular surgery. We defined AKI using KDIGO (Kidney Disease: Improving Global Outcomes) criteria as change in creatinine ≥ 0.3 mg/dl or ≥50% increase from the reference value. CKD was determined from medical history. Regression models were adjusted for demographic and socio-economic characteristics, comorbid conditions, surgery type, and postoperative complications.
Results
The prevalence of kidney disease among vascular surgery patients is high with 49% of patients developing AKI during hospitalization and 17% presenting with CKD on admission. In risk-adjusted logistic regression analysis, perioperative AKI (OR 2.2, 95% CI 1.5-3.3) was the most significant predictor of ninety-day mortality. The risk-adjusted average cost was significantly higher for patients with any type of kidney disease. The incremental cost of having any type of kidney disease ranged from $9,100 to $19,100, even after adjustment for underlying comorbidities and other postoperative complications.
Conclusions
Kidney disease after major vascular surgery is associated with significant increase in ninety-day mortality and cost with the highest risk observed among patients with AKI regardless of previous CKD.
Keywords: Acute Kidney Injury, chronic kidney disease, hospital mortality, ninety-day mortality, resource utilization, hospital cost
1. Introduction
Chronic kidney disease (CKD) is a well-known risk factor for adverse short-term outcomes after major vascular surgery. CKD is an independent predictor for 30-day mortality, and its estimated absolute risk increase of death ranges from 2.1% for carotid endarterectomy procedures to 4.7% for thoracoabdominal and abdominal aortic aneurysmal repair.1-6 Similarly, CKD is associated with increased length of stay and major postoperative complications following vascular surgery, with reported incidence of postoperative cardiovascular events as high as 28.6%. 1,4,5
Acute kidney injury (AKI) is a common postoperative complication, and our group has shown that small changes in serum creatinine are associated with increased hospital and intensive care unit lengths of stay as well as hospital and ninety-day mortality rates after major surgery.7-10 With the emergence of consensus criteria to standardize the definition of this postoperative complication, especially with mild and moderate degrees of AKI, recent studies within the vascular literature have demonstrated that AKI prevalence ranges from 13%-76% and is associated with adverse perioperative outcomes.11-14 Many of these studies have focused on the prevalence of AKI after a specific procedure using small patient cohorts.
We sought to characterize the health care burden of postoperative AKI after vascular surgery within the context of chronic kidney disease. In a large, single-center cohort of patients undergoing major inpatient vascular surgical procedures, we assessed the outcomes, resource utilization, and costs associated with both acute and chronic kidney disease in the perioperative period.
2. Materials and Methods
2.1 Data source
Using the University of Florida Integrated Data Repository we have previously assembled a single center cohort of perioperative patients by integrating multiple existing clinical and administrative databases at UF Health.8 We included all adult patients (age greater or equal to 18 years at admission) admitted to the hospital for longer than 24 hours following any type of inpatient operative procedure between January 1, 2000 and November 30, 2010. The study was designed and approved by the Institutional Review Board of the University of Florida and the University of Florida Privacy Office.
2.2 Participants
We identified vascular surgical patients as those in whom the primary admission or discharge service was vascular or cardiothoracic surgery and who received a primary or secondary International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) vascular surgical procedure code.15 We subdivided procedure codes into categories as described previously and excluded patients receiving Dialysis Access Procedures and Other Procedures (Thoracic-subclavian surgeries, n=1023; Open abdominal aortic surgeries, n=806; Peripheral vascular surgeries, n=629; Endovascular aortic surgeries, n= 545; Percutaneous surgeries, n=277; Lower extremity amputations, n=246; Open carotid surgeries, n=120)(Table A.1).15 For patients with multiple surgeries we choose the first procedure. The final cohort consisted of 3,646 patients.
2. 3 Outcomes and Covariates
Main outcomes included hospital and ninety-day mortality and hospital cost. Patient survival status was determined using hospital discharges and Social Security Death Index. All performed procedures and hospital charges were obtained from the billing database, and cost of hospitalization was estimated by applying the ratio of cost-to-charge for urban hospitals in the South Atlantic division to the amount charged for each hospitalization.16 We converted all costs to 2010 dollars using the Consumer Price Index for Medical Services to adjust for inflation over the years. We defined postoperative complications using previously described criteria.17,18 For the definition of sepsis, we followed the criteria by the Agency for Healthcare Research and Quality for the patient safety indicators “Postoperative Sepsis”.19 Organ failure associated with sepsis was identified by adding ICD-9-CM codes for acute organ dysfunction to those used for sepsis.20 The exact dates were used to calculate the duration of mechanical ventilation (MV), intensive care unit (ICU) and hospital stay. We determined need for vasopressor therapy for longer than 24 hours for each patient using administration times and doses from the pharmacy database.
The main covariate of interest was the occurrence of kidney disease during index hospitalization. Chronic kidney disease at the time of admission was determined using validated approach that combines diagnostic and procedural ICD-9-CM codes. Using the consensus Kidney Disease: Improving Global Outcomes (KDIGO) criteria, we defined AKI during hospitalization as at least a 50% or 0.3 mg/dl increase in serum creatinine during admission relative to the reference value.21 For all calculations, we defined reference serum creatinine as either the minimum of all values available within six months prior to admission or as the minimum and mean of the creatinine values available within seven days prior to admission (used for sensitivity analyses).22 We also applied the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) criteria that defines postoperative AKI as a rise in sCr greater than 2 mg/dl from the preoperative value or as acute postoperative requirement for renal replacement therapy (RRT) within 30 days of the operation. 23
The presence of underlying comorbidities was identified by ICD-9-CM codes based on previously validated criteria.24 We calculated the Charlson-Deyo comorbidity index and grouped patients into score categories of 0, 1, 2, ≥3.25,26 Emergent surgery was defined as either non-elective surgery or emergent admission using clinical data. Using residency zip code, we linked to US Census data27 to calculate residing neighborhood characteristics and distance from hospital.28
2.4 Statistical analyses
The analytical plan has followed the STROBE recommendations for transparent reporting of observational studies.29 The Pearson χ2 test or Fisher's exact test were used to test independence between categorical variables while Student's T-test, analysis of variance and Kruskal-Wallis test were used for comparison of continuous variables as appropriate. All significance tests were two-sided with α<0.05 considered statistically significant. Bonferroni correction was used to adjust for multiple comparisons.
We constructed multivariable regression models for hospital and ninety-day mortality and hospital cost using occurrence of kidney disease as the main independent predictor. All models were risk-adjusted for preoperative covariates including age, gender, ethnicity, primary payer, number of comorbidities, emergent surgery status, weekend admission, type of surgery and estimated glomerular filtration rate (GFR) on admission as well as postoperative complications. We selected explanatory variables based on their significance in a prior univariate analysis and any previously reported association in the literature.
For models where hospital cost was primary outcome we used logarithmic transformation of cost to account for skewness of the distribution while for mortality model we used logistic regression. The exponentiated coefficients were used to calculate risk-adjusted odds ratios for mortality and relative ratios for cost while comparing patients with a certain type of kidney disease to patients with no kidney disease. For each postoperative complication we calculated incremental risk-adjusted average cost with 95% confidence intervals (CI) using generalized linear models30 while stratifying patients by the occurrence of kidney disease. Standard errors were calculated using the smearing estimate to adjust for bias due to transformation of cost to original scale.31 We used the area under the receiver operating characteristics curve values (AUC) and Hosmer–Lemeshow goodness-of-fit test to assess model discrimination and fit of logistic regression models. We performed sensitivity analyses by comparing the effects of a) different definitions for reference serum creatinine and b) inclusion of patients with missing values for sCr and c) omission of different covariates on model fit for each outcome. Statistical analyses were performed with SAS (v.9.3, Cary, N.C.).
3. Results
3. 1 Prevalence of Kidney Disease
Baseline characteristics of the cohort stratified by the occurrence of kidney disease during hospitalization are shown in Table 1. At the time of admission 17% of the patient cohort had documented chronic kidney disease. During hospitalization 49% of the patient cohort developed acute kidney injury, and over 80% of these patients did not have a history of chronic kidney disease prior to admission. Only 43% of the cohort did not have evidence of either acute or chronic kidney disease during hospitalization. The commonly used NSQIP criteria for AKI definition captured only 17% (311/1801) of the patients with AKI defined using consensus KDIGO criteria. Only 20% of all AKI patients had a diagnosis of AKI listed in their discharge summaries (Table 1).
Table 1.
Clinical characteristics of the patients stratified by the occurrence of kidney disease.
| Variables | No Kidney Disease (n=1557, 43%) | Acute Kidney Injury | Chronic Kidney Disease without Acute Kidney Injury (n=288, 8%) | |
|---|---|---|---|---|
|
| ||||
| Without Chronic Kidney Disease (n=1465, 40%) | With Chronic Kidney Disease (n=336, 9%) | |||
| Age, Mean (SD) | 62 (14) | 66 (14)* | 68 (12)* | 66 (12)* |
| Female gender, n (%) | 575 (36.9) | 555 (37.9) | 94 (28.0)* | 103 (35.8) |
| African-American ethnicity, n (%) | 116 (7.5) | 157 (10.7)* | 53 (15.8)* | 58 (20.1)* |
| Rural area residency, n (%) | 457 (29.4) | 366 (25.1)* | 79 (23.6) | 83 (29.0) |
| Distance from residing neighborhood to hospital (km), Median (25th,75th) | 71 (31, 140) | 79 (38, 160) | 87 (38, 162) | 70 (29, 150) |
| Population living in poverty in residing neighborhood, % (SD) | 13.8 (7.5) | 13.8 (7.7) | 13.2 (7.2) | 14.5 (7.4) |
| Insurance, n (%) | ||||
| Medicare | 832 (53.4) | 939 (64.1)* | 262 (78.0)* | 211 (73.3)* |
| Medicaid | 163 (10.5) | 138 (9.4) | 32 (9.5) | 28 (9.7) |
| Private | 483 (31.0) | 340 (23.2)* | 37 (11.0)* | 43 (14.9)* |
| Uninsured | 79 (5.1) | 48 (3.3) | 5 (1.5)* | 6 (2.1) |
| Emergent surgery, n (%) | 498 (32.0) | 607 (41.4)* | 156 (46.4)* | 147 (51.0)* |
| Weekend admission, n (%) | 139 (8.9) | 172 (11.7)* | 39 (11.6) | 41 (14.2)* |
| Weekend Discharge, n (%) | 328 (21.1) | 284 (19.4) | 54 (16.1) | 49 (17.0) |
| Charlson's Comorbidity Index, Median (25th, 75th) | 2 (1, 3) | 2 (1, 3) | 3 (2, 4)* | 4 (3, 5)* |
| Comorbid conditions on admission, n (%) | ||||
| Peripheral vascular disease | 1307 (83.9) | 1289 (88.0)* | 303 (90.2)* | 226 (78.5) |
| Congestive heart failure | 115 (7.4) | 177 (12.1)* | 75 (22.3)* | 71 (24.7)* |
| Myocardial infarction | 205 (13.2) | 208 (14.2) | 62 (18.5)* | 43 (14.9) |
| Cerebrovascular disease | 189 (12.1) | 164 (11.2) | 50 (14.9) | 47 (16.3) |
| Chronic pulmonary disease | 450 (28.9) | 429 (29.3) | 112 (33.3) | 82 (28.5) |
| Diabetes mellitus | 336 (21.6) | 281 (19.2) | 76 (22.6) | 103 (35.8)* |
| Admission serum creatinine (mg/dl), Median (25th, 75th) | 0.8 (0.7, 0.9) | 1.1 (0.9, 1.3)* | 1.5 (1.2, 2.1)* | 1.8 (1.3, 4.1)* |
| AKI by NSQIP definition, n (%) | 0 (0) | 180 (12.3)* | 131 (39.0)* | 67 (23.3)* |
| ICD-9-CM AKI diagnostic code in discharge summary, n (%) | 22 (1.4) | 227 (15.5)* | 138 (41.1)* | 43 (14.9)* |
Abbreviations. AKI, Acute kidney injury; NSQIP, National Surgical Quality Improvement Project; ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification.
p-value < 0.05 for comparison with respect to no kidney disease group using Bonferroni adjustment.
Patients with either acute or chronic kidney disease were more likely to be older and of African American ethnicity and to be admitted for emergent surgery. Patients who developed AKI were more likely to have underlying peripheral vascular disease and congestive heart failure, while patients with chronic kidney disease, regardless whether it was complicated by AKI or not, had significantly higher number of comorbidities on admission.
3. 2 Adverse Hospital Outcomes and Acute Kidney Injury
Overall hospital and ninety-day mortality among 3,636 patients in the cohort were 6.0% and 9.2%, respectively. Noticeably, more than one thirds of all deaths in the first ninety days of admission occurred after hospital discharge. Patients with any form of kidney disease had multi-fold increases in both hospital and ninety-day mortality (Table 2). Hospital mortality was 0.8% in patients with no kidney disease whereas the mortality rates ranged between 7.3% and 14.6% for patients with acute or chronic kidney disease. Ninety-day mortality was 2.6% in patients with no kidney disease whereas the mortality rates ranged between 12.8% and 18.5% for patients with any form of kidney disease. Patients with both AKI and CKD had the highest rate of unadjusted hospital (14.6%) and ninety-day (18.5%) mortality.
Table 2.
Postoperative complications and hospital outcomes of the patients stratified by the occurrence of kidney disease.
| Variables | No Kidney Disease (n=1557, 43%) | Acute Kidney Injury | Chronic Kidney Disease without Acute Kidney Injury (n=288, 8%) | |
|---|---|---|---|---|
|
| ||||
| Without Chronic Kidney Disease (n=1465, 40%) | With Chronic Kidney Disease (n=336, 9%) | |||
| Hospital Outcomes | ||||
| Hospital mortality, n (%) | 12 (0.8) | 138 (9.4)* | 49 (14.6)* | 21 (7.3)* |
| Ninety-day mortality, n (%) | 41 (2.6) | 188 (12.8)* | 62 (18.5)* | 45 (15.6)* |
| Days in hospital, Median (25th, 75th) | 6 (4, 9) | 10 (6, 18)* | 15 (8, 30)* | 8 (5, 20)* |
| Discharge to home, n (%) | 1293 (83.3) | 903 (61.9)* | 147 (44.3)* | 173 (60.5)* |
| Resource Utilization | ||||
| Intensive care unit admission, n (%) | 1178 (75.7) | 1296 (88.5)* | 299 (89.0)* | 197 (68.4)* |
| Days in intensive care unit, Median (25th, 75th) | 3 (2, 5) | 5 (3, 10)* | 8 (3, 20)* | 4 (2, 10)* |
| Mechanical ventilation, n (%) | 523 (33.6) | 937 (64.0)* | 226 (67.3)* | 104 (36.1) |
| Days on mechanical ventilation, Median (25th, 75th) | 2 (1, 2) | 3 (2, 9)* | 6 (2, 23)* | 3 (2, 12.5)* |
| Postoperative Complications | ||||
| Pulmonary complications and/or mechanical ventilation, n (%) | 590 (37.9) | 981 (67.0)* | 241 (71.7)* | 112 (38.9) |
| Cardiovascular complications and/or need for vasopressors, n (%) | 488 (31.3) | 790 (53.9)* | 213 (63.4)* | 112 (38.9)* |
| Procedural complications, n (%) | 138 (8.9) | 250 (17.1)* | 69 (20.5)* | 39 (13.5)* |
| Neurological complications and/or delirium, n (%) | 79 (5.1) | 176 (12.0)* | 48 (14.3)* | 32 (11.1)* |
| Mechanical wound complications and/or surgical infections, n (%) | 49 (3.2) | 124 (8.5)* | 32 (9.5)* | 17 (5.9) |
| Severe sepsis, n (%) | 16 (1.0) | 109 (7.4)* | 56 (16.7)* | 26 (9.0)* |
| Gastrointestinal complications, n (%) | 21 (1.4) | 45 (3.1)* | 15 (4.5)* | 7 (2.4) |
| Venous thromboembolism, n (%) | 54 (3.5) | 84 (5.7)* | 25 (7.4)* | 29 (10.1)* |
| Number of postoperative complications, n (%) | ||||
| None | 693 (44.5) | 306 (20.9)* | 54 (16.1)* | 121 (42.0) |
| 1 | 392 (25.2) | 329 (22.5) | 64 (19.1) | 57 (19.8) |
| >=2 | 472 (30.3) | 830 (56.7)* | 218 (64.9)* | 110 (38.2) |
p-value < 0.05 for comparison with respect to no known kidney disease group using Bonferroni adjustment.
The presence of acute or chronic kidney disease was associated with increasing rates of most postoperative complications, including cardiovascular complications, procedural complications, neurologic complications, severe sepsis, and venous thromboembolism (Table 2). Pulmonary complications and mechanical ventilation, mechanical wound complications and surgical infections, and gastrointestinal complications were most frequent in patients who developed AKI, regardless of underlying CKD. Patients with AKI and CKD had the highest rate of all postoperative complications except for the development of venous thromboembolism, which was highest in patients with stable CKD. While 80% of patients with AKI had at least one postoperative complication close to half of the patients without kidney disease had no other postoperative complications.
In the risk-adjusted logistic regression models the occurrence of acute kidney injury, regardless of the underlying chronic kidney disease, was independently associated with two-fold increase in the odds of hospital and ninety-day mortality (Table 3 and 4). The models had good discrimination and fit (AUC 0.92, 95% CI 0.91-0.93 for hospital mortality, AUC 0.85, 95% CI 0.83-0.87 for ninety-day mortality, Hosmer-Lemeshow test non-significant). Patients with chronic kidney disease at admission who did not develop AKI during their hospitalization had higher odds of dying at ninety days (OR 2.09, 95% CI 1.17-3.72) but the association with hospital mortality was not significant (OR 2.28, 95% CI 0.94-5.53, p=0.07) likely because most of the deaths in this group occurred after hospital discharge.
Table 3. Risk-adjusted association between acute and chronic kidney disease and hospital and ninety-day mortality.
| Variables | Hospital mortality Adjusted Odds Ratio (95% Confidence Interval) | Ninety-day mortality Adjusted Odds Ratio (95% Confidence Interval) |
|---|---|---|
| No known kidney disease | 1 (Reference) | 1 (Reference) |
| Acute Kidney Injury without chronic kidney disease | 3.62 (1.9, 6.87)* | 2.21 (1.49, 3.28)* |
| Acute kidney injury with chronic kidney disease | 2.93 (1.38, 6.23)* | 1.8 (1.07, 3.04)* |
| Chronic kidney disease with no acute kidney injury | 2.28 (0.94, 5.53) | 2.09 (1.17, 3.72)* |
| Female gender (vs. male) | 1.1 (0.79, 1.54) | 1.11 (0.85, 1.44) |
| Age, per 1-year increase | 1.04 (1.02, 1.06)* | 1.05 (1.03, 1.06)* |
| Charlson Comorbidity Index Score, per unit increase | 1.23 (1.1, 1.38)* | 1.25 (1.15, 1.36)* |
| Emergent surgery (vs. Elective) | 1.99 (1.43, 2.77)* | 2.56 (1.97, 3.33)* |
| Estimated glomerular filtration rate | 0.99 (0.99, 1)* | 0.99 (0.99, 1) |
| Postoperative Complications | ||
| Pulmonary complications and/or mechanical ventilation (vs. None) | 5.06 (2.42, 10.56)* | 1.68 (1.17, 2.4)* |
| Cardiovascular complications and/or need for vasopressors (vs. None) | 9.83 (5.13, 18.86)* | 2.53 (1.83, 3.51)* |
| Procedural complications (vs. None) | 1.98 (1.39, 2.82)* | 1.74 (1.28, 2.35)* |
| Infectious complications (vs. None)† | 3.26 (2.32, 4.58)* | 2.45 (1.8, 3.32)* |
| Other complications (vs. None)‡ | 1.77 (1.26, 2.48)* | 1.94 (1.47, 2.56)* |
p-value<0.05.
Infectious complications include severe sepsis and mechanical wound complications and/or surgical infections.
Other complications include neurological complications, delirium, gastrointestinal complications and venous thromboembolism. Area under the receiving operating characteristics curves are 0.92 (95% confidence interval 0.91-0.93), and 0.85 (95% confidence interval 0.82- 0.87) for hospital and ninety-day mortality, respectively.
Table 4.
Risk-adjusted association between the occurrence of acute and chronic kidney disease and mortality and hospital cost after major vascular surgery.
| Ninety-day Mortality | Hospital Cost | |||||
|---|---|---|---|---|---|---|
| Unadjusted odds ratio (95% CI) | Risk-adjusted odds ratio (95% CI) | Risk-adjusted mean % (95% CI) | Risk-adjusted relative cost ratio (95% CI) | Risk-adjusted incremental cost per patient (95% CI) | Risk-adjusted average cost per patient (95% CI) | |
| No Kidney Disease | 1 (Reference) | 1 (Reference) | 5.8 (4.1, 7.5) | 1 (Reference) | 0 (Reference) | $39,200 ($37,800, $40,600) |
| Acute Kidney Injury without Chronic Kidney Disease | 5.44 (3.85, 7.69)* | 2.21 (1.49, 3.28)* | 10.7 (9.3, 12.0)* | 1.23 (1.18, 1.29)* | $9,100 ($7,200, $11,000)* | $48,300 ($46,900, $49,600)* |
| Acute Kidney Injury with Chronic Kidney Disease | 8.37 (5.53, 12.67)* | 1.80 (1.07, 3.04)* | 9.2 (6.9, 11.5)* | 1.49 (1.38, 1.60)* | $19,100 ($15,000, $23,100)* | $58,300 ($54,700, $61,800)* |
| Chronic Kidney Disease without Acute Kidney Injury | 6.85 (4.39, 10.68)* | 2.09 (1.17, 3.72)* | 10.2 (7.1, 13.3)* | 1.23 (1.13, 1.33)* | $8,900 ($5,100, $12,700)* | $48,100 ($44,700, $51,500)* |
Abbreviations. CI, confidence interval.
The risk-adjusted ratios and means were derived using generalized linear models adjusted for age, gender, ethnicity, primary payer, Charlson Comorbidity Index, emergent surgery status, weekend admission, estimated GFR (glomerular filtration rate), and all postoperative complications.
P<0.05 using generalized linear models to compare to no known kidney disease group.
3. 3 Utilization of Hospital Resources
Patients with any form of kidney disease had significantly longer hospitalizations, and those with AKI and CKD had a median length of stay over a week longer than the median for patients with no known kidney disease (Table 2). Discharge to home was also significantly less in patients with any form of kidney disease. Patients with AKI, regardless of history of underlying chronic kidney disease, were more likely to be admitted to ICU and to have longer ICU stay. Patients with AKI and underlying chronic kidney disease had ICU stay that was almost triple that for patients with no known kidney disease. Interestingly, patients with chronic kidney disease on admission who did not develop AKI had actually a significantly lower rate of ICU admission. The occurrence of AKI, regardless of underlying chronic kidney disease, was associated with more frequent requirement for mechanical ventilation with significantly longer median duration compared to patients without kidney disease.
In multivariable regression analysis of hospital cost taking into account underlying comorbidities and postoperative complications other than AKI, relative hospital costs for patients with any form of kidney disease were significantly higher than for patients with no known kidney disease (Table 4). Patients with CKD but no AKI and those with AKI with no CKD had costs that were 23% higher, and those with concomitant AKI and CKD had costs that were 49% higher than the costs for patients with no known kidney disease. These increased adjusted relative cost ratios were associated with adjusted incremental costs of $8,900, $9,100, and $19,100 for patients with CKD with no AKI, AKI with no CKD, and AKI with CKD, respectively. The risk-adjusted average hospital cost of care for patients undergoing vascular surgery was $39,200, $48,100, $48,300, and $58,300 for patients with no known kidney disease, CKD with no AKI, AKI with no CKD, and AKI with CKD respectively (Table 4). Serious postoperative complications resulted in increased average costs of care for all patients; this increase, however, was more pronounced in those with any form of kidney disease compared with patients with no known kidney disease (Figure 1). We further examined effect of survival status and age on adjusted average costs when stratified by the occurrence of acute or chronic kidney disease. At all age groups, average adjusted costs were highest and very similar across all age groups for patients who did not survive and who had either acute or chronic kidney disease (Figure 2).
Figure 1.
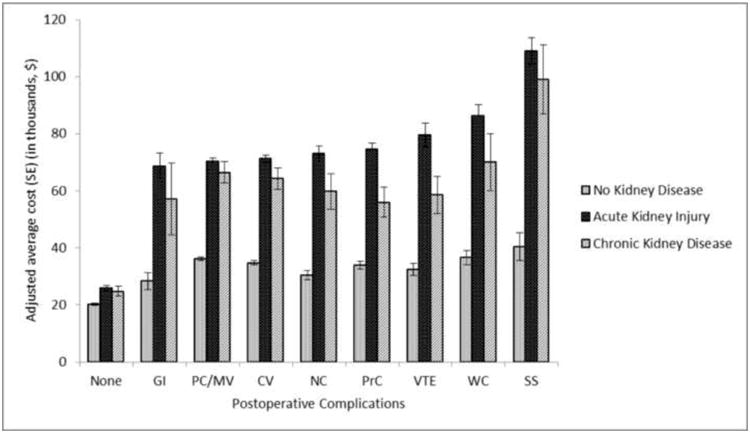
Risk-adjusted average cost of postoperative complications stratified by the occurrence of kidney disease. PC indicates postoperative complications; GI, gastrointestinal complications; PC/MV, pulmonary complications and/or mechanical ventilation; CV, cardiovascular complications and/or need for vasopressors; NC, neurological complications and/or delirium; PrC, procedural complications; VTE, venous thromboembolism; SS, severe sepsis; WC mechanical wound complications and/or surgical infections.
Figure 2.
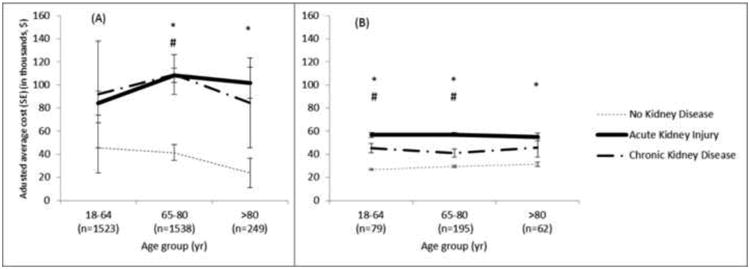
Risk-adjusted average cost for patients stratified by occurrence of kidney disease among patients who (A) died within 90 days of admission (B) did not die within 90 days of admission.
*denotes significance difference between no kidney disease and AKI groups.
#denotes significance difference between no kidney disease and CKD groups.
4. Discussion
In a large single-center cohort of patients undergoing major vascular surgery we have demonstrated that both acute and chronic kidney disease commonly occur during hospitalization for major inpatient vascular surgery. Postoperative acute kidney injury was the most common postoperative complication occurring in almost half of all patients. While our cohort consisted of patients receiving an array of diverse vascular surgical procedures, the prevalence of AKI using consensus criteria in our cohort appears consistent with the reported values in the vascular surgical literature: 12.7% of patients developed AKI after surgical intervention for peripheral arterial disease 11 while 76% of patients developed AKI after ruptured abdominal aortic aneurysmal repair. 14 Postoperative AKI, when defined using the KDIGO consensus criteria, is a common postoperative complication after major inpatient vascular surgery. The American College of Surgeons National Surgical Quality Improvement Program criteria for AKI grossly underestimated the incidence of this complication in our cohort and captured only 17% of the patients with KDIGO-defined AKI. These results confirm studies in other surgical populations demonstrating the inability of this definition to estimate the risk associated with mild and moderate acute kidney injury 8,32 and speak to the importance of using consensus KDIGO criteria in this clinical setting. The fact that only 20% of all AKI patients had any mention of AKI occurrence in discharge summaries illustrates well that, although rising, awareness for this serious postoperative complication is still low in clinical practice. Interestingly, recent study evaluating the effectiveness of electronic alert notifying providing physician of the onset of acute kidney injury using real-time serum creatinine changes demonstrated that physicians receiving the alert did nothing different compared to physicians not receiving them resulting in no improvement in the rate of dialysis or death among patients whose physicians received the alert.33 The diagnosis of AKI was documented in only 46% of the cases and compliance with the appropriate management of AKI recommended in consensus guidelines to which the alert had directed physicians was low. Thus both knowledge and perception of AKI importance among physicians involved in perioperative care needs to improve in order to affect physicians' behavior when it comes to consistent implementation of consensus guidelines for best practices. Together with growing body of literature our study helps to overcome the misconception that acute kidney injury after major vascular surgery only serves as a marker for overall illness burden rather than being a complication that independently contributes to poor surgical outcomes.
Our risk-adjusted models have demonstrated that patients who develop AKI are at a greater risk for ninety-day mortality than even patients with chronic kidney disease, an established predictor of poor outcomes in the vascular literature. Chronic kidney disease is a known risk factor for both perioperative and 1-year mortality following vascular surgery.1,5,34-36 Our study demonstrates that close to half of CKD patients will develop AKI as a postoperative complication leading to further increase in mortality, resource utilization and cost compared to patients with stable chronic kidney disease. Our study reinforces the importance of AKI in the vascular surgery population by demonstrating both the high prevalence and the poor outcomes associated with this complication. Those outcomes emphasize the need to stratify patients according to their preoperative risk of developing this complication, and the importance of early identification and management of those patients who develop AKI that has become more feasible with the newly validated and FDA approved urinary biomarkers for AKI risk stratification. 37,38
The occurrence of either acute or chronic kidney disease was independently associated with two-fold increase in the odds of ninety-day mortality after vascular surgery, even after adjusting for other postoperative complications. Interestingly most of the deaths among patients with both acute and chronic kidney disease occurred after hospital discharge and before ninety-day follow-up emphasizing importance of extending follow-up for surgical patients with postoperative complications.39,40 Khuri et al. have hypothesized that the postoperative complications induce a cascade of inflammatory processes that ultimately influence survival beyond hospital discharge.41 Several studies have demonstrated that postoperative acute kidney injury, even after it was resolved, was associated with increased risks for chronic kidney disease, hemodialysis and death years after surgery. 9,10,42-44 Other serious postoperative complications like mechanical ventilation and sepsis are also associated with increased long-term mortality and development of chronic critical illness and disability.45-47 Hence postoperative complications can affect a diverse group of patients imposing an acute burden of death and suffering, and even after resolved can affect future health and quality of life mandating that follow-up of these patients occur in prolonged and more structured manner.
Our study has some limitations. The large sample size of the vascular surgery cohort and our multivariate adjustment for known associations in the literature minimize the selection bias inherent to this design, but we cannot discount the possibility that other variables are contributing to the strengths of the associations we have demonstrated. The use of administrative database also brings about the potential for incorrect reporting of procedures and hospital costs. The vascular surgical cohort was defined retrospectively from the combination of admission and discharge services and validated groupings of ICD-9-CM codes. 15 Despite these clear selection criteria, there is the potential for misclassification and misrepresentation of patients in our cohort. In addition, despite the long follow-up period after AKI episode, the number of patients that were completely lost to follow-up due to invalid social security numbers was less than 1%. Nevertheless, this could have led to additional underestimation of the risk.
5. Conclusions
In summary, the prevalence of CKD at hospitalization for major inpatient vascular surgery was high, and nearly half of these patients developed AKI. Postoperative AKI occurred among 49% of the patients and was associated with higher hospital and ninety-day mortality, increased resource utilization, and hospital cost of care, even after adjustment for preoperative variables and other postoperative complications. Perioperative risk stratification for AKI using clinical scoring and novel urinary biomarkers and timely and standardized prevention and management of AKI in this patient population are urgently warranted.
Acknowledgments
We want to thank Gigi Lipori, Christine Bono and Yue Du for assistance with data retrieval. AB is supported by Center for Sepsis and Critical Illness Award P50 GM-111152 from the National Institute of General Medical Sciences and has received research grants from Astute Medical, Inc. MH was supported by the University of Florida Medical Student Summer Research fellowship.
Conflicts of Interest and Source of Funding: AB is supported by Center for Sepsis and Critical Illness Award P50 GM-111152 from the National Institute of General Medical Sciences and has received research grants from Astute Medical, Inc. MH was supported by the University of Florida Medical Student Summer Research fellowship.
Table A.1. Inpatient vascular procedures categories by International Classification and Diagnosis, 9th Revision, procedure codes.
| Open carotid |
| 3812 Endarterectomy, other vessels of head and neck |
| 3802 Incision of vessels, other vessels of head and neck |
| 3832 Resection of vessel with anastomosis, other vessels of head and neck |
| 3842 Resection of vessel with replacement, other vessels of head and neck |
| 3862 Other excision of vessels, other vessels of head and neck |
| 3882 Other surgical occlusion of vessels, other vessels of head and neck |
| 398 Operations on carotid body, carotid sinus and other vascular bodies |
| Open abdominal aortic |
| 3804 Incision of vessel, aorta |
| 3806 Incision of vessels, abdominal arteries |
| 3814 Endarterectomy, aorta |
| 3816 Endarterectomy, abdominal arteries |
| 3834 Resection of vessel with anastomosis, aorta |
| 3836 Resection of vessel with anastomosis, abdominal arteries |
| 3844 Resection of vessel with replacement, aorta |
| 3846 Resection of vessel with replacement, abdominal arteries |
| 3864 Other excision of vessels, aorta |
| 3866 Other excision of vessels, abdominal arteries |
| 3884 Other surgical occlusion of vessels, aorta, abdominal |
| 3886 Other surgical occlusion of vessels, abdominal arteries |
| 3924 Aorta-renal bypass |
| 3925 Aorta-iliac-femoral bypass |
| 3926 Other intra-abdominal vascular shunt or bypass |
| 3954 Re-entry operation (aorta) |
| 3955 Reimplantation of aberrant renal vessel |
| Thoracic-subclavian |
| 3805 Incision of vessels, other thoracic vessels |
| 3815 Endarterectomy, other thoracic vessels |
| 3835 Resection of vessel with anastomosis, other thoracic vessels |
| 3845 Resection of vessel with replacement, other thoracic vessels |
| 3865 Other excision of vessels, thoracic vessel |
| 3885 Other surgical occlusion of vessels, aorta, thoracic vessel |
| 3922 Aorta-subclavian-carotid bypass |
| 3923 Other intrathoracic vascular shunt or bypass |
| Endovascular thoracic/abdominal aortic |
| 3971 Endovascular implantation of graft in abdominal aorta |
| 3973 Endovascular implantation of graft in thoracic aorta |
| Peripheral |
| 3929 Other (peripheral) vascular shunt or bypass |
| (Lower extremity vascular procedure) |
| 3808 Incision of vessels, lower limb arteries |
| 3818 Endarterectomy, lower limb arteries |
| 3838 Resection of vessel with anastomosis, lower limb arteries |
| 3839 Resection of vessel with anastomosis, lower limb veins |
| 3848 Resection of vessel with replacement, lower limb arteries |
| 3849 Resection of vessel with replacement, lower limb veins |
| 3868 Other excision of vessels, lower limb arteries |
| 3869 Other excision of vessels, lower limb veins |
| 3888 Other surgical occlusion of vessels, lower limb arteries |
| 3889 Other surgical occlusion of vessels, lower limb veins |
| (Upper extremity vascular procedure) |
| 3803 Incision of vessels, upper limb vessels |
| 3813 Endarterectomy, upper limb vessels |
| 3833 Resection of vessel with anastomosis, upper limb vessels |
| 3843 Resection of vessel with replacement, upper limb vessels |
| 3863 Other excision of vessels, upper limb vessels |
| 3883 Other surgical occlusion of vessels, upper limb vessels |
| Percutaneous |
| 0063 Percutaneous insertion of carotid artery stent(s) |
| 3950 Angioplasty or atherectomy of noncoronary vessel |
| 3972 Endovascular repair or occlusion of head and neck vessel |
| 3979 Other endovascular repair (of aneurysm) of other vessels |
| 3990 Insertion of nondrug-eluting, noncoronary artery stent(s) |
| 9910 Injection or infusion of thrombolytic agent |
| Dialysis access |
| 3927 Arteriovenostomy for renal dialysis |
| 3942 Revision of arteriovenous shunt for renal dialysis |
| 3943 Removal of arteriovenous shunt for renal dialysis |
| 3953 Repair of arteriovenous fistula |
| Lower extremity amputations |
| 8410 Lower limb amputation, not otherwise specified |
| 8411 Amputation of toe |
| 8412 Amputation of through foot |
| 8413 Disarticulation of ankle |
| 8414 Amputation of ankle through malleoli of tibia and fibula |
| 8415 Other amputation below knee |
| 8416 Disarticulation of knee |
| 8417 Amputation above knee |
| 843 Revision of amputation stump |
| Other procedures |
| 387 Interruption of vena cava |
| 3949 Other revision of vascular procedure |
| 3952 Other repair of aneurysm |
| 3956 Repair of blood vessel with tissue patch graft |
| 3957 Repair of blood vessel with synthetic patch graft |
| 3958 Repair of blood vessel with unspecified type of patch graft |
| 3991 Freeing of vessel |
| 3999 Other operations on vessels |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gaber AO, Moore LW, Aloia TA, et al. Cross-sectional and case-control analyses of the association of kidney function staging with adverse postoperative outcomes in general and vascular surgery. Ann Surg. 2013;258:169–77. doi: 10.1097/SLA.0b013e318288e18e. [DOI] [PubMed] [Google Scholar]
- 2.Lomazzi C, Mariscalco G, Piffaretti G, et al. Endovascula treatment of elective abdominal aortic aneurysms: indpendent predictors of early and late mortality. Ann Vasc Surg. 2011;25:299–305. doi: 10.1016/j.avsg.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Marrocco-Trischitta MM, Melissano G, Kahlberg A, Calori G, Setacci F, Chiesa R. Chronic kidney disease classfication stratifies mortality risk after elective stent graft repair of the thoracic aorta. J Vasc Surg. 2009;49:296–301. doi: 10.1016/j.jvs.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 4.Sidawy AN, Adnian G, Johnson ON, 3rd, White PW, DeZee KJ, Henderson WG. Effect of chronic renal insuffciency on outcomes of carotid endarterectomy. J Vasc Surg. 2008;48:1423–30. doi: 10.1016/j.jvs.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Mathew A, Devereaux PJ, O'Hare A, et al. Chronic kidney disease and postoperative mortality: a systematic review and mete-analysis. Kidney Int. 2008;73:1069–81. doi: 10.1038/ki.2008.29. [DOI] [PubMed] [Google Scholar]
- 6.Debing E, Van den Brande P. Chronic renal insufficiency and risk of early mortality in patients undergoing carotid endarterectomy. Ann Vasc Surg. 2006;20:609–13. doi: 10.1007/s10016-006-9080-5. [DOI] [PubMed] [Google Scholar]
- 7.Hobson C, Ozrazgat-Baslanti T, Kuxhausen A, et al. Cost and Mortality Associated With Postoperative Acute Kidney Injury. Ann Surg. 2014 doi: 10.1097/SLA.0000000000000732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bihorac A, Brennan M, Ozrazgat-Bslanti T, et al. National surgical quality improvement program underestimates the risk associated with mild and moderate postoperative acute kidney injury. Crit Care Med. 2013;41:2570–83. doi: 10.1097/CCM.0b013e31829860fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bihorac A, Yavas S, Subbiah S, et al. Long-term risk of mortality and acute kidney injury during hospitalization after major surgery. Ann Surg. 2009;249:851–8. doi: 10.1097/SLA.0b013e3181a40a0b. [DOI] [PubMed] [Google Scholar]
- 10.Hobson CE, Yavas S, Segal MS, et al. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation. 2009;119:2444–53. doi: 10.1161/CIRCULATIONAHA.108.800011. [DOI] [PubMed] [Google Scholar]
- 11.Adalbert S, Adelina M, Romulus T, et al. Acute kidney injury in peripheral arterial surgery patients:a cohort study. Ren Fail. 2013;35:1236–9. doi: 10.3109/0886022X.2013.823830. [DOI] [PubMed] [Google Scholar]
- 12.Yue JN, Luo Z, Guo DQ, et al. Evaluation of acute kidney injury as defined by the risk, injury, failure, loss, and end-stage criteria in critically ill patients undergoing abdominal aortic aneurysm repair. Chin Med J (Engl) 2013;126:431–6. [PubMed] [Google Scholar]
- 13.van Beek SC, Legemate DA, Vahl A, et al. Acute kidney injury defined according to the ‘Risk,’ ‘Injury,’ ‘Failure,’ ‘Loss,’ and ‘End-stage’ (RIFLE) criteria after repair for a ruptured abdominal aortic aneurysm. J Vasc Surg. 2014;60:1159–67 e1. doi: 10.1016/j.jvs.2014.04.072. [DOI] [PubMed] [Google Scholar]
- 14.Kopolovic I, Simmonds K, Duggan S, Ewanchuk M, Stollery DE, Bagshaw SM. Risk factors and outcomes associated with acute kidney injury following ruptured abdominal aortic aneurysm. BMC Nephrol. 2013;14:99. doi: 10.1186/1471-2369-14-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jim J, Owens PL, Sanchez LA, Rubin BG. Population-based analysis of inpatient vascular procedures and predicting future workload and implications for training. J Vasc Surg. 2012;55:1394–9. doi: 10.1016/j.jvs.2011.11.061. discussion 9-400. [DOI] [PubMed] [Google Scholar]
- 16.(CCR). HC-t-CRF. Healthcare Cost and Utilization Project (HCUP) Rockville, MD: Agency for Healthcare Research and Quality; pp. 200–1020. [PubMed] [Google Scholar]
- 17.LaPar DJ, Bhamidipati CM, Mery CM, et al. Primary payer status affects mortality for major surgical operations. Ann Surg. 2010;252:554–50. doi: 10.1097/SLA.0b013e3181e8fd75. discussion 50-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guller U, Hervey S, Purves H, et al. Laparoscopic versus open appendectomy: outcomes comparison based on a large administrative database. Ann Surg. 2004;239:43–52. doi: 10.1097/01.sla.0000103071.35986.c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mason MA. Better educating our new breadwinners. In: Boushey H, O'Leary A, editors. The Shriver Report: A Woman's Nation Changes Everything. Washington, DC: Center for American Progress 2009; 2009. [Google Scholar]
- 20.Dombrovskiy VY, Martin AA, Sunderam J, Paz HL. Facing the challenge: decreasing case fatality rates in severe sepsis despite increasing hospitalizations. Crit Care Med. 2005;33:2555–62. doi: 10.1097/01.ccm.0000186748.64438.7b. [DOI] [PubMed] [Google Scholar]
- 21.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. Clinical Practice Guideline for Acute Kidney Injury. Kidney inter, Suppl. 2012;2:1–138. [Google Scholar]
- 22.Siew ED, Ikizler TA, Matheny ME, et al. Estimating baseline kidney function in hospitalized patients with impaired kidney function. Clin J Am Soc Nephrol. 2012;7:712–9. doi: 10.2215/CJN.10821011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American College of Surgeons National Surgical Quality Improvement Program. User Guide for the 2010 Participant Use Data File. Chicago, IL 60611 -3211: American College of Surgeons; 2010. [Google Scholar]
- 24.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity Measures for Use with Administrative Data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 26.Daskivich TJ, Fan KH, Koyama T, et al. Effect of age, tumor risk, and comorbidity on competing risks for survival in a U.S. population-based cohort of men with prostate cancer. Ann Intern Med. 2013;158:709–17. doi: 10.7326/0003-4819-158-10-201305210-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.American FactFinder. [Accessed 04/02/2011];2010 at http:/www2.census.gov/
- 28.Pebesma EJ, Bivand RS. Classes and methods for spatial data in R. R News; 2005. [Google Scholar]
- 29.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147:573–7. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 30.Pasta DJ. Estimating Standard Errors for CLASS Variables in Generalized Linear Models Using PROC IML. SAS Users Group International Proceedings. 2003;28:364–28. [Google Scholar]
- 31.Duan N. Smearing Estimate: A Nonparametric Retransformation Method. J Am Stat Assoc. 1983;78:605–10. [Google Scholar]
- 32.Vaught A, Ozrazgat-Baslanti T, Javed A, Morgan L, Hobson C, Bihorac A. Acute kidney injury in major gynaecological surgery: an observational study. BJOG. 2014 doi: 10.1111/1471-0528.13026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson FP, Shashaty M, Testani J, et al. Automated, electronic alerts for acute kidney injury: a single-blind, parallel-group, randomised controlled trial. The Lancet. doi: 10.1016/S0140-6736(15)60266-5. Published Online: 25 February 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel VI, Mukhopadhyay S, Guest JM, et al. Impact of severe chronic kidney disease on outcomes of infrainguinal peripheral arterial intervention. J Vasc Surg. 2014;59:368–75. doi: 10.1016/j.jvs.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 35.Tallarita T, Oderich GS, Gloviczki P, et al. Patient survival after open and endovascular mesenteric revascularization for chronic mesenteric ischemia. J Vasc Surg. 2013;57:747–55. doi: 10.1016/j.jvs.2012.09.047. discussion 54-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Howell NJ, Keogh BE, Bonser RS, et al. Mild renal dysfunction predicts in-hospital mortality and post-discharge survival following cardiac surgery. Eur J Cardiothorac Surg. 2008;34:390–5. doi: 10.1016/j.ejcts.2008.04.017. discussion 5. [DOI] [PubMed] [Google Scholar]
- 37.Bihorac A, Chawla LS, Shaw AD, et al. Validation of Cell-Cycle Arrest Biomarkers for Acute Kidney Injury Using Clinical Adjudication. Am J Respir Crit Care Med. 2014 doi: 10.1164/rccm.201401-0077OC. [DOI] [PubMed] [Google Scholar]
- 38.Kashani K, Al-Khafaji A, Ardiles T, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013;17:R25. doi: 10.1186/cc12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee PH, Gawande AA. The number of surgical procedures in an American lifetime in 3 states. J Am Coll Surg. 2008;207:S75–S. [Google Scholar]
- 40.Weiser TG, Regenbogen SE, Thompson KD, et al. An estimation of the global volume of surgery: a modelling strategy based on available data. The Lancet. 2008;372:139–44. doi: 10.1016/S0140-6736(08)60878-8. [DOI] [PubMed] [Google Scholar]
- 41.Khuri SF, Henderson WG, DePalma RG, et al. Determinants of Long-Term Survival After Major Surgery and the Adverse Effect of Postoperative Complications. Ann Surg. 2005;242:326–43. doi: 10.1097/01.sla.0000179621.33268.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bihorac A, Delano MJ, Schold JD, et al. Incidence, clinical predictors, genomics, and outcome of acute kidney injury among trauma patients. Ann Surg. 2010;252:158–65. doi: 10.1097/SLA.0b013e3181deb6bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cuthbertson BH, Roughton S, Jenkinson D, Maclennan G, Vale L. Quality of life in the five years after intensive care: a cohort study. Crit Care. 2010;14:R6. doi: 10.1186/cc8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ishani A, Nelson D, Clothier B, et al. The magnitude of acute serum creatinine increase after cardiac surgery and the risk of chronic kidney disease, progression of kidney disease, and death. Arch Intern Med. 2011;171:226–33. doi: 10.1001/archinternmed.2010.514. [DOI] [PubMed] [Google Scholar]
- 45.Hopkins RO, Jackson JC. Short- and long-term cogntive outcomes in intensive care unit survivors. Clin Chest Med. 2009;30:143–53. ix. doi: 10.1016/j.ccm.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 46.Unroe M, Kahn JM, Carson SS, et al. One-year trajectories of care and resource utilization for recipients of prolonged mechanical ventilation:a cohort study. Ann Intern Med. 2010;153:167–75. doi: 10.1059/0003-4819-153-3-201008030-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gentile LF, Cuenca AG, Efron PA, et al. Persistent inflammation and immunosuppression: A common syndrome and new horizon for surgical intensive care. J Trauma Acute Care Surg. 2012;72:1491–501. doi: 10.1097/TA.0b013e318256e000. [DOI] [PMC free article] [PubMed] [Google Scholar]


