Abstract
Previous in vitro studies showed that glutamine (Gln) prevents acetaldehyde-induced disruption of tight junctions and adherens junctions in Caco-2 cell monolayers and human colonic mucosa. In the present study, we evaluated the effect of Gln supplementation on ethanol-induced gut barrier dysfunction and liver injury in mice in vivo. Ethanol feeding caused a significant increase in inulin permeability in distal colon. Elevated permeability was associated with a redistribution of tight junction and adherens junction proteins and depletion of detergent-insoluble fractions of these proteins, suggesting that ethanol disrupts apical junctional complexes in colonic epithelium and increases paracellular permeability. Ethanol-induced increase in colonic mucosal permeability and disruption of junctional complexes were most severe in mice fed Gln-free diet. Gln supplementation attenuated ethanol-induced mucosal permeability and disruption of tight junctions and adherens junctions in a dose-dependent manner, indicating the potential role of glutamine in nutritional intervention to alcoholic tissue injury. Gln supplementation dose-dependently elevated reduced-protein thiols in colon without affecting the level of oxidized-protein thiols. Ethanol feeding depleted reduced protein thiols and elevated oxidized protein thiols. Ethanol-induced protein thiol oxidation was most severe in mice fed Gln-free diet and absent in mice fed Gln-supplemented diet, suggesting that antioxidant effect is one of the likely mechanisms involved in Gln-mediated amelioration of ethanol-induced gut barrier dysfunction. Ethanol feeding elevated plasma transaminase and liver triglyceride, which was accompanied by histopathologic lesions in the liver; ethanol-induced liver damage was attenuated by Gln supplementation. These results indicate that Gln supplementation ameliorates alcohol-induced gut and liver injury.
Keywords: Alcohol, tight junction, adherens junction, occludin, oxidative stress, ZO-1, claudin, cadherin, catenin, fatty liver, steatosis
1 Introduction
Epithelial tight junctions form a selective barrier for the diffusion of solutes and macromolecules [1]. In the gastrointestinal tract, epithelial tight junctions prevent the diffusion of toxins, allergens and pathogens from the intestinal lumen into the mucosa and systemic circulation. Disruption of tight junctions and diffusion of bacterial toxins play an important role in the pathogenesis of many gastrointestinal diseases. Recent studies indicated that colonic epithelial barrier dysfunction impacts the systemic organs, beyond the gastrointestinal tract, due to impact of endotoxemia on different organ systems. One of such conditions is alcoholic liver disease (ALD), which is associated with the intestinal mucosal barrier dysfunction and endotoxemia [2, 3]. Endotoxemia was detected not only in alcoholics with the symptoms of liver disease [3–5], but also in the experimental models of ALD [2, 3, 6–8]. Endotoxin-mediated Kupffer cell activation and subsequent inflammatory reactions are involved in the pathogenesis of ALD [9]. Therefore, alcohol-induced gut barrier dysfunction is a crucial event in the initiation and progression of ALD.
Tight junctions are multi protein complexes consisting of transmembrane proteins such as occludin, claudins, tricellulin and junctional adhesion molecules [10]. The intracellular domains of transmembrane proteins bind to intracellular adapter proteins such as ZO1, ZO2 and ZO3 [11]. These protein complexes interact with other tight junction-specific proteins such as cingulin, AF6, 7H6 and catenins [12–14]. These protein-protein interactions are essential for the assembly and maintenance of tight junctions. Adherens junctions, the junctional complexes lie beneath the tight junctions, are also multi protein complexes composed of E-cadherin (the transmembrane protein) and catenins (the adapter proteins) [15]. Adherens junctions are not diffusion barriers for macromolecules, but they indirectly regulate the integrity of tight junctions and therefore the barrier function [16]. Both tight junctions and adherens junctions interact with numerous signaling proteins and are regulated by the activity of intracellular signaling cascades. Tight junction and adherens junction protein complexes interact with the actin cytoskeleton [17, 18], which is essential for the assembly and maintenance of tight junctions and adherens junctions.
Factors that protect tight junctions and adherens junctions against injurious agents are of importance in developing therapeutics for the prevention and treatment of various gastrointestinal diseases. Growth factors and nutritional components are known to help maintain the gastrointestinal mucosal homeostasis and preserve the epithelial barrier function [19]. Glutamine (Gln) is one such nutritional components that play important role in preservation of intestinal mucosal homeostasis [20]. Gln is the most abundant amino acid in blood stream accounting for 30–35% of plasma amino acid nitrogen, and is classified as a non-essential amino acid as it is readily synthesized in muscle, liver, brain and stomach tissue [20]. However, it has been relabeled as a conditionally essential amino acid, as the body depends on dietary Gln under pathophysiologic conditions. Gln is an essential source of fuel for the gastrointestinal tract [21]. Therefore, gastrointestinal tract is the first organ that responds to depletion of plasma Gln. Gln supplementation was found to be beneficial in the treatment of burn injury, malnutrition, radiation injury and critically ill patients [22, 23]. Evidence indicates that Gln plays a critical role in maintenance of the intestinal epithelial barrier function [24–26]. Depletion of Gln by Gln synthase increases paracellular permeability in Caco-2 cell monolayers [27], and Gln treatment protects tight junctions and preserves barrier function from acetaldehyde in Caco-2 cell monolayers and human colonic mucosa in vitro [28, 29].
In the present study, we evaluated the influence of dietary Gln on ethanol-induced intestinal mucosal barrier dysfunction and liver injury. Results of this study provide evidence to the beneficial effect of dietary Gln supplementation in alcoholic gut and liver injury.
2 METHODS AND MATERIALS
2.1 Chemicals
Maltose dextrin was purchased from Bioserv (Flemington, NJ). Regular Lieber DeCarli ethanol diet (Dyet # 710260) and Gln free diet (Dyet # 717781) were purchased from Dyets Inc. (Bethlehem, PA). EnzyChrom Alanine transaminase (EALT-100) and EnzyChrom Aspartate transaminase (EAST-100) assay kits were purchased from BioAssay systems (Hayward, CA). Triglyceride assay kit was purchased from Pointe Scientific Inc., (Canton, MI). Hoechst 33342 dye and BODIPY FL-N-(2-aminoethyl) maleimide were purchased from Life technologies (Grand Island, NY). N-ethylmaleimide and tris(2-carboxyethyl)phosphine were from Sigma Aldrich (St Louis, MO). All other chemicals were purchased from either Sigma Aldrich (St. Louis, MO) or Thermo Fisher Scientific (Tustin, CA).
2.2 Antibodies
Anti-ZO-1, anti-occludin, and anti-claudin-3 antibodies were purchased from Invitrogen (Carlsbad, CA). Anti E-Cadherin and anti β-catenin antibodies were purchased from BD Biosciences (Billerica, MA). Horseradish peroxidase (HRP)-conjugated anti-mouse IgG, HRP-conjugated anti-rabbit IgG and anti-β-actin antibodies were obtained from Sigma Aldrich (St. Louis, MO). AlexaFlour-488-conjugated anti-mouse IgG and Cy3-conjugated anti-rabbit IgG were purchased from Molecular Probes (Eugene, OR).
2.3 Animals and diets
Female C57BL/6 mice (12–14 weeks, Harlan Laboratories, Houston, TX) were used for all experiments. All animal experiments were performed according to the protocols approved by UTHSC Institutional Animal Care and Use Committee. Animals were housed in institutional animal care facility with 12 hours light and dark cycles. All mice had free access to regular laboratory chow and water until the start of experiments.
2.4 Study Protocol
In the first study, adult female (12–14 weeks age) mice were fed Lieber-DeCarli liquid diet (#710260), with or without ethanol (0% for 2 days, 1% for 2 days, 2% for 2 days, 4% for one week, 5% for one week and 6% for one week), and with (GN) or without (GS) L-Gln (8.4 g/L) supplementation for 4 weeks. The diet without ethanol was supplemented with isocaloric maltose dextrin. In the second set of the studies, animals were fed Gln-free diet (Dyet # 717782), a modified Lieber-DeCarli ethanol diet, with no Gln (GF) or with L-Gln, 8.4 g/L (GN) or 16.8 g/L (GS). Corresponding control groups received isocaloric maltose dextrin in place of ethanol. Diet intake was recorded daily and body weights recorded twice a week. The diet intake of mice fed ethanol-containing diet was 10–15% lower than that in mice fed control diets. Therefore, control mice were pair fed to maintain constant diet and caloric intakes in all groups. At the end of experiment, gut permeability was measured as described below. Intestinal segments and liver were collected for further analyses.
2.5 Gut permeability in vivo
Mucosal barrier dysfunction was evaluated by measuring gut permeability to FITC-inulin (6 kDa). On the last day of experiment, mice were intravenously injected with FITC-inulin (50 mg/ml solution; 2 μl/g body weight) via tail vein. One hour after injection, blood samples were collected by cardiac puncture under isoflurane anesthesia for plasma preparation. Luminal contents from intestinal segments were flushed with 0.9% saline. Fluorescence in plasma and luminal flushing was measured using fluorescence plate reader. Fluorescence values in the luminal flushing were normalized to fluorescence values in corresponding plasma samples and calculated as percent of amount injected.
2.6 Immunofluorescence microscopy
Cryo-sections of intestine (10 μm thickness) were fixed in acetone methanol mixture (1:1) at −20°C for 2 min and rehydrated in phosphate buffered saline (PBS). Sections were permeabilized with 0.2% Triton X-100 in PBS for 15 min and blocked in 4% non-fat milk in TBST (20mM Tris, pH 7.2 and 150 mM NaCl). It was then incubated for one hour with primary antibodies (mouse monoclonal anti-occludin and rabbit polyclonal anti-ZO-1 antibodies or mouse monoclonal E-cadherin and rabbit polyclonal anti-β-catenin antibodies), followed by incubation for one hour with secondary antibodies (AlexaFluor-488-conjugated anti-mouse IgG and Cy3-conjugated anti-rabbit IgG antibodies from Molecular Probes, Eugene, OR) containing Hoechst 33342. The fluorescence was examined by using a confocal microscope (Zeiss 710) and images from x–y sections (1 μm) were collected using Zen software. Images were stacked using the Image J software (NIH) and processed by Adobe Photoshop (Adobe Systems Inc., San Jose, CA).
2.7 Preparation of detergent-insoluble fraction
Actin-rich detergent-insoluble fraction was prepared as described previously [30, 31]. Mucosal scrapping from distal colon and ileum were incubated on ice for 15 min with lysis buffer-CS (Tris buffer containing 1% Triton-X100, 2 μg/ml leupeptin, 10 μg/ml aprotinin, 10 μg/ml bestatin, 10 μg/ml pepstatin-A, 10 μl/ml of protease inhibitor cocktail, 1 mM sodium vanadate and 1 mM PMSF). Briefly, mucosal lysates were centrifuged at 15,600 × g for 4 min at 4°C to sediment the high-density actin-rich detergent-insoluble fraction. Supernatant was used a detergent soluble fraction. The pellet was suspended in 100 μl of preheated lysis buffer-D (20 mM Tris buffer, pH 7.2, containing 10 μl/ml of protease inhibitor cocktail, 10 mM sodium fluoride, 1 mM sodium vanadate and 1 mM PMSF) and sonicated to homogenize the actin cytoskeleton, and heated at 100°C. Protein content was measured by BCA method (Pierce Biotechnology, Rockford, IL). Triton-insoluble and soluble fractions were mixed with equal volume of Laemmli’s sample buffer (2× concentrated), heated at 100°C for 5 min and 25–40 μg protein sample was used for immunoblot analysis.
2.6 Immunoblot analysis
Triton soluble and insoluble fractions were separated by SDS-polyacrylamide gel (7%) electrophoresis and transferred to PVDF membranes as described before [30, 32, 33]. Membranes were immunoblotted for different proteins using specific antibodies for different tight junction and adherens junction proteins with β-actin as house keeping protein in combination with HRP-conjugated anti-mouse IgG or anti-rabbit IgG secondary antibodies. The blots were developed using ECL chemiluminescence method (Pierce) and quantitated by densitometry using Image J software. The density for each band was normalized to density of corresponding actin band.
2.7 Protein thiol assay
Protein thiols in colonic sections was assessed as described before [34]. Reduced protein thiols were evaluated by staining cryosections colon with BODIPY FL-N-(2-aminoethyl) maleimide (Flm) and confocal microscopy at excitation and emission wavelengths, 490 nm and 534 nm, respectively. For oxidized protein thiols, the reduced protein thiol was first alkylated with N-ethylmaleimide (NEM) followed by reduction of oxidized protein thiols with tris(2-carboxyethyl)phosphine prior to staining with Flm. Control staining is done after NEM treatment. Fluorescence images collected and fluorescence quantitated by Image J software.
2.8 Liver histopathology
Liver tissue was fixed in 10% buffered formalin and 8μm thick sections were stained with hematoxylin and Eosin. Stained sections were imaged in a Nikon 80Ti microscope using 10× objective lens and a color camera.
2.9 Plasma transaminase assay
Plasma aspartate transaminase (AST) and alanine transaminase (ALT) activities were measured by colorimetric assay using EnzyChrom AST/ALT assay kits (EASL-100/EAST-100, Bioassay systems) according to vendor’s instructions.
2.10 Oil Red-O staining
To detect fat deposition in the liver, frozen liver sections from both control and ethanol treated groups were fixed for 10 min in 4% Paraformaldehyde, stained with oil red O (Sigma-Aldrich, St. Louis, MO, USA) and then rinsed with 60% isopropanol. Nuclei were lightly stained with hematoxylin stain and later on rinsed with distilled water. Later on, images were collected in a Nikon 80Ti microscope using 10X objective lens and a color camera.
2.11 Triglyceride assay
Liver triglyceride was measured by Triglyceride (GPO) method using a kit from Beckman Coulter (Brea, CA). Briefly, liver tissues were digested in 3M potassium hydroxide (in 65% ethanol) at 70° C for 1hr, followed by incubation at room temperature for 24 hrs. Triglycerides in extract were measured by enzymatic hydrolysis to glycerol and free fatty acids followed by colorimetric estimation of free glycerol at 540 nm, and calculated as mgs of triglycerides/g tissue weight.
2.12 Statistical analyses
Values are expressed as mean ± SE of 4–8 animals. Statistical analysis was performed by Student’s t-test. Statistical significance assessed at P values <0.05.
3 RESULTS
3.1 Chronic ethanol feeding and body weights
Our previous studies showed that L-Gln attenuates acetaldehyde-induced increase in paracellular permeability in Caco-2 cell monolayers [35, 36]. Here we evaluated the effect of Gln feeding on chronic ethanol feeding-induced increase in intestinal mucosal permeability and liver injury in mice in vivo. Two different dietary conditions were used. In the first, Gln was added to regular Lieber-DeCarli liquid diet to double the amount of dietary Gln. In the second, Lieber-DeCarli diet was custom modified by replacing the proteins with the amino acid mixture to create a Gln-free diet. Gln was added to Gln-free diet at one or two times the regular dietary dose. Ethanol feeding slightly reduced diet intake, while Gln feeding significantly increased diet intake in ethanol-fed mice. Therefore, animals in different groups were pair fed to maintain a similar diet intake and calorie intake in all groups (Fig. 1A & B). Under the pair fed conditions, Gln feeding significantly increased body weight in the absence of ethanol feeding (Fig 1C), whereas Gln supplementation showed no significant effect on the body weights of ethanol fed mice. On the other hand, Gln supplementation in mice fed Gln-free diet, showed no significant change in body weights in non-ethanol group, whereas Gln supplementation dose-dependently ameliorated ethanol-induced body weight loss (Fig. 1D).
Figure 1. Effect of Gln supplementation on chronic ethanol feeding in mice.
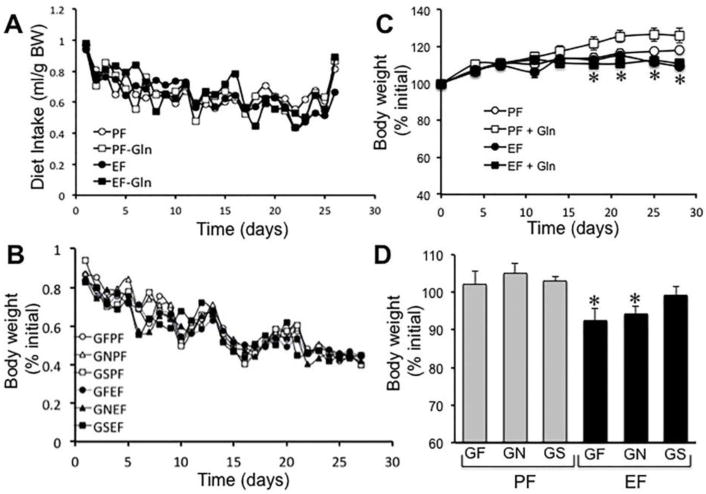
A & B: Adult mice were fed Lieber-DeCarli liquid diet with (● EF & ■ EF-Gln) or without (○ PF & □ PF-Gln) ethanol (1–6% over 4 weeks) and with (○ PF & ● EF) or without (□ PF-Gln & ■ EF-Gln) Gln supplementation. Animals in all groups were pair fed to control a similar diet intake in all groups. Diet intake was measured daily (A). Body weight of animals recorded twice a week (B). Values are mean ± SE (n = 6). C & D: Mice were fed a Gln-free amino acid diet, a modification of Lieber-DeCarli liquid diet with (● GFEF, ▲ GNEF & ■ GSEF) or without (○ GFPF, △ GNPF & □ GSPF) ethanol (1–6% over 4 weeks) and without (○ GFPF & ● GFEF) or with 8.4 g/L diet (△ GNPF & ▲ GNEF) or 16.8 g/L diet (□ GSPF & ■ GSEF) Gln supplementation. Animals in all groups were pair fed to control a similar diet intake in all groups. Diet intake was measured daily (A), and body weights recorded twice a week (B). Values are mean ± SE (n = 6). Asterisks indicate the values that are significantly (p<0.05) different from values for corresponding non-ethanol groups.
3.2 Intestinal mucosal permeability
Measurement of vascular-to-luminal flux of FITC-inulin showed that chronic ethanol feeding significantly increased mucosal permeability in the distal colon (Fig. 2A), but not in the proximal colon (Fig. 2B) or ileum (data not shown). Gln supplementation in diet significantly reduced the ethanol-induced increase in inulin permeability in distal colon. Gln by itself showed no significant influence on the intestinal mucosal permeability. Gln supplementation in mice fed Gln-free diet indicated that ethanol-induced inulin permeability in distal colon was most severe in mice fed Gln-free diet (Fig. 2C), and that ameliorating effect of Gln was dose-dependent. In the proximal colon, ethanol or Gln had no significant effect on mucosal permeability (Fig. 2D). In the absence of ethanol feeding, Gln caused no significant effect on inulin permeability in proximal or distal colon.
Figure 2. Gln supplementation ameliorates chronic ethanol-induced increase in colonic mucosal permeability.
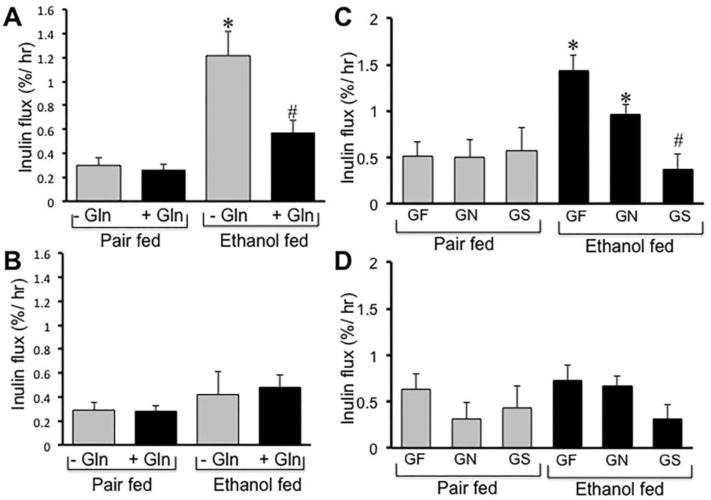
A & B: Adult mice were fed Lieber-DeCarli liquid diet with (Ethanol fed) or without (Pair fed) ethanol (1–6% over 4 weeks) and with (+ Gln) or without (− Gln) Gln supplementation. Intestinal permeability was evaluated by measuring plasma-to-luminal flux of FITC-inulin. Fluorescence recovered in the luminal contents of distal colon (A) and proximal colon (B) was measured. Values are mean ± SE (n = 6). Asterisk indicates the value that is significantly (p<0.05) different from the value for corresponding pair fed group, and the hash tag indicates the value that is different from the value for corresponding “− Gln” group. C & D: Mice were fed a Gln-free amino acid diet with (Ethanol fed) or without (Pair fed) ethanol (1–6% over 4 weeks) and without (GF) or with 8.4 g/L diet (GN) or 16.8 g/L diet (GS) Gln supplementation. Plasma-to-luminal flux of FITC-inulin was measured in distal colon (A) and proximal colon (B). Values are mean ± SE (n = 6). Asterisks indicate the values that are significantly (p<0.05) different from the value for corresponding pair fed group, and the hash tag indicates the value that is different from the value for corresponding GF and GN groups.
3.3 Redistribution of tight junction and adherens junction proteins
Confocal immunofluorescence microscopy of intestinal sections for tight junction proteins showed that chronic ethanol feeding reduces the levels of occludin and ZO1 at the intercellular junctions of epithelium in distal colon (Fig. 3A). Ethanol feeding caused no effect on the distribution of tight junction proteins in proximal colon or ileum (data not shown). Gln supplementation preserved the junctional organization of occludin and ZO1 in the distal colon of ethanol fed mice.
Figure 3. Ethanol-induced redistribution of tight junction proteins in the distal colon is blocked by Gln supplementation.
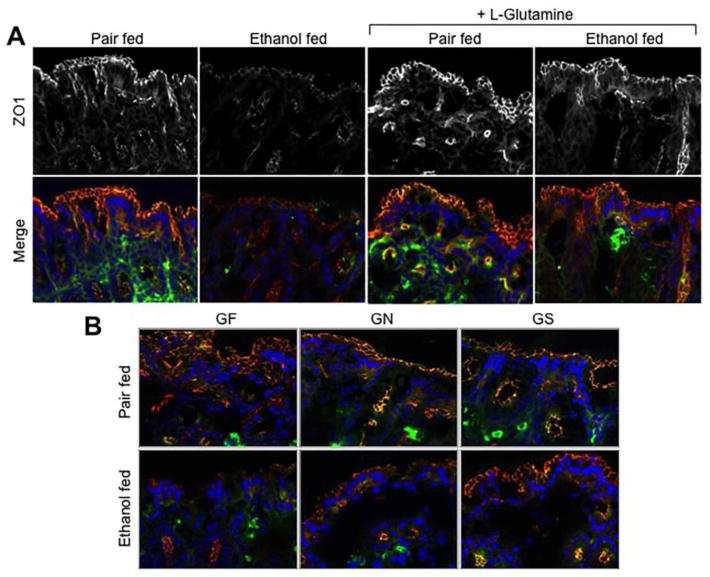
A: Adult mice were fed Lieber-DeCarli liquid diet with (Ethanol fed) or without (Pair fed) ethanol and with or without Gln supplementation. Cryosections of distal colon were stained for occludin (green) and ZO1 (red; gray in the upper panels) by immunofluorescence method, and the nucleus stained with Hoechst dye (blue). B: Mice were fed a Gln-free amino acid diet with (Ethanol fed) or without (Pair fed) ethanol (1–6% over 4 weeks) and without (GF) or with 8.4 g/L (GN) or 16.8 g/L (GS) Gln supplementation. Cryosections of distal colon were stained for occludin (green) and ZO1 (red) by immunofluorescence method, and the nucleus stained with Hoechst dye (blue).
Gln-free diet caused no obvious effect of the junctional distribution of occludin and ZO1 in the absence of ethanol feeding (Fig. 3B). However, ethanol feeding induced a most severe loss of occludin and ZO1 from the epithelial junctions in mice fed Gln-free diet. Addition of Gln to diet, dose-dependently ameliorated the ethanol-induced redistribution of tight junction proteins in distal colon.
Similarly, ethanol feeding reduced junctional distribution of adherens junction proteins, E-cadherin and β-catenin, in the distal colon (Fig. 4A), and Gln supplementation blocked this effect of ethanol on adherens junction. Gln-free diet showed a slight reduction in junctional distribution of E-cadherin and β-catenin in the distal colonic epithelium in the absence of ethanol feeding (Fig. 4B). Ethanol feeding however caused much severe loss of E-cadherin and β-catenin at the colonic epithelial junctions. Gln replacement in Gln-free diet dose-dependently ameliorated this effect of ethanol on adherens junction protein distribution (Fig. 4B).
Figure 4. Ethanol-induced redistribution of adherens junction proteins in the distal colon is blocked by Gln supplementation.
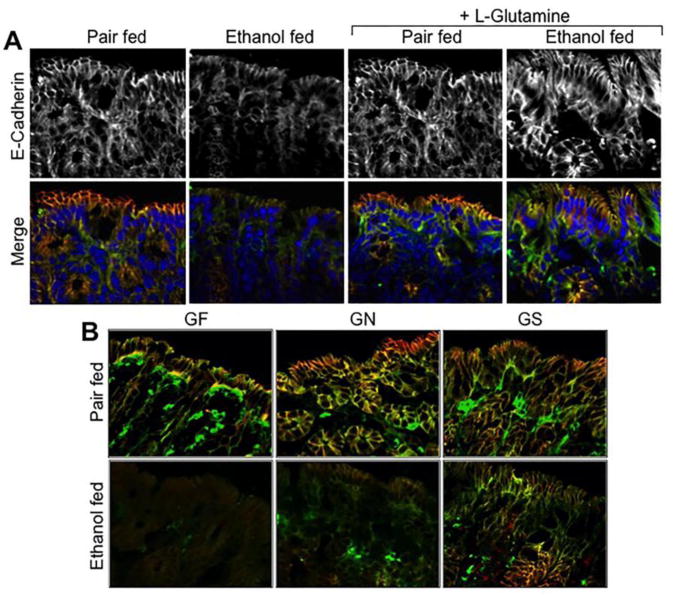
A: Adult mice were fed Lieber-DeCarli liquid diet with (Ethanol fed) or without (Pair fed) ethanol and with or without Gln supplementation. Cryosections of distal colon were stained for E-cadherin (green) and β-catenin (red; gray in the upper panels) by immunofluorescence method, and the nucleus stained with Hoechst dye (blue). B: Mice were fed a Gln-free amino acid diet with (Ethanol fed) or without (Pair fed) ethanol (1–6% over 4 weeks) and without (GF) or with 8.4 g/L (GN) or 16.8 g/L (GS) Gln supplementation. Cryosections of distal colon were stained for E-cadherin (green) and β-catenin (red) by immunofluorescence method.
3.4 Detergent-insoluble fractions of tight junction and adherens junction proteins
Tight junction and adherens junction protein complexes interact with the actin cytoskeleton, and this association is crucial for the assembly and maintenance of tight junctions and adherens junctions. Therefore, tight junction and adherens junction proteins are pulled down along with the actin cytoskeletal fractions (detergent insoluble) of epithelial cell monolayers. Disruption of tight junctions and adherens junctions is associated with the loss of this interaction with the actin cytoskeleton and loss of detergent-insoluble fractions of these proteins [30]. The detergent-insoluble fractions of mucosa from distal colon were immunoblotted for tight junction and adherens junction proteins and quantitated by densitometric analysis. Results show that ethanol feeding reduced the levels of detergent-insoluble fractions of tight junction and adherens junction proteins (Fig. 5A). Detergent-insoluble fractions of occludin (Fig. 5B), but not ZO-1 (Fig. 5C) were significantly reduced by ethanol feeding. Ethanol feeding also reduced the levels of detergent-insoluble E-cadherin (Fig. 5D) and β-catenin (Fig. 5E). Gln replacement in Gln-free diet dose-dependently blocked ethanol-induced reduction of detergent-insoluble fractions of occludin, E-cadherin and β-catenin.
Figure 5. Attenuation of ethanol-induced depletion of detergent-insoluble fractions of tight junction and adherens junction proteins in the distal colon by Gln supplementation.
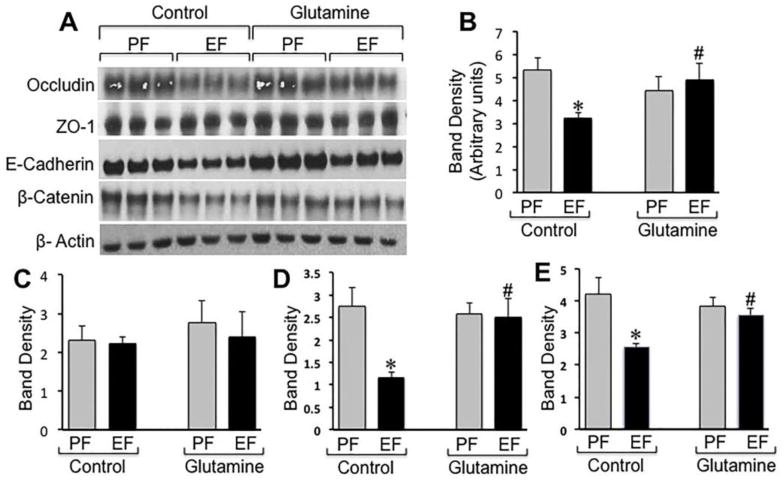
Adult mice were fed Lieber-DeCarli liquid diet with (EF) or without (PF) ethanol and with or without Gln supplementation. Triton-insoluble fractions prepared from distal colonic mucosa were immunoblotted for tight junction and adherens junction proteins (A). Immunoblot bands for occludin (B), ZO1 (C), E-cadherin (D) and β-catenin (E) were quantitated by densitometric analysis and normalized to band density of corresponding actin bands. Values are mean ± SE (n = 3). Asterisks indicate the values that are significantly (p<0.05) different from values for corresponding PF group, and the hash tags indicate the values that are significantly (p<0.05) different from values for corresponding non-Gln control group.
Ethanol-induced depletion of Triton-insoluble fractions of tight junction and adherens junction proteins in distal colonic mucosa was most severe in mice fed Gln-free diet (Fig. 6A). Gln supplementation in Gln-free diet dose-dependently attenuated ethanol-induced depletion of occludin (Fig. 6B), claudin-3 (Fig. 6C), E-cadherin (Fig. 6D) and β-catenin (Fig. 6E). Gln supplementation showed no significant effect on the Triton-insoluble fractions of tight junction and adherens junction proteins in non-ethanol, pair fed mice.
Figure 6. Gln supplementation dose-dependently attenuates ethanol-induced depletion of detergent-insoluble fractions of tight junction and adherens junction proteins in distal colon.
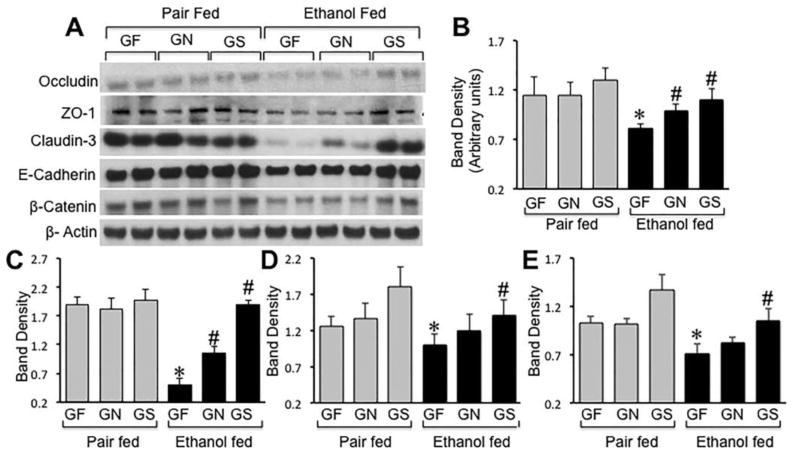
Mice were fed a Gln-free amino acid diet with (Ethanol fed) or without (Pair fed) ethanol (1–6% over 4 weeks) and without (GF) or with 8.4 g/L (GN) or 16.8 g/L (GS) Gln supplementation. Triton-insoluble fractions prepared from distal colonic mucosa were immunoblotted for tight junction and adherens junction proteins (A). Immunoblot bands for occludin (B), claudin-3 (C), E-cadherin (D) and β-catenin (E) were quantitated by densitometric analysis and normalized to band density of corresponding actin bands. Values are mean ± SE (n = 4). Asterisks indicate the values that are significantly (p<0.05) different from values for corresponding pair fed group, and the hash tags indicate the values that are significantly (p<0.05) different from values for corresponding GF group.
3.5 Protein thiol oxidation
In addition to serving as a preferred source of energy in the intestine, Gln also plays a role in biosynthesis of the antioxidant, glutathione. Previous studies demonstrated that oxidative stress caused by hydrogen peroxide and glutathione oxidation disrupts intestinal epithelial tight junctions leading to barrier dysfunction [37, 38]. Therefore, we evaluated the levels of reduced and oxidized-protein thiols in distal colon. Confocal microscopy showed that ethanol feeding depletes the level of reduced-protein thiols (Fig. 7A) with a concomitant elevation of oxidized-protein thiols (Fig. 7B). In pair fed animals, dietary Gln showed no influence on the levels of oxidized protein thiols (Fig. 7B & 7D), but it significantly elevated reduced-protein thiols (Fig. 7A & 7C). In ethanol fed mice, Gln replacement in Gln-free increased reduced-protein thiols and reduced oxidized-protein thiols in a dose-dependent manner.
Figure 7. Gln dose-dependently attenuates ethanol-induced protein thiol oxidation in colon.
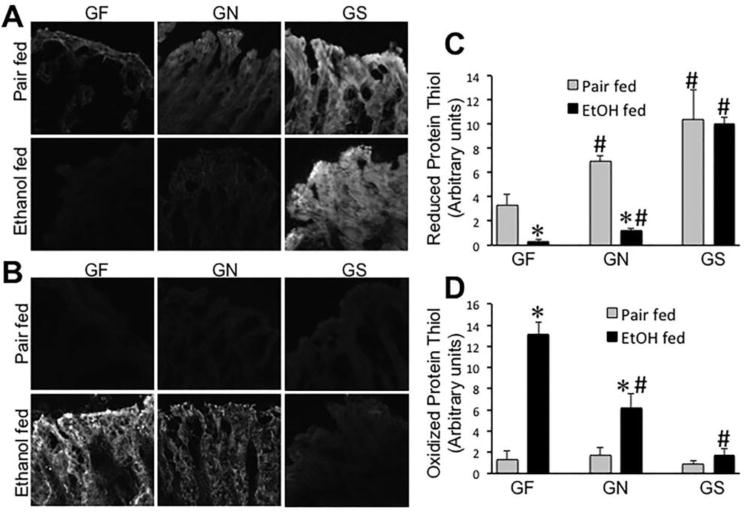
Mice were fed a Gln-free amino acid diet with (Ethanol fed) or without (Pair fed) ethanol (1–6% over 4 weeks) and without (GF) or with 8.4 g/L (GN) or 16.8 g/L (GS) Gln supplementation. Cryosections of colon were stained for reduced (A) and oxidized (B) protein thiols. Fluorescence was evaluated by using Image J software (C & D). Values are mean ± SE (n = 4). Asterisks indicate that the values are significantly (p<0.05) different from corresponding values for non-ethanol pair fed group, and the hash tags indicate the values that are significantly (p<0.05) different from corresponding values for “GF” group.
3.6 Liver damage
Liver weight per unit body weight was slightly elevated by ethanol feeding, which was unaffected by Gln supplementation (Fig. 8A). Plasma ALT was elevated nearly 3-fold by ethanol feeding (Fig. 8B), which was significantly blunted by Gln supplementation. Histopathologic examination indicated the presence of lesions in the liver of ethanol-fed mice (Fig. 8C). Such lesions were not detectable in ethanol-fed mice supplemented with Gln in diet. Staining liver sections with Oil Red-O showed deposits of lipid droplets in the liver of ethanol-fed mice (Fig. 8D). Fat deposits were considerably low in the liver of Gln-supplemented, ethanol-fed mice. Total triglyceride level in the liver was nearly 3-fold higher in ethanol-fed mice (Fig. 8E), which was significantly attenuated by Gln supplementation.
Figure 8. Gln supplementation ameliorates chronic ethanol-induced liver injury.
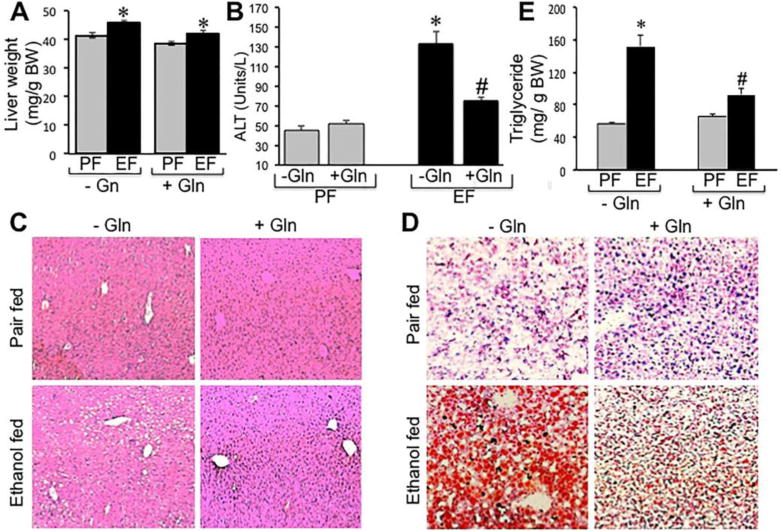
Adult mice were fed Lieber-DeCarli liquid diet with (EF) or without (PF) ethanol and with or without Gln supplementation. Liver weights (A) were recorded, plasma ALT levels measured (B) and H & E stained paraffin sections of liver were imaged by light microscopy (C). Cryosections of liver were stained with Oil Red-O and imaged by light microscopy (D), and liver extracts were assayed for triglyceride content. Values are mean ± SE (n = 6). Asterisks indicate the values that are significantly (p<0.05) different from values for corresponding non-ethanol pair fed group, and the hash tags indicate the values that are significantly (p<0.05) different from corresponding values for “− Gln” group.
Ethanol feeding significantly elevated liver weight in mice fed Gln-free diet (Fig. 9A). Gln supplementation blocked ethanol-induced changes in liver weight. Similarly, plasma ALT was significantly elevated by ethanol feeding in mice fed Gln-free diet with or without regular dietary dose of Gln (Fig. 9B), but not in mice fed Gln-supplemented diet. Histopathologic examination indicated that ethanol feeding induced more severe lesions in the liver of mice fed Gln-free diet compared to that in mice fed regular Gln diet (Fig. 9C). No lesion was detected in the liver of mice fed Gln-supplemented diet. Oil Red-O staining indicated that ethanol feeding induced fat deposition in the liver of mice fed Gln-free with or without regular dietary dose of Gln (Fig. 9D), whereas ethanol failed to induce fat deposition in liver of mice fed Gln-supplemented diet. This beneficial effect of Gln in liver was confirmed by measuring total triglyceride content in the liver (Fig. 9E). Ethanol-induced increase in liver triglyceride was significantly low in mice fed Gln-supplemented diet compared to those in mice fed Gln free diet with or without regular dietary dose of Gln.
Figure 9. Gln dose-dependently attenuates ethanol-induced liver injury.
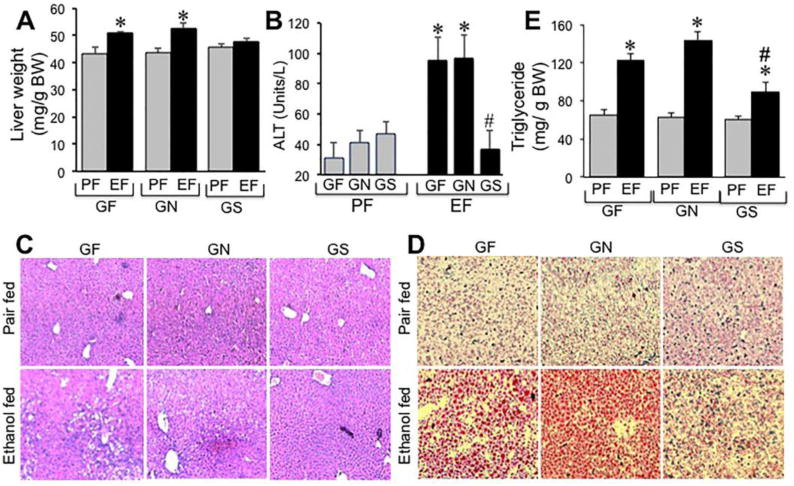
Mice were fed a Gln-free amino acid diet with (Ethanol fed) or without (Pair fed) ethanol (1–6% over 4 weeks) and without (GF) or with 8.4 g/L (GN) or 16.8 g/L (GS) Gln supplementation. Liver weights (A) were recorded, plasma ALT levels measured (B) and H & E stained paraffin sections of liver were imaged by light microscopy (C). Cryosections of liver were stained with Oil Red-O and imaged by light microscopy (D), and liver extracts were assayed for triglyceride content. Values are mean ± SE (n = 6). Asterisks indicate the values that are significantly (p<0.05) different from values for corresponding non-ethanol pair fed group, and the hash tags indicate the values that are significantly (p<0.05) different from corresponding values for “GF” group.
4. Discussion
Gln, a conditionally essential amino acid, is well known to play a role in the maintenance of gastrointestinal mucosal homeostasis [20]. Gln supplementation is beneficial in the treatment of critically ill, burn injury and total parenteral nutrition patients [22, 23, 39]. In the present study, we provide evidence to the beneficial effect of Gln supplementation in diet in alcoholic tissue injury.
Acute or chronic alcohol consumption has been well documented to disrupt intestinal epithelial barrier function leading to enhanced endotoxin absorption from the colon and endotoxemia. Evidence indicates that endotoxin plays a crucial role in the pathogenesis of ALD [3, 40]. Alcoholics with liver disease show elevated intestinal permeability to macromolecules and plasma endotoxin level. Experimental studies in animal models of alcoholic liver damage also showed increased intestinal permeability to macromolecules and endotoxemia. Results of the present study confirm that chronic ethanol feeding causes an increased mucosal permeability to macromolecules in the distal colon, indicating the disruption of colonic mucosal barrier function. Gln supplementation in these mice showed a significant amelioration of ethanol-induced colonic mucosal permeability. These data indicate that Gln supplementation protects the intestinal mucosa from ethanol-induced mucosal injury and help preserve the mucosal barrier function. Our previous studies in Caco-2 cell monolayers showed that Gln ameliorates acetaldehyde-induced increase in paracellular permeability in an intestinal epithelium in vitro [29]. Acetaldehyde is a metabolic intermediate of ethanol metabolism, which is accumulated at high level in the colonic lumen following alcohol consumption due to alcohol dehydrogenases in the mucosa and luminal micro flora. The results of present study demonstrate that dietary Gln is beneficial in preventing alcohol-induced intestinal mucosal barrier dysfunction in vivo.
Interestingly, alcohol-induced increase in mucosal permeability was confined to distal colon. The reason for restriction of alcohol-induced mucosal permeability to distal colon is unclear at this time. It is possible that the barrier function of the proximal colon and small intestine is also compromised by ethanol, but the barrier is restored in these segments much faster than the distal colon. It is also possible that the mucosal defense mechanism in distal colon is less effective compared to that in the proximal colon and small intestine. Ulcerative colitis [41] and azoxymethane-induced colonic tumorigenesis [42] were also found to be greater in the distal colon. Additionally, differences in the microbiome population in different segments of colon may contribute to permeability differences. The metabolic profiles of microbiome in the proximal colon are distinctly different from those in distal colon [43]. For instance, saccharolytic bacteria that produce short chain fatty acids are high in the proximal colon. Therefore, proximal and distal segments of colon exhibit distinct properties in many respects.
Ethanol-induced increase in colonic mucosal permeability was most severe in mice fed Gln-free diet, and Gln replacement in Gln-free diet dose-dependently blocked the effect of chronic ethanol consumption on gut barrier function. This observation suggests that Gln in regular diet is protective against ethanol-induced gut barrier dysfunction, and that the Gln supply in the regular diet is insufficient to completely prevent the ethanol effect. Gln supplementation at 2-fold the regular dietary dose is required for more effective amelioration of ethanol-induced gut barrier dysfunction. Gln produced no significant influence on gut permeability in pair fed mice in the absence of ethanol, suggesting that dietary Gln may not be required for the maintenance of gut barrier function under normal conditions. However, dietary Gln becomes beneficial when epithelial barrier is challenged by chronic alcohol consumption. This observation supports the classification of Gln as a conditionally essential amino acid.
The epithelial tight junctions form the primary component of intestinal mucosal barrier function. Tight junctions in the gastrointestinal epithelium form a selective barrier that prevents diffusion of molecules with less than 4A° diameter in size [44]. Therefore, epithelial tight junctions form a strong barrier for absorption of endotoxins from the colonic lumen into mesenteric circulation. Previous in vitro studies in Caco-2 cell monolayers showed that Gln prevents acetaldehyde-induced disruption of tight junctions and adherens junctions by a mechanism involving the activities of EGF receptor tyrosine kinase, protein kinase C and ERK1/2 [45]. Our subsequent studies showed that Gln also prevents acetaldehyde-induced disruption of tight junctions and adherens junctions in the human colonic mucosa in vitro [36]. The results of present study show that chronic ethanol feeding induces redistribution of the tight junction proteins, occludin and ZO1 from the intercellular junctions of colonic epithelium, indicating that ethanol disrupts colonic epithelial tight junctions in vivo. This observation was supported by a loss of detergent-insoluble fractions of tight junction proteins. Chronic ethanol-induced redistribution of occludin and ZO1 from the intercellular junctions of colonic epithelium and depletion of these proteins in the detergent-insoluble fractions of mucosa were almost completely blocked by Gln supplementation, indicating the role of Gln in preserving colonic epithelial tight junction integrity in ethanol fed mice. These data demonstrate that Gln supplementation protects the colonic epithelial tight junctions and barrier function from alcohol-induced damage.
Adherens junction is not a physical barrier for diffusion of macromolecules across the epithelium. However, adherens junctions are known to indirectly regulate the integrity of tight junctions and therefore the barrier function. The present study shows that chronic ethanol feeding induces redistribution of E-cadherin and β-catenin from the epithelial junctions, indicating the ethanol-induced disruption of adherens junctions. Gln supplementation attenuated ethanol-induced redistribution of E-cadherin and β-catenin from the intercellular junctions of colonic epithelium and depletion of these proteins in the detergent-insoluble fractions of mucosa, indicating that Gln supplementation prevents ethanol-induced disruption of adherens junctions. Therefore, Gln supplementation protects both tight junctions and adherens junctions in the colonic epithelium of ethanol fed mice. Previous in vitro studies in Caco-2 cell monolayers [45] and human colonic mucosa [36] showed a similar Gln-mediated protection of adherens junctions from acetaldehyde-induced damage.
Ethanol-induced disruption of tight junctions and adherens junctions was most severe in mice fed Gln-free diet, and Gln dose-dependently prevented it. This suggests that Gln in the regular diet is protective against alcohol-induced disruption of tight junctions and adherens junctions in the colonic mucosa. However, Gln supplementation is required for a most effective protection from ethanol damage. Once again, Gln had no significant influence on the basal tight junction integrity, indicating the conditionally essential nature of Gln. Staining for E-cadherin and β-catenin showed a slight reorganization of adherens junctions by Gln-free diet in the absence of ethanol, which is supported by slightly lower levels of detergent-insoluble fractions of E-cadherin and β-catenin in the colon of mice fed Gln-free diet; the effect may become more obvious if the animals are continued in Gln-free diet for a longer period. This observation suggests that dietary Gln may be required for maintenance of adherens junction integrity under normal physiologic conditions.
The mechanism of Gln-mediated prevention of ethanol-induced gut barrier dysfunction is unclear. Previous studies showed that one of beneficial effect of Gln is to serve as precursor for biosynthesis of glutathione and therefore offers antioxidant function in the cell [46, 47]. The present study shows that ethanol feeding induces a dramatic increase in protein thiol oxidation, suggesting that ethanol feeding induces oxidative stress in colonic mucosa. Our previous studies have demonstrated that oxidative stress disrupts intestinal epithelial tight junctions and induces barrier dysfunction [37, 38]. In the present study we show that dietary Gln dose-dependently prevents ethanol-induced protein thiol oxidation. Therefore, it is likely that one of the mechanisms involved in Gln-mediated preservation of gut barrier function in ethanol fed mice is elevation of protein thiols and prevention of oxidative stress-induced tight junction disruption.
Pathogenesis of ALD involves progression from fatty liver to hepatitis and finally to cirrhosis. Endotoxemia and endotoxin-mediated activation of Kupffer cells and other hepatic cells is a crucial step in the pathogenesis of ALD. The source of plasma endotoxins in alcoholics is the colonic luminal micro flora. Alcohol-induced mucosal barrier dysfunction and increased intestinal permeability play crucial role in alcoholic endotoxemia and liver injury. As shown before, the histopathologic examination of liver in the present study show that chronic ethanol feeding elevates plasma ALT and induces lesions in liver tissue. Gln supplementation significantly attenuated ethanol-induced plasma ALT elevation and liver lesions. As shown before, the chronic ethanol feeding induced fatty liver as indicated by deposition of lipid droplets and elevated liver triglyceride content. Gln supplementation significantly reduced the ethanol-induced fat deposition and elevation of liver triglyceride. These data demonstrate that dietary Gln supplementation ameliorates alcoholic liver injury.
Interestingly, ethanol-induced elevation of plasma ALT and liver triglyceride was similar in mice fed Gln free diet and Gln supplementation at normal dietary dose. This observation suggests that dietary Gln under regular dietary dose is not high enough to suppress alcoholic fatty liver. However, Gln supplementation at 2-fold the regular dietary dose significantly ameliorated ethanol-induced elevation of plasma ALT and liver triglyceride content. Gln is normally synthesized in the tissues, but is conditionally essential and dependent on dietary Gln under pathophysiologic conditions. Dietary Gln may not be required for basal cellular functions. However, dietary Gln is required under pathophysiologic conditions such as burn injury, total parenteral nutrition and critically ill patients [39]. In this study, we show that Gln at regular dietary dose is insufficient to prevent ethanol-induced liver injury. This may be explained by the fact that, at regular dietary dose, Gln does not completely block ethanol-induced gut permeability. Gln supplementation blocks ethanol-induced gut permeability and ameliorates alcoholic fatty liver in mice.
In conclusion, this study shows that dietary Gln supplementation protects colonic epithelial tight junctions and adherens junctions and prevents alcohol-induced gut barrier dysfunction and liver injury. Therefore, Results dietary Gln supplementation may offer beneficial effects in prevention and/or control of alcoholic gut and liver injury.
Acknowledgments
This was supported by NIH grants AA12307 and DK55532 (RKR) and P20 AA017837, R01 AA019673 & 1U01AA021890 (LEN).
Abbreviations
- ALD
alcoholic liver disease
- ALT
alanine aminotransferase
- ECL
enhanced chemiluminescent
- EF
ethanol fed
- EGF
epidermal growth factor
- ERK
extracellular receptor activated kinase
- FITC
fluorescein isothiocyanate
- Gln
glutamine
- GF
Gln free
- GN
Gln at regular dietary dose
- GS
Gln supplemented
- HRP
horseradish peroxidase
- PF
pair fed
- PMSF
phenylmethanesulfonyl fluoride
- ZO-1
zona occludens-1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anderson C, Andersson T, Molander M. Ethanol absorption across human skin measured by in vivo microdialysis technique. Acta Derm Venereol. 1991;71:389–93. [PubMed] [Google Scholar]
- 2.Rao R. Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatology. 2009;50:638–44. doi: 10.1002/hep.23009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rao RK, Seth A, Sheth P. Recent Advances in Alcoholic Liver Disease I. Role of intestinal permeability and endotoxemia in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2004;286:G881–4. doi: 10.1152/ajpgi.00006.2004. [DOI] [PubMed] [Google Scholar]
- 4.Bode JC, Bode C, Heidelbach R, Durr HK, Martini GA. Jejunal microflora in patients with chronic alcohol abuse. Hepatogastroenterology. 1984;31:30–4. [PubMed] [Google Scholar]
- 5.Schafer C, Parlesak A, Schutt C, Bode JC, Bode C. Concentrations of lipopolysaccharide-binding protein, bactericidal/permeability-increasing protein, soluble CD14 and plasma lipids in relation to endotoxaemia in patients with alcoholic liver disease. Alcohol Alcohol. 2002;37:81–6. doi: 10.1093/alcalc/37.1.81. [DOI] [PubMed] [Google Scholar]
- 6.Jokelainen K, Reinke LA, Nanji AA. Nf-kappab activation is associated with free radical generation and endotoxemia and precedes pathological liver injury in experimental alcoholic liver disease. Cytokine. 2001;16:36–9. doi: 10.1006/cyto.2001.0930. [DOI] [PubMed] [Google Scholar]
- 7.Mathurin P, Deng QG, Keshavarzian A, Choudhary S, Holmes EW, Tsukamoto H. Exacerbation of alcoholic liver injury by enteral endotoxin in rats. Hepatology. 2000;32:1008–17. doi: 10.1053/jhep.2000.19621. [DOI] [PubMed] [Google Scholar]
- 8.Nanji AA, Su GL, Laposata M, French SW. Pathogenesis of alcoholic liver disease–recent advances. Alcohol Clin Exp Res. 2002;26:731–6. [PubMed] [Google Scholar]
- 9.Duryee MJ, Klassen LW, Freeman TL, Willis MS, Tuma DJ, Thiele GM. Lipopolysaccharide is a cofactor for malondialdehyde-acetaldehyde adduct-mediated cytokine/chemokine release by rat sinusoidal liver endothelial and Kupffer cells. Alcohol Clin Exp Res. 2004;28:1931–8. doi: 10.1097/01.alc.0000148115.90045.c5. [DOI] [PubMed] [Google Scholar]
- 10.Anderson JM, Van Itallie CM. Physiology and function of the tight junction. Cold Spring Harb Perspect Biol. 2009;1:a002584. doi: 10.1101/cshperspect.a002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furuse M, Itoh M, Hirase T, Nagafuchi A, Yonemura S, Tsukita S. Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J Cell Biol. 1994;127:1617–26. doi: 10.1083/jcb.127.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodgers LS, Fanning AS. Regulation of epithelial permeability by the actin cytoskeleton. Cytoskeleton (Hoboken) 2011;68:653–60. doi: 10.1002/cm.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turner JR. Molecular basis of epithelial barrier regulation: from basic mechanisms to clinical application. Am J Pathol. 2006;169:1901–9. doi: 10.2353/ajpath.2006.060681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madara JL. Intestinal absorptive cell tight junctions are linked to cytoskeleton. Am J Physiol. 1987;253:C171–5. doi: 10.1152/ajpcell.1987.253.1.C171. [DOI] [PubMed] [Google Scholar]
- 15.Lilien J, Balsamo J, Arregui C, Xu G. Turn-off, drop-out: functional state switching of cadherins. Dev Dyn. 2002;224:18–29. doi: 10.1002/dvdy.10087. [DOI] [PubMed] [Google Scholar]
- 16.Gumbiner B, Stevenson B, Grimaldi A. The role of the cell adhesion molecule uvomorulin in the formation and maintenance of the epithelial junctional complex. J Cell Biol. 1988;107:1575–87. doi: 10.1083/jcb.107.4.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madara JL, Barenberg D, Carlson S. Effects of cytochalasin D on occluding junctions of intestinal absorptive cells: further evidence that the cytoskeleton may influence paracellular permeability and junctional charge selectivity. J Cell Biol. 1986;102:2125–36. doi: 10.1083/jcb.102.6.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wittchen ES, Haskins J, Stevenson BR. Protein interactions at the tight junction. Actin has multiple binding partners, and ZO-1 forms independent complexes with ZO-2 and ZO-3. J Biol Chem. 1999;274:35179–85. doi: 10.1074/jbc.274.49.35179. [DOI] [PubMed] [Google Scholar]
- 19.Jacobi SK, Odle J. Nutritional factors influencing intestinal health of the neonate. Adv Nutr. 2012;3:687–96. doi: 10.3945/an.112.002683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reeds PJ, Burrin DG. Glutamine and the bowel. J Nutr. 2001;131:2505S–8S. doi: 10.1093/jn/131.9.2505S. discussion 23S–4S. [DOI] [PubMed] [Google Scholar]
- 21.Evans MA, Shronts EP. Intestinal fuels: glutamine, short-chain fatty acids, and dietary fiber. J Am Diet Assoc. 1992;92:1239–46. 49. [PubMed] [Google Scholar]
- 22.Wilmore DW. The effect of glutamine supplementation in patients following elective surgery and accidental injury. J Nutr. 2001;131:2543S–9S. doi: 10.1093/jn/131.9.2543S. discussion 50S-1S. [DOI] [PubMed] [Google Scholar]
- 23.Windle EM. Glutamine supplementation in critical illness: evidence, recommendations, and implications for clinical practice in burn care. Journal of burn care & research : official publication of the American Burn Association. 2006;27:764–72. doi: 10.1097/01.BCR.0000245417.47510.9C. [DOI] [PubMed] [Google Scholar]
- 24.Feng Y, Ralls MW, Xiao W, Miyasaka E, Herman RS, Teitelbaum DH. Loss of enteral nutrition in a mouse model results in intestinal epithelial barrier dysfunction. Ann N Y Acad Sci. 2012;1258:71–7. doi: 10.1111/j.1749-6632.2012.06572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mao Y, Wang SQ, Mao XB, Zeng QT, Li YS. Intestinal barrier function in patients with acute myocardial infarction and the therapeutic effect of glutamine. Int J Cardiol. 2011;146:432–3. doi: 10.1016/j.ijcard.2010.10.102. [DOI] [PubMed] [Google Scholar]
- 26.Ewaschuk JB, Murdoch GK, Johnson IR, Madsen KL, Field CJ. Glutamine supplementation improves intestinal barrier function in a weaned piglet model of Escherichia coli infection. Br J Nutr. 2011;106:870–7. doi: 10.1017/S0007114511001152. [DOI] [PubMed] [Google Scholar]
- 27.Li N, Lewis P, Samuelson D, Liboni K, Neu J. Glutamine regulates Caco-2 cell tight junction proteins. Am J Physiol Gastrointest Liver Physiol. 2004;287:G726–33. doi: 10.1152/ajpgi.00012.2004. [DOI] [PubMed] [Google Scholar]
- 28.Basuroy S, Sheth P, Mansbach CM, Rao RK. Acetaldehyde disrupts tight junctions and adherens junctions in human colonic mucosa: protection by EGF and L-glutamine. Am J Physiol Gastrointest Liver Physiol. 2005;289:G367–75. doi: 10.1152/ajpgi.00464.2004. [DOI] [PubMed] [Google Scholar]
- 29.Seth A, Basuroy S, Sheth P, Rao RK. L-Glutamine ameliorates acetaldehyde-induced increase in paracellular permeability in Caco-2 cell monolayer. Am J Physiol Gastrointest Liver Physiol. 2004;287:G510–7. doi: 10.1152/ajpgi.00058.2004. [DOI] [PubMed] [Google Scholar]
- 30.Rao RK, Basuroy S, Rao VU, Karnaky KJ, Jr, Gupta A. Tyrosine phosphorylation and dissociation of occludin-ZO-1 and E-cadherin-beta-catenin complexes from the cytoskeleton by oxidative stress. Biochem J. 2002;368:471–81. doi: 10.1042/BJ20011804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Basuroy S, Sheth P, Kuppuswamy D, Balasubramanian S, Ray RM, Rao RK. Expression of kinase-inactive c-Src delays oxidative stress-induced disassembly and accelerates calcium-mediated reassembly of tight junctions in the Caco-2 cell monolayer. J Biol Chem. 2003;278:11916–24. doi: 10.1074/jbc.M211710200. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki T, Seth A, Rao R. Role of phospholipase Cgamma-induced activation of protein kinase Cepsilon (PKCepsilon) and PKCbetaI in epidermal growth factor-mediated protection of tight junctions from acetaldehyde in Caco-2 cell monolayers. J Biol Chem. 2008;283:3574–83. doi: 10.1074/jbc.M709141200. [DOI] [PubMed] [Google Scholar]
- 33.Sheth P, Basuroy S, Li C, Naren AP, Rao RK. Role of phosphatidylinositol 3-kinase in oxidative stress-induced disruption of tight junctions. J Biol Chem. 2003;278:49239–45. doi: 10.1074/jbc.M305654200. [DOI] [PubMed] [Google Scholar]
- 34.Iwasaki T, Terrill J, Shavlakadze T, Grounds MD, Arthur PG. Visualizing and quantifying oxidized protein thiols in tissue sections: a comparison of dystrophic mdx and normal skeletal mouse muscles. Free Radic Biol Med. 2013;65:1408–16. doi: 10.1016/j.freeradbiomed.2013.09.024. [DOI] [PubMed] [Google Scholar]
- 35.Sheth P, Seth A, Atkinson KJ, Gheyi T, Kale G, Giorgianni F, et al. Acetaldehyde dissociates the PTP1B-E-cadherin-beta-catenin complex in Caco-2 cell monolayers by a phosphorylation-dependent mechanism. Biochem J. 2007;402:291–300. doi: 10.1042/BJ20060665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Basuroy S, Sheth P, Mansbach CM, Rao RK. Acetaldehyde disrupts tight junctions and adherens junctions in human colonic mucosa: protection by EGF and L-glutamine. Am J Physiol Gastrointest Liver Physiol. 2005;289:G367–75. doi: 10.1152/ajpgi.00464.2004. [DOI] [PubMed] [Google Scholar]
- 37.Rao RK, Li L, Baker RD, Baker SS, Gupta A. Glutathione oxidation and PTPase inhibition by hydrogen peroxide in Caco-2 cell monolayer. Am J Physiol Gastrointest Liver Physiol. 2000;279:G332–40. doi: 10.1152/ajpgi.2000.279.2.G332. [DOI] [PubMed] [Google Scholar]
- 38.Rao R. Oxidative stress-induced disruption of epithelial and endothelial tight junctions. Front Biosci. 2008;13:7210–26. doi: 10.2741/3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tremel H, Kienle B, Weilemann LS, Stehle P, Furst P. Glutamine dipeptide-supplemented parenteral nutrition maintains intestinal function in the critically ill. Gastroenterology. 1994;107:1595–601. doi: 10.1016/0016-5085(94)90797-8. [DOI] [PubMed] [Google Scholar]
- 40.Rao RK. Acetaldehyde-induced barrier disruption and paracellular permeability in Caco-2 cell monolayer. Methods Mol Biol. 2008;447:171–83. doi: 10.1007/978-1-59745-242-7_13. [DOI] [PubMed] [Google Scholar]
- 41.Koutroubakis IE. Recent advances in the management of distal ulcerative colitis. World J Gastrointest Pharmacol Ther. 2010;1:43–50. doi: 10.4292/wjgpt.v1.i2.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Robertis M, Massi E, Poeta ML, Carotti S, Morini S, Cecchetelli L, et al. The AOM/DSS murine model for the study of colon carcinogenesis: From pathways to diagnosis and therapy studies. J Carcinog. 2011;10:9. doi: 10.4103/1477-3163.78279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martin FP, Wang Y, Yap IK, Sprenger N, Lindon JC, Rezzi S, et al. Topographical variation in murine intestinal metabolic profiles in relation to microbiome speciation and functional ecological activity. J Proteome Res. 2009;8:3464–74. doi: 10.1021/pr900099x. [DOI] [PubMed] [Google Scholar]
- 44.Anderson JM, Van Itallie CM. Tight junctions and the molecular basis for regulation of paracellular permeability. Am J Physiol. 1995;269:G467–75. doi: 10.1152/ajpgi.1995.269.4.G467. [DOI] [PubMed] [Google Scholar]
- 45.Seth A, Basuroy S, Sheth P, Rao RK. L-Glutamine ameliorates acetaldehyde-induced increase in paracellular permeability in Caco-2 cell monolayer. Am J Physiol Gastrointest Liver Physiol. 2004;287:G510–7. doi: 10.1152/ajpgi.00058.2004. [DOI] [PubMed] [Google Scholar]
- 46.Amores-Sanchez MI, Medina MA. Glutamine, as a precursor of glutathione, and oxidative stress. Molecular genetics and metabolism. 1999;67:100–5. doi: 10.1006/mgme.1999.2857. [DOI] [PubMed] [Google Scholar]
- 47.Mates JM, Perez-Gomez C, Nunez de Castro I, Asenjo M, Marquez J. Glutamine and its relationship with intracellular redox status, oxidative stress and cell proliferation/death. The international journal of biochemistry & cell biology. 2002;34:439–58. doi: 10.1016/s1357-2725(01)00143-1. [DOI] [PubMed] [Google Scholar]


