Abstract
Background & Aims
Overactivation of the innate immune response underlies many forms of liver injury including that caused by hepatotoxins. Recent studies have demonstrated that macrophage autophagy regulates innate immunity and resultant tissue inflammation. Although hepatocyte autophagy has been shown to modulate hepatic injury, little is known about the role of autophagy in hepatic macrophages during the inflammatory response to acute toxic liver injury. Our aim therefore was to determine whether macrophage autophagy functions to down regulate hepatic inflammation.
Methods
Mice with a LysM-CRE-mediated macrophage knockout of the autophagy gene ATG5 were examined for their response to toxin-induced liver injury from D-galactosamine/lipopolysaccharide (GalN/LPS).
Results
Knockout mice had increased liver injury from GalN/LPS as determined by significant increases in serum alanine aminotransferase, histological evidence of liver injury, positive terminal deoxynucleotide transferase-mediated deoxyuridine triphosphate nick end-labeling, caspase activation and mortality as compared to littermate controls. Levels of proinflammatory tumor necrosis factor and interleukin (IL)-6 hepatic mRNA and serum protein were unchanged, but serum IL-1β was significantly increased in knockout mice. The increase in serum IL-1β was secondary to elevated hepatic caspase 1 activation and inflammasome-mediated cleavage of pro-IL-1β to its active form. Cultured hepatic macrophages from GalN/LPS-treated knockout mice had similarly increased IL-1β production. Dysregulation of IL-1β was the mechanism of increased liver injury as an IL-1 receptor antagonist prevented injury in knockout mice in concert with decreased neutrophil activation.
Conclusions
Macrophage autophagy functions to limit acute toxin-induced liver injury and death by inhibiting the generation of inflammasome-dependent IL-1β.
Keywords: Apoptosis, Galactosamine, Inflammasome, Innate immunity, Kupffer cell, Lipopolysaccharide, Macrophage
Introduction
Liver injury results not only from the direct effects of injurious agents on hepatocytes, but also from cytokines generated by the accompanying innate immune response [1]. Although resident and recruited macrophages mediate the resolution and repair of liver damage, excessive macrophage activation underlies many forms of hepatocyte injury including that from the toxin alcohol and hepatic steatosis [2-4]. The liver presents unique challenges for the proper control of immune responses as this organ contains the vast majority (80-90%) of the resident macrophages in the body. In addition, the intestinal blood supply delivers gut-derived lipopolysaccharide (LPS) and other bacterial products directly to the liver where they can trigger immune cell activation. LPS induces proinflammatory M1 macrophage polarization and production of tumor necrosis factor (TNF) which is cytotoxic to hepatocytes [5]. Additional proinflammatory cytokines may contribute to liver injury including interleukin (IL)-1β and interferon-γ (IFNγ) [6-9]. The large complement of liver macrophages and their exposure to high LPS levels requires that hepatic immune responses be carefully regulated to prevent excessive and injurious macrophage activation.
Recent studies have demonstrated essential protective functions for hepatocyte macroautophagy (hereafter referred to as autophagy) during liver injury [10-12]. In contrast, little is known about the role of autophagy in macrophages [13]. Autophagy was initially linked to inflammatory responses through the ability of this pathway to sequester and eliminate microbial pathogens [14, 15]. Autophagy also regulates macrophage responses in sterile inflammation. Recently we demonstrated that in obesity-induced hepatic steatosis impaired macrophage autophagy increases liver inflammation and injury from LPS by promoting proinflammatory M1 macrophage polarization [16]. In non-hepatic inflammatory models autophagy inhibits inflammation by down regulating caspase 1-dependent inflammasome cleavage of pro-IL-1β to its active, secreted form [17, 18], suggesting another mechanism by which autophagy may limit hepatic inflammation.
This study examined whether macrophage autophagy regulates acute toxin-induced liver injury from D-galactosamine (GalN) and LPS. GalN/LPS liver injury results from GalN-mediated hepatocyte sensitization to cytotoxicity from LPS-induced cytokines [6, 9, 19]. In mice with a myeloid cell-specific knockout of the autophagy gene ATG5, decreased macrophage autophagy amplified GalN/LPS liver injury. With decreased autophagy there was a heightened inflammatory response restricted to increased generation of IL-1β IL-18 and IFNγ. Higher levels of IL-1β resulted from inflammasome-mediated caspase 1 cleavage of pro-IL-1β and promoted liver injury. These findings establish a new function for macrophage autophagy in protecting against IL-1β-dependent, acute hepatotoxic injury.
Materials and methods
Animal model
Male mice 10-14 weeks old were maintained under 12 h light/dark cycles with unlimited access to food and water. Wild-type C57BL/6 mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Atg5f/f mice [20] containing floxed alleles for the autophagy gene Atg5 were crossed with LysM-CRE mice [21] to generate Atg5Δmye mice with a myeloid cell-specific knockout of Atg5-dependent autophagy [16]. Littermate Atg5f/f mice lacking the CRE transgene served as controls. Liver injury was induced by intraperitoneal injections of 100 μg/kg of LPS (E. coli 0111:B4) and 700 mg/kg of GalN (Sigma, St. Louis, MO) dissolved in phosphate-buffered saline (PBS), as previously performed [22]. Some mice were pretreated with PBS vehicle or the IL-1 receptor antagonist (IL-1Ra) anakinra (Amgen, Thousand Oaks, CA) 0.5 h before GalN/LPS administration. All animal studies were approved by the Animal Care and Use Committee of the Albert Einstein College of Medicine and followed the National Institutes of Health guidelines for animal care.
Alanine aminotransferase (ALT) assay
Serum ALTs were measured by commercial kit (TECO Diagnostics, Anaheim, CA).
Histology
Mouse livers were fixed in 10% neutral formalin and stained with hematoxylin and eosin. The percentage of apoptosis/necrosis was graded semi-quantitatively in a blinded fashion by a single pathologist on a sliding scale of: 0, absent; 0.5, minimal; 1, mild; 1.5, mild to moderate; 2, moderate; 2.5, moderate to marked; and 3, marked.
Terminal deoxynucleotide transferase-mediated deoxyuridine triphosphate nick end-labeling (TUNEL) assay
The numbers of TUNEL positive cells were detected in liver sections with the DeadEnd Colorimetric System (Promega, Madison, WI) kit. The numbers of TUNEL positive cells were counted under light microscopy in 10 randomly selected high power fields (400X magnification).
Protein isolation and Western blotting
Total mouse liver protein was isolated and Western blotting preformed, as previously described [23, 24]. Membranes were exposed to antibodies that recognized caspase 3 (Cell Signaling, Beverly, MA; #9665), caspase 7 (Cell Signaling, #9492), poly (ADP-ribose) polymerase (Cell Signaling, #9542), tubulin (Cell Signaling, #2148), caspase 1 (Santa Cruz Biotechnology, Dallas, TX; #SC-514) and IL-1β (R&D Systems, Minneapolis, MN; #AF-401-NA).
Caspase 8 activity
Hepatic caspase 8 activity was measured by commercial kit (BioVision, Milpitas, CA). Reactions were carried out with 200 μg of protein at 37°C for 2 h and fluorescence measured with 400 nm excitation and 505 nm emission filters.
Immunofluorescence microscopy
Livers were frozen in 2-methylbutane, sectioned, blocked for 1 h in 2% donkey serum, 1% bovine serum albumin (Sigma) and 0.05% Tween 20 (Fisher), and incubated overnight with anti-CD68 (Abd Serotec, Raleigh, NC; #MCA1957GA) or anti-Ly6G (Biolegend, San Diego, CA; #127602) antibody at 4°C. The tissues were then washed with PBS and incubated for 1 h with Cy3-conjugated secondary antibody (Jackson ImmunoResearch, West Grove, PA; #111-165-152). After washing with PBS, tissues were mounted in anti-fading medium containing 4′,6-diamidino-2-phenylindole (Life Technologies, Carlsbad, CA) and visualized by fluorescence microscopy.
Quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR)
Liver and macrophage RNA were isolated with the RNeasy Plus kit (QIAGEN, Valencia, CA). Reverse transcription with 1 μg of RNA was carried out in an Eppendorf Mastercycler (Hamburg, Germany) using a high capacity cDNA reverse transcription kit (ABI, Foster City, CA). qRT-PCR was performed in a 7500 Fast Real-Time PCR System (ABI). The primers in Supplemental Table S1 were purchased from Integrated DNA Technologies (Coralville, IA). Data analysis was performed using the 2−ΔΔCT method for relative quantification and normalized to levels of glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Cytokine assay
Serum and medium cytokines were measured with a multiplex kit (MCYTOMAG-70K) from EMD Millipore (Billerica, MA) and Luminex® xMAP® Technology with the exception of IL-18 which was measured by a standard ELISA (Roche, Basal, Switzerland).
Hepatic macrophage isolation and culture
Mouse liver nonparenchymal cells were isolated by Liberase (Roche) perfusion and centrifuged at 50 g to isolate and remove the hepatocytes. Hepatic macrophages were isolated from the nonparenchymal cell population by differential centrifugation through a 29% Nycodenz (Accurate Chemical & Scientific Corp., Westbury, NY) gradient at 1,380 g. Cells were washed with DMEM (Hyclone, Logan, UT) and repelleted at 1380 g. The resuspended cells were plated in DMEM, 10% fetal bovine serum (Atlanta Biological, Atlanta, GA), 2% hepes (Sigma), 1% penicillin/streptomycin (Mediatech, Manassas, VA), 1% non-essential amino acids (Sigma) and 1% sodium pyruvate (Lonza, Walkersville, MD). Non-adherent cells were removed after 1 h by changing the medium to RPMI-1640 (Hyclone) with the same supplements. Some cells were treated with 10 ng/ml of LPS and 5 mM ATP (Sigma) at the time of the medium change, and medium and cells harvested 1 h later. Other cells were left untreated for 18 h prior to harvesting medium and cells. Purity of the macrophage population was insured by mRNA enrichment for macrophage genes and absence of expression for hepatocyte and hepatic stellate cell genes by RT-PCR.
In additional studies hepatic macrophages were separated into Kupffer cells and recruited macrophages by FACS. The nonparenchymal cell fraction was blocked by CD16/32 antibody (eBioscience, #14-0161-82), and then incubated with the following primary antibodies from eBioscience: CD45-FITC (#11-0451-82), CD45-PerCP-Cyanine5.5 (#45-0451-80), Ly6G-PerCP-Cyanine5.5 (#45-5931-80), CD3-PerCP-Cyanine5.5 (#45-0031-80), NK1.1-PerCP-Cyanine5.5 (#45-5941-80), B220-PerCP-Cyanine5.5 (#45-0452-80), F4/80-APC (#17-4801-82), and CD11b-PE (#12-0112-82). Kupffer cells were defined as CD45+ Ly6G− NK1.1− B220− F4/80high CD11blow and infiltrating monocyte-derived macrophages as CD45+ Ly6G− NK1.1− B220− F4/80low CD11bhigh. The FACS sorting was performed using a BD FACS Aria Cell Sorter (BD Biosciences, San Jose, CA). Cells were cultured as above, and the medium assayed for IL-1β 1 h after the medium change.
Neutrophil isolation and culture
Mouse livers were flushed with PBS, minced into small pieces, digested with Liberase at 37°C for 30 min, passed through a cell strainer and cells collected by centrifugation at 400 g for 10 min. Neutrophils were isolated from the other cell types by differential Percoll (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) gradient (60%-30%) centrifugation at 1,300 g. Cells were treated with ammonium-chloride-potassium lysing buffer (Life Technologies, Grand Island, NY) to remove the red blood cells and centrifuged to obtain a neutrophil pellet. Neutrophils were cultured in RPMI-1640, 10% FBS and 1% penicillin/streptomycin for 1 h following which cells were collected for mRNA and the supernatant for protein.
To isolate bone marrow neutrophils, mouse tibias and femurs was flushed with neutrophil buffer (PBS, 0.5% BSA, 0.5 mM EDTA) that was centrifuged at 1,300 g for 10 min to pellet the cells. Cells were separated by differential Percoll gradient (85%-65%-55%) centrifugation at 1,500 g for 30 min, and the neutrophil cell fraction resuspended with ammonium-chloride-potassium lysing buffer and pelleted. Neutrophils were resuspended in 0.7 ml of RPMI-1640, 10% FBS and 1% penicillin/streptomycin and left untreated or treated with 0.1 ml of Kupffer cell medium for 1 h. Medium and cells were collected for RNA and protein. Neutrophil purity was insured by mRNA enrichment for neutrophil genes and absence of expression for macrophage, hepatocyte and hepatic stellate cell genes by RT-PCR.
Statistical analysis
Numerical results are reported as means ± S.E. and derived from at least three independent experiments unless otherwise indicated. The unpaired Student’s t-test was used to assess significance between control and treated groups. Survival rates between control and knockout mice were analyzed by Kaplan-Meier survival and log-rank tests. Statistical significance was defined as P<0.05.
Results
Inhibition of macrophage autophagy magnifies GalN/LPS liver injury
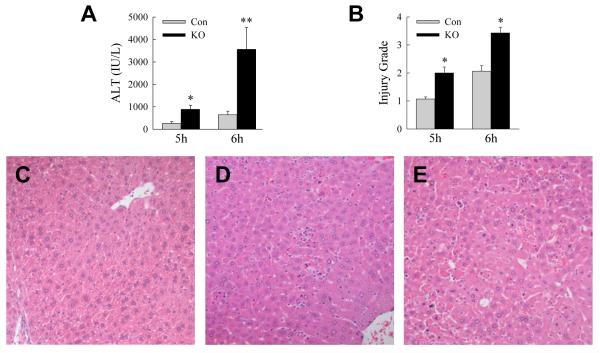
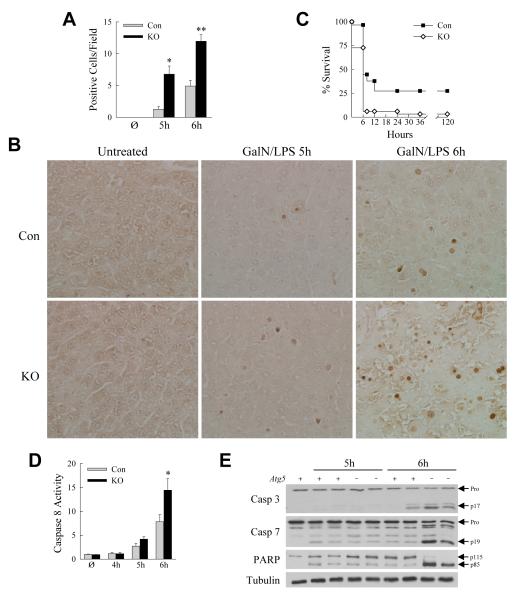
To determine the function of macrophage autophagy in acute toxic liver injury, Atg5Δmye mice with a myeloid cell specific knockout of autophagy were examined for their response to GalN/LPS. Serum ALT levels were significantly increased in Atg5Δmye mice as compared to littermate controls at 5 and 6 h after GalN/LPS administration (Fig. 1A). Histological grading of H&E sections confirmed greater liver injury in Atg5Δmye mice at both 5 and 6 h (Fig. 1B-E). Hepatocyte death resulted in the knockout mice as increased numbers of TUNEL-positive cells were present in the livers of Atg5Δmye mice at 5 and 6 h (Fig. 2A and 2B). Hepatocyte injury and death from GalN/LPS were increased by the loss of Atg5-dependent macrophage autophagy.
1. Inhibition of macrophage autophagy increases GalN/LPS liver injury.
(A) Serum ALT levels in littermate control (Con) and Atg5Δmye knockout (KO) mice at 5 and 6 h after GalN/LPS administration (*P<0.01, **P<0.005 as compared to control mice; n=6-8). (B) Histological grade of liver injury at the same times (*P<0.002 as compared to control mice; n=7-8). (C-E) Hematoxylin and eosin sections of an untreated control mouse liver (C), and control (D) and Atg5Δmye (E) mouse livers 6 h after GalN/LPS (200X).
Fig. 2. Knockout mice have increased apoptosis and mortality.
(A) Numbers of TUNEL-positive cells per high power field in the livers of untreated (Ø) and 5 and 6 h GalN/LPS-treated littermate control (Con) and knockout (KO) mice (*P<0.003, **P<0.0003, as compared to control mice with the same treatment; n=3-7). (B) Representative TUNEL images (400X). (C) Percentage survival in control and knockout mice (P<0.0003; n=29-33). (D) Relative caspase 8 activity in littermate control (Con) and Atg5Δmye knockout (KO) mice untreated (Ø) and GalN/LPS treated for the indicated hours (*P<0.04; n=3-7). (E) Immunoblots of total hepatic protein from an untreated control (Atg5 +) mouse and 5 and 6 h GalN/TNF-treated control and knockout (Atg5 −) mice probed for caspase 3 (Casp 3), caspase 7 (Casp 7), PARP and the loading control tubulin. Arrows indicate the procaspases (Pro), cleaved caspase 3 (p17) and 7 (p19), and intact (p115) and cleaved (p85) forms of PARP.
GalN/LPS-treated Atg5Δmye mice have increased mortality
GalN/LPS liver injury progresses to lethality from fulminant hepatic failure. To determine whether increased liver injury translated into an effect on mortality, survival after GalN/LPS treatment was examined. Within 8 h of GalN/LPS administration 94% of the Atg5Δmye mice died, whereas almost half of the control mice were still alive (Fig. 2C). Long-term survival occurred in 27% of control but only 3% of knockout mice (Fig. 2C). Loss of Atg5-dependent macrophage autophagy therefore magnified both liver injury and mortality from GalN/LPS.
Apoptotic cell death pathway activation is accelerated in Atg5Δmye mice
To determine whether decreased macrophage autophagy affected hepatocyte apoptosis, caspase activation was examined. Initiator caspase 8 activity was elevated in knockout mice relative to controls, although not significantly until 6 h (Fig. 2D). Although downstream effector caspases were activated in all mice 6 h after GalN/LPS treatment as detected by protein cleavage on immunoblots, levels of cleaved caspase 3 and 7 and the caspase substrate PARP were significantly higher in knockout mice (Fig. 2E). Therefore increased activation of caspase-dependent death pathways occurred in knockout mice consistent with greater apoptotic death signaling.
Decreased autophagy alters macrophage cytokine production
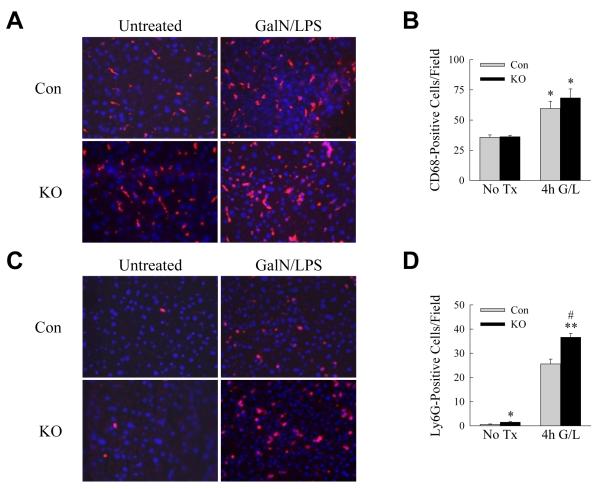
GalN/LPS liver injury results from GalN-mediated hepatocyte sensitization to TNF cytotoxicity, although other cytokines have been implicated in this injury model [6, 9]. Our recent studies demonstrated that decreased macrophage autophagy in steatotic livers does not alter macrophage number but promotes a proinflammatory M1 phenotype with increased TNF and IL-6 production [16]. These findings suggested that increased cytokine generation may similarly underlie the sensitivity of Atg5Δmye mice to GalN/LPS. Initially we examined the extent of inflammation by immunofluorescence staining for macrophages and neutrophils at the 4 h onset of liver injury. CD68 macrophage staining revealed equivalent 70% increases in hepatic macrophage number in control and knockout mice (Fig. 3A and 3B). The numbers of Ly6G-positive neutrophils were also markedly increased in control and knockout mouse livers, and modestly but significantly greater in knockout mice (Fig. 3C and 3D).
Fig. 3. Atg5Δmye mouse livers have equivalent numbers of macrophages but increased neutrophils.
(A) Immunofluorescence staining of livers from control (Con) and knockout (KO) mice untreated or 4 h post-GalN/LPS administration (200X). (B) Quantification of the numbers of CD68 positive cells in untreated (No Tx) and 4 h post-GalN/LPS (4h G/L) livers (*P<0.02 as compared to untreated control mice; n=3-4). (C) Immunofluorescence for Ly6G in the same livers. (D) Numbers of Ly6G positive staining cells (*P<0.03, **P<0.0001 as compared to untreated control mice; #P<0.005 as compared to GalN/LPS-treated controls; n=3-4).
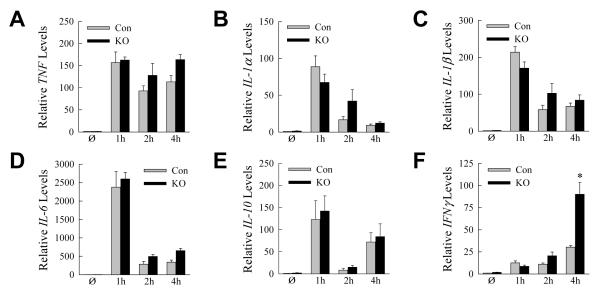
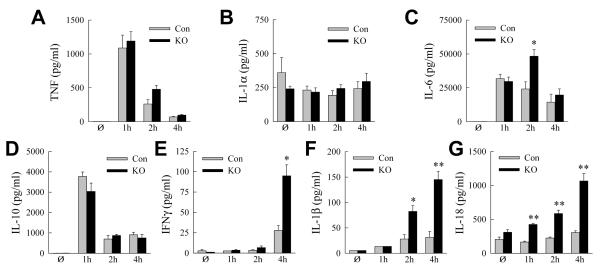
To assess the nature of the inflammatory response, hepatic cytokine gene induction was assayed by qRT-PCR over the 4 h prior to injury. Macrophage proinflammatory (TNF, IL-1α, IL-1β and IL-6) and anti-inflammatory (IL-10) cytokines were induced equally in control and knockout mice (Fig. 4A-E). The absence of an increase in TNF and IL-6 in the knockout mouse livers indicated that the mechanism of liver injury was not increased proinflammatory M1 macrophage polarization as occurred with LPS-induced inflammation in steatotic livers with inhibition of macrophage autophagy [16]. An increase in IFNγ occurred in knockout mice at 4 h, indicating a later activation of lymphocytes (Fig. 4F).
Fig. 4. Cytokine gene induction in GalN/LPS-treated control and knockout mice.
(A-F) Relative hepatic mRNA levels for TNF (A), IL-1α (B), IL-1β (C), IL-6 (D), IL-10 (E) and IFNγ (F) determined by qRT-PCR in control (Con) and Atg5Δmye knockout (KO) mice that were untreated (Ø) or treated for the indicated hours with GalN/LPS (*P<0.03, as compared to control mice at the same time point; n=4).
In concert with the mRNA findings, serum protein levels of TNF, IL-1α, IL-6 and IL-10 increased markedly but equivalently in GalN/LPS-treated control and knockout mice with the exception of a 2-fold greater increase in IL-6 in knockouts at 2 h (Fig. 5A-D). Serum IFNγ was significantly increased in knockout mice at 4 and 6 h, also consistent with the mRNA findings (Fig. 5E). Despite the lack of change in IL-1β mRNA in knockout mice, IL-1β serum protein was increased significantly at 2 h, a time point well before the onset of hepatocyte injury, and at 4 h (Fig. 5F). In contrast to the other cytokines, IL-1β production is inflammasome dependent, indicating that increased inflammasome activation occurred in knockout mice. Consistent with this conclusion was the finding that a second inflammasome-dependent cytokine, IL-18, was increased in the serum of knockout but not control mice after GalN/LPS treatment (Fig. 5G).
Fig. 5. Knockout mice have increased serum IL-1β.
(A-G) Serum cytokine levels in control (Con) and knockout (KO) mice untreated and GalN/LPS-treated for the indicated number of hours. Serum levels of TNF (A), IL-1α (B), IL-6 (C), IL-10 (D), IFNγ (E), IL-1β (F) and IL-18 (G) (*P<0.01, **P<0.004, as compared to control mice at the same time point; n=3-4).
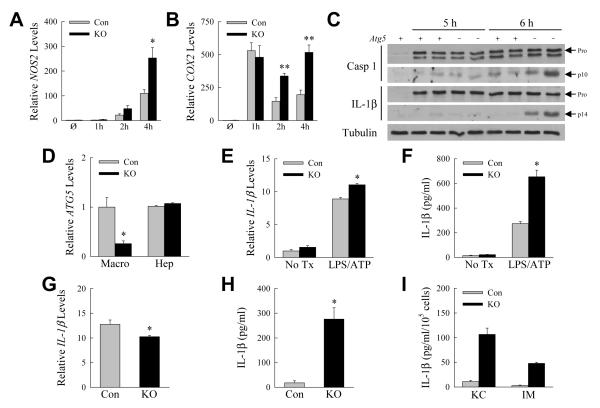
IL-1β induces the proinflammatory factors nitric oxide synthetase 2 (NOS2) and cyclooxygenase 2 (COX2) [25, 26]. Levels of NOS2 and COX2 mRNA were significantly increased in knockout mouse livers in concert with the elevation in IL-1β (Fig. 6A and 6B), indicating that increased inflammasome activation and IL-1β had significant biological consequences in the knockout mice. Thus, the only cytokines with a temporal increase consistent with mediating increased GalN/LPS liver injury in knockout mice were the inflammasome-dependent cytokines IL-1β and IL-18.
Fig. 6. IL-1β is increased from inflammasome overactivation.
(A-B) Hepatic mRNA levels for NOS2 (A) and COX2 (B) in control (Con) and Atg5Δmye knockout (KO) mice untreated (Ø) or GalN/LPS-treated for the indicated hours (*P<0.03, **P<0.003, as compared to control mice at the same time point; n=3-4). (C) Immunoblots of total hepatic protein from an untreated control (Atg5 +) mouse and 5 and 6 h GalN/TNF-treated control and knockout (Atg5 −) mice for caspase 1 (Casp 1), IL-1β and tubulin. Arrows indicate the procaspase and pro-IL-1β forms (Pro) and the cleaved caspase 1 (p10) and IL-1β (p14) forms. The images of the cleaved proteins are longer exposures of the same immunoblots as for the pro-forms. (D) Relative ATG5 mRNA levels in hepatic macrophages (Macro) and hepatocytes (Hep) from control and knockout mouse livers (*P<0.006; n=3-6). (E) Relative IL-1β mRNA levels in freshly isolated control and knockout hepatic macrophages untreated (No Tx) or co-stimulated with LPS and ATP for 1 h (*P<0.003, as compared to treated control macrophages; n=2-4). (F) Amounts of secreted IL-β from the same cells (*P<0.0005, as compared to treated control macrophages; n=2-4). (G) mRNA levels for IL-1β in control and knockout hepatic macrophages left untreated for 18 h (*P<0.02; n=3-4). (H) IL-1β secreted by the same cells (*P<0.0002; n=7-8). (I) IL-1β secretion by cultured Kupffer cells (KC) and infiltrating macrophages (IM) (n=2-5).
Increased inflammasome activation occurs with GalN/LPS treatment in the absence of Atg5-dependent autophagy
Findings of elevated serum IL-1β protein but not mRNA suggested that hepatic inflammasome-generated IL-1β cleavage and secretion was increased in response to GalN/LPS in knockout mice. Both genotypes of GalN/LPS-treated mice had detectable cleaved caspase 1 on liver immunoblots, but levels were higher in knockout mice (Fig. 6C). Levels of pro-IL-1β were markedly and equivalently increased in control and knockout mice with GalN/LPS treatment, but significant levels of cleaved IL-1β were detected only in the livers of knockout mice (Fig. 6C). Elevated inflammasome activation occurred with decreased macrophage autophagy, leading to increased generation of the cleaved, active form of IL-1β.
Hepatic macrophages from Atg5Δmye mice secrete increased amounts of IL-1β
Hepatic macrophages were isolated from control and knockout mice to examine directly the effects of decreased autophagy on inflammasome activation in these cells. Initially we confirmed that Atg5Δmye mice had a macrophage-specific ATG5 knockout. Hepatic macrophages isolated from Atg5Δmye mice had a 75% decrease in ATG5 mRNA as compared to control macrophages, but hepatocyte ATG5 levels were unaltered (Fig. 6D). We then examined inflammasome activation in hepatic macrophages isolated from GalN/LPS-injected mice. First, we determined the responsiveness of freshly isolated hepatic macrophages from GalN/LPS-treated mice to inflammasome activation by LPS and ATP. Similar to findings in total liver of equivalent IL-1β gene induction in control and knockout mice, 1 h after LPS/ATP treatment IL-β mRNA was strongly induced in hepatic macrophages from both types of mice, although to a slightly greater extent in knockout cells (Fig. 6E). IL-1β protein secretion, however, was 2.5-fold greater from the knockout cells (Fig. 6F). Second, IL-1β mRNA and protein levels were determined after cells were left untreated in culture for 18 h. IL-1β mRNA was highly and similarly induced in control and knockout macrophages with levels slightly lower in knockout cells (Fig. 6G). However, levels of secreted IL-1β protein were significantly increased 15-fold in the culture medium of the knockout cells (Fig. 6H). Thus, despite similar IL-1β gene induction, increased inflammasome-generated IL-1β was produced by autophagy-deficient hepatic macrophages from GalN/LPS-treated livers. These data are consistent with the in vivo findings that decreased autophagic function in macrophages leads to increased inflammasome activation and IL-1β processing and secretion in GalN/LPS-induced liver injury.
To further define the source of IL-1β, hepatic macrophages were FACS separated into Kupffer cells and recruited macrophages. Kupffer cells were defined as CD45+ Ly6G− CD3− NK1.1− B220− F4/80high CD11blow and infiltrating, monocyte-derived macrophages as CD45+ Ly6G− CD3− NK1.1− B220− F4/80low CD11bhigh (Supplemental Fig. S1). Both types of macrophages from knockout mice produced increased amounts of IL-1β (Fig. 6I), indicating that both populations undergo increased inflammasome activation in response to GalN/LPS.
Inhibition of IL-1β blocks liver injury in knockout mice
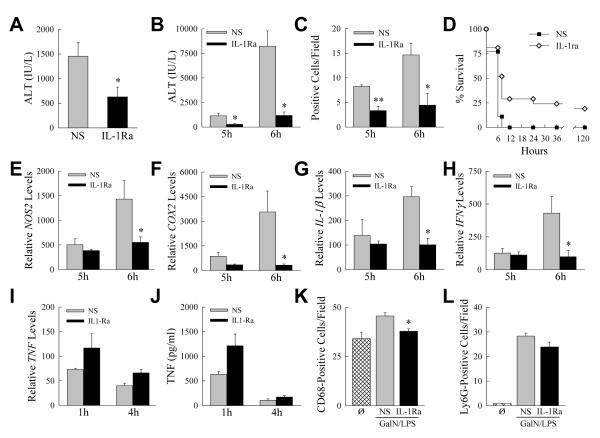
The finding that the primary cytokine abnormality in knockout mice was increased IL-1β together with the fact that IL-1β cytotoxicity has been implicated in liver injury [7-9, 27], suggested that increased IL-1β may be the mechanism of injury from impaired macrophage autophagy. We therefore examined whether IL-1β inhibition reduced liver injury in knockout mice by means of an IL-1Ra that competes with IL-1α and IL-1β for receptor binding to block IL-1 signaling [28]. Initially we confirmed that IL-1β was mechanistically involved in GalN/LPS-induced liver injury in mice with intact macrophage autophagic function. Wild-type C57BL/6 mice were pretreated with PBS vehicle or IL-1Ra 0.5 h before GalN/LPS administration. Inhibition of IL-1β activity significantly decreased liver injury from GalN/LPS as determined by a 57% reduction in 6 h serum ALT levels (Fig. 7A), confirming the contribution of IL-β to GalN/LPS liver injury. Blockade of IL-1 activity in knockout mice led to an even greater reduction in liver injury as measured by serum ALT at both 5 and 6 h (Fig. 7B). Numbers of TUNEL-positive cells were significantly decreased in IL-1Ra-treated Atg5Δmye mice (Fig. 7C), and their survival time significantly increased (Fig. 7D). These findings demonstrate that the induction in IL-1β that occurs with the loss of Atg5-dependent macrophage autophagy amplifies liver injury in these animals by the mechanism of increased IL-1β cytotoxicity.
Fig. 7. Liver injury in knockout mice is blocked by an IL-1Ra.
(A) Serum ALT levels in C57BL6 mice pretreated with normal saline (NS) or IL-1Ra 6 h after GalN/LPS administration (*P<0.04 as compared to normal saline; n=6). (B) Serum ALT levels in Atg5Δmye mice pretreated with normal saline (NS) or IL-1Ra at the indicated times after GalN/LPS (*P<0.004 as compared to normal saline; n=6-7). (C) Numbers of TUNEL positive cells per high power field (*P<0.03, **P<0.002, as compared to normal saline; n=3-5). (D) Percentage survival (P<0.001; n=18-21). (E-H) Hepatic mRNA levels at 5 and 6 h for (E) NOS2 (*P<0.02), (F) COX2 (*P<0.02), (G) IL-1β (*P<0.002), and (H) IFNγ (*P<0.04) (n=5-7). (I) Hepatic TNF mRNA levels at 1 and 4 h (n=3). (J) Serum TNF levels in the same mice (n=3). (K) Number of CD68 positive cells in untreated (Ø) and 4 h post-GalN/LPS-treated livers (*P<0.005 as compared to normal saline; n=3-6). (L) Numbers of Ly6G positive staining cells in the same livers (n=3-6).
The reduction in liver injury by IL-1Ra occurred in concert with decreases in the IL-1β-dependent inflammatory mediators NOS2 and COX2 (Fig. 7E and 7F). mRNA levels of IL-1β were also significantly decreased indicating that IL-1β amplified its own production (Fig. 7G). IL-1Ra treatment completely blocked the increase in INFγ demonstrating that IFNγ induction was secondary to IL-1β (Fig. 7H). However, blocking IL-1 activity had no effect on the induction of TNF mRNA (Fig. 7I), or serum protein (Fig. 7J), indicating that inhibition of liver injury by IL-1Ra was not secondary to a TNF effect. IL-1Ra treatment significantly reduced the increase in macrophage number by 68% in GalN/LPS-treated knockout mice (Fig. 7K). In contrast, IL-1Ra treatment had no significant effect on neutrophil infiltration (Fig. 7L). IL-1β therefore increased the inflammatory response to GalN/LPS injury.
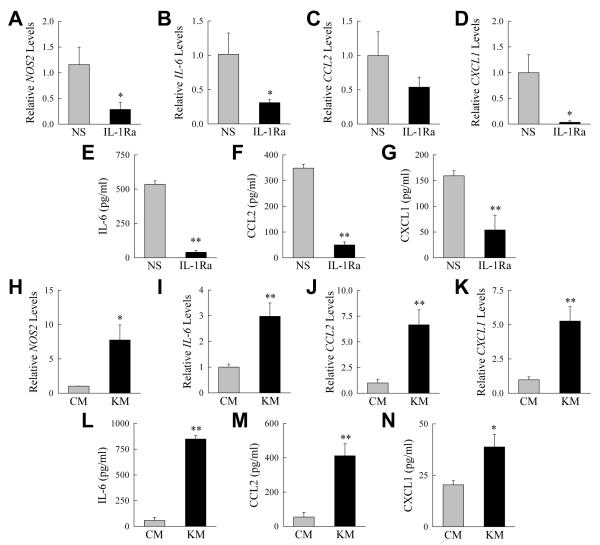
Despite the absence of an effect of IL-1 blockade on neutrophil number, the possibility remained that the elevated production of IL-1β by macrophages with impaired autophagy increased hepatic inflammation by amplifying neutrophil activation. The effect of in vivo IL-1 inhibition on the inflammatory state of neutrophils was therefore examined in knockout mice. Mice were pretreated with normal saline or IL-1Ra, injected with GalN/LPS, and hepatic neutrophils isolated 4 h later for analysis of proinflammatory cytokine gene induction and protein secretion. Neutrophils from IL-1Ra-treated knockout mice had decreased mRNA induction for the proinflammatory gene NOS2 (Fig. 8A) and the cytokines IL-6, CCL2 and CXCL1 (Fig. 8B-D). Similarly neutrophils from IL-1-Ra-treated knockout mice secreted significantly reduced amounts of all three cytokines (Fig. 8E-G). Increased Il-1β production resulting from impaired macrophage autophagy therefore increased the proinflammatory state of hepatic neutrophils despite not affecting the amount of cellular infiltration.
Fig. 8. Decreased macrophage autophagy promotes neutrophil activation.
(A-D) Relative mRNA levels for the indicated genes in hepatic neutrophils isolated from GalN/LPS-treated knockout mice that had been pretreated with normal saline (NS) or IL-1Ra. (E-G) Levels of secreted cytokines from the same neutrophils. (H-K) mRNA levels for the indicated genes in bone marrow neutrophils cultured for 1 h in conditioned medium from control (CM) and knockout macrophages (KM). (L-N) Amounts of cytokine secretion from the neutrophils treated with conditioned medium (*P<0.05, **P<0.01; n=3-6).
To directly establish that autophagy-deficient macrophages stimulate neutrophil inflammation through a paracrine mechanism, bone marrow-derived neutrophils isolated from untreated, wild-type mice were cultured in conditioned medium from control and knockout hepatic macrophages. Medium from knockout macrophages led to a greater increase in both neutrophil inflammatory gene expression (Fig. 8H-K) and cytokine secretion (Fig. 8L-N). These findings demonstrate that loss of Atg5-dependent macrophage autophagy results in increased neutrophil as well as macrophage inflammation.
Discussion
Autophagy protects cells against death from injurious stress. In the liver, autophagy in hepatocytes limits liver injury [29], including that from the hepatotoxin GalN [12]. Our recent finding that macrophage autophagy down regulates LPS-induced inflammation in hepatic steatosis [16], suggested that macrophage autophagy may similarly block injurious inflammation in LPS dependent toxin-induced liver injury [30, 31]. The present findings demonstrate that macrophage autophagy limits GalN/LPS injury by modulating cytokine production, but by the mechanism of inhibiting inflammasome-mediated IL-1β cleavage which is distinct from the anti-inflammatory effect of macrophage autophagy in steatotic liver injury. Together these findings indicate a central but context specific function of macrophage autophagy in regulating injury-associated hepatic inflammation.
Several features of the hepatic inflammatory response were unaffected by the loss of Atg5-dependent macrophage autophagy. First, the increase in hepatic macrophage number in response to GalN/LPS was not influenced by a decrease in macrophage autophagy. Second, there was no difference in the induction of proinflammatory macrophage cytokines such as TNF and IL-6. These findings indicate that a decrease in macrophage autophagy failed to affect macrophage recruitment into the damaged liver or the activation of hepatic macrophages into M1 polarized cells.
The significant effect on hepatic inflammation from decreased macrophage autophagy was a selective increase in the inflammasome-dependent macrophage cytokines IL-1β and IL-18. With decreased autophagy inflammasome activation was increased as demonstrated by higher levels of cleaved caspase 1 and IL-1β in the livers of GalN/LPS-treated knockout mice. This effect occurred in hepatic macrophages as cells isolated from GalN/LPS-injected knockout mice produced more IL-1β in cell culture. GalN/LPS liver injury is dependent on LPS-induced cytokines, however it is TNF which has been primarily implicated as the cytotoxic cytokine [19]. GalN/LPS injury has been reported to be both unaffected and blocked in caspase 1 knockout mice that are unable to process IL-1β [9, 32]. Our study demonstrates that with decreased autophagy macrophage inflammasome activation is increased, leading to caspase 1-dependent cleavage of pro-IL-1β to its active form and liver injury. Autophagy is therefore a critical regulator of hepatic inflammasome activation, an inflammatory pathway that has been linked to several forms of liver injury [33]. These findings, along with recent investigations in acetaminophen- and alcohol-induced liver injury [7, 8], provide increasing evidence for a previously unappreciated cytotoxic role for IL-1β in toxin-induced liver injury. Variations in macrophage autophagic function may explain in part differences in IL-1β involvement among individuals or different types of liver injury with a concomitant impairment in macrophage autophagy promoting IL-1β hepatotoxicity.
Although findings in LysM-CRE knockout mouse models are attributed to macrophage effects, the promoter is also expressed in neutrophils. After GalN/LPS treatment there was an increase in hepatic neutrophil number, suggesting the possibility that the knockout phenotype was secondary to neutrophil and not macrophage effects. However, the mechanistic involvement of IL-1β proves that the knockout phenotype is the result of macrophage effects as IL-1β is a macrophage product in the liver [34, 35]. IL-1Ra effectively reduced liver injury without altering neutrophil infiltration, however the proinflammatory state of hepatic neutrophils was decreased by inhibition of macrophage-produced IL-1β. That autophagy-impaired hepatic macrophages directly stimulate increased proinflammatory neutrophil activation was demonstrated by studies with conditioned medium from hepatic macrophages isolated from GalN/LPS-treated control and knockout mice. The lymphocyte cytokine IFNγ was also increased in knockouts and has been implicated in GalN/LPS liver injury based on studies in IFNγ receptor knockout mice [6]. However, receptor knockouts had early effects on TNF and CD14 long before IFNγ induction, suggesting that the findings were not mediated by IFNγ. In our study, IFNγ increased after the onset of injury indicating that IFNγ did not mediate liver injury in knockouts. In contrast, IL-1β increased within 2 h, before the onset of liver injury. In addition, the IL-1Ra completely blocked the IFNγ increase in knockout mice demonstrating that induction was secondary to IL-1β signaling. TNF also did not mediate the increase in liver injury as the IL-1Ra prevented liver injury in the absence of any decrease in TNF. The mechanism of injury was therefore increased toxicity from macrophage-generated IL-1β that promoted secondary proinflammatory neutrophil activation.
The mechanism of the increased inflammation/injury in this toxin-induced liver injury model contrasts to that described in a mouse nonalcoholic steatosis model [16]. In both models the defect in macrophage autophagy amplified hepatic inflammation and liver injury. Despite LPS being the inflammatory stimulus in both models, the mechanisms by which decreased macrophage autophagy increased hepatic inflammation and liver injury were distinctly different. In steatotic liver injury, inflammasome-independent proinflammatory cytokines such as TNF were increased due to a distortion of macrophage polarization to a more proinflammatory M1 phenotype, whereas IL-1β levels were unaffected [16]. In contrast, the mechanism of increased inflammation in toxin-induced liver injury was due to an effect on inflammasome-dependent IL-1β. Depending on the form of liver injury, macrophage autophagy can shape the nature of the hepatic immune response through multiple mechanisms including effects on macrophage polarization and inflammasome degradation. These findings point to the need to further define the role of macrophage autophagy in hepatic inflammation and injury as this pathway may be an important therapeutic target in human liver disease. Agents that increase cellular levels of autophagy may be of double therapeutic benefit by both increasing hepatocyte resistance to cell death and limiting harmful macrophage activation.
Supplementary Material
Acknowledgements
We thank Xianzhang Meng for help with the statistical analysis.
Financial support
Supported by NIH Grants R01DK044234 to MJC and F32DK096791 to KL and an American Liver Foundation Postdoctoral Research Fellowship Award to KL.
Abbreviations
- LPS
lipopolysaccharide
- TNF
tumor necrosis factor
- IL
interleukin
- IFNγ
interferon-γ
- GalN
D-galactosamine
- PBS
phosphate-buffered saline
- IL-1Ra
IL-1 receptor antagonist
- ALT
alanine aminotransferase
- TUNEL
terminal deoxynucleotide transferase-mediated deoxyuridine triphosphate nick end-labeling
- qRT-PCR
quantitative real-time reverse transcription polymerase chain reaction
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- PARP
poly (ADP-ribose) polymerase
- NOS2
nitric oxide synthetase 2
- COX2
cyclooxygenase 2
Footnotes
Conflict of interest
The authors who have taken part in this study declared that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
Authors’ contributions
G. Ilyas: Acquisition, analysis and interpretation of the data, drafting of the article. E. Zhao and K. Liu: Acquisition and analysis of the data. Y. Lin, L. Tesfa and K. Tanaka: Acquisition of the data. M. Czaja: Conception and design, acquisition, analysis, and interpretation of the data, obtained funding and drafting of the article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Laskin DL, Sunil VR, Gardner CR, Laskin JD. Macrophages and tissue injury: agents of defense or destruction? Annu Rev Pharmacol Toxicol. 2011;51:267–288. doi: 10.1146/annurev.pharmtox.010909.105812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Maher JJ, Leon P, Ryan JC. Beyond insulin resistance: Innate immunity in nonalcoholic steatohepatitis. Hepatology. 2008;48:670–678. doi: 10.1002/hep.22399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Adams DH, Ju C, Ramaiah SK, Uetrecht J, Jaeschke H. Mechanisms of immune-mediated liver injury. Toxicol Sci. 2010;115:307–321. doi: 10.1093/toxsci/kfq009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Szabo G, Petrasek J, Bala S. Innate immunity and alcoholic liver disease. Dig Dis. 2012;30(Suppl 1):55–60. doi: 10.1159/000341126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Schwabe RF, Brenner DA. Mechanisms of Liver Injury. I. TNF-α-induced liver injury: role of IKK, JNK, and ROS pathways. Am J Physiol Gastrointest Liver Physiol. 2006;290:G583–G589. doi: 10.1152/ajpgi.00422.2005. [DOI] [PubMed] [Google Scholar]
- [6].Car BD, Eng VM, Schnyder B, Ozmen L, Huang S, Gallay P, et al. Interferon γ receptor deficient mice are resistant to endotoxic shock. J Exp Med. 1994;179:1437–1444. doi: 10.1084/jem.179.5.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Imaeda AB, Watanabe A, Sohail MA, Mahmood S, Mohamadnejad M, Sutterwala FS, et al. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J Clin Invest. 2009;119:305–314. doi: 10.1172/JCI35958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Petrasek J, Bala S, Csak T, Lippai D, Kodys K, Menashy V, et al. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J Clin Invest. 2012;122:3476–3489. doi: 10.1172/JCI60777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hoque R, Farooq A, Ghani A, Gorelick F, Mehal WZ. Lactate reduces liver and pancreatic injury in Toll-like receptor- and inflammasome-mediated inflammation via GPR81-mediated suppression of innate immunity. Gastroenterology. 2014;146:1763–1774. doi: 10.1053/j.gastro.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ding WX, Li M, Chen X, Ni HM, Lin CW, Gao W, et al. Autophagy reduces acute ethanol-induced hepatotoxicity and steatosis in mice. Gastroenterology. 2010;139:1740–1752. doi: 10.1053/j.gastro.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ni HM, Bockus A, Boggess N, Jaeschke H, Ding WX. Activation of autophagy protects against acetaminophen-induced hepatotoxicity. Hepatology. 2012;55:222–232. doi: 10.1002/hep.24690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Amir M, Zhao E, Fontana L, Rosenberg H, Tanaka K, Gao G, et al. Inhibition of hepatocyte autophagy increases tumor necrosis factor-dependent liver injury by promoting caspase-8 activation. Cell Death Differ. 2013;20:878–887. doi: 10.1038/cdd.2013.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Czaja MJ. Functions of autophagy in hepatic and pancreatic physiology and disease. Gastroenterology. 2011;140:1895–1908. doi: 10.1053/j.gastro.2011.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Deretic V, Saitoh T, Akira S. Autophagy in infection, inflammation and immunity. Nat Rev Immunol. 2013;13:722–737. doi: 10.1038/nri3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011;469:323–335. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Liu K, Zhao E, Ilyas G, Lalazar G, Lin Y, Haseeb M, et al. Impaired macrophage autophagy increases the immune response in obese mice by promoting proinflammatory macrophage polarization. Autophagy. 2015;11:271–284. doi: 10.1080/15548627.2015.1009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Saitoh T, Fujita N, Jang MH, Uematsu S, Yang BG, Satoh T, et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1β production. Nature. 2008;456:264–268. doi: 10.1038/nature07383. [DOI] [PubMed] [Google Scholar]
- [18].Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12:222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Nowak M, Gaines GC, Rosenberg J, Minter R, Bahjat FR, Rectenwald J, et al. LPS-induced liver injury in D-galactosamine-sensitized mice requires secreted TNF-α and the TNF-p55 receptor. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1202–R1209. doi: 10.1152/ajpregu.2000.278.5.R1202. [DOI] [PubMed] [Google Scholar]
- [20].Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- [21].Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8:265–277. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- [22].Wang Y, Singh R, Xiang Y, Greenbaum LE, Czaja MJ. Nuclear factor κB up-regulation of CCAAT/enhancer-binding protein β mediates hepatocyte resistance to tumor necrosis factor α toxicity. Hepatology. 2010;52:2118–2126. doi: 10.1002/hep.23929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wang Y, Singh R, Lefkowitch JH, Rigoli RM, Czaja MJ. Tumor necrosis factor-induced toxic liver injury results from JNK2-dependent activation of caspase-8 and the mitochondrial death pathway. J Biol Chem. 2006;281:15258–15267. doi: 10.1074/jbc.M512953200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wang Y, Schattenberg JM, Rigoli RM, Storz P, Czaja MJ. Hepatocyte resistance to oxidative stress is dependent on protein kinase C-mediated down-regulation of c-Jun/AP-1. J Biol Chem. 2004;279:31089–31097. doi: 10.1074/jbc.M404170200. [DOI] [PubMed] [Google Scholar]
- [25].Geller DA, de Vera ME, Russell DA, Shapiro RA, Nussler AK, Simmons RL, et al. A central role for IL-1β in the in vitro and in vivo regulation of hepatic inducible nitric oxide synthase. IL-1β induces hepatic nitric oxide synthesis. J Immunol. 1995;155:4890–4898. [PubMed] [Google Scholar]
- [26].Ristimaki A, Garfinkel S, Wessendorf J, Maciag T, Hla T. Induction of cyclooxygenase-2 by interleukin-1α. Evidence for post-transcriptional regulation. J Biol Chem. 1994;269:11769–11775. [PubMed] [Google Scholar]
- [27].Miura K, Kodama Y, Inokuchi S, Schnabl B, Aoyama T, Ohnishi H, et al. Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1β in mice. Gastroenterology. 2010;139:323–334. doi: 10.1053/j.gastro.2010.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- [29].Czaja MJ, Ding WX, Donohue TM, Friedman SL, Kim JS, Komatsu M, et al. Functions of autophagy in normal and diseased liver. Autophagy. 2013;9:1131–1158. doi: 10.4161/auto.25063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Czaja MJ, Xu J, Ju Y, Alt E, Schmiedeberg P. Lipopolysaccharide-neutralizing antibody reduces hepatocyte injury from acute hepatotoxin administration. Hepatology. 1994;19:1282–1289. [PubMed] [Google Scholar]
- [31].Adachi Y, Moore LE, Bradford BU, Gao W, Thurman RG. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology. 1995;108:218–224. doi: 10.1016/0016-5085(95)90027-6. [DOI] [PubMed] [Google Scholar]
- [32].van Molle W, Brouckaert P, Libert C. Caspase-1 is not involved in experimental hepatitis in mouse. FEBS Lett. 1999;445:115–118. doi: 10.1016/s0014-5793(99)00109-x. [DOI] [PubMed] [Google Scholar]
- [33].Kubes P, Mehal WZ. Sterile inflammation in the liver. Gastroenterology. 2012;143:1158–1172. doi: 10.1053/j.gastro.2012.09.008. [DOI] [PubMed] [Google Scholar]
- [34].Bilzer M, Roggel F, Gerbes AL. Role of Kupffer cells in host defense and liver disease. Liver Int. 2006;26:1175–1186. doi: 10.1111/j.1478-3231.2006.01342.x. [DOI] [PubMed] [Google Scholar]
- [35].Ramadori G, Armbrust T. Cytokines in the liver. Eur J Gastroenterol Hepatol. 2001;13:777–784. doi: 10.1097/00042737-200107000-00004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.