Abstract
MicroRNAs (miRNAs) inhibit RNA targets and may contribute to postpartum CNS gene expression changes, although this has never been tested. In the present study, we directly evaluated miRNA levels using RNA sequencing during reproduction in female mice in lateral septum (LS). We found the reliable and robust changes of miRNAs away from the virgin stage at the three other stages, namely pregnant, day 1 postpartum, and day 8 postpartum. For a given miRNA that was significantly different from the virgin condition in more than one group, the direction of change was always the same. Overall, we identified 32 upregulated miRNAs and 25 downregulated miRNAs that were consistently different from the virgin state. ‘Arm switching’ occurs for miR-433-3 and miR-7b. Unexpectedly, a third of upregulated miRNAs (relative to virgin) were highly localized within the 12qF1 region of chromosome 12 that includes the Dlk1-Dio3 gene cluster implicated in stem cell and neuronal differentiation. Over 1500 genes were targeted by multiple upregulated miRNAs with about 100 genes targeted by 5 or more miRNAs. Over 1000 genes were targeted by multiple downregulated miRNAs with about 50 genes targeted by 5 or more miRNAs. Half of the target genes were regulated by up and downregulated miRNAs, indicating homeostatic regulation. Transcriptional regulation was the most enriched pathway for genes linked to up or down regulated miRNAs. Other enriched pathways included protein kinase activity (e.g., MAP kinase), CNS development, axon guidance, neurotrophin signaling, neuron development/differentiation, and neurogenesis. Previously published postpartum LS gene expression changes were enrichment for LS miRNA targets, as expected. Surprisingly, postpartum gene expression changes from other regions were also enriched against LS miRNA targets, suggesting a core group of miRNAs may act across the CNS during reproduction. Together, we directly examine miRNAs and find significant alterations in the postpartum brain.
Keywords: maternal, postpartum, 14q32, microRNA, lateral septum, Dlk1-Dio3
1. Introduction
MicroRNAs (miRNAs) play an important role in gene expression by inhibiting RNA targets (Bartel, 2009). The genes for these small, non-coding RNAs are found throughout the genome, including in intergenic regions and in genes (both in exons and in introns) (Bartel, 2004). Active miRNAs are 22 nucleotides long and during processing either the 3p or 5p form (but not both) is strongly favored with each form having distinct targets (Ha and Kim, 2014). The RNA targets for a given miRNA are determined using both computational analysis and direct testing (Bartel, 2009, Thomas et al., 2010) and the number of RNA targets for a single miRNA can range from a few to over a thousand (Griffiths-Jones et al., 2006, Sethupathy et al., 2006, Lu et al., 2012). Thus, the actions of small number of miRNAs could range from focused to widespread, depending on the miRNAs.
In recent studies we have identified large scale gene expression (mRNA) changes in the postpartum brain across in multiple regions (Eisinger et al., 2013b, Driessen et al., 2014b, Eisinger et al., 2014, Zhao et al., 2014). These large changes typically involve over a thousand genes in a given region and support the maternal phenotype. Our bioinformatics analysis of these expression changes suggested a strong influence of miRNAs on postpartum gene expression, including within whole septum, that included lateral septum (LS) (Zhao et al., 2012b), medial prefrontal cortex (mPFC) (Eisinger et al., 2014), and medial preoptic area (MPOA) (Driessen et al., 2014b). Despite indirect evidence of an important role for miRNAs in sculpting the maternal brain, to date no studies have directly examined miRNA changes in the maternal CNS.
In this study we used small RNA-seq approaches to directly examine whether or how miRNA expression changes occur in the postpartum CNS. We chose to examine LS because this region contributes to multiple aspects of the postpartum phenotype and emotional state. Lesions of LS have a major disruptive effect on maternal care, including retrieval, nest building, and pup survival (Slotnick and Nigrosh, 1975) and LS is thought to be part of an activation circuit for various maternal behaviors (Olazábal et al., 2013). LS is involved in regulation of emotional state (Sheehan et al., 2004) and contributes to both protection of offspring and alterations in anxiety (D’Anna and Gammie, 2009, Lee and Gammie, 2009, Scotti et al., 2011). Further, we have already identified in LS large scale gene expression changes (Eisinger et al., 2013b) and neurotransmission changes (Zhao and Gammie, 2014). Using small RNA sequencing, we evaluated miRNA expression across reproduction in the virgin, pregnant, day 1 postpartum, and day 8 postpartum brain. For any significant miRNA identified, we determined the genome region of the gene and the likely mRNA targets using web-based bioinformatics tools. We further conducted enrichment analysis of miRNA targets using a range of tools and compared miRNA targets to genes with known expression changes from our previous microarray study on LS. Together, the study provides insights for the first time into whether miRNAs are altered in the postpartum brain.
2. Experimental Procedures
2.1 Animals
All virgin female mice (outbred hsd:ICR strain, Harlan, Madison, WI) were ~70 days old at time of arrival to the animal facility. Virgin females were housed in pairs in our colony. After one week of habituation, females were pair-housed with a breeder male (hsd:ICR strain) for mating. Vaginal plugs were checked every morning between 09:00–10:00 CST. Once a plug was detected (gestational Day 0), the male was removed from the female to ensure that the beginning of gestation was accurate. At the same time, virgin females were housed individually and all mice received precut nesting material until dissections. The timing of cohousing and isolation was performed to minimize the effects of isolation-induced stress while also providing similar housing across groups to help control for the effects of housing on gene expression. The paradigm has been used previously in our lab to examine expression alterations associated with the collective experience, including mating, pregnancy, parturition, and postpartum (Zhao et al., 2012b, Eisinger et al., 2013b, Zhao et al., 2014). The maternal-virgin comparison is a proven approach for examining a wide variety of markers that characterize the maternal phenotype (Mann et al., 1997, Neumann et al., 1999, Kinsley and Amory-Meyer, 2011, Maeng and Shors, 2012, Shams et al., 2012). However, to understand the contribution of any given input (e.g., parturition), additional follow up studies would be needed. Polypropylene cages were changed once weekly. When pups were born (postpartum Day 0), cages were not changed for the postpartum females or the age matched virgin controls for the remainder of the experiment. Female mice were given ad lib access to breeder chow (Harlan) and tap water, and were housed in the same room under a 12:12 light/dark cycle with lights on at 06:00 h CST. All experiments were performed in compliance with the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and were approved by the University of Wisconsin Animal Care and Use Committee.
2.2 Tissue collection and RNA extraction
On designated days (gestation day 15, postpartum Days 1 and 8) between 10:00 and 12:00, pregnant and lactating animals were lightly anaesthetized with isoflurane, decapitated, and whole brains were removed as previously described (Eisinger et al., 2013b, Driessen et al., 2014a, Zhao et al., 2014). Brains from age-matched, virgin females were also removed on the same day. Following decapitation, stage of estrous cycle in virgin females was examined using a vaginal lavage. Virgin mice in only vaginal diestrus were included in this study. The removed brains were snap frozen in isopentane on dry ice, and then stored at −80°C until sliced. Brain sections at 150 microns were sliced on a cryostat (Leica, CM1850, Bannockburn, IL, USA) and then mounted on glass slides. Microdissection of frozen brain sections was made with Brain Punch Set (Stoelting, Wood Dale, IL, USA) under a dissecting microscope and tissue from LS was collected bilaterally from Bregma 1.10 to 0.14. The approaches and dissection were exactly as for our microarray study of LS and Figure 1 of that study indicates dissection of LS as performed here (Eisinger et al., 2013b). Microdissected tissues from 6 animals in each group were collected and stored at −80°C until being processed for miRNA sequencing and real-time PCR analysis. Total RNA including miRNA was isolated and purified using a Fatty Tissue RNA Purification Kit (Norgen Biotek Corporation, Thorold, ON, Canada) according to the manufacturer’s specifications. Following isolation, the integrity of RNA was assessed using Agilent RNA 6000 Nano Chips with Agilent Bioanalyzer 2100 (Agilent Technologies, Palo Alto, CA). The purity of RNA was tested, and the yield of RNA was determined using NanoDrop 2000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA). Purified total RNA was stored at 80°C until processed.
2.3 Small RNA sequencing, alignment, and statistical analysis
Sequencing was performed by the UW-Madison Biotechnology Center’s DNA Synthesis Facility. Small RNA libraries were prepared from total RNA with an Illumina TruSeq small RNA sample prep kit according to the manufacturer’s specifications (Illumina, San Diego, CA, USA). These libraries were sequenced in multiplex as single end 50 bp reads on two lanes of an Illumina HiSeq 2000 at the manufacturer’s recommended depth, resulting in a median of 12,959,978 reads per sample (minimum of 8,517,307 reads, maximum of 19,327,427 reads). Before downstream informatics work, raw runs were demultiplexed and converted to FASTQ files in CASAVA v. 1.8.2.
All informatics analysis was performed in OS X v. 10.9. Before alignment to miRBase, we filtered the dataset. Because miRNAs are usually shorter than the read length of 50 bp, we used Trimmomatic v. 0.22 to trim adapter sequences from both the 5′ and 3′ end, to remove low-quality (score < 3) elements from either end, to remove low-quality quadruplets (average quality score < 15) from either end, and to filter reads whose final lengths were shorter than 15 bp. We aligned the remaining small RNA libraries using bowtie v. 0.12.8 against a contaminant file containing rRNA, mitochondrial RNA, E. coli RNA, tRNA, and adapter sequences, preserving remaining reads for downstream alignment to sequences of interest. Contaminant RNA files were derived from the iGenomes mm10 contaminant folder as well as an Illumina Customer Sequence Letter dated 2012-09-07. The reads that did not align to tRNA were then aligned to miRNA sequences from miRBase 20, first for the mature miRNA sequences and then for the precursor miRNA sequences. Remaining reads were aligned to the Illumina mm10 iGenome. Alignments was performed using bowtie v. 0.12.8. Alignment files were converted to BAM files using samtools v. 0.1.18. Reads in each miRNA feature were counted using htseq-count v. 0.5.4p3. Statistical analysis was done in edgeR v. 3.0 with TMM normalization and tagwise dispersion. We performed both an ANOVA across all biological conditions as well as all pairwise comparisons.
2.4 Verification of sequencing results with quantitative real-time PCR (qPCR)
Five miRNAs (mmu-miR-433-5p, mmu-miR-488-5p, mmu-miR-532-5p, mmu-miR-574-3p, and mmu-miR-676-3p) that showed altered expression using deep sequencing were evaluated using qPCR. These miRNAs were chosen because they displayed consistent expression changes in the three non-virgin conditions (pregnant, postpartum day 1, postpartum day 8) compared to the virgin state. The first strand cDNA was synthesized from 10 ng of total RNA using a miRCURY LNATM Universal RT microRNA PCR Universal cDNA Synthesis kit II (Exiqon, Vedbæk, Denmark) in an T100 Thermal Cycler (Bio-Rad, Hercules, CA, USA) according to the manufacturer’s protocol. Real-time PCR was performed in triplicate using an ExiLENT SYBR Green master mix (Exiqon, Denmark) in a CFX96 Touch real-time PCR Detection system (Bio-Rad). The amplification mixtures (10 μL) contained 1× microRNA LNA™ PCR master mix, diluted cDNA template, and 0.1× primer mix for each specific target miRNA. The cycling parameters used are recommended by Exiqon as follows: an initial polymerase activation/denaturation step at 95ºC for 10 min followed by 40 cycles of a 95ºC melting step for 10 sec, a 60ºC annealing step except miR-574-3p (at 62 ºC) for 1 min. Mature miRNA-specific LNATM-enhanced forward and reverse primers (mmu-miR-433-5p#205430, mmu-miR-488-5p#205441, mmu-miR-532-5p#204221, mmu-miR-574-3p#204365, and mmu-miR-676-3p#205098) were purchased from Exiqon (Denmark). Rnu5g (RNU5G#203908) and Snord68 (SNORD68#203911) (Exiqon) were used as reference genes, as both small nuclear RNAs were found to be among the most stable genes in rodent brain and to be similar among virgin, pregnant, and lactating females in our deep sequencing results. Following amplification, a melting curve analysis was performed to verify the specificity of amplicon. The expression ratio of miRNA in lactating relative to virgin (normalized to the reference genes Rnu5g and Snord68) was calculated using a relative expression software tool qBase (Hellemans et al., 2007).
2.5 Identification of target genes for miRNAs
For the 32 miRNAs that were consistently upregulated and for the 25 miRNAs that were consistently downregulated relative to virgin, the gene targets for each were identified using the on-line tool, miRSystem, that uses 7 known target gene prediction programs, including DIANA, MiRanda, PicTar, PITA, rna22, and TargetScan (Lu et al., 2012). We used the default settings such that only targets were identified if they were found in three or more of the seven databases for a given miRNA.
2.6 Enrichment analysis of miRNA target genes
For enrichment analysis, gene targets were determined separately for both up and down regulated miRNAs and ranked based first on number of microRNAs for a given gene and then the observed/expected ratio (O/E ratio) provided by MiRSystem. The gene targets of upregulated and downregulated miRNAs were analyzed for enrichment separately. In addition to using MirSystem enrichment analysis that incorporated information from all gene targets, we also used NIH DAVID (Huang da et al., 2009) and ToppCluster (Kaimal et al., 2010) enrichment programs. For the latter two, we used the top 1500 genes in order to stay within the limits of the analysis programs.
2.7 Analysis of miRNA target genes and enrichment for known LS gene expression changes
MiRNA targets were compared to known large scale postpartum gene expression changes (via microarray) in LS (relative to virgin) (Eisinger et al., 2013b). For the microarray, we used the p-value cutoff of p<0.01 and this provided 1028 unique genes with just over half of these being downregulated in maternal females. We previously developed the software program, Modular Single-set Enrichment Test (MSET), which runs in R and provides enrichment testing of large scale gene expression studies using a simulation approach of sampling without replacement in the true microarray background to generate a null distribution (Eisinger et al., 2013a). However, one limitation of this approach is that when the target gene lists get larger (e.g., over 500), there is an increasing likelihood of a false positive (Newton et al., 2007). Because of the large number of miRNA targets here, we used the basics of MSET to develop a new program, Bigtest, that allows for testing against any large target list. The innovation of Bigtest is that the target can be broken into smaller pieces, which overcomes the issue of false positives. The user can define the size of the target pieces and the program will randomly break the larger list into smaller lists. The program then uses MSET to analyze each piece for enrichment using a number of simulations defined by the user (e.g., 2000). The user also determines how many times to run the test (i.e., number of loops), whereby for each loop the larger list is again randomly broken into smaller pieces. Each loop provides multiple p-values. For statistics, Bigtest provides a p-value for each small piece within a loop, the overall medial p-value for a given loop, and the distribution of all the median p-values generated by each loop. The end p-value is the median of all the median p-values for each loop. For Bigtest in this study, we used the following parameters: 2000 simulations per test (for each piece) and 20 loops. We also explored other settings, such as 1000 simulations per test and 50 loops, but the results were almost identical. We set the maximum size of the targets to be less than 500. For example, for all miRNA targets, there were 4,624 genes. Thus, we set the small piece maximum to 463 genes and Bigtest produced 10 smaller target bins (nine with 463 and one with 462). Similar to MSET, Bigtest identifies all the genes in common between the gene expression study and the target. The advantage of Bigtest is that it allows for large target list testing, while also allowing the background of the gene expression study to be used. However, as an alternative approach, we also used in R the hypergeometric test, “phyper”. The hypergeometric test allows for comparisons of large lists, but does not include background information (Goeman and Bühlmann, 2007). In this case, we used 16,000 as the universe size which is less than the number of known genes, but is closer to the likely number of genes expression for a given region in the rodent brain (Stansberg et al., 2007).
In addition to analysis of all miRNA targets together, we analyzed targets from upregulated and downregulated miRNAs separately. We also examined separately upregulated and downregulated LS genes against various targets. We further evaluated targets from up or down regulated miRNAs based on whether there was a net higher number of up or downregulated miRNAs for a given target gene. For example, Tnrc6b (described below) would be considered a target of upregulated miRNAs because it was targeted by 10 upregulated miRNAs and only 4 downregulated miRNAs, so there was a net up of 6.
2.8 Analysis of miRNA targets and gene expression in other postpartum brain regions and other models
Using Bigtest, miRNA targets were also evaluated against gene expression results from other postpartum brain regions, including nucleus accumbens (NAC), MPOA, and mPFC. Further, we compared the targets to postpartum mammary gland gene expression changes (pregnant day 1 to postpartum day 1) (Anderson et al., 2007). Finally, to test specificity of our results, we made comparisons to other known gene expression changes in the CNS, including heroin effects on striatum (Piechota et al., 2012), clozapine effects on forebrain (Rizig et al., 2012), engrailed2 knockout effects on hippocampus (Sgadò et al., 2013), methylphenidate effect on substantia nigra (Sadasivan et al., 2012), and gene background effects of a mouse model for mania on hippocampus (Saul et al., 2012). For all comparisons, we use the exact same parameters in Bigtest as used when evaluating LS.
3. Results
3.1 miRNAs with altered expression during reproduction in LS
Following sequencing, 551 unique sequences were matched to annotated mouse miRNAs. Of these, 53 (or about 10% of total) showed a significant change in expression when using an overall ANOVA for all four groups (FDR p-value <0.05). Posthoc tests indicated that all of these included significant changes away from the baseline virgin group by either pregnant, Day 1 or Day 8 postpartum females. Fig. 1 shows the number of miRNAs and the direction of change away from the baseline virgin group using a FDR pvalue <0.05 for each of the reproductive groups. The direction of movement away from virgin was remarkably consistent whereby when more than one non-virgin groups showed significant differences from virgin, the direction of change was always identical. We identified 32 miRNAs that were consistently upregulated and 25 miRNAs that were consistently downregulated relative to the virgin state based on either 1) an overall ANOVA with FDR p-value <0.05 and at least two of three groups being significantly different from virgin state (Table 1) or 2) an average p value of <0.05 for all three groups versus virgin. For four of the miRNAs (mmu-miR-433-3p and 433-5p; and mmu-miR-7b-3p and 7b-5p) ‘arm switching’ occurs whereby the guide strand and passenger strand are switched as evidenced by expression of the 3p and 5p strands moving in opposite directions away from the virgin state. Four of the downregulated miRNAs were examined for expression changes using qPCR and in each case significance and relative change from virgin condition was confirmed (Fig. 2).
Fig. 1.
Overview of miRNA changes with reproduction in LS. Bars indicate the number of miRNAs and the direction of change away from the baseline virgin group (blue = up; red = down) based on significant differences relative to virgin for each group using FDR pvalue <0.05. The direction of movement of miRNA expression away from virgin was remarkably consistent whereby when more than one non-virgin groups showed significant differences from virgin for the same miRNA, the direction of change was always identical.
Table 1.
Summary of consistently upregulated or downregulated miRNAs in LS relative to virgin condition.
| Upregulated miRNAs | Downregulated miRNAs |
|---|---|
|
| |
| mmu-miR-1a-3p | mmu-miR-7b-3p |
| mmu-miR-7a-5p | mmu-let-7b-3p |
| mmu-miR-7b-5p | mmu-let-7f-5p |
| mmu-let-7b-5p | mmu-miR-19b-3p |
| mmu-let-7c-5p | mmu-miR-92b-3p |
| mmu-miR-15b-3p | mmu-miR-107-5p |
| mmu-miR-99b-5p | mmu-miR-124-5p |
| mmu-miR-146b-5p | mmu-miR-128-3p |
| mmu-miR-183-5p | mmu-miR-129-1-3p |
| mmu-miR-185-5p | mmu-miR-129-2-3p |
| mmu-miR-193b-3p | mmu-miR-148a-3p |
| mmu-miR-210-5p | mmu-miR-152-5p |
| mmu-miR-212-5p | mmu-miR-192-5p |
| mmu-miR-215-5p | mmu-miR-340-5p |
| mmu-miR-296-5p | mmu-miR-376b-3p |
| mmu-miR-323-5p | mmu-miR-423-5p |
| mmu-miR-331-3p | mmu-miR-429-3p |
| mmu-miR-345-5p | mmu-miR-433-5p |
| mmu-miR-346-5p | mmu-miR-448-3p |
| mmu-miR-370-3p | mmu-miR-488-5p |
| mmu-miR-374-5p | mmu-miR-532-5p |
| mmu-miR-379-5p | mmu-miR-574-3p |
| mmu-miR-409-5p | mmu-miR-676-3p |
| mmu-miR-411-3p | mmu-miR-1843-3p |
| mmu-miR-425-3p | mmu-miR-1964-3p |
| mmu-miR-433-3p | |
| mmu-miR-495-3p | |
| mmu-miR-501-3p | |
| mmu-miR-541-5p | |
| mmu-miR-770-3p | |
| mmu-miR-1224-5p | |
| mmu-miR-1981-5p | |
Fig. 2.
Quantitative real-time PCR confirmation of expression changes for miRNAs of interest in pregnant, postpartum day 1 (PPD1), and postpartum day 8 (PPD8). Relative expression distribution (Y-axis) is shown for each of the four groups (n = 6 per group) for four miRNAs, mir-433-5p, mir-532-5p, mir-574-3p, and mir-676-3p, normalized against two references genes, Rnu5g and Snord68. Shown are mean ± SE. *p < 0.05 relative to virgin.
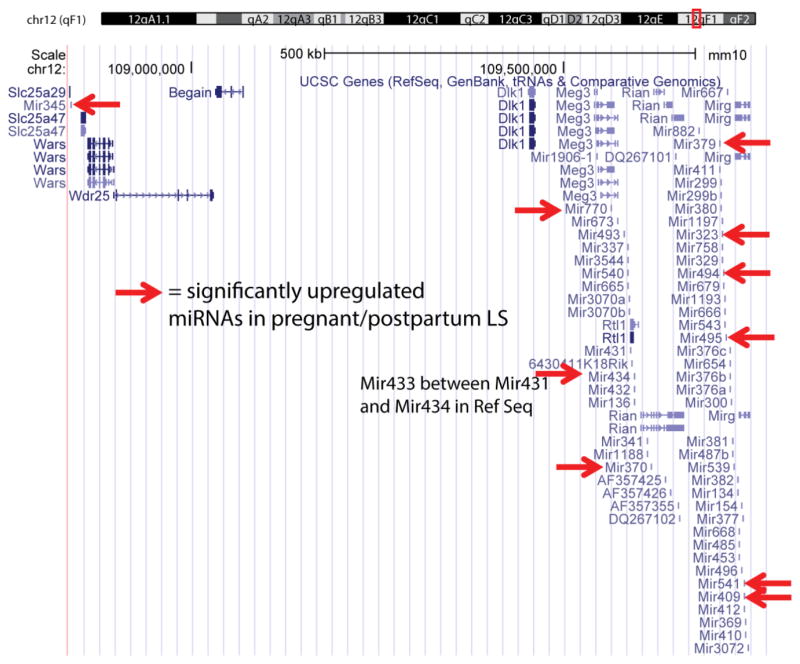
The locations of miRNAs genes across the chromosomes were fairly evenly distributed with one notable exception. Namely, 10 of the 32 upregulated miRNAs (relative to virgin) were highly localized within a small region within the 12qF1 region of chromosome 12 (Fig. 3). MiRNAs from within this subregion have previously been implication in stem cell and neuronal differentiation (Stadtfeld et al., 2010). Further, three other miRNAs (mir-154-5p, mir-673-3p, and mir-382-5p) are found in this region that are significantly upregulated relative to virgin for two of three groups, but the overall ANOVA is greater than 0.05.
Fig. 3.
Localization of upregulated miRNAs in pregnant and postpartum LS in region 12qF1. 10 of the 32 consistently upregulated miRNAs (relative to virgin) are highly localized within a small region within the 12qF1 region of chromosome 12 (red arrows). This region includes the well-studied Dlk1-Dio3 gene cluster (Dio3 not shown, but just right of shown miRNAs) and miRNAs from within this subregion have previously been implication in stem cell and neuronal differentiation. Further, three other miRNAs (mir-154-5p, mir-673-3p, and mir-382-5p) in this region are significantly upregulated relative to virgin for two of three groups, although the overall ANOVA is greater than 0.05. Background image taken from UCSC Genome Browser (http://genome.ucsc.edu) (Kent et al., 2002).
In terms of locations of miRNA genes relative to typical, coding genes, 14 of 32 upregulated miRNAs were found within known genes (exon or intron). 15 of 25 downregulated miRNAs were found within known genes (exon or intron).
3.2 Identification of miRNA target genes
For the 32 miRNAs that were consistently upregulated and for the 25 miRNAs that were consistently downregulated relative to virgin, gene targets were identified using the on-line tool, miRSystem (Lu et al., 2012). We used the setting whereby miRSystem calls a gene a target if it is found in three or more lists from 7 established miRNA databases. Using this conservative approach, 26 (of the 32) upregulated microRNAs were found to have high quality gene targets and 14 (of the 25) downregulated miRNAs were found to have gene targets. As shown in Fig. 4, upregulated miRNAs targeted 3,250 genes. A number of genes were targeted by more than one upregulated miRNA, including 1,611 genes that were targeted by 2 or more miRNAs. 643 genes were targeted by 3 or more miRNAs (Fig. 4). The downregulated miRNAs targeted 3,310 genes and 1,154 genes were targeted by 2 or more miRNAs. 424 genes were targeted by 3 or more miRNAs (Fig. 4). Some genes were found to be targeted by both up and down regulated miRNAs. For example, Tnrc6b was targeted by 10 upregulated miRNAs and 4 downregulated miRNAs. In contrast, Zbtb34a was targeted by 7 downregulated miRNAs and 3 upregulated miRNAs. Approximately 60% of the target genes for either up or down regulated miRNAs were found in both of the two lists.
Fig. 4.
Overview of miRNA target genes. For the 32 consistently upregulated miRNAs (pregnant/postpartum relative to virgin) (blue bar), 3250 target genes were identified using miRSystem. Of these, 1611 genes were targeted by 2 or more miRNAs and 91 genes were targeted by more than 5 different upregulated miRNAs. For the 25 consistently downregulated miRNAs (relative to virgin) (red bar), 3310 genes were targeted. Of these, 1154 were targeted by two or more miRNAs and 50 genes were targeted by 5 or more downregulated miRNAs. Approximately 60% of the target genes for either up or down regulated miRNAs were found in both of the two lists, suggesting homeostatic regulation.
3.3 Enrichment analysis of miRNA target genes
Enrichment analysis of target genes yielded highly consistent results using the three enrichment tools, NIH DAVID, ToppCluster, and MirSystem. Analysis for NIH DAVID and ToppCluster was performed using the top 1500 genes from up or downregulated miRNA targets to stay within the limits of the analysis programs (see Methods for details). Transcriptional regulation was the most enriched pathway or process for genes linked to up or down regulated miRNAs. Protein kinase activity, including for MAP kinase, was also enriched for genes modulated by up or down regulated miRNAs. Additionally, CNS development, including axon guidance, neurotrophin signaling, neuron development/differentiation, and neurogenesis, was also enriched for both groups.
Because of the high enrichment for transcriptional regulation, we used the Animal Transcription Factor Database (ATFD) (Zhang et al., 2012) to identify all genes related to transcriptional regulation that were targeted by up or downregulated miRNAs. We identified 574 genes (out of 3250) from upregulated miRNA targets to be involved in transcriptional regulation (~18% of total). We found 531 genes (out of 3310) from downregulated miRNA targets to be involved in transcriptional regulation (~18% of total).
3.4 Analysis of miRNA target genes and LS gene expression data
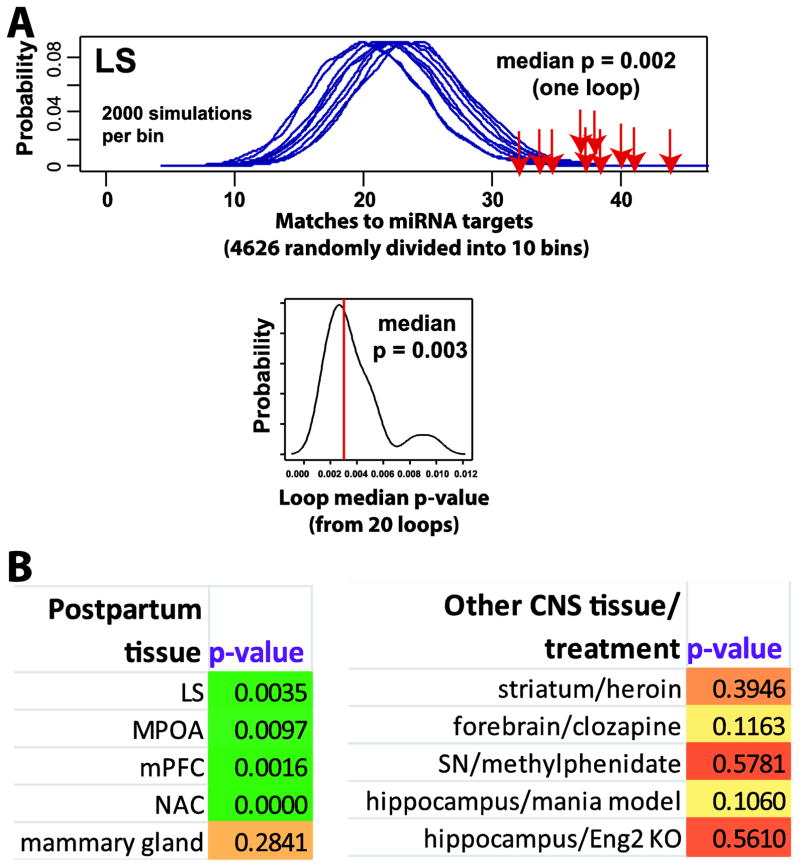
We previously identified large scale gene expression changes in postpartum LS (relative to virgin) (Eisinger et al., 2013b) and found that LS postpartum genes were enriched for all miRNA target genes (Bigtest median p-value = 0.0035; Fig. 5A). Overall, 334 genes from the top 1028 postpartum LS genes (32%) were also targeted by miRNAs with altered expression in postpartum LS. Using the hypergeometric test as an alternative approach, enrichment was also found (p=0.006). Of these, 56 were involved in transcriptional regulation. When examining separately genes that were upregulated or downregulated in LS, upregulated genes were not enriched (median p-value = 0.1245), while downregulated genes were significantly enriched (median p-value = 0.0087), suggesting downregulated genes received more miRNA regulation. To explore this issue further, we separately analyzed targets from up and down regulated miRNAs (see Methods for details). Downregulated genes were significantly enriched for upregulated miRNAs targets (median p-value = 0.0027). Downregulated LS genes were also enriched downregulated miRNA targets (median p-value = 0.0247), suggesting homeostatic regulation of downregulated genes. Upregulated LS genes did not show an overall enrichment (average p > 0.05) for targets from either up or downregulated miRNAs.
Fig. 5.
Overview of enrichment of known significantly altered LS postpartum gene (mRNA) expression against targets for altered miRNAs in LS during reproduction. Known LS gene expression changes are significantly enriched when evaluated for all miRNA targets together (A). Y-axis represents the probability of X matches to miRNA targets (divided into 10 bins) appearing in a randomly generated set of simulated results from previously published postpartum gene expression changes in LS (Eisinger et al., 2013b). The red arrows show how many matches were found in the actual significant postpartum LS expression changes and where that number falls on the probability density distribution. Images were created using MSET. The enrichment p-value is derived from the number of simulated results (2000 simulations each) that contained at least as many matches to database as the actual results. Bottom image shows probability from running the same overall test 20 times and identifies the median p-value using Bigtest. A key advantage of Bigtest is that by dividing larger lists into smaller pieces (such as for miRNAs targets here), the issue of false positives from large target lists is avoided. B) Summary of Bigtest p-values when using miRNA targets against significant postpartum NAC, MPOA, mPFC, and NAC genes. Also, shown (on right) p-values of miRNA targets against significant gene expression changes from various CNS manipulations, including heroin action on striatum, clozapine action on forebrain, methylphenidate action on substantia nigra (SN), mouse model for mania in hippocampus, and effects of engrailed2 (Eng2) on hippocampus (see Methods for details).
As an additional step, we evaluated targets from up or down regulated miRNAs based on whether there was a net higher number of up or downregulated miRNAs for a given target gene. Using this approach, downregulated postpartum LS genes were significantly associated with net downregulated targets (median p-value = 0.0015) and net upregulated miRNA targets (average p-value = 0.021), again suggesting strong homeostatic regulation of downregulated miRNAs. For upregulated LS genes, no enrichment was found for either net up or down regulated miRNA targets.
3.5 Analysis of miRNA targets and gene expression in other postpartum brain regions and other models
In addition to microarray analysis of LS, we have examined large scale gene expression of three other brain regions in the postpartum condition, namely MPOA, mPFC, and NAC (Driessen et al., 2014b, Eisinger et al., 2014, Zhao et al., 2014). Common genetic hallmarks of the maternal brain have been found across these regions as well as genes unique to each. miRNAs can be transported as extracellular signals and because of our finding of high enrichment of LS genes to miRNA targets, we wanted to test whether maternal gene expression in other regions bore any connection to targets from LS postpartum miRNAs. Enrichment was found for MPOA (median p = 0.0097), mPFC (median p = 0.0016), and NAC (median p = 0.0002) (Fig. 5). For NAC, though, upregulated genes were enriched for miRNA, while for MPOA and mPFC, both up and down regulated mRNAs showed enrichment (data not shown). Enrichment included both genes common across multiple regions (e.g., Nr1d1, Uhrf2, Lipa, Penk, Rbfox2, and Adcy6) and genes unique to a region, suggesting the possibility that a relatively small number of miRNAs may be used globally across the postpartum brain. We found no enrichment for gene expression changes in mouse mammary gland for postpartum brain miRNAs (median p = 0.2841), suggesting CNS postpartum miRNAs are likely exclusive to the CNS (Fig. 5B). As an additional control, we evaluated microarray gene expression in the rodent CNS in association with various non-maternal manipulations (from publicly available databases) and in no cases did we find any enrichment (Fig. 5B).
4. Discussion
In this study we directly examine for the first time miRNA changes in association with reproduction in the female brain. By examining LS miRNA expression at four stages (virgin, pregnant, Day 1 postpartum, and Day 8 postpartum) we were able to capture insights into dynamic miRNA changes.
4.1 Consistent miRNA changes relative to virgin baseline
One major finding is the reliable and robust changes of miRNA away from the virgin stage at the three other stages (Fig. 1). For most significant miRNA findings, the changes were found in either two or three other groups relative to virgin and these changes (increased or decreased) were always in the same direction. We were able to identify 32 miRNAs that were reliably increased and 25 miRNAs that were reliable decreased relative to virgin (Fig. 4). Thus, for a given maternal miRNA, it is highly predictable in that direction of change from baseline virgin will either remain up or downregulated or return to baseline. The findings of consistent changes always included one or both postpartum groups (Day 1 or Day 8), so the consistent changes include contributions to the postpartum brain. Further, miRNAs are a step in altering expression of other genes, including numerous transcription factors (as seen in this study), that would in turn alter expression of other genes. Thus, any change observed at a given time point would be expected to be having a prolonged effect on gene expression, so even miRNA changes beginning in the pregnant state could have lasting effects towards shaping the maternal brain.
4.2 Arm switching
A given miRNA gene can produce two miRNA (3p or 5p). The one miRNA arm produced in larger abundance (usually greater than 90% of total) is considered the guide strand, while the lesser produced arm is the passenger strand (Ha and Kim, 2014). ‘Arm switching’ involves a switch between guide and passenger strand, whereby the abundance of the 3p or 5p products are reversed (Chiang et al., 2010). In the postpartum LS, we observed this happening for two miRNAs, miR-433 and miR-7b. Compared to the virgin state miR-433-3p and miR-7b-5p were elevated while miR-433-5p and 7b-3p were decreased. ‘Arm switching’ may result from changes in processing of miRNAs by the protein Drosha (Chiang et al., 2010) and it has been implicated in evolutionary events (Griffiths-Jones et al., 2011). Whether ‘arm switching’ of mir-433 and mir-7b is a key event for production of the postpartum brain remains to be addressed.
4.3 12qF1 as a Chromosome 12 hotspot for upregulated miRNAs
One unexpected finding was that 10 of the 32 upregulated miRNAs (relative to virgin) were highly localized within a small region within the 12qF1 region of chromosome 12 (Fig. 3). Other significant miRNAs were spread across the genome. We also found within this small region three other miRNAs (mir-154-5p, mir-673-3p, and mir-382-5p) that were significantly upregulated relative to virgin for two of three groups, but the overall ANOVA was greater than 0.05, so they were not part of our original analysis. The clustering of these upregulated miRNAs suggests a tight, coordinated regulation of a subset of miRNAs. Interestingly, miRNAs from within 12qF1, referred to as the Dlk1-Dio3 gene cluster, have previously been implicated in stem cell and neuronal differentiation (Stadtfeld et al., 2010). In particular, silencing of miRNAs in this region were associated with the cellular status of induced pluripotent stem cell (Stadtfeld et al., 2010). Thus, the elevated miRNA expression here may reflect a differentiation event. Many genes within 12qF1 are imprinted and this region has shared synteny with 14q32 in humans (I Laufer and M Singh, 2012), suggesting a conserved regulation of genes and miRNAs in this region. Alterations in imprinting and gene expression in this region in humans have been linked to deficits in fetal and postnatal development (Howard and Charalambous, 2015) as well as other pathological processes, including cancer (Benetatos et al., 2013). While the contribution of the region to child development has been well established, this study is the first to suggest important changes in activation of the region in association with reproduction in the mother. How this region contributes to the postpartum brain and emergence of maternal care or physiology is not known, but could be of great importance.
4.4 mRNA targets of miRNAs
Using the conservative miRSystem, we identified just over 3,000 target genes for both the LS miRNAs that were upregulated or downregulated relative to the virgin state. In both cases, a number of genes were targeted by multiple miRNAs, indicating convergent control. For example, upregulated miRNA targets included over 1,600 genes that were targeted by 2 or more miRNAs while downregulated miRNAs targets had over 1,100 genes that were targeted by 2 or more miRNAs. Approximately 60% of the target genes were associated with at least one up and down regulated miRNAs, suggesting homeostatic regulation of these genes. One of the heavily regulated genes was Tnrc6b (targeted by 10 upregulated miRNAs and 4 downregulated miRNAs) which itself is involved in mediated mRNA decay by miRNAs (Chen et al., 2009). Another strongly regulated gene was Zbtb34a (targeted by 7 downregulated miRNAs and 3 upregulated miRNAs) which is involved in transcriptional regulation (Qi et al., 2006). Because a given miRNA can have targets that range from the single digits to over a thousand, it is not necessarily surprising that we find cooperative and competitive regulation of a subset of genes and the finding is consistent with other studies (Grimson et al., 2007, Lewohl et al., 2011).
4.5 Transcriptional regulation enrichment
In terms of enrichment analysis of miRNA target genes, by far the most significant pathways or processes were linked with transcriptional regulation and this was true for both up and down regulated miRNAs. Using ATFD, we identified 574 genes from upregulated miRNA targets to be involved in transcriptional regulation (~18% of total) as well as 531 genes from downregulated miRNA targets (~18% of total). miRNAs and transcription factors can interact with one another in multiple and complex ways as they can affect expression of one another and/or common target genes (Zhou et al., 2007, Friard et al., 2010, Wang et al., 2010, Arora et al., 2013, Le et al., 2013). To our knowledge, this is one of the few studies of miRNA targets to find transcriptional regulation as the top pathway for enrichment analysis. The finding is consistent with the large and dramatic mRNA changes we have identified as occurring across the maternal brain and suggests a complex interaction of transcription factors and miRNAs sculpt the postpartum brain.
4.6 Other Enriched Pathways, including CNS development
We also identified enrichment for other pathways, such as protein kinase activity, including for MAP kinase, and CNS development, including axon guidance, neurotrophin signaling, neuron development/differentiation, and neurogenesis. The latter finding of enrichment of CNS development/differentiation is consistent with our recent findings of enrichment for these pathways in association with gene expression in the maternal brain (Eisinger et al., 2013b), and suggests the postpartum brain may be a developmental endpoint that includes a high level of plasticity and developmental events. Interestingly, the elevated expression of miRNAs found in 12qF1 (see above) is consistent with cellular differentiation events (Stadtfeld et al., 2010) and may contribute to CNS plasticity in the postpartum brain. While studies on neurogenesis alterations in the maternal brain have mostly focused on hippocampus and the subventricular zone (Pawluski and Galea, 2007, Levy et al., 2011, Galea et al., 2013), to our knowledge no study has examined neurogenesis within LS during the peripartum period. Further, structural changes have been identified in the maternal brain (Leuner and Gould, 2010, Shams et al., 2012), but LS has not been a focus of research. In terms of general plasticity (e.g., expression changes, neurotransmitter changes), though, we and others have documented numerous changes in LS (Francis et al., 2000, Caughey et al., 2011, Curley et al., 2012, Zhao et al., 2012a, Eisinger et al., 2013b, Driessen et al., 2014a, Zhao and Gammie, 2014, 2015). Understanding how the miRNA alterations lead to specific neurodevelopmental events would need to be addressed in follow up studies.
4.7 Comparison of LS miRNA targets to genes with altered expression in postpartum LS
Because we had previously identified large scale gene expression changes in postpartum LS (relative to virgin) (Eisinger et al., 2013b), we were able to compare these gene expression changes with inferred gene expression regulation by the miRNAs. As expected, we identified postpartum LS genes as significantly enriched for the miRNA targets. Overall, 334 genes from the top 1028 postpartum LS genes (32%) were also targeted by miRNAs with altered expression in postpartum LS. Downregulated LS genes were responsible for most of the significant association, suggesting downregulated genes received more miRNA regulation. These genes were significantly associated with both up and downregulated miRNA targets, suggesting strong homeostatic regulation of downregulated miRNAs.
4.8 Indirect evidence for core miRNAs acting across the postpartum CNS
Because we have examined postpartum gene expression in multiple maternal brain regions in addition to LS, including MPOA, mPFC, and NAC, we were able to evaluate whether expression in these regions was enriched for miRNA targets. The finding that each region was significantly associated with LS miRNA targets suggests the interesting possibility that core postpartum miRNAs act across the CNS to help produce the maternal brain. This finding is interesting given the enrichment for development/plasticity pathways and the number of studies documenting plasticity changes throughout the maternal CNS (see above). Further, we found that while some of the miRNA-mRNA matches across the maternal brain were similar, each region also had a distinct association. This is consistent with our prior work identifying both conserved gene expression changes across the CNS and region specific changes (Driessen et al., 2014b, Eisinger et al., 2014, Zhao et al., 2014). Similarly, common and unique miRNAs may be found in various reproductive CNS regions, but this needs to be tested. Mouse mammary glands gene expression was not associated with the LS miRNA targets suggesting that the population of miRNAs acting centrally and peripherally are different. Our finding of no association among multiple CNS manipulations and the maternal CNS miRNA targets suggests again that the collection of miRNAs used for producing the postpartum brain is unique.
While the present studies due not address causality, the original rationale for this study on miRNAs came from enrichment analysis of large scale gene expression studies that suggested miRNAs were involved in producing the maternal brain. In the present studies, we found that the miRNA targets were in turn highly enriched for known gene expression changes in the postpartum brain, which provides additional support that the miRNAs are indeed contributing to the maternal brain and are not simply a consequence of the maternal experience. However, to address this question more directly, future studies could involve manipulations of these miRNAs (overexpression or knockdown) and examination of subsequent changes in the CNS.
Highlights.
A subset of up and downregulated miRNAs are a component of the maternal brain.
A number of miRNAs downregulated during female reproduction are located in 12qF1 region that includes the Dlk1-Dio3 cluster.
Reproductive miRNA gene targets are highly enriched for genes involved in transcriptional regulation.
Reproductive miRNA gene targets are consistent with known postpartum gene expression changes across multiple CNS regions.
A new research tool provides enrichment analysis of large scale gene expression datasets against large target datasets.
Acknowledgments
The authors wish to thank Sharon Stevenson for managerial support, Wayne Davis and the University of Wisconsin-Madison Gene Expression Center for microarray technical assistance, and Kate Skogen and Jeff Alexander for animal care. This work was supported by the United States National Institutes of Health Grant R01 MH 085642 to Stephen Gammie.
Abbreviations
- mPFC
medial prefrontal cortex
- MPOA
medial preoptic area
- miRNAs
microRNAs
- MSET
Modular Single-set Enrichment Test
- LS
lateral septum
- qPCR
quantitative real-time PCR
- NAC
nucleus accumbens
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson SM, Rudolph MC, McManaman JL, Neville MC. Secretory activation in the mammary gland: it’s not just about milk protein synthesis. Breast Cancer Res. 2007;9:204–217. doi: 10.1186/bcr1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora S, Rana R, Chhabra A, Jaiswal A, Rani V. miRNA–transcription factor interactions: a combinatorial regulation of gene expression. Molecular Genetics and Genomics. 2013;288:77–87. doi: 10.1007/s00438-013-0734-z. [DOI] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benetatos L, Hatzimichael E, Londin E, Vartholomatos G, Loher P, Rigoutsos I, Briasoulis E. The microRNAs within the DLK1-DIO3 genomic region: involvement in disease pathogenesis. Cellular and Molecular Life Sciences. 2013;70:795–814. doi: 10.1007/s00018-012-1080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caughey S, Klampfl S, Bishop V, Pfoertsch J, Neumann I, Bosch O, Meddle S. Changes in the intensity of maternal aggression and central oxytocin and vasopressin V1a receptors across the peripartum period in the rat. Journal of Neuroendocrinology. 2011;23:1113–1124. doi: 10.1111/j.1365-2826.2011.02224.x. [DOI] [PubMed] [Google Scholar]
- Chen C-YA, Zheng D, Xia Z, Shyu A-B. Ago–TNRC6 triggers microRNA-mediated decay by promoting two deadenylation steps. Nature structural & molecular biology. 2009;16:1160–1166. doi: 10.1038/nsmb.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang HR, Schoenfeld LW, Ruby JG, Auyeung VC, Spies N, Baek D, Johnston WK, Russ C, Luo S, Babiarz JE. Mammalian microRNAs: experimental evaluation of novel and previously annotated genes. Genes Dev. 2010;24:992–1009. doi: 10.1101/gad.1884710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curley J, Jensen C, Franks B, Champagne F. Variation in maternal and anxiety-like behavior associated with discrete patterns of oxytocin and vasopressin 1a receptor density in the lateral septum. Hormones and Behavior. 2012;61:454–461. doi: 10.1016/j.yhbeh.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Anna KL, Gammie SC. Activation of corticotropin-releasing factor receptor 2 in lateral septum negatively regulates maternal defense. Behavioral neuroscience. 2009;123:356–368. doi: 10.1037/a0014987. [DOI] [PubMed] [Google Scholar]
- Driessen T, Zhao C, Whittlinger A, Williams H, Gammie SC. Endogenous CNS expression of neurotensin and neurotensin receptors is altered during the postpartum period in outbred mice. Public Library of Science ONE. 2014a doi: 10.1371/journal.pone.0083098. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen TM, Eisinger BE, Zhao C, Stevenson SA, Saul MC, Gammie SC. Genes showing altered expression in the medial preoptic area in the highly social maternal phenotype are related to autism and other disorders with social deficits. BMC neuroscience. 2014b doi: 10.1186/1471-2202-15-11. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisinger BE, Driessen TM, Zhao C, Gammie S. Medial prefrontal cortex: genes linked to bipolar disorder and schizophrenia have altered expression in the highly social maternal phenotype. Frontiers in Behavioral Neuroscience. 2014;8 doi: 10.3389/fnbeh.2014.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisinger BE, Saul MC, Driessen TM, Gammie SC. Development of a versatile enrichment analysis tool reveals associations between the maternal brain and mental health disorders, including autism. BMC neuroscience. 2013a;14:147. doi: 10.1186/1471-2202-14-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisinger BE, Zhao C, Driessen TM, Saul MC, Gammie SC. Large scale expression changes of genes related to neuronal signaling and developmental processes found in lateral septum of postpartum outbred mice. PLoS One. 2013b;8:e63824. doi: 10.1371/journal.pone.0063824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis DD, Champagne FC, Meaney MJ. Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. Journal of Neuroendocrinology. 2000;12:1145–1148. doi: 10.1046/j.1365-2826.2000.00599.x. [DOI] [PubMed] [Google Scholar]
- Friard O, Re A, Taverna D, De Bortoli M, Corá D. CircuitsDB: a database of mixed microRNA/transcription factor feed-forward regulatory circuits in human and mouse. BMC bioinformatics. 2010;11:435. doi: 10.1186/1471-2105-11-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea LA, Wainwright SR, Roes MM, Duarte-Guterman P, Chow C, Hamson DK. Sex, Hormones and Neurogenesis in the Hippocampus: Hormonal Modulation of Neurogenesis and Potential Functional Implications. Journal of Neuroendocrinology. 2013;25:1039–1061. doi: 10.1111/jne.12070. [DOI] [PubMed] [Google Scholar]
- Goeman JJ, Bühlmann P. Analyzing gene expression data in terms of gene sets: methodological issues. Bioinformatics. 2007;23:980–987. doi: 10.1093/bioinformatics/btm051. [DOI] [PubMed] [Google Scholar]
- Griffiths-Jones S, Grocock RJ, Van Dongen S, Bateman A, Enright AJ. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006;34:D140–D144. doi: 10.1093/nar/gkj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths-Jones S, Hui JH, Marco A, Ronshaugen M. MicroRNA evolution by arm switching. EMBO reports. 2011;12:172–177. doi: 10.1038/embor.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimson A, Farh KK-H, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Molecular cell. 2007;27:91–105. doi: 10.1016/j.molcel.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha M, Kim VN. Regulation of microRNA biogenesis. Nature Reviews Molecular Cell Biology. 2014;15:509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome biology. 2007;8:R19. doi: 10.1186/gb-2007-8-2-r19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard M, Charalambous M. Molecular basis of imprinting disorders affecting chromosome 14: lessons from murine models. Reproduction. 2015;149:R237–R249. doi: 10.1530/REP-14-0660. [DOI] [PubMed] [Google Scholar]
- Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature protocols. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Laufer IB, Singh MS. A macro role for imprinted clusters of MicroRNAs in the brain. MicroRNA. 2012;1:59–64. doi: 10.2174/2211536611201010059. [DOI] [PubMed] [Google Scholar]
- Kaimal V, Bardes EE, Tabar SC, Jegga AG, Aronow BJ. ToppCluster: a multiple gene list feature analyzer for comparative enrichment clustering and network-based dissection of biological systems. Nucleic Acids Res. 2010;38:W96–102. doi: 10.1093/nar/gkq418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, Haussler D. The human genome browser at UCSC. Genome Res. 2002;12:996–1006. doi: 10.1101/gr.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsley CH, Amory-Meyer E. Why the maternal brain? Journal of Neuroendocrinology. 2011;23:974–983. doi: 10.1111/j.1365-2826.2011.02194.x. [DOI] [PubMed] [Google Scholar]
- Le TD, Liu L, Liu B, Tsykin A, Goodall GJ, Satou K, Li J. Inferring microRNA and transcription factor regulatory networks in heterogeneous data. BMC bioinformatics. 2013;14:92. doi: 10.1186/1471-2105-14-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G, Gammie SC. GABA(A) receptor signaling in the lateral septum regulates maternal aggression in mice. Behavioral neuroscience. 2009;123:1169–1177. doi: 10.1037/a0017535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Gould E. Dendritic growth in medial prefrontal cortex and cognitive flexibility are enhanced during the postpartum period. The Journal of neuroscience. 2010;30:13499–13503. doi: 10.1523/JNEUROSCI.3388-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy F, Gheusi G, Keller M. Plasticity of the parental brain: a case for neurogenesis. J Neuroendocrinol. 2011;23:984–993. doi: 10.1111/j.1365-2826.2011.02203.x. [DOI] [PubMed] [Google Scholar]
- Lewohl JM, Nunez YO, Dodd PR, Tiwari GR, Harris RA, Mayfield RD. Up-Regulation of MicroRNAs in Brain of Human Alcoholics. Alcoholism: Clinical and Experimental Research. 2011;35:1928–1937. doi: 10.1111/j.1530-0277.2011.01544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T-P, Lee C-Y, Tsai M-H, Chiu Y-C, Hsiao CK, Lai L-C, Chuang EY. miRSystem: an integrated system for characterizing enriched functions and pathways of microRNA targets. PLoS One. 2012;7:e42390. doi: 10.1371/journal.pone.0042390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng LY, Shors TJ. Once a mother, always a mother: maternal experience protects females from the negative effects of stress on learning. Behavioral Neuroscience. 2012;126:137. doi: 10.1037/a0026707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann PE, Rubin BS, Bridges RS. Differential proopiomelanocortin gene expression in the medial basal hypothalamus of rats during pregnancy and lactation. Molecular brain research. 1997;46:9–16. doi: 10.1016/s0169-328x(96)00267-7. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Torner L, Wigger A. Brain oxytocin: differential inhibition of neuroendocrine stress responses and anxiety-related behaviour in virgin, pregnant and lactating rats. Neuroscience. 1999;95:567–575. doi: 10.1016/s0306-4522(99)00433-9. [DOI] [PubMed] [Google Scholar]
- Newton MA, Quintana FA, Den Boon JA, Sengupta S, Ahlquist P. Random-set methods identify distinct aspects of the enrichment signal in gene-set analysis. The Annals of Applied Statistics. 2007:85–106. [Google Scholar]
- Olazábal DE, Pereira M, Agrati D, Ferreira A, Fleming AS, González-Mariscal G, Lévy F, Lucion AB, Morrell JI, Numan M. Flexibility and adaptation of the neural substrate that supports maternal behavior in mammals. Neuroscience & Biobehavioral Reviews. 2013;37:1875–1892. doi: 10.1016/j.neubiorev.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Pawluski J, Galea L. Reproductive experience alters hippocampal neurogenesis during the postpartum period in the dam. Neuroscience. 2007;149:53–67. doi: 10.1016/j.neuroscience.2007.07.031. [DOI] [PubMed] [Google Scholar]
- Piechota M, Korostynski M, Sikora M, Golda S, Dzbek J, Przewlocki R. Common transcriptional effects in the mouse striatum following chronic treatment with heroin and methamphetamine. Genes, Brain and Behavior. 2012;11:404–414. doi: 10.1111/j.1601-183X.2012.00777.x. [DOI] [PubMed] [Google Scholar]
- Qi J, Zhang X, Zhang H-K, Yang H-M, Zhou Y-B, Han Z-G. ZBTB34, a novel human BTB/POZ zinc finger protein, is a potential transcriptional repressor. Molecular and cellular biochemistry. 2006;290:159–167. doi: 10.1007/s11010-006-9183-x. [DOI] [PubMed] [Google Scholar]
- Rizig MA, McQuillin A, Ng A, Robinson M, Harrison A, Zvelebil M, Hunt SP, Gurling HM. A gene expression and systems pathway analysis of the effects of clozapine compared to haloperidol in the mouse brain implicates susceptibility genes for schizophrenia. Journal of psychopharmacology. 2012;26:1218–1230. doi: 10.1177/0269881112450780. [DOI] [PubMed] [Google Scholar]
- Sadasivan S, Pond BB, Pani AK, Qu C, Jiao Y, Smeyne RJ. Methylphenidate exposure induces dopamine neuron loss and activation of microglia in the basal ganglia of mice. 2012 doi: 10.1371/journal.pone.0033693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saul MC, Gessay GM, Gammie SC. A new mouse model for mania shares genetic correlates with human bipolar disorder. PLoS One. 2012;7:e38128. doi: 10.1371/journal.pone.0038128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotti MA, Lee G, Gammie SC. Maternal defense is modulated by beta adrenergic receptors in lateral septum in mice. Behavioral neuroscience. 2011;125:434–445. doi: 10.1037/a0023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethupathy P, Corda B, Hatzigeorgiou AG. TarBase: A comprehensive database of experimentally supported animal microRNA targets. Rna. 2006;12:192–197. doi: 10.1261/rna.2239606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgadò P, Provenzano G, Dassi E, Adami V, Zunino G, Genovesi S, Casarosa S, Bozzi Y. Transcriptome profiling in engrailed-2 mutant mice reveals common molecular pathways associated with autism spectrum disorders. Molecular autism. 2013;4:5. doi: 10.1186/2040-2392-4-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shams S, Pawluski JL, Chatterjee-Chakraborty M, Oatley H, Mastroianni A, Fleming AS. Dendritic morphology in the striatum and hypothalamus differentially exhibits experience-dependent changes in response to maternal care and early social isolation. Behavioural Brain Research. 2012;233:79–89. doi: 10.1016/j.bbr.2012.04.048. [DOI] [PubMed] [Google Scholar]
- Sheehan TP, Chambers RA, Russell DS. Regulation of affect by the lateral septum: implications for neuropsychiatry. Brain research Brain research reviews. 2004;46:71–117. doi: 10.1016/j.brainresrev.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Slotnick BM, Nigrosh BJ. Maternal behavior of mice with cingulate cortical, amygdala, or septal lesions. J Comp Physiol Psychol. 1975;88:118–127. doi: 10.1037/h0076200. [DOI] [PubMed] [Google Scholar]
- Stadtfeld M, Apostolou E, Akutsu H, Fukuda A, Follett P, Natesan S, Kono T, Shioda T, Hochedlinger K. Aberrant silencing of imprinted genes on chromosome 12qF1 in mouse induced pluripotent stem cells. Nature. 2010;465:175–181. doi: 10.1038/nature09017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansberg C, Vik-Mo AO, Holdhus R, Breilid H, Srebro B, Petersen K, Jørgensen HA, Jonassen I, Steen VM. Gene expression profiles in rat brain disclose CNS signature genes and regional patterns of functional specialisation. BMC genomics. 2007;8:94. doi: 10.1186/1471-2164-8-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M, Lieberman J, Lal A. Desperately seeking microRNA targets. Nature structural & molecular biology. 2010;17:1169–1174. doi: 10.1038/nsmb.1921. [DOI] [PubMed] [Google Scholar]
- Wang J, Lu M, Qiu C, Cui Q. TransmiR: a transcription factor–microRNA regulation database. Nucleic Acids Res. 2010;38:D119–D122. doi: 10.1093/nar/gkp803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H-M, Chen H, Liu W, Liu H, Gong J, Wang H, Guo A-Y. AnimalTFDB: a comprehensive animal transcription factor database. Nucleic Acids Res. 2012;40:D144–D149. doi: 10.1093/nar/gkr965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Driessen T, Gammie SC. Glutamic acid decarboxylase 65 and 67 expression in the lateral septum is up-regulated in association with the postpartum period in mice. Brain research. 2012a;1470:35–44. doi: 10.1016/j.brainres.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Eisinger BE, Driessen TM, Gammie SC. Addiction and reward-related genes show altered expression in the postpartum nucleus accumbens. Frontiers in Behavioral Neuroscience. 2014;8:388. doi: 10.3389/fnbeh.2014.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Gammie SC. Glutamate, GABA, and glutamine are synchronously upregulated in the mouse lateral septum during the postpartum period. Brain Research. 2014 doi: 10.1016/j.brainres.2014.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Gammie SC. Metabotropic Glutamate Receptor 3 Is Downregulated and Its Expression Is Shifted From Neurons to Astrocytes in the Mouse Lateral Septum During the Postpartum Period. Journal of Histochemistry & Cytochemistry 0022155415578283. 2015 doi: 10.1369/0022155415578283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Saul MC, Driessen T, Gammie SC. Gene expression changes in the septum: possible implications for microRNAs in sculpting the maternal brain. PLoS One. 2012b;7:e38602. doi: 10.1371/journal.pone.0038602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Ferguson J, Chang JT, Kluger Y. Inter-and intra-combinatorial regulation by transcription factors and microRNAs. BMC genomics. 2007;8:396. doi: 10.1186/1471-2164-8-396. [DOI] [PMC free article] [PubMed] [Google Scholar]