Abstract
The catechol-O-methyltransferase (COMT) val158met single nucleotide polymorphism (SNP) alters metabolic activity of the COMT enzyme regulating catecholamines, with the Val (valine) allele resulting in 40% greater enzymatic activity than the Met (methionine) allele. Previous research has identified systematic inter-individual differences in cognitive and behavioral phenotypes related to this polymorphism, often attributed to the fact that extracellular dopamine in the prefrontal cortex is strongly affected by the COMT enzyme. The neurophysiological mechanisms mediating these inter-individual differences in specific brain systems and task contexts remain to be established however. In the current study, we examined the extent to which physio-mechanistic differences by COMT genotype affect somato-visceral and visual cortical responses to learned threat cues. Classical aversive differential conditioning was implemented using rapidly phase-reversing grating stimuli, previously shown to engage retinotopic visual cortex. Differential response patterns in sensory and autonomic systems were elicited by pairing one grating (CS+, conditioned stimulus), but not the other (CS-), with a noxious stimulus. Dense-array electroencephalography and somato-visceral measures of defensive reactivity were recorded in addition to self-report data. Individuals of the Val/Val genotype, compared to Met allele carriers, reliably showed greater initial enhancement in their visuocortical response to the CS+, accompanied by stronger defensive engagement, indexed by heart rate acceleration and startle potentiation. The finding that COMT polymorphism status affects threat cue reactivity at the visuocortical level is consistent with the notion that sensory processing of threat is facilitated by strong re-entrant bias signals from anterior areas, including the prefrontal cortex.
Keywords: classical conditioning, EEG, ssVEP, physiology, genotyping
Graphical abstract
Introduction
The catechol-O-methyltransferase (COMT) rs4680 single nucleotide polymorphism (SNP) G>A results in the substitution of valine (Val) to methionine (Met) at codon 158 (Lachman et al., 1996). This polymorphism impacts the effectiveness of COMT, a regulatory enzyme in the metabolic pathways of catecholamines (dopamine, epinephrine and norepinephrine) in the central nervous system and peripherally in red blood cells and liver of both rodents and humans (Weinshilboum & Dunnette, 1981). The amino acid change resulting from the COMT val158met polymorphism alters metabolic activity of the COMT enzyme, where the Val form has a three to four-fold increase in enzymatic activity over the Met form (Weinshilboum, 1999). Physiologically, increased COMT activity leads to a more efficient elimination of extracellular dopamine, particularly in prefrontal cortex (PFC) due to a paucity of dopamine transporters in this region (Hirvonen et al., 2010; Slifstein et al., 2008; Yavich et al., 2007). The tonic-phase dopamine hypothesis relates varying COMT enzymatic activity levels to systematic changes in dopamine transmission in ventral striatal and PFC neurons (Bilder et al., 2004). High-activity COMT (Val allele) results in decreased tonic dopamine activity in striatum, therefore lowering threshold for phasic dopamine through D2 auto-receptors. In PFC on the other hand, high-activity COMT results in lower extracellular dopamine availability. In vivo PET studies however have demonstrated that D2 levels in human cortex and striatum do not differ as a function of COMT genotype (Hirvonen et al., 2010), concluding that the COMT SNP impacts D1 receptors instead.
Given this complexity in fronto-striatal dopamine signaling, it is not surprising that contending hypotheses have been discussed regarding the impact of COMT status on cognitive functioning. Behavioral studies using executive control and working memory paradigms have found that Met allele carriers tend to report task items more accurately compared to Val homozygotes (Barnett et al., 2007; Egan et al., 2001). Opposite findings have been reported for tasks entailing reversal learning, task-switching, and emotional distraction, in which Val homozygotes performed more accurately than individuals carrying at least one Met allele (Bishop et al., 2006; Calzato et al., 2010; Nolan et al., 2004). These findings implicate Met carriers to have an advantage, cognitively, in maintaining task specific demands in the context of working memory, whereas Val carriers appear to display an advantage in updating task demands (Bellander et al., 2015; Rosa et al., 2010). Although this evidence suggests that COMT genotype status may affect behavior, identification of specific neurophysiological mechanisms mediating this link has been difficult. Well-defined experimental protocols that challenge known neurophysiological processes may be a suitable avenue towards this goal. Classical differential conditioning incorporates task demands in the context of changing contingencies and may therefore be ideally suited to extend the COMT literature. The aim of the current study was to examine the extent to which COMT genotype affects aversive learning during classical differential aversive conditioning.
Classical differential aversive conditioning in the laboratory has been extensively studied in both animal and human models, demonstrating the fundamental process of an initially neutral stimulus acquiring an aversive quality through pairing with a noxious stimulus (Miskovic & Keil, 2012). The acquisition of an aversive quality has been shown neurophysiologically to involve learning induced changes in sensory systems, such as retinotopic visual cortex (McTeague et al., 2015; Stolarova et al., 2009), as well as systematic changes in autonomic reflex physiology and behavior (Hamm & Weike, 2005; Hodes et al., 1985). Neuroimaging studies have shown several brain regions to be involved during aversive learning including sensory and motor cortices, as well as limbic structures such as amygdala, insula, striatum and hippocampus (Buchel et al., 1998; LeDoux, 2000). Cortical and subcortical regions are thought to modulate primary sensory cortices through reentrant feedback projections, leading to learning induced changes impacting perception (Lamme & Roelfsema, 2000).
Furthermore, stimulus expectancy and anticipation have been theorized to play an essential role in the process of contingency learning (Rescorla & Wagner, 1972), implicating the involvement of anterior brain regions such PFC (Delgado et al., 2006). In a recent study, rapid CS discrimination was found specifically in PFC regions during the acquisition phase of an aversive conditioning paradigm (Rehbein et al., 2014). The PFC may be crucial in aversive learning due to its involvement in attention, associative learning and working memory through top-down modulation of higher-order sensory cortices (Asaad et al., 1998; Barcelo et al., 2000; Desimone, 1996; B.T. Miller et al., 2011; E.K. Miller & Cohen 2001; E.K. Miller et al., 1996; Rainer & Miller, 2000). The modulatory role of the dopaminergic system has been suggested as one mechanism by which anterior brain regions contribute to the acquisition and extinction of aversive pairings (Abraham et al., 2014; Horvitz, 2000; Wendler, 2014).
In summary, these findings imply that fronto-striatal regions may play a modulatory role in aversive learning, potentially reflected in learning-related changes of cognitive processes such as attention, anticipation, working memory. Previous work partly supports this notion: During viewing of aversive stimuli, as well as during emotional memory tasks, Met carriers tend to show heightened amygdala and hippocampal activation compared to Non-Met carriers (Drabant et al., 2006; Smolka et al., 2007; Smolka et al., 2005; Williams et al., 2010). In terms of autonomic engagement, Met carriers show enhanced startle potentiation during passive viewing of aversive stimuli (Montag et al., 2008). Additionally, Lonsdorf and colleagues (2009) found Met carriers to have failed startle suppression during the extinction phase of an aversive conditioning paradigm, suggesting that Met carriers may have an increased proneness to anxiety. Although these findings can be taken as evidence for altered emotional reactivity based on COMT genotype, results are mixed when individual differences are taken into account such as psychiatric diagnosis, gender and ethnicity (Domschke et al., 2012; Lee & Prescott, 2014). In addition, Wang and colleagues (2013) demonstrated that COMT by ethnicity interactions as observed for Caucasians versus Asians (Domschke et al., 2007) may be attributed to altered brain physiology between ethnic groups. The neurophysiological mechanisms that explain the cognitive, autonomic and behavioral differences reported for the COMT val158met polymorphism are scarce in the literature and warrant more systematic investigations that challenge these processes.
An increasingly used neuroimaging tool in the cognitive neurosciences is the steady-state visually evoked potential (ssVEP). In this technique, visual cortical neurons are entrained at a given frequency by an external luminance- or contrast-modulated stimulus, captured as oscillatory scalp voltage changes through electroencephalography (EEG). Recorded activity is thought to reflect the evoked, synchronous activity of large masses of neurons in response to a stimulus. The ssVEP is modulated by experimental manipulations such as selective attention, feature selection, and motivational/affective relevance of the stimulus (Keil et al., 2009; Moratti et al., 2004; Muller et al., 2006; Silberstein et al., 1995; Song & Keil, 2014). The increase of ssVEP amplitude with motivational relevance, especially during aversive engagement, has been attributed to top-down influences on visual cortex exerted by anterior structures involved in the neurocomputation of aversive or appetitive value (Lang & Bradley, 2010).
The aim of the current study was to explore the extent to which COMT genotype status affects defensive reactivity to threat cues during aversive learning. We implemented a differential aversive conditioning paradigm in which a visual stimulus (conditioned stimulus, CS+) attained aversive quality by repeated and consistent pairing with a noxious stimulus (unconditioned stimulus, US), whereas a different visual stimulus (CS-) is never paired with the noxious stimulus. By recording ssVEPs elicited by the visual stimuli (CSs), peripheral physiological measures, and self-report data, we aimed to capture learning-induced perceptual, autonomic and behavioral changes, respectively, in order to assess COMT genotype differences. As aversive learning engages processes underlying both emotional reactivity and cognitive functioning, two alternative hypotheses are plausible in light of the current literature on the COMT val158met polymorphism: Met allele carriers have been shown to exhibit heightened defensive reactivity during passive viewing of aversive stimuli. In the context of aversive learning, this may translate to not only heightened defensive engagement, but also enhanced perceptual processing of the aversively cued stimulus (CS+). On the other hand, as Val/Val homozygotes have been demonstrated to show an advantage in updating task demands, individuals of this genotype may present with more pronounced differential adaptation to the changing contingencies of the differential conditioning paradigm, when comparing the CS+ and CS- during habituation, acquisition and extinction.
Methods
Participants
Participants were students taking General Psychology courses at the University of Florida who received course credit. A total of 83 participants were originally included in the study. Fourteen individuals were excluded from the analysis due to poor data quality in the electroencephalography (EEG) recording. Poor data quality was determined by excessive loss of trials due to movement artifacts and low signal-to-noise ratio of the steady-state visually evoked potential (ssVEP), measured using the circular T-square statistic proposed by Victor and Mast (1991). Of the remaining 69 individuals, 63 were successfully genotyped for the COMT val158met polymorphism. Demographics are reported for all participants run in the study. Participants ranged in age from 18-23 (M = 18.9, SD = 1.3), with the majority being right-handed, female Caucasians (94.5%, 67.0% and 64.8%, respectively). The breakdown of minorities was as follows: 17.6% Hispanic, 11.0% Asian, 5.5% African-American and 1.1% Middle Eastern. All participants had normal or corrected vision, and reported no personal or family history of epilepsy or photic seizures. No additional inclusion/exclusion criteria were applied.
Stimuli
Visual stimuli for the study were sinusoidal gratings multiplied with a Gaussian envelope (Gabor patches). For each Gabor patch we generated two, phase inverted, versions and these were reversed at a rapid, fixed rate to elicit steady-state visually evoked potentials (ssVEPs) in the visual cortex. Previous research has found these gratings to effectively elicit differential responding in primary visual cortex in a classical conditioning paradigm (Keil et al., 2013).
Presentation between a Gabor grating and its phase-reversed counterpart alternated at a rate of either 13.3 Hz, 14 Hz or 15 Hz (manipulated between participants), with one cycle length (containing both patterns) equating to 150.00 ms, 142.86 ms, or 133.33 ms, respectively.1
Luminance Gabor gratings were alternating black and gray patterns of 6 cycles, with a Michelson contrast of .98, subtending a vertical visual angle of 7.4°, a horizontal visual angle of 10.9°, and a spatial frequency 0.6 cycles per degree (cpd). Two orientations for each of the Gabor gratings (+15° and -15°) were presented using Psychtoolbox software (Brainard, 1997) for each trial iteration, implemented through Matlab. These orientations served as CS+ and CS- conditions during the acquisition phase. In an additional part of the experiment, participants viewed chromatic gratings, but these data are not reported on in this manuscript.
All acoustic stimuli were presented through speakers placed behind the participant's head, at a distance of approximately 40 cm. The unconditioned stimulus (US) was white noise filtered between 1 and 1000 Hz, presented at 96 dB for 1400 ms. Startle probes were 50 ms bursts of white noise presented during the Gabor flicker (4 or 5 s post onset) or 1 s following the US.
Design
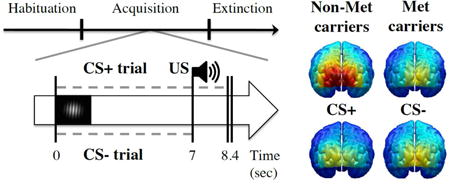
The current design was a classical, differential aversive conditioning paradigm containing three phases: habituation, acquisition and extinction. Pairing of the US with the CS+ (Gabor grating oriented +15°) occurred only during the acquisition phase with a 100% reinforcement schedule. The CS- (Gabor grating oriented -15°) was never paired with the US. Additionally, no CS-US pairing occurred and no US was present at any time during the habituation and extinction phases. Before the start of both acquisition and extinction phases, participants were instructed on the contingencies of the CS-US pairing. Each phase contained 12 trials of the luminance Gabor gratings with a balanced, pseudo-randomized trial order that was kept the same for all participants. During habituation, extinction, and CS- trials in acquisition, trial length was 7000 ms in duration, whereas the CS+ trials extended for an additional 1400 ms during which the US was simultaneously presented (delay conditioning). One of three startle probes was presented every trial: at 4 sec, 5 sec or 1 sec after the end of the trial. Startle probes occurring during the trial (4 and 5 sec) were averaged together for later analyses. In terms of the flickering Gabor gratings, a 7000 ms trial contained an average of 49 phase-reversed cycles. Furthermore, acquisition and extinction phases were broken down into two blocks each for data analysis in order to examine the temporal dynamics of aversive conditioning. Experimental phases and trial timing are illustrated in Figure 1.
Fig 1.
Experimental design for classical, discriminant aversive conditioning. The experiment consisted of three phases (habituation, acquisition and extinction), in which CS+ was paired with the US (loud noise) only during acquisition (100% reinforcement schedule). Trials in habituation, extinction and CS- trials in acquisition lasted 7 sec. CS+ trials in acquisition lasted an additional 1.4 sec, with US onset starting at 7 sec. Dotted gray line signifies flickering Gabor patch (CS), crosses signify startle probes (trial and ITI probes). Example of the CS+ Gabor patch shown on the left.
Procedure
Upon arriving in the laboratory, participants provided informed consent, were led to the recording chamber and seated in a chair at a distance of 100 cm between their eyes and the presentation monitor. Experimental procedures were then explained and participants were given the State and Trait Anxiety Inventory (STAI) questionnaire (Spielberger et al., 1983), which they completed in the absence of the experimenter. Subsequently, participants were instructed on how to use the mouthwash (1.5 fl Oz Scope® bottle) for the saliva sample: vigorously swishing half the mouthwash for a minute, then spitting into the pre-labeled test tube provided, and repeating with the other half. The test tube with the saliva sample was then immediately taken by the experimenter wearing non-latex gloves and placed in a cold-storage box in the laboratory. Electrodes for recording peripheral physiological measures were placed, in order, on the left palm (skin conductance response), on either arm (heart rate response) and underneath the left eye (startle response). The EEG net was applied next and an impedance check was run. Lights were turned off completely before starting the experiment. After each phase there was a brief pause in the experiment where the participant completed ratings with the Self-Assessment Manikin (SAM) (Bradley & Lang, 1994) for the Gabor gratings, reporting perceived hedonic valence and arousal. After the conditioning phase participants additionally completed the State Anxiety portion (form Y1) of the STAI questionnaire again, to obtain pre and post measures of self-reported state anxiety. Upon conclusion of the study, participants were asked to rate the US on a scale of 1-10 (higher scores being more unpleasant) and were subsequently debriefed. All procedures were approved by the institutional review board of the University of Florida.
Data Collection and Processing
Electroencephalography (EEG)
Collection
EEG was continuously recorded from either a GSN 200 257 channel sensor net or a HCGSN 129 channel sensor net by Electrical Geodesic (EGI), with Cz as the reference electrode. EEG signal was digitized at 250Hz and band-pass filtered online from 0.1 to 48 Hz, with impedances being kept below 60 kΩ for the 257 channel nets and 40 kΩ for the 129 channel nets.
Processing
Offline preprocessing of the data was implemented through EMEGS software (Peyk et al., 2011). Data were filtered with an 18 Hz lowpass filter (cut-off at 3 dB point, 45 dB/octave, 16th order Butterworth) and a 7 Hz highpass filter (cut-off at 3 dB point; 24 dB/octave, 5th order Butterworth), segmented (epochs of 7600ms, including 400 ms pre-trigger onset), and submitted to artifact rejection. The procedure for artifact rejection identifies artifacts in individual channels by referencing Cz (recording reference) and detecting deviations based on the distribution of the mean, the standard deviation as well the gradient of the voltage amplitude (Junghöfer et al., 2000). Data were then re-referenced to a global average and artifacts such as blinks, eye movements and other movements were eliminated. Channels contaminated by such artifacts were interpolated using a statistically weighted, spherical spline interpolation from the entire channel set. After artifact rejection, an average of 75% of the trials across all conditions were kept for further analyses.
Scalp voltage data were converted to source space data through the minimum norm estimation (MNE) method using the implementation proposed by Hauk and colleagues (Hauk, 2004; Hauk et al., 2002). For this method, we used a 3D source space consisting of four concentric spheres, on which the dipoles were placed equidistantly to approximate the brain volume. This source space contained 655 source locations (i.e. the model sources). At each model source location, currents were modeled for three spatial orientations (1 radial, two tangential with respect to the scalp surface; orientations were orthogonal relative to each other). This was done to capture voltage gradients in all possible directions (Hauk, 2004). The four shells had the radii 0.8, 0.6, 0.4, and 0.2 relative to the electrode radius of 1, respectively. For regularization, we used the Tikhonov-Philips approach, which is optimized to suppress uncorrelated noise (Hauk, 2004), and a regularization factor of 0.8. Paralleling previous work (Keil et al., 2007), source density estimates from the radial component in the outer shell were considered best approximations of the cortical currents contributing to the recorded signal. Accordingly, these data were utilized for all further analyses. Subsequently, source space data were converted to the frequency domain by means of discrete Fourier transformation (DFT) on the last two seconds of the evoked potential leading up to US onset (Moratti et al., 2006). For standardization, signal-to-noise ratios (SNR) were then computed on the frequency domain data by specifying the bin of the ssVEP frequency and dividing by the average of the surrounding 5 bins to either side of the target bin. Trials of the same condition were averaged together, forming condition-specific representations of the evoked response in source space. To examine temporal dynamics during the acquisition and extinction phases, trials were partitioned into 2 blocks per phase resulting in 6 trials per condition per block. For illustration, source data were projected back onto a 257 channel net in the topographies presented in figures. As the main dependent variable for all subsequent analyses, ssVEP source strength was defined as a regional average of signal-to-noise ratios over an occipital sensor cluster that included site Oz of the international 10-20 system and its 8 nearest neighbors, including POz, O1, O2, and Iz. See Figure 2 for depiction of ssVEP time-domain data, frequency spectrum, as well as occipital sensor cluster chosen.
Figure 2.
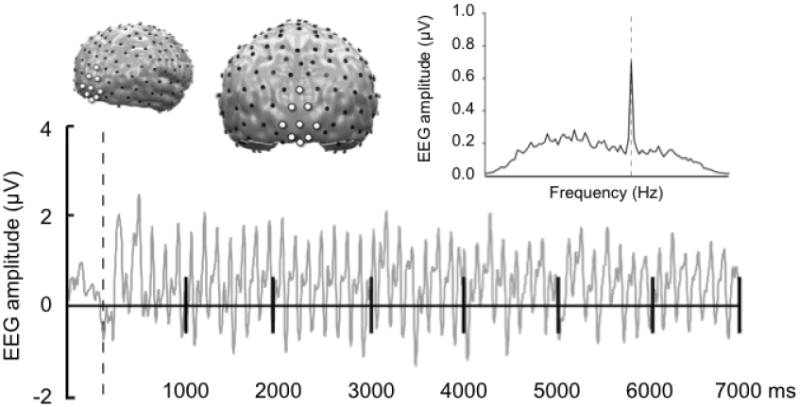
Visuocortical evoked response in the time and frequency domain from a subsample illustrating 14Hz pattern-reversal ssVEP. After transformation into the frequency domain, evoked power of the ssVEP signal is clearly distinguishable against noise (indicated by dashed gray line, top right). Sensor cluster used in ssVEP analyses indicated by white sensors at occipital pole, viewed from the back of the brain, with Oz in the center (top left).
Peripheral Physiological Measures
A computer running VPM software (Cook, 2002) controlled data acquisition for all peripheral physiological measures. Skin conductance was recorded from 8mm Ag-AgCl electrodes filled with 0.05 M NaCl paste on the hypothenar eminence of the left palm and continuously sampled at 20 Hz. Skin conductance was converted to microSiemens and averaged into half-second bins. Maximum skin conductance change during the 7 sec trial deviated from baseline (1000 ms prior stimulus onset) were then log transformed and averaged over condition specific trials. Heart rate was recorded through 8mm Ag-AgCl electrodes on each forearm. Inter-beat (R-R) intervals were reduced into half-second bins using the procedure proposed by (Graham, 1978). Left orbicularis oculi EMG was recorded using 4mm Ag-AgCl electrodes, which was sampled at 1000 Hz, amplified, band-pass filtered (90-250 Hz), and integrated (20 ms time constant). Startle magnitude of the eye blink response was then processed offline in VPM using a peak-scoring algorithm (Balaban et al., 1986) that utilizes onset latency and amplitude of each blink elicited. Trials with no startle responses were not included in the analysis. Subsequently, nonresponders (total average responding < 14%) who failed to elicit a sufficient amount of startle reflexes for all given experimental conditions were eliminated from further analyses (Blumenthal et al., 2005). Z scores were calculated within experimental phase using mean and standard deviations within individuals, trimmed by 3 standard deviations and subsequently converted to t scores. Trial probes represented an average of the probe positions 4 and 5 sec, with post-US probe being the startle probe 1 sec after the end of the trial. All non-startle autonomic data were processed as change scores from the baseline (1000 ms prior stimulus onset) and averaged as condition-specific responses over 2 blocks per phase (6 blocks in total), with the exception of startle responding that was averaged over the entire phase (3 in total).
Self-Report Measures
The State and Trait Anxiety Inventory (STAI) questionnaire contained two forms, one assessing self-reported State Anxiety (anxiety felt at the current moment) and the other Trait Anxiety (anxiety felt on a daily basis). The entire questionnaire was given before the start of the experiment, as well as the State Anxiety part again after undergoing conditioning as previously described in the procedure. After each phase of the experiment SAM ratings were taken for the Gabor gratings. US ratings were noted at the end of the experiment.
Genotyping
The genomic DNA (gDNA) was isolated from buccal cells using a commercially available kit (QiaGen, MD, USA). Genotyping for catechol-O-methyltransferase (COMT rs4680) was carried out using TaqMan 7900 allele discrimination assays (Life Technologies, CA, USA) and Pyrosequencing (Langaee & Ronaghi, 2005) PSQ HS 96 System (QiaGen, MD, USA), at the University of Florida Center for Pharmacogenomics. Saliva samples for genotyping were collected over a span of two years and analyzed at irregular intervals during data collection. Six samples were not successfully genotyped due to poor quality, despite multiple attempts, conceivably due to DNA degradation over time. Fisher's exact tests revealed that genotype frequencies for COMT rs4680 did not deviate from Hardy-Weinberg Equilibrium (p = .794). Individuals of the Met/Met genotype (n=11, 6 female) and the Met/Val genotype (n=29, 18 females) were collapsed into a group of Met carriers (n=40, 24 females) to be analyzed against the Non-Met carriers (Val/Val genotype, n=23, 14 females).
Statistical Analyses
Repeated measures ANOVA was conducted on ssVEP amplitudes (signal-noise-ratio scores) across participants, with CS condition (2; CS +/-) and block (3; acquisition block 1 and 2, extinction block 1) as the within-subjects factors, and genotype (2; Met vs Non-Met carriers) as the between-subjects factor. Both acquisition blocks and the first block of extinction were a priori selected, as conditioning effects were expected to occur during these three blocks, to keep ANOVA models within a manageable size and to account for the fact that null-results were expected for habituation. Significant effects were followed up with repeated measures ANOVA on each block separately.
Subsequent analyses of the peripheral physiological and self-report measures were conducted using repeated measures ANOVAs with within-subjects factors of condition (2; CS +/-), time segment (see Results section for description for each measure) and the between-subjects factor of genotype (3; Met vs Non-Met carriers). In addition, a repeated measures ANOVA was conducted on startle separately for each startle probe position (2; trial probe and post-US probe) as empty cells in the ANOVA table for post-US probes resulted in the loss of individuals as more artifacts occurred directly after US presentation.
Due to consistent findings of gender differences in the COMT literature (Domschke et al., 2012; Lee & Prescott, 2014), gender was added as a covariate in the analyses of all dependent measures.
Results
Occipital electrocortical signals (ssVEP amplitude)
Genotype differences were found for the COMT val158met polymorphism grouped by Met and Non-Met allele carriers. Across conditioning blocks and the first block of extinction, a two-way interaction was found for CS condition × genotype [F(1,61) = 4.73, p < .05], in addition to a three-way interaction of block × CS condition × genotype [F(2,122) = 5.03, p <.05, partial eta squared = .076]. Follow-up ANOVA revealed that during the first block of acquisition, NonMet allele carriers showed significant visuocortical enhancement of the CS+, compared to the CS- [F(1,61) = 11.03, p < .05, partial eta squared = .153].2
In the scatter plot of the individual distributions, group separation of COMT allele carriers is shown for differences scores (CS+ minus CS-) in visuocortical activation (Figure 3). This enhancement when viewing the CS+ was limited to the first block of acquisition, and disappeared during the second block [F(1,61) = 1.81, n.s.]. Follow-up t-tests were conducted testing CS+ versus CS- activation and was found to be exclusive to the Non-Met group (Val/Val) [t(23) = 2.72, p < .05] (Figure 4).
Figure 3.
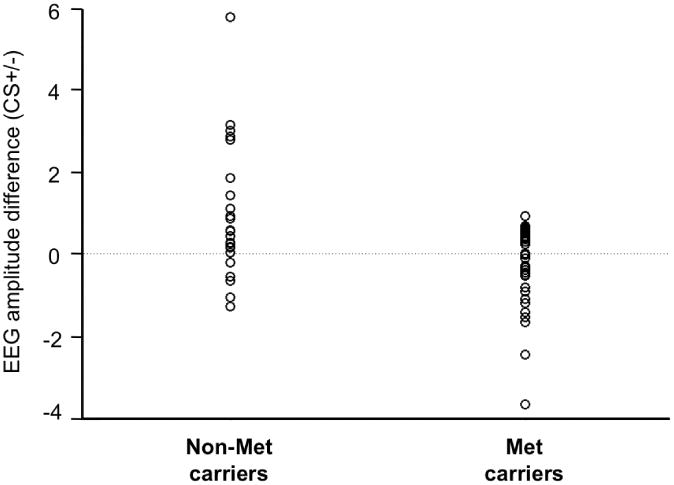
Distribution of conditioning effects of EEG scalp potentials captured through the ssVEP by genotype grouping of the COMT val158met polymorphism during the first half of conditioning. Ns of genotypes are as followed: Non-Met carriers (23), Met carriers (40). Positive values indicate greater amplitude enhancement to the CS+ compared to the CS-. Circles represent single individuals within a group.
Figure 4.
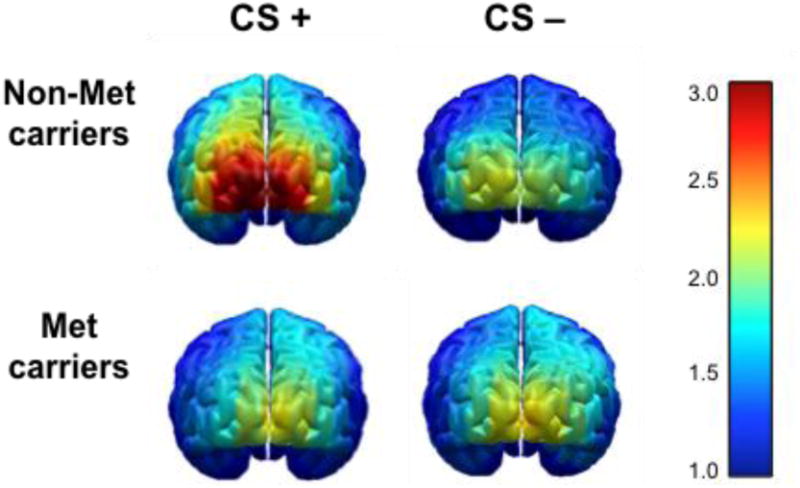
Activation over occipital pole to CS stimuli measured through ssVEP during first half of conditioning. Non-Met carriers (n=23) showed significant enhancement to the CS+ compared to CS-, whereas Met carriers (n=40) showed no CS differentiation (scale in μV, SNR corrected and source estimated).
Somato-visceral measures
Difference scores were computed for mean heart rate (HR) reactivity in time intervals 0-3 sec (D1), 3-5 sec (A1) and 5-7 sec (D2), with the first difference score being A1-D1 (accelerative window) and the second D2-A1 (decelerative window). Both COMT groups showed HR deceleration to the CS+ for the first half of conditioning [F(1,54) = 4.4, p < .05] (Figure 5). In the second block, main effects were observed for the two difference scores [F(1,54) = 4.29, p < .05], condition (CS+/-) [F(1,54) = 4.96, p < .05], as well as a three-way interaction of difference scores × condition × COMT group (Met vs Non-Met). In the follow-up analysis, a two-way interaction of difference scores × COMT group [F(1,54) = 8.39, p < .05] was found. An independent-samples t-test for the between-subject factor of COMT group showed significant HR increase in response to the CS+ during the accelerative window in Non-Met individuals, whereas Met individuals showed HR deceleration [t(54) = 2.37, p < .05] (Figure 5 and Table 1).
Figure 5.
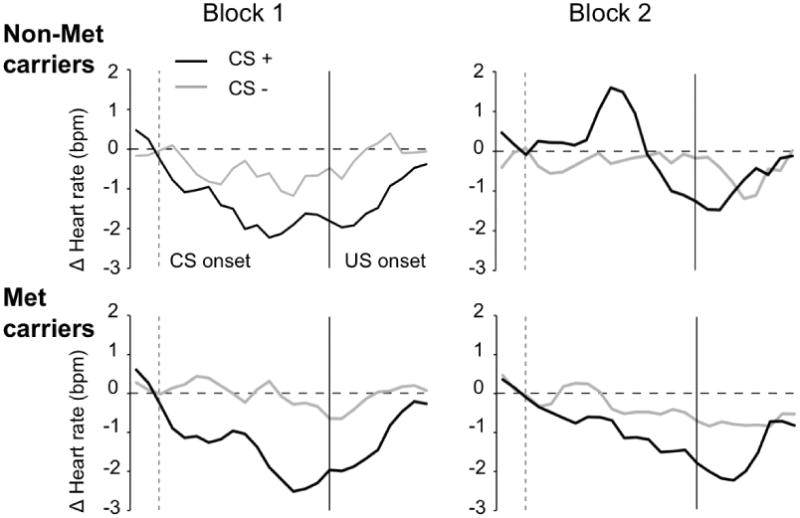
Heart rate reactivity over conditioning phase, split into two blocks. US onset is at 7 sec (thick line) for 1.4 sec. Black line signifies response to CS+, gray to CS-. Non-Met carriers (n=19) and Met carriers (n=37) do not differ in first block of conditioning in their response to the CS+, but show significant deceleration compared to CS-. In the second block Non-Met carriers show significant increase in heart rate to the CS+.
Table 1. Heart rate reactivity in acquisition during accelerative window.
Heart rate reactivity is shown as the difference score between acceleration and first deceleration (A1-D1). Standard error of the mean is shown in parentheses.
| COMT Genotype | Block 1 | Block 2 | ||
|---|---|---|---|---|
|
| ||||
| CS + | CS - | CS + | CS - | |
| Non-Met Carriers | - 1.0 (0.4) - | 0.2 (0.3) | 0.7 (0.5)* | 0.1 (0.3) |
| Met Carriers | - 0.4 (0.3) | -0.1 (0.2) | - 0.5 (0.3) | -0.3 (0.2) |
Bold value indicates where Val/Val individuals showed significant heart rate acceleration to CS+ compared to CS-
Startle response during the acquisition phase to the trial probe showed no main effect of condition [F(1,42) = 3.31, n.s.], but an interaction of condition × COMT group emerged [F(1,42) = 4.24, p < .05]. A follow up independent-samples t-test showed significantly greater startle potentiation to the CS+ in Non-Met individuals compared to the Met carriers [t(45) = 2.02, p < .05], with no group difference for the CS- [t(42) = 1.51, n.s.] (Figure 6 and Table 2).
Figure 6.
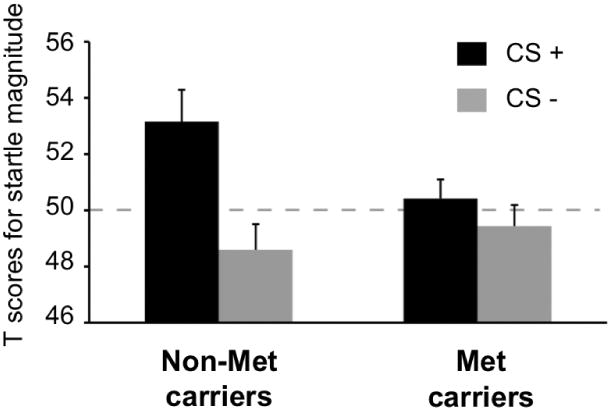
Startle magnitude to trial probe during acquisition. Black bars indicate startle response to CS+, gray bars to CS-. Non-Met carriers (n=16) showed significantly greater startle potentiation to the CS+ during acquisition than Met carriers (n=31). Standard error of the mean is shown as error bars.
Table 2. Startle blink magnitude in acquisition to startle probes.
T scores are shown with standard error of the mean in parentheses.
| COMT Genotype | Trial probe | Post-US probe | ||
|---|---|---|---|---|
|
| ||||
| CS + | CS - | CS + | CS - | |
| Non-Met Carriers | 53.2 (1.1)* | 48.6 (0.9) | 44.9 (1.5) | 49.3 (1.5) |
| Met Carriers | 50.4 (0.7) | 49.4 (0.7) | 48.0 (1.2) | 50.3 (1.1) |
Bold value indicates where Val/Val individuals showed significant startle potentiation to CS+ compared to CS-
Startle response to the post-US probe showed neither a main effect of condition [F(1,37) = 2.05, n.s.] nor an interaction of condition × COMT group [F(1,37) = .23, n.s.]. Startle response to the post-US probe during extinction, however, revealed a significant condition × COMT group interaction [F(1,31) = 7.34, p < .05], by which Non-Met carriers showed greater potentiation to the CS- compared to Met carriers [t(32) = 2.94, p < .01].3
In response to the CS presentation, there was a main effect of condition (CS+/-) for skin conductance response (SCL) in the first block of acquisition, with greater SCL to the CS+ [F(1,55) = 9.00, p < .01] (Table 3). No significant interaction was found for condition × COMT group [F(1,55) = 1.13, n.s.]. The main effect of condition disappeared by the second half of acquisition. In response to the US presentation, across both acquisition blocks, there was a main effect of condition [F(1,55) = 34.18, p < .001], with greater SCL to the CS+, as well as a main effect of block [F(1,55) = 16.38, p < .001], with greater SCL during the first block. In addition, an interaction of condition × block [F(1,55) = 37.94, p < .001] showed that from the first to the second block of acquisition, SCL significantly decreased in response to the CS+ [t(56) = 6.83, p < .001], but not to the CS- [t(56) = -1.38, n.s.].
Table 3. Skin conductance responding (SCL) in acquisition to CS presentation.
Significant enhancement to the CS+ over the CS- during the first block of acquisition was observed for SCL, with no COMT genotype differences. Standard error of the mean is shown in parentheses.
| COMT Genotype | Block 1 | Block 2 | ||
|---|---|---|---|---|
|
| ||||
| CS + | CS - | CS + | CS - | |
| Non-Met Carriers | 0.04 (0.01) | 0.01 (0.01) | 0.01 (0.01) | 0.01 (0.00) |
| Met Carriers | 0.08 (0.01) | 0.02 (0.01) | 0.02 (0.01) | 0.01 (0.00) |
Follow-up linear regression analyses were performed on measures of EEG, heart rate and startle response: Enhanced visuocortical discrimination during the first block of acquisition significantly predicted increased heart rate acceleration to the CS+ in the second block of acquisition [F(1,54) = 4.45, p < .05; R2 = .08; β = .28], but did not predict startle potentiation to the CS+ in acquisition [F(1,38) = .27, n.s.; R2 = .007]. EEG discrimination predicting HR acceleration did not appear to be mediated by COMT genotype (Sobel t-statistic: 1.65, p = .098).
Self-Report
For ratings of the US, SAM ratings of the stimuli, and the State Anxiety portion of the STAI questionnaire, no significant group differences were found for COMT genotype groups. The Trait Anxiety portion of the STAI questionnaire, however, showed significant group differences by COMT genotype [F(1,53) = 8.61, p < .05], with Non-Met individuals more strongly endorsing anxiety. US ratings indicated that all participants experienced the US as unpleasant (mean = 6.1 on a 1-10 scale), but with no COMT group differences (F(1,62) = .80, n.s.]. Participants reported higher state anxiety scores after acquisition, compared to before acquisition [F(1,48) = 8.49, p < .05], but again no COMT group difference was observed [F(1,48) = .1, n.s.]. For the SAM ratings, participants overall rated the CS+ as more arousing during conditioning [F(1,54) = 57.15, p < .001] and more unpleasant than the CS- [F(1,54) = 37.15, p < .001], compared to their ratings during habituation. No COMT group differences were found for arousal [F(1,54) = 1.60, n.s.] nor for valence [F(1,54) = .81, n.s.]. All these measures indicated that conditioning occurred across individuals (Table 4).
Table 4. Self-report measures by COMT genotype grouping.
SAM ratings are reported in change from habituation to conditioning for CS+ stimulus only. STAI questionnaire has two ratings for State portion (S-R1 before, S-R2 after conditioning) and Trait scores (T). US rating given on a 1-10 scale, 10 being very unpleasant. Group differences for COMT were only found for Trait Anxiety, with Val/Val individuals (Non-Met) reporting higher Trait Anxiety scores (indicated in bold).
| SAM | STAI | US | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| Arousal | Valence | S-R1 | S-R2 | T | ||
| Non-Met | 1.5 (1.3) | -1.8 (1.6) | 33.5 (10.3) | 37.7 (13.5) | 39.2 (9.8) | 6.2 (2.1) |
| Met | 1.5 (1.9) | -1.2 (1.3) | 32.2 (9.2) | 37.4 (10.2) | 33.3 (7.0) | 6.0 (1.7) |
No gender differences were found in any of the recorded measures.
Discussion
The current study set out to characterize the relation between an individual's COMT val158met polymorphism status and indices of visual processing, reflex physiology and evaluative self-report during aversive conditioning. The conditioning regime reliably prompted defensive responses across COMT genotype groups, shown by a decelerative, orienting response for CS+ trials during initial conditioning. Pronounced inter-individual differences were also observed: Across measures, observers homozygous on the Val allele (Non-Met carriers) showed evidence of rapid and flexible adaptation to the experimental contingencies, with selective visuocortical responses to the CS+ after few conditioning trials, followed by heightened somatovisceral responses – greater heart rate (HR) acceleration and startle potentiation – selectively to the CS+. By contrast, the Met carriers did not display selective HR acceleration or visuocortical facilitation. Although no COMT group difference was found for skin conductance responding, findings of HR acceleration and enhanced startle potentiation during CS+ presentation suggest that individuals homozygous on the Val allele displayed stronger defensive engagement than Met individuals.
The current findings show substantial CS discrimination over occipital cortex during initial stages of aversive conditioning for individuals of the Val/Val genotype. This discrimination remained significant after various data transformations (normalization methods, outlier rejection), supporting the robustness of the observation of COMT-specific visuocortical change. Rapid signal enhancement of the ssVEP in visual cortex to motivationally relevant stimuli has been hypothesized to result from reentrant modulation by anterior brain regions, such as limbic and fronto-temporal cortical areas (Gruber et al., 2004; Keil et al., 2009). Rehbein and colleagues (2014) additionally found that rapid CS discrimination during aversive conditioning was localized specifically to prefrontal cortical (PFC) regions in the acquisition phase. In line with research in selective attention (Barcelo et al., 2000) these findings suggest that the PFC has a role in biasing the sensitivity of visual neurons towards motivationally relevant cues. These findings lend credence to the notion that anterior regions of the brain, including widespread cortical areas, play an active role during aversive learning (Miskovic & Keil, 2012). Furthermore, our findings support the hypothesis that Val/Val homozygotes display more pronounced adaptation to the changing contingencies of the paradigm, compared to the Met carriers: Following habituation, Met carriers failed to show perceptual changes reflecting the contingencies of instructed, differential conditioning, whereas Non-Met carriers displayed rapid perceptual discrimination between the CS+ and CS-. Heightened, selective attention may be one of the processes contributing to the greater perceptual discrimination found in Non-Met carriers and future work may manipulate selective attention to address this point.
COMT genotype-dependent action has been suggested to involve dorsolateral prefrontal regions (Green et al., 2013; Mier et al., 2010). The fact that varying levels of enzymatic COMT activity affect extracellular dopamine in PFC has given rise to theoretical notions in which variable dopamine signal-to-noise in PFC is linked to activity in subcortical structures such as amygdala, hippocampus, ventral striatum and ventral tegmental area (Akil et al., 2003; Bertolino et al., 2006; Bilder et al., 2004; Winterer et al., 2006). Further research suggests fundamentally altered cortical-subcortical connectivity by COMT genotype. For example, Met allele carriers show greater activation and connectivity between the amygdaloid body and subcortical regions during viewing of unpleasant stimuli (Smolka et al., 2007). Surguladze and colleagues (2012) demonstrated Met allele carriers to have reduced reciprocal connectivity between the amygdala and various cortical regions (including prefrontal and visuocortical) when observers view fearful faces. Other findings suggest altered network coupling by COMT genotype not only for aversive and fearful stimuli, but also related to working memory in the parahippocampal cortex (Zhang et al. 2015). Future research in the animal model, particularly non-human primates, may aim to characterize how the COMT polymorphism affects dopaminergic signaling within these networks, in particular in the context of aversive conditioning.
In terms of reflexive physiology, individuals of the Val/Val genotype showed HR acceleration to the CS+ in the later half of acquisition, as well as increased startle potentiation to probes presented during CS+ trials, compared to Met carriers. These findings support the notion that individuals homozygous on the Val allele exhibit stronger defensive engagement than Met allele carriers to the aversively cued stimulus, raising questions regarding potential mechanisms of such differences in autonomic physiology, specifically differential involvement of the sympathetic versus parasympathetic branch. Supporting a potential direct mechanism by which COMT may affect autonomic reactivity, work by Jabbi and colleagues (2007) has linked variable enzymatic activity due to the COMT val158met polymorphism to not only central catecholamine, but peripheral plasma catecholamine regulation, such as epinephrine. Recent work has expanded this concept of differential autonomic system activity varying as a function of COMT genotype in children (Mueller et al., 2012): During exposure to a social stressor, individuals homozygous on the Val allele displayed increased HR acceleration (marker of sympathetic activity) followed by quick recovery, as well as blunting of heart rate variability (HRV, a marker of parasympathetic activity) during stress recovery. Furthermore, work by Hansen and colleagues (Hansen et al., 2003) has demonstrated that individuals with greater HRV perform better on tasks involving executive functioning such as working memory and attention. This suggests that the COMT polymorphism may impact both branches of the autonomic nervous system (ANS). The current study found that activation of the sympathetic branch, as captured by skin conductance responding, did not vary by COMT genotype. Few studies have investigated defensive engagement in relation to COMT polymorphism status, finding Met carriers to show enhanced startle potentiation during viewing of aversive stimuli or reporting failed suppression of startle during extinction (Lonsdorf et al., 2009; Montag et al., 2008). Future studies are needed to investigate whether ANS dominance may vary as a function of COMT genotype and to what extent attention plays a role in this.
A large body of work on the COMT polymorphism has focused research into clinical disorders of dysfunctional dopamine signaling and prefrontal systems such as schizophrenia, ADHD, PTSD, anxiety disorders, drug abuse and Parkinson's (Domschke et al., 2012; Eisenberg et al., 1999; Goldman et al., 2005; Harrison & Weinberger, 2005; Norrholm et al., 2013; Weinberger et al., 2001). Some of this work proposes a warrior/worrier model (Goldman et al., 2005), suggesting a trade-off effect between emotional resilience and cognitive performance (Alexander et al., 2011). COMT genotype on its own is not a sufficient risk factor for the anxiety disorders spectrum. The COMT polymorphism impacts complex behavioral and cognitive phenotypes through varying enzymatic metabolisms of dopamine (Mier et al., 2010). Furthermore, relating the COMT polymorphism to inter-individual differences in higher-order cognitive or affective phenotypes has been complicated by a spectrum of confounds such as ethnicity and gender (Lee & Prescott, 2014; Wang et al., 2013). The present study reports on a largely female Caucasian sample, in which no gender effects were observed for any of the measures. Although gender effects are reported in the COMT literature, these findings are often quite heterogeneous: Panic-disorder proclivity may be female-specific, but measures of neuroticism and avoidance male-specific (Domschke et al., 2007; Lee & Prescott, 2014). As only State and Trait Anxiety questionnaire data were taken in an unselected sample of undergraduates, the gender distribution of potential behavioral dispositions as well as susceptibility to anxiety disorders is unknown in the current study. Future research may benefit from preselecting a sample based on these factors, while examining specific neurocognitive processes, ideally focusing on processes for which well established animal models exist.
In the current study, Val/Val individuals displayed significant CS discrimination in their visuocortical response, interpretable as a heightened attentional orienting response during instructed aversive conditioning. Its co-occurrence with enhanced defensive engagement and endorsement of higher trait anxiety scores suggests that heightened orienting to threat is part of a stable cluster of defensive behaviors characteristic of Val/Val individuals. In light of research finding a greater presence of Met carriers in various anxiety disorders, the current findings may instead suggest that Met carriers show diminished aversive discrimination, visuocortically, when undergoing aversive learning compared to Val/Val carriers. In the clinical case of dysfunctional processing this may be augmented, with Met carriers showing a lack of fear discrimination and a failure to learn safety cues (Lissek, 2012). Differential perceptual orienting during aversive learning, as a function of COMT genotype, may therefore be an appropriate avenue for future investigations to further expand the neurophysiological impact of the COMT val158met polymorphism in humans.
Highlights.
- We investigated COMT val158met genotype differences in aversive learning
- Visuocortical, autonomic and behavioral measures were recorded
- Non-Met carriers showed rapid visuocortical enhancement during conditioning
- Non-Met carriers rapidly adapt to task demands reflected as heightened attention
Acknowledgments
This research was funded by the National Institute of Health (MH097320). Thanks to Margaret Bradley for assistance with the peripheral psychophysiological data acquisition and analysis. Thanks also to Gabrielle Gordon for assistance in data collection.
Abbreviations
- COMT
catechol-O-methyltransferase
- CS
conditioned stimulus
- EEG
electroencephalography
- HR
heart rate
- PFC
prefrontal cortex
- SAM
Self-Assessment Manikin
- SCL
skin conductance response
- SNP
single nucleotide polymorphism
- ssVEP
steady-state visually evoked potential
- STAI
State and Trait Anxiety Inventory
- US
unconditioned stimulus
Footnotes
Due to a change in the presentation monitor in the later half of data collection, multiple stimulus frequencies were used, as these are dependent on monitor refresh rate.
Presentation rate of the Gabor grating was added as a between-subjects factor and was found to be non-significant [F(2,62) = .03, n.s.].
EEG was reanalyzed for individuals who were startle responders only and were genotyped for the COMT SNP (n=43). Findings of Non-Met individuals showing enhanced visuocortical activation to the CS+ compared to CS- during block 1 of acquisition remained significant in this smaller sample [F(1,42) = 7.34, p < .01].
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham AD, Neve KA, Lattal KM. Dopamine and extinction: a convergence of theory with fear and reward circuitry. Neurobiol Learn Mem. 2014;108:65–77. doi: 10.1016/j.nlm.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akil M, Kolachana BS, Rothmond DA, Hyde TM, Weinberger DR, Kleinman JE. Catechol-O-methyltransferase genotype and dopamine regulation in the human brain. J Neurosci. 2003;23(6):2008–2013. doi: 10.1523/JNEUROSCI.23-06-02008.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander N, Osinsky R, Mueller E, Schmitz A, Guenthert S, Kuepper Y, et al. Genetic variants within the dopaminergic system interact to modulate endocrine stress reactivity and recovery. Behav Brain Res. 2011;216(1):53–58. doi: 10.1016/j.bbr.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Behniea H, Kelly JL. Topographic organization of projections from the amygdala to the visual cortex in the macaque monkey. Neuroscience. 2003;118(4):1099–1120. doi: 10.1016/s0306-4522(02)01001-1. [DOI] [PubMed] [Google Scholar]
- Asaad WF, Rainer G, Miller EK. Neural activity in the primate prefrontal cortex during associative learning. Neuron. 1998;21(6):1399–1407. doi: 10.1016/s0896-6273(00)80658-3. [DOI] [PubMed] [Google Scholar]
- Balaban M, Losito B, Simons R, Graham F. Off-line latency and amplitude scoring of the human reflex eye blink with Fortran IV. Psychophysiology. 1986;23(5):612. [Google Scholar]
- Barcelo F, Suwazono S, Knight RT. Prefrontal modulation of visual processing in humans. Nat Neurosci. 2000;3(4):399–403. doi: 10.1038/73975. [DOI] [PubMed] [Google Scholar]
- Barnett JH, Jones PB, Robbins TW, Müller U. Effects of the catechol-Omethyltransferase Val158Met polymorphism on executive function: a meta-analysis of the Wisconsin Card Sort Test in schizophrenia and healthy controls. Mol Psychiatry. 2007;12(5):502–509. doi: 10.1038/sj.mp.4001973. [DOI] [PubMed] [Google Scholar]
- Bellander M, Bäckman L, Liu T, Schjeide BM, Bertram L, Schmiedek F, et al. Lower baseline performance but greater plasticity of working memory for carriers of the val allele of the COMT Val158 Met polymorphism. Neuropsychology. 2015;29(2):247–254. doi: 10.1037/neu0000088. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Rubino V, Sambataro F, Blasi G, Latorre V, Fazio L, et al. Prefrontal-hippocampal coupling during memory processing is modulated by COMT val158met genotype. Biol Psychiatry. 2006;60(11):1250–1258. doi: 10.1016/j.biopsych.2006.03.078. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Volavka J, Lachman HM, Grace AA. The catechol-O-methyltransferase polymorphism: relations to the tonic-phasic dopamine hypothesis and neuropsychiatric phenotypes. Neuropsychopharmacology. 2004;29(11):1943–1961. doi: 10.1038/sj.npp.1300542. [DOI] [PubMed] [Google Scholar]
- Bishop SJ, Cohen JD, Fossella J, Casey BJ, Farah MJ. COMT genotype influences prefrontal response to emotional distraction. Cogn Affect Behav Neurosci. 2006;6(1):62–70. doi: 10.3758/cabn.6.1.62. [DOI] [PubMed] [Google Scholar]
- Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Lipp OV, van Boxtel A. Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiology. 2005;42(1):1–15. doi: 10.1111/j.1469-8986.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: the Self-Assessment Manikin and the Semantic Differential. J Behav Ther Exp Psychiatry. 1994;25(1):49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spat Vis. 1997;10(4):433–436. [PubMed] [Google Scholar]
- Büchel C, Morris J, Dolan RJ, Friston KJ. Brain systems mediating aversive conditioning: an event-related fMRI study. Neuron. 1998;20(5):947–957. doi: 10.1016/s0896-6273(00)80476-6. [DOI] [PubMed] [Google Scholar]
- Colzato LS, Waszak F, Nieuwenhuis S, Posthuma D, Hommel B. The flexible mind is associated with the catechol-O-methyltransferase (COMT) Val158Met polymorphism: evidence for a role of dopamine in the control of task-switching. Neuropsychologia. 2010;48(9):2764–2768. doi: 10.1016/j.neuropsychologia.2010.04.023. [DOI] [PubMed] [Google Scholar]
- Cook EW., 3rd . VPM reference manual. Birmingham, AL: Author; 2002. [Google Scholar]
- Delgado MR, Olsson A, Phelps EA. Extending animal models of fear conditioning to humans. Biol Psychol. 2006;73(1):39–48. doi: 10.1016/j.biopsycho.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Desimone R. Neural mechanisms for visual memory and their role in attention. Proc Natl Acad Sci U S A. 1996;93(24):13494–13499. doi: 10.1073/pnas.93.24.13494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domschke K, Baune BT, Havlik L, Stuhrmann A, Suslow T, Kugel H, et al. Catechol-O-methyltransferase gene variation: impact on amygdala response to aversive stimuli. Neuroimage. 2012;60(4):2222–2229. doi: 10.1016/j.neuroimage.2012.02.039. [DOI] [PubMed] [Google Scholar]
- Domschke K, Deckert J, O'donavan MC, Glatt SJ. Meta-analysis of COMT val158met in panic disorder: ethnic heterogeneity and gender specificity. Am J Med Genet B Neuropsychiatr Genet. 2007;144B(5):667–673. doi: 10.1002/ajmg.b.30494. [DOI] [PubMed] [Google Scholar]
- Drabant EM, Hariri AR, Meyer-Lindenberg A, Munoz KE, Mattay VS, Kolachana BS, et al. Catechol O-methyltransferase val158met genotype and neural mechanisms related to affective arousal and regulation. Arch Gen Psychiatry. 2006;63(12):1396–1406. doi: 10.1001/archpsyc.63.12.1396. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, et al. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A. 2001;98(12):6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg J, Mei-Tal G, Steinberg A, Tartakovsky E, Zohar A, Gritsenko I, et al. Haplotype relative risk study of catechol-O-methyltransferase (COMT) and attention deficit hyperactivity disorder (ADHD): association of the high-enzyme activity Val allele with ADHD impulsive-hyperactive phenotype. Am J Med Genet. 1999;88(5):497–502. [PubMed] [Google Scholar]
- Goldman D, Oroszi G, Ducci F. The genetics of addictions: uncovering the genes. Nat Rev Genet. 2005;6(7):521–532. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- Graham FK. Constraints on measuring heart rate and period sequentially through real and cardiac time. Psychophysiology. 1978;15(5):492–495. doi: 10.1111/j.1469-8986.1978.tb01422.x. [DOI] [PubMed] [Google Scholar]
- Green AE, Kraemer DJ, Deyoung CG, Fossella JA, Gray JR. A gene-brain-cognition pathway: prefrontal activity mediates the effect of COMT on cognitive control and IQ. Cereb Cortex. 2013;23(3):552–559. doi: 10.1093/cercor/bhs035. [DOI] [PubMed] [Google Scholar]
- Gruber T, Malinowski P, Müller MM. Modulation of oscillatory brain activity and evoked potentials in a repetition priming task in the human EEG. Eur J Neurosci. 2004;19(4):1073–1082. doi: 10.1111/j.0953-816x.2004.03176.x. [DOI] [PubMed] [Google Scholar]
- Hamm AO, Weike AI. The neuropsychology of fear learning and fear regulation. Int J Psychophysiol. 2005;57(1):5–14. doi: 10.1016/j.ijpsycho.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Hansen AL, Johnsen BH, Thayer JF. Vagal influence on working memory and attention. Int J Psychophysiol. 2003;48(3):263–274. doi: 10.1016/s0167-8760(03)00073-4. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2005;10(1):40–68. doi: 10.1038/sj.mp.4001558. image 45. [DOI] [PubMed] [Google Scholar]
- Hauk O. Keep it simple: a case for using classical minimum norm estimation in the analysis of EEG and MEG data. Neuroimage. 2004;21(4):1612–1621. doi: 10.1016/j.neuroimage.2003.12.018. [DOI] [PubMed] [Google Scholar]
- Hauk O, Keil A, Elbert T, Muller MM. Comparison of data transformation procedures to enhance topographical accuracy in time-series analysis of the human EEG. J Neurosci Methods. 2002;113(2):111–122. doi: 10.1016/s0165-0270(01)00484-8. [DOI] [PubMed] [Google Scholar]
- Hirvonen MM, Nagren K, Rinne JO, Pesonen U, Vahlberg T, Hagelberg N, et al. COMT Val158Met genotype does not alter cortical or striatal dopamine D2 receptor availability in vivo. Mol Imaging Biol. 2010;12(2):192–197. doi: 10.1007/s11307-009-0257-5. [DOI] [PubMed] [Google Scholar]
- Hodes RL, Cook EW, 3rd, Lang PJ. Individual differences in autonomic response: conditioned association or conditioned fear? Psychophysiology. 1985;22(5):545–560. doi: 10.1111/j.1469-8986.1985.tb01649.x. [DOI] [PubMed] [Google Scholar]
- Horvitz JC. Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience. 2000;96(4):651–656. doi: 10.1016/s0306-4522(00)00019-1. [DOI] [PubMed] [Google Scholar]
- Jabbi M, Kema IP, van der Pompe G, te Meerman GJ, Ormel J, den Boer JA. Catechol-o-methyltransferase polymorphism and susceptibility to major depressive disorder modulates psychological stress response. Psychiatr Genet. 2007;17(3):183–193. doi: 10.1097/YPG.0b013e32808374df. [DOI] [PubMed] [Google Scholar]
- Junghofer M, Elbert T, Tucker DM, Rockstroh B. Statistical control of artifacts in dense array EEG/MEG studies. Psychophysiology. 2000;37(4):523–532. [PubMed] [Google Scholar]
- Keil A, Miskovic V, Gray MJ, Martinovic J. Luminance, but not chromatic visual pathways, mediate amplification of conditioned danger signals in human visual cortex. Eur J Neurosci. 2013;38(9):3356–3362. doi: 10.1111/ejn.12316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil A, Sabatinelli D, Ding M, Lang PJ, Ihssen N, Heim S. Re-entrant projections modulate visual cortex in affective perception: evidence from Granger causality analysis. Hum Brain Mapp. 2009;30(2):532–540. doi: 10.1002/hbm.20521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil A, Stolarova M, Moratti S, Ray WJ. Adaptation in visual cortex as a mechanism for rapid discrimination of aversive stimuli. Neuroimage. 2007;36:472–479. doi: 10.1016/j.neuroimage.2007.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics and Genomics. 1996;6(3):243–250. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- Lamme VA, Roelfsema PR. The distinct modes of vision offered by feedforward and recurrent processing. Trends Neurosci. 2000;23(11):571–579. doi: 10.1016/s0166-2236(00)01657-x. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM. Emotion and the motivational brain. Biol Psychol. 2010;84(3):437–450. doi: 10.1016/j.biopsycho.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langaee T, Ronaghi M. Genetic variation analyses by Pyrosequencing. Mutat Res. 2005;573(1-2):96–102. doi: 10.1016/j.mrfmmm.2004.07.023. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lee LO, Prescott CA. Association of the catechol-O-methyltransferase val158met polymorphism and anxiety-related traits: a meta-analysis. Psychiatr Genet. 2014;24(2):52–69. doi: 10.1097/YPG.0000000000000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S. Toward an account of clinical anxiety predicated on basic, neurally mapped mechanisms of Pavlovian fear-learning: the case for conditioned overgeneralization. Depress Anxiety. 2012;29(4):257–263. doi: 10.1002/da.21922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdorf TB, Weike AI, Nikamo P, Schalling M, Hamm AO, Ohman A. Genetic gating of human fear learning and extinction: possible implications for gene-environment interaction in anxiety disorder. Psychol Sci. 2009;20(2):198–206. doi: 10.1111/j.1467-9280.2009.02280.x. [DOI] [PubMed] [Google Scholar]
- McTeague LM, Gruss LF, Keil A. Aversive learning shapes neuronal orientation tuning in human visual cortex. Nat Commun. 2015;6:7823. doi: 10.1038/ncomms8823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mier D, Kirsch P, Meyer-Lindenberg A. Neural substrates of pleiotropic action of genetic variation in COMT: a meta-analysis. Mol Psychiatry. 2010;15(9):918–927. doi: 10.1038/mp.2009.36. [DOI] [PubMed] [Google Scholar]
- Miller BT, Vytlacil J, Fegen D, Pradhan S, D'Esposito M. The prefrontal cortex modulates category selectivity in human extrastriate cortex. J Cogn Neurosci. 2011;23(1):1–10. doi: 10.1162/jocn.2010.21516. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Miller EK, Erickson CA, Desimone R. Neural mechanisms of visual working memory in prefrontal cortex of the macaque. J Neurosci. 1996;16(16):5154–5167. doi: 10.1523/JNEUROSCI.16-16-05154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskovic V, Keil A. Acquired fears reflected in cortical sensory processing: a review of electrophysiological studies of human classical conditioning. Psychophysiology. 2012;49(9):1230–1241. doi: 10.1111/j.1469-8986.2012.01398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montag C, Buckholtz JW, Hartmann P, Merz M, Burk C, Hennig J, et al. COMT genetic variation affects fear processing: psychophysiological evidence. Behav Neurosci. 2008;122(4):901–909. doi: 10.1037/0735-7044.122.4.901. [DOI] [PubMed] [Google Scholar]
- Moratti S, Keil A, Miller GA. Fear but not awareness predicts enhanced sensory processing in fear conditioning. Psychophysiology. 2006;43(2):216–226. doi: 10.1111/j.1464-8986.2006.00386.x. [DOI] [PubMed] [Google Scholar]
- Moratti S, Keil A, Stolarova M. Motivated attention in emotional picture processing is reflected by activity modulation in cortical attention networks. Neuroimage. 2004;21(3):954–964. doi: 10.1016/j.neuroimage.2003.10.030. [DOI] [PubMed] [Google Scholar]
- Mueller A, Strahler J, Armbruster D, Lesch KP, Brocke B, Kirschbaum C. Genetic contributions to acute autonomic stress responsiveness in children. Int J Psychophysiol. 2012;83(3):302–308. doi: 10.1016/j.ijpsycho.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Muller MM, Andersen S, Trujillo NJ, Valdes-Sosa P, Malinowski P, Hillyard SA. Feature-selective attention enhances color signals in early visual areas of the human brain. Proc Natl Acad Sci U S A. 2006;103(38):14250–14254. doi: 10.1073/pnas.0606668103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan KA, Bilder RM, Lachman HM, Volavka J. Catechol O-methyltransferase Val158Met polymorphism in schizophrenia: differential effects of Val and Met alleles on cognitive stability and flexibility. Am J Psychiatry. 2004;161(2):359–361. doi: 10.1176/appi.ajp.161.2.359. [DOI] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T, Smith AK, Binder E, Klengel T, Conneely K, et al. Differential Genetic and Epigenetic Regulation of catechol-O-methyltransferase is Associated with Impaired Fear Inhibition in Posttraumatic Stress Disorder. Front Behav Neurosci. 2013;7:30. doi: 10.3389/fnbeh.2013.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyk P, De Cesarei A, Junghofer M. ElectroMagnetoEncephalography software: overview and integration with other EEG/MEG toolboxes. Comput Intell Neurosci. 2011;2011:861705. doi: 10.1155/2011/861705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainer G, Miller EK. Effects of visual experience on the representation of objects in the prefrontal cortex. Neuron. 2000;27(1):179–189. doi: 10.1016/s0896-6273(00)00019-2. [DOI] [PubMed] [Google Scholar]
- Rehbein MA, Steinberg C, Wessing I, Pastor MC, Zwitserlood P, Keuper K, et al. Rapid plasticity in the prefrontal cortex during affective associative learning. PLoS One. 2014;9(10):e110720. doi: 10.1371/journal.pone.0110720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasy WF, editors. Classical conditioning II: current research and theory. New York: Appleton-Century-Crofts; 1972. pp. 64–99. [Google Scholar]
- Rosa EC, Dickinson D, Apud J, Weinberger DR, Elvevåg B. COMT Val158Met polymorphism, cognitive stability and cognitive flexibility: an experimental examination. Behav Brain Funct. 2010;6:53. doi: 10.1186/1744-9081-6-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberstein RB, Ciorciari J, Pipingas A. Steady-state visually evoked potential topography during the Wisconsin card sorting test. Electroencephalogr Clin Neurophysiol. 1995;96(1):24–35. doi: 10.1016/0013-4694(94)00189-r. [DOI] [PubMed] [Google Scholar]
- Slifstein M, Kolachana B, Simpson EH, Tabares P, Cheng B, Duvall M, et al. Mol Psychiatry. Vol. 13. England: 2008. COMT genotype predicts cortical-limbic D1 receptor availability measured with [11C]NNC112 and PET; pp. 821–827. [DOI] [PubMed] [Google Scholar]
- Smolka MN, Buhler M, Schumann G, Klein S, Hu XZ, Moayer M, et al. Gene-gene effects on central processing of aversive stimuli. Mol Psychiatry. 2007;12(3):307–317. doi: 10.1038/sj.mp.4001946. [DOI] [PubMed] [Google Scholar]
- Smolka MN, Schumann G, Wrase J, Grusser SM, Flor H, Mann K, et al. Catechol-O-methyltransferase val158met genotype affects processing of emotional stimuli in the amygdala and prefrontal cortex. J Neurosci. 2005;25(4):836–842. doi: 10.1523/JNEUROSCI.1792-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song I, Keil A. Differential classical conditioning selectively heightens response gain of neural population activity in human visual cortex. Psychophysiology. 2014;51(11):1185–1194. doi: 10.1111/psyp.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene PR, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press, Inc; 1983. [Google Scholar]
- Stolarova M, Keil A, Moratti S. Modulation of the C1 visual event-related component by conditioned stimuli: evidence for sensory plasticity in early affective perception. Cereb Cortex. 2006;16(6):876–887. doi: 10.1093/cercor/bhj031. [DOI] [PubMed] [Google Scholar]
- Surguladze SA, Radua J, El-Hage W, Gohier B, Sato JR, Kronhaus DM, et al. Interaction of the catechol O-methyltransferase and serotonin transporter genes modulates effective connectivity in a facial emotion-processing circuitry. Transl Psychiatry. 2012;2:e70. doi: 10.1038/tp.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor JD, Mast J. A new statistic for steady-state evoked potentials. Electroencephalogr Clin Neurophysiol. 1991;78(5):378–388. doi: 10.1016/0013-4694(91)90099-p. [DOI] [PubMed] [Google Scholar]
- Wang Y, Li J, Chen C, Zhu B, Moysis RK, Lei X, et al. COMT rs4680 Met is not always the ‘smart allele’: Val allele is associated with better working memory and larger hippocampal volume in healthy Chinese. Genes Brain Behav. 2013;12(3):323–329. doi: 10.1111/gbb.12022. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Egan MF, Bertolino A, Callicott JH, Mattay VS, Lipska BK, et al. Prefrontal neurons and the genetics of schizophrenia. Biol Psychiatry. 2001;50(11):825–844. doi: 10.1016/s0006-3223(01)01252-5. [DOI] [PubMed] [Google Scholar]
- Weinshilboum R, Dunnette J. Thermal stability and the biochemical genetics of erythrocyte catechol-O-methyl-transferase and plasma dopamine-beta-hydroxylase. Clin Genet. 1981;19(5):426–437. doi: 10.1111/j.1399-0004.1981.tb00740.x. [DOI] [PubMed] [Google Scholar]
- Weinshilboum RM, Otterness DM, Szumlanski CL. Methylation pharmacogenetics: catechol O-methyltransferase, thiopurine methyltransferase, and histamine N-methyltransferase. Annu Rev Pharmacol Toxicol. 1999;39:19–52. doi: 10.1146/annurev.pharmtox.39.1.19. [DOI] [PubMed] [Google Scholar]
- Wendler E, Gaspar JC, Ferreira TL, Barbiero JK, Andreatini R, Vital MA, et al. The roles of the nucleus accumbens core, dorsomedial striatum, and dorsolateral striatum in learning: performance and extinction of Pavlovian fear-conditioned responses and instrumental avoidance responses. Neurobiol Learn Mem. 2014;109:27–36. doi: 10.1016/j.nlm.2013.11.009. [DOI] [PubMed] [Google Scholar]
- Williams LM, Gatt JM, Grieve SM, Dobson-Stone C, Paul RH, Gordon E, et al. COMT Val(108/158)Met polymorphism effects on emotional brain function and negativity bias. Neuroimage. 2010;53(3):918–925. doi: 10.1016/j.neuroimage.2010.01.084. [DOI] [PubMed] [Google Scholar]
- Winterer G, Musso F, Vucurevic G, Stoeter P, Konrad A, Seker B, et al. COMT genotype predicts BOLD signal and noise characteristics in prefrontal circuits. Neuroimage. 2006;32(4):1722–1732. doi: 10.1016/j.neuroimage.2006.05.058. [DOI] [PubMed] [Google Scholar]
- Yavich L, Forsberg MM, Karayiorgou M, Gogos JA, Mannisto PT. Site-specific role of catechol-O-methyltransferase in dopamine overflow within prefrontal cortex and dorsal striatum. J Neurosci. 2007;27(38):10196–10209. doi: 10.1523/JNEUROSCI.0665-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Li J, Qin W, Yu C, Liu B, Jiang T. The catechol-o-methyltransferase Val158Met polymorphism modulates intrinsic functional network centrality of the parahippocampal cortex in healthy subjects. Sci Rep. 2015;5:10105. doi: 10.1038/srep10105. [DOI] [PMC free article] [PubMed] [Google Scholar]


