Abstract
The basolateral amygdala (BLA) plays crucial roles in stimulus value coding, as well as drug and alcohol dependence. Ethanol alters synaptic transmission in the BLA, while endocannabinoids (eCBs) produce presynaptic depression at BLA synapses. Recent studies suggest interactions between ethanol and eCBs that have important consequences for alcohol drinking behavior. To determine how ethanol and eCBs interact in the BLA, we examined the physiology and pharmacology of GABAergic synapses onto BLA pyramidal neurons in neurons from young rats. Application of ethanol at concentrations relevant to intoxication increased, in both young and adult animals, the frequency of spontaneous and miniature GABAergic inhibitory postsynaptic currents, indicating a presynaptic site of ethanol action. The potentiation by ethanol was prevented by inhibition by adenylyl cyclase, and reduced by inhibition by protein kinase A. Activation of type 1 cannabinoid receptors (CB1) in the BLA inhibited GABAergic transmission via an apparent presynaptic mechanism, and prevented ethanol potentiation. Surprisingly, ethanol potentiation was also prevented by CB1 antagonists/inverse agonists. Brief depolarization of BLA pyramidal neurons suppressed GABAergic transmission (depolarization-induced suppression of inhibition [DSI]), an effect previously shown to be mediated by postsynaptic eCB release and presynaptic CB1 activation. A CB1-mediated suppression of GABAergic transmission was also produced by combined afferent stimulation at 0.1 Hz (LFS), and postsynaptic loading with the eCB arachidonoyl ethanolamide (AEA). Both DSI and LFS-induced synaptic depression were prevented by ethanol. Our findings indicate antagonistic interactions between ethanol and eCB/CB1 modulation at GABAergic BLA synapses that may contribute to eCB roles in ethanol seeking and drinking.
Keywords: alcohol, CB1 receptor, synapse, inhibition, arachidonoyl ethanolamide, cyclic AMP
Introduction
The basolateral amygdala (BLA) plays key roles in value coding, affect, and actions of abused drugs, including conditioned place preference for ethanol and other substances (Brown & Fibiger, 1993; Davis, Rainnie, & Cassell, 1994; Everitt, Morris, O’Brien, & Robbins, 1991; Gremel & Cunningham, 2009; Hiroi & White, 1991; LeDoux, 1993, 2000; Olmstead & Franklin, 1997; Sarter & Markowitsch, 1985; See, Fuchs, Ledford, & McLaughlin, 2003; Stuber et al., 2011). The BLA is also implicated in ethanol withdrawal-induced increases in anxiety, as well as other withdrawal/abstinence-related behaviors (Läck, Christian, Diaz, & McCool, 2009; Läck, Diaz, Chappell, DuBois, & McCool, 2007). Acute ethanol exposure alters BLA neuron excitability and increases GABAergic synaptic transmission onto BLA projection neurons, including increasing GABA release at synapses made by local interneurons (Silberman, Ariwodola, & Weiner, 2009; Silberman, Shi, Brunso-Bechtold, & Weiner, 2008; Zhu & Lovinger, 2006). GABAergic transmission at interneuron synapses onto BLA projection neurons is also altered after chronic ethanol exposure (Diaz, Christian, Anderson, & McCool, 2011). It is important to elucidate ethanol actions in BLA to gain a better understanding of the basis of cellular and circuit-level effects of this drug.
Presynaptic, Gi/o-interacting G-protein-coupled receptors (GPCRs) inhibit neurotransmitter release and prevent ethanol effects at GABAergic synapses in several brain regions (Ariwodola & Weiner, 2004; Kang-Park, Kieffer, Roberts, Siggins, & Moore, 2007; Kelm, Criswell, & Breese, 2008; Offermanns, 2003; Roberto et al., 2010; Silberman et al., 2009; Theile, Morikawa, Gonzales, & Morrisett, 2008; Wan, Berton, Madamba, Francesconi, & Siggins, 1996; Zhu & Lovinger, 2006), suggesting that endogenous neurotransmitter actions limit ethanol-induced increases in release. A possible point of convergence for the ethanol and Gi/o-GPCRs is adenylyl cyclase (AC). This cyclic AMP-generating enzyme can be activated by ethanol (Tabakoff, Nelson, Yoshimura, Hellevuo, & Hoffman, 2001) and inhibited by the GPCRs of interest. Determining which neurotransmitters produce these ethanol potentiation-limiting effects at which synapses will help us to understand acute drug actions under different physiological conditions.
Cannabinoid type 1 (CB1) receptors are predominantly presynaptic and decrease neurotransmitter release (Freund, Katona, & Piomelli, 2003; Lòpez-Moreno, González-Cuevas, Moreno, & Navarro, 2008; Mackie, 2008; Matsuda, Bonner, & Lolait, 1993; Wilson & Nicoll, 2001). CB1 expression is robust in the BLA, and strongly localized to axon terminals of CCK-expressing GABAergic interneurons (Katona et al., 2001). Endocannabinoids (eCBs), endogenous lipid metabolites, activate CB1 receptors (Alger, 2009). Arachidonoyl ethanolamide (AEA) and 2-arachidonoyl glycerol (2-AG) are the best characterized eCBs (Heifets & Castillo, 2009). Receptor activation by eCBs usually involves “retrograde’ postsynaptic-to-presynaptic signaling that produces either short- or long-term synaptic depression (Alger, 2009; Alger et al., 1996; Heifets & Castillo, 2009; Lovinger, 2007, 2008; Ohno-Shosaku, Maejima, & Kano, 2001; Wilson & Nicoll, 2001), both of which have been observed at GABAergic synapses onto BLA principal neurons (Marsicano et al., 2002; Patel, Kingsley, Mackie, Marnett, & Winder, 2009; Szabo & Schlicker, 2005; Zhu & Lovinger, 2005). The eCB-mediated reduction of GABA release enhances excitatory output from BLA (Perra, Pillola, Luchicchi, & Pistis, 2008).
Past studies have described interactions between eCB/CB1 signaling and acute ethanol effects, in which CB1 activation depresses GABAergic transmission and prevents ethanol potentiation of GABA release (Kelm et al., 2008; Roberto et al., 2010). Signaling by eCBs is also implicated in behavioral actions of ethanol, including intake and preference, as well as conditioned place preference (Alén et al., 2009; Arnone et al., 1997; Basavarajappa, Ninan, & Arancio, 2008; Basavarajappa, Yalamanchili, Cravatt, Cooper, & Hungund, 2006; Blednov, Cravatt, Boehm, Walker, & Harris, 2007; Cippitelli et al., 2005, 2007; Colombo et al., 2007; Colombo, Serra, Vacca, Carai, & Gessa, 2005; Economidou et al., 2006; Freedland, Sharpe, Samson, & Porrino, 2001; Houchi et al., 2005; Hungund & Basavarajappa, 2000, 2004; Manzanares, Ortiz, Oliva, Pérez-Rial, & Palomo, 2005; Pava et al., 2012; Thanos, Dimitrakakis, Rice, Gifford, & Volkow, 2005; Vinod, Sanguino, Yalamanchili, Manzanares, & Hungund, 2008; Vinod, Yalamanchili, et al., 2008). Chronic ethanol treatment produces tolerance to the behavioral effects of cannabinoids (Pava et al., 2012). This evidence suggests important eCB roles in regulation of the CNS effects of ethanol.
In the present study, we examine interactions between ethanol and eCB/CB1 signaling at GABAergic synapses in rat BLA. In addition, studies have indicated that adolescent rats are less sensitive than adults are to several ethanol actions in both electrophysiological and behavioral experiments (Little, Kuhn, Wilson, & Swartzwelder, 1996; Silveri & Spear, 1998; Van Skike et al., 2010). Administration of low to moderate ethanol doses induces social facilitation in young animals, while the same doses reduce social behavior in adults (Varlinskaya & Spear, 2002). The different response to ethanol in adolescents may play an important role in the development of ethanol-related problems (Spear & Varlinskaya, 2005). For this reason, we evaluated, in a separate set of adult animals, some of the effects observed in young rats regarding the interaction between ethanol and the eCB system. Our findings indicate a general antagonistic effect between ethanol and cannabinoidergic transmission that has strong implications for acute intoxication.
Materials and methods
Brain slice preparation
All experiments were approved by the NIAAA Animal Care and Use Committee. Postnatal day 15–19 (young) or day 50–60 (adult) Sprague Dawley rats were anesthetized by halothane inhalation. After trans-cardial perfusion with ice-cold modified artificial cerebrospinal fluid (aCSF) containing (in mM): 194 sucrose, 30 NaCl, 4.5 KCl, 1 MgCl2, 26 NaHCO3, 1.2 NaH2PO4, and 10 glucose (pH 7.4 with 95% O2/5% CO2), they were then decapitated, and their brains rapidly transferred into ice-cold modified aCSF. Coronal sections (300 μm thickness) were cut in ice-cold modified aCSF using a Leica VT1200S vibratome (Leica Microsystem Nussloch GmbH, Heidelberg, Germany). Slices were transferred immediately to a nylon net submerged in normal aCSF containing (in mM): 124 NaCl, 4.5 KCl, 2 CaCl2, 1 MgCl2, 26 NaHCO3, 1.2 NaH2PO4, and 10 D-glucose, with osmolarity set to 320 mOsm (pH 7.4), at 33 °C for at least 30 min. After an additional 1-h incubation at room temperature, hemi-slices were transferred to a recording chamber, submerged in normal aCSF with a constant flow of ~2 mL/min. For all experiments, the temperature of the bath was maintained at ~31 °C during any given experiment.
Neuronal isolation
Mechanical isolation of BLA neurons was performed as previously described (Jun, Cuzon Carlson, Ikeda, & Lovinger, 2011; Zhu & Lovinger, 2005). Briefly, coronal brain slices containing BLA were transferred to a 35-mm diameter culture dish containing (in mM) 150 NaCl, 5 KCl, 10 HEPES, 1 MgCl2, 2.5 CaCl2, 10 D-glucose, pH adjusted to 7.4 using NaOH, osmolarity set to ~300 mOsm with sucrose. Using a piezoelectric bimorph (Burleigh Instruments, NY), the tip of a flame-sealed micropipette was vibrated laterally at 30 Hz as it descended through the BLA. The slice was then removed from the dish, leaving the isolated neurons.
Whole-cell voltage-clamp recording
In brain slices, whole-cell recordings were performed from BLA principal neurons. Recording pipettes were pulled from borosilicate glass on a Flaming Brown micropipette puller (Novato, CA). Pipette resistance ranged from 2.5–4.5 MΩ, when filled with a CsCl-based internal solution containing (in mM): 150 CsCl, 10 HEPES, 5 lidocaine N-ethyl bromide (QX314), 2 MgCl2, 3 Mg-ATP, 0.3 Na-GTP, and 0.2 BAPTA-4K, pH adjusted to 7.2 with CsOH, and osmolarity set to 298 mOsm with sucrose. To exclude the presence of glutamatergic synaptic responses, 50 μM AP5 and 5 μM NBQX were added to the solution to block NMDA and AMPA receptor-mediated currents, respectively. Cells were voltage-clamped at −60 mV. We analyzed only recordings with series resistance values ranging from 9 to 25 MΩ without compensation, and if this value changed by more than 20% during the course of an experiment, the cell was discarded.
Synaptic currents were recorded with an Axopatch 700A amplifier (Axon Instruments, Foster City, CA), filtered at 2 kHz, and digitized at 5 kHz. Spontaneous IPSC amplitude and frequency were measured using peak and event detection software in pCLAMP9.2 and analyzed by Minianalysis software (Synaptosoft, Decatur, GA, USA). After initiation of the whole-cell recording, stable baseline responses were observed within ~10 min. After 2–3 additional minutes of baseline recording, we started drug perfusion or the DSI protocol. For evoked IPSC recordings, the concentric bipolar stimulating electrode was placed approximately 200 μm medial to the recording site. This would certainly stimulate the inhibitory afferents arising from local interneurons, but stimulation of afferents from paracapsular cells cannot be ruled out. For DSI experiments, after a recording period of approximately 3 min, the membrane potential was stepped from −60 to 0 mV for 5 sec under voltage clamp. The magnitude of DSI was calculated as the sIPSC amplitude and frequency after the depolarization divided by the average during a 3-min pre-depolarization period. In a different set of experiments, AEA (50 μM) was loaded in the patch electrode to increase the pool of endocannabinoid available for release during DSI. The low-frequency stimulation (LFS) protocol consisted of 0.1-Hz stimulation for 3 min using a concentric bipolar electrode (FHC, Bowdoin, ME). Stimulation intensity was set to induce an eIPSC with an amplitude ~50% of the maximal response.
In some experiments examining ethanol effects on DSI, the DSI protocol was repeated three times in each neuron, once during the baseline period, once in the presence of ethanol, and once after washout. In other experiments the DSI protocol was run only once per cell, and SR or ethanol were present throughout the recording. In the experiments involving ethanol wash-on and washout, we applied the DSI protocol after 3 min of stable baseline and continued to record for an additional 3 min after DSI. We then applied ethanol to the slice for a total of 5 min. Three minutes into the ethanol application, if the response was stable, we applied the DSI protocol again. Two minutes after this second DSI protocol, we began the washout of ethanol. After a total of 4 min of washout, we again applied the DSI protocol, and recorded for a further 3 min. Whole-cell voltage-clamp recordings from isolated neurons were performed on the stage of an inverted Nikon T200 microscope as previously described (Zhu & Lovinger, 2006), using micropipettes with resistances of 2–4 MΩ filled with the CsCl-based solution. Solutions containing ethanol and other compounds were applied via local perfusion using an array of fused square-tipped glass tubes, with lateral movement driven by the Faststep stepper motor system (Warner Instruments, Hamden, CT). Recordings were performed in the presence of 5-μM NBQX and 25-μM AP5 to block fast glutamatergic transmission, allowing for measurement of spontaneous GABAA receptor-mediated IPSCs.
Drugs
The following drugs were used: ethanol (Pharma corporation), WIN 55,212, SR161417A, AEA, 2AG, H-89, BSE, and DDA (Sigma-Aldrich, San Diego, CA), NBQX, D-AP5, TTX (Tocris, Ellisville, MO).
Statistical analysis
Statistical comparisons of pooled data were performed by t test, or one-way ANOVA followed by the Tukey or Neuman-Keuls post hoc tests, or repeated-measures two-way ANOVA. In all cases, a p value of <0.05 was considered statistically significant.
Results
Effect of ethanol on sIPSCs recorded from BLA principal neurons of young rats
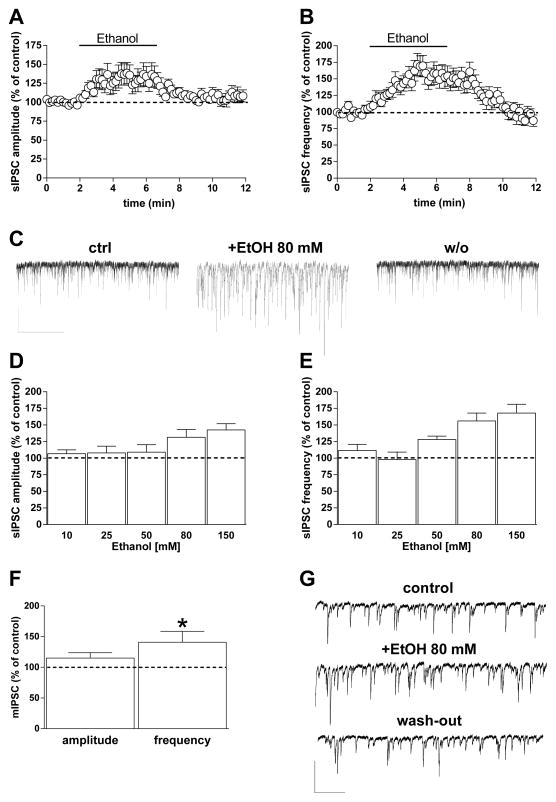
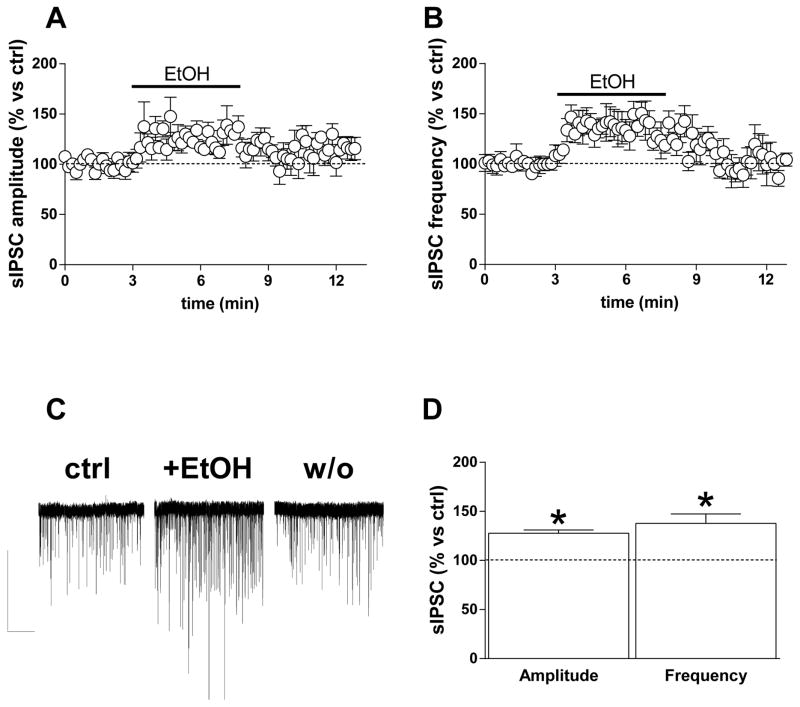
GABAergic sIPSCs occur with reliable frequency and amplitude (Ampl. 56.3 ± 6 pA; Freq. 8.4 ± 0.8 Hz; n = 73) in pyramidal neurons examined in BLA brain slices (Fig. 1) from young rats, as previously reported (Diaz, Chappell, Christian, Anderson, & McCool, 2011; Diaz, Christian, et al., 2011; Silberman et al., 2008; Zhu & Lovinger, 2006). Consistent with previous studies (Silberman et al., 2009; Zhu & Lovinger, 2006), application of 80-mM ethanol induced a significant increase in sIPSC amplitude and frequency that developed within 3–4 min of the onset of ethanol application (Amp: 32 ± 12% increase; Freq: 56 ± 12% increase; p < 0.05, paired t test), (Fig. 1A, B, C). The potentiation reversed within 5 min after cessation of ethanol application. Potentiation of sIPSC frequency by ethanol was concentration-dependent (F[4,58] = 3.89, p = 0.007), without any significant concentration-dependence of the change in event amplitude, where only the higher concentrations were significant (p < 0.05, paired t test) (Fig. 1D, E). In another set of neurons from young rats, we examined action potential-independent miniature IPSCs (mIPSCs) in the presence of the voltage-dependent sodium channel blocker TTX (1 μM) (basal amplitude 45.3 ± 6.2 pA; basal frequency 4 ± 0.9 Hz; n = 11). When ethanol (80 mM) was perfused into the slice it increased mIPSC frequency by 41 ± 18% (p < 0.05, paired t test vs control) without any significant change in amplitude (15 ± 8.5%), (Fig. 1F, G).
Fig. 1. Ethanol increases GABAergic transmission onto BLA principal neurons.
A, B) Graphs showing the effect of 5-min 80 mM ethanol perfusion on both sIPSC amplitude (A) and frequency (B). C) Representative current traces obtained from a single neuron before, during, and 5 min after ethanol perfusion (scale bar 100 pA, 10 sec). D, E) Bar graph showing the average ethanol effect on sIPSCs at different concentrations (10, 25, 50, 80, and 150 mM). The extent of the ethanol effect was calculated during the 2 min in which the drug showed its maximal effect. Data are expressed as mean ± SEM (n = 5, 9, 11, 27, and 11 cells, respectively). F) Bar graph showing 80-mM ethanol effects on amplitude and frequency of TTX-insensitive sIPSCs (mIPSCs) (n = 11 cells) (*p < 0.05 vs. baseline, paired t test). G) Representative traces of mIPSCs recorded from a single neuron before, during, and after ethanol slice perfusion (scale bar 50 pA, 5 sec).
Effect of adenylyl cyclase and PKA inhibitors on ethanol potentiation of sIPSCs
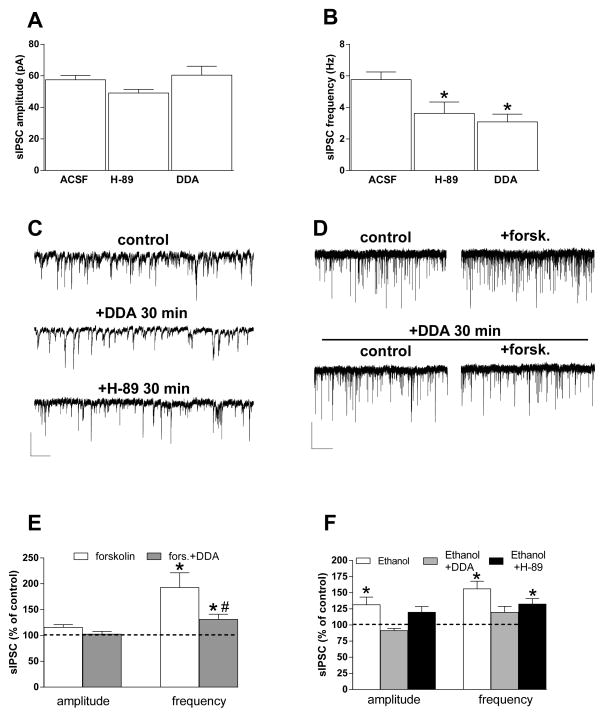
Previous studies evaluated the adenylyl cyclase (AC) and protein kinase A (PKA) effect on ethanol potentiation of GABA release (Kelm et al., 2008; Roberto et al., 2010). Ethanol can activate AC (Luthin & Tabakoff, 1984; Rabin & Molinoff, 1981), and we thus examined effects of AC activation and inhibition, as well as PKA inhibition, on GABAergic sIPSCs and ethanol potentiation in BLA slices from young animals. As previously described in hippocampus (Chevaleyre, Heifets, Kaeser, Südhof, & Castillo, 2007) and prefrontal cortex (Chiu, Puente, Grandes, & Castillo, 2010), a 30-min pre-incubation with the 10-μM AC inhibitor dideoxy-adenosine (DDA) or the PKA inhibitor H-89 (10 μM) decreased basal sIPSC frequency without affecting amplitude (Fig. 2A, B, C). Bath application of the AC activator forskolin (10 μM) potentiated sIPSC frequency. The forskolin effect was prevented by previous incubation of slices in 10-μM DDA (Fig. 2D, E). The increases in sIPSC amplitude and frequency induced by 80-mM ethanol were reduced in slices incubated with DDA (Fig. 2F), as previously reported (Kelm et al., 2008). However, in slices treated with H-89, ethanol was still able to induce an increase in sIPSC frequency that was not distinguishable from the effect of ethanol alone (Fig. 2F). A significant difference among the three treatment groups in ethanol effects on sIPSC amplitude was observed (one-way ANOVA, F[2,14] = 8.28, p = 0.0042), with post hoc Tukey multiple comparisons indicating significant differences only between the ethanol alone and DDA + ethanol treatments. Analysis of the sIPSC frequency data indicated a significant effect of treatment (F[2,14] = 7.004, p = 0.0078), with post hoc comparisons indicating significant differences only between the ethanol alone versus DDA + ethanol group. One-sample t tests indicated that ethanol produced significant increases in sIPSC frequency in all groups, and thus DDA did not completely eliminate ethanol potentiation of GABAergic transmission.
Fig. 2. Roles of adenylyl cyclase and protein kinase A in ethanol potentiation of sIPSCs.
A, B) Bar graphs showing the effect of 30-min incubation of slices with the PKA inhibitor H-89 (10 μM) or the AC inhibitor DDA (10 μM). Neither compound altered sIPSC amplitude (A), but both produced a decrease in sIPSC frequency (B) (n = 54, 11, 21) (*p < 0.05 vs. aCSF unpaired t test. C) Representative traces obtained from different neurons incubated in normal aCSF, or after 30 min of H-89 or DDA incubation. Scale bar = 50 pA, 10 sec. D, E) Forskolin (20 μM) increased sIPSC frequency, and this effect was dramatically decreased in slices incubated for 30 min with DDA. Representative current traces in D show forskolin effects on sIPSCs in the absence (top) and presence (bottom) of DDA. Scale bar = 50 pA, 10 sec. Bar graphs in E show the forskolin and forskolin + DDA effects on sIPSC amplitude and frequency (n = 5, 3) (*p < 0.05 vs. baseline paired t test; #p < 0.05 vs. forskolin, unpaired t test). F) The AC inhibitor DDA decreases ethanol potentiation of the amplitude and frequency of sIPSCs recorded from BLA-projecting neurons, while the PKA inhibitor H-89 did not significantly alter ethanol actions (n = 27, 7, 5) (*p < 0.05 vs. baseline, paired t test).
CB1R interactions with ethanol-induced increases in GABA release
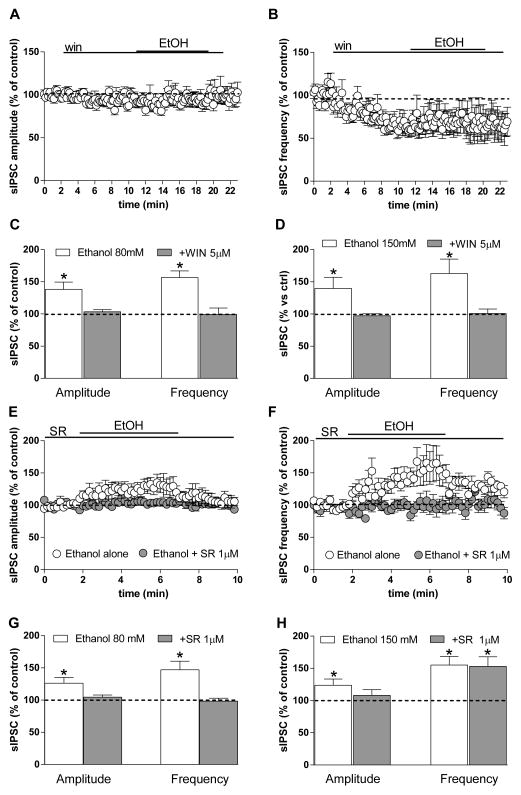
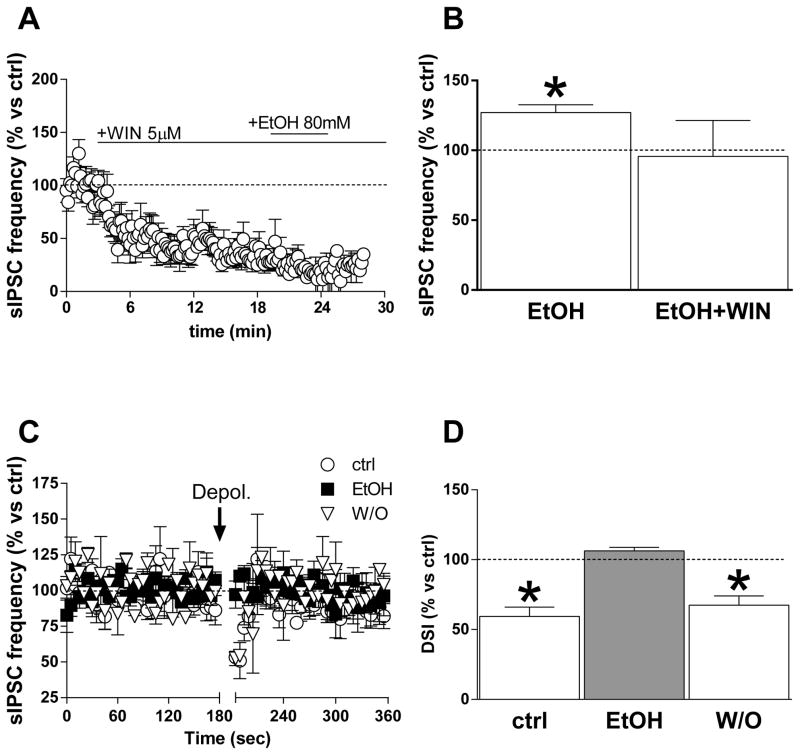
We next determined whether CB1 receptor activation alters synaptic transmission and ethanol potentiation at GABAergic terminals in BLA principal neurons recorded from brain slices made from young animals, as has been observed at other synapses (Roberto et al., 2010). The CB1 agonist WIN 55,212 (WIN) (5 μM) significantly decreased sIPSC frequency (35.7 ± 7% below baseline, p < 0.01), but not amplitude (6.7 ± 4.4) (Fig. 3A, B). Once sIPSC frequency had reached a stable level in the presence of WIN, we applied 80-mM ethanol in the continued presence of CB1 agonist. No significant change in sIPSC frequency or amplitude was observed during ethanol (80 and even 150 mM) application in the presence of WIN (Fig. 3C, D) (*p < 0.05, t test vs. baseline).
Fig. 3. CB1 agonists and antagonists modulate ethanol effects on sIPSCs.
A, B) sIPSC amplitude (A) and frequency (B) plotted as a function of time during an experiment in which the CB1 agonist WIN 55,212 (5 μM) was applied to BLA slices prior to application of 80 mM ethanol. The agonist reduced sIPSC frequency and abolished potentiation by ethanol. C, D) Bar graphs showing the average ethanol effect on sIPSC amplitude and frequency at two different concentrations (80 mM, C; and 150 mM, D) in the presence and absence of WIN. Data were collected from 5–11 cells (*p < 0.05 vs. baseline, paired t test). E, F) Graphs showing the time course of 80-mM ethanol effects on sIPSC amplitude and frequency, respectively, in the presence and absence of the CB1 antagonist SR (1 μM). G, H) Bar graph showing the average ethanol effect at two different concentrations (80 and 150 mM) in the presence and absence of the CB1 antagonist/inverse agonist. Data were collected from 8–12 cells (*p < 0.05 vs. baseline, paired t test).
We next evaluated the effect of ethanol in the presence of the CB1 antagonist/inverse agonist SR141716A (SR) (1 μM) in the slice preparation. The compound was applied for 3 min prior to 5-min co-application of ethanol (80 mM), and SR did not alter sIPSC amplitude or frequency, but prevented the increase induced by ethanol (Fig. 3E, F, G) (*p < 0.05 vs. baseline, t test). The increase in sIPSC frequency induced by 80-mM ethanol was intact in the presence of DMSO (dimethyl sulfoxide, 0.01%), the carrier used to dilute SR (Amp: 27.6 ± 4.8%, Freq: 56.9 ± 6.5% increase; data not shown). Another CB1 antagonist/inverse agonist, AM251 (1 μM) also prevented ethanol (80 mM) potentiation of sIPSC frequency and amplitude (amp = 0.57 ± 1.3%, paired t test, p > 0.05, freq = 2.3 ± 4.6%, paired t test, p > 0.05, n = 11). However, when a high concentration of ethanol (150 mM) was used, SR did not reduce ethanol potentiation of sIPSC frequency (paired t test, p < 0.05, t = 3.58, df = 6; unpaired t test ethanol + vehicle vs. ethanol + SR, p = 0.92, t = 0.098, df = 19) (Fig. 3H).
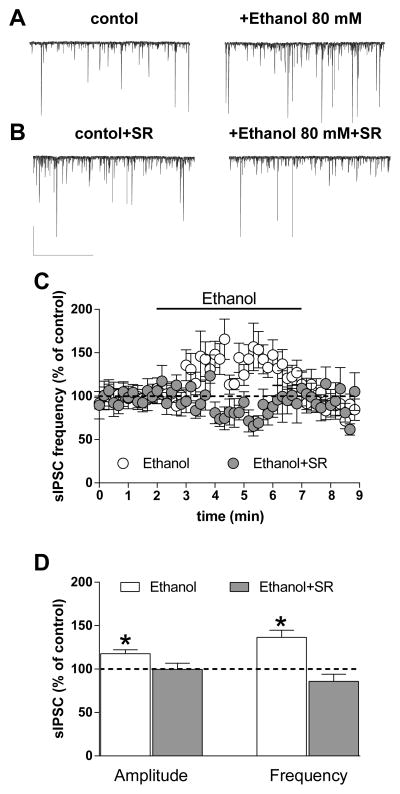
In mechanically isolated BLA principal neurons from young animals, we observed sIPSCs, and application of 80-mM ethanol increased sIPSC amplitude and frequency (Fig. 4A, C, D) (*p < 0.05 vs. baseline, t test), consistent with our previous observations (Zhu & Lovinger, 2005). In a separate set of neurons we applied SR (1 μM) throughout the recording. Similar to what was observed by Zhu and Lovinger (2005), SR perfusion induced an increase in sIPSC frequency of 52 ± 20% above baseline in 58% (4 of 7) of cells tested. However, no potentiation was observed in the remaining cells. In the presence of SR, ethanol 80-mM failed to increase the frequency and amplitude of sIPSCs (Fig. 4B, C, D). Thus, the ability of the CB1 antagonist/inverse agonist to prevent potentiation by ethanol is not dependent on intact circuitry or the slice milieu, and is likely due to a more direct effect on ethanol-sensitive axon terminals.
Fig. 4. Ethanol potentiation is prevented by a CB1 antagonist/inverse agonist in vibrodissociated BLA projection neurons.
A, B) Representative traces obtained from single neurons before and during ethanol application using fast perfusion in the absence and presence of the CB1 antagonist SR (1 μM). (scale bar 200 pA, 60 sec). C) Time course of the effect of 80-mM ethanol on sIPSC frequency in the presence and absence of the CB1 antagonist SR (n = 12 cells). D) Bar graph showing the average ethanol effect on sIPSC amplitude and frequency in the presence and absence of the CB1 antagonist/inverse agonist (*p < 0.05 vs. baseline, paired t test).
Ethanol reduces DSI at synapses on BLA principal neurons
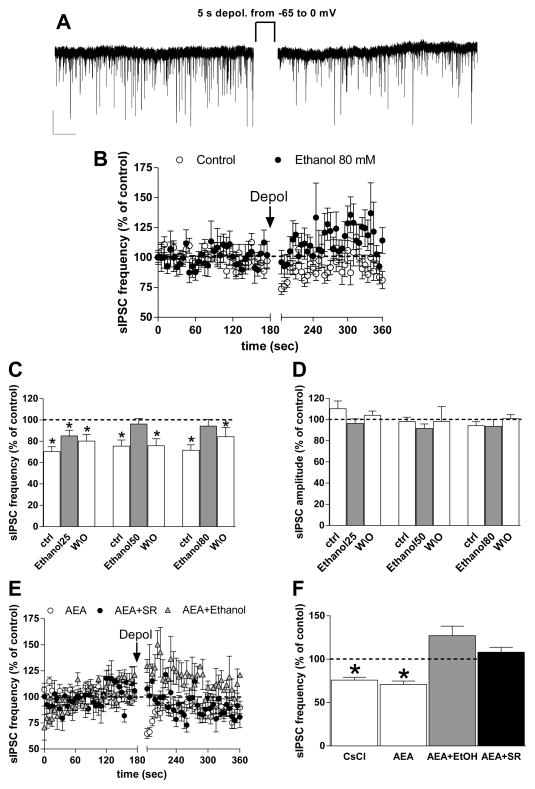
To determine if ethanol alters eCB actions at BLA GABAergic synapses, we first examined depolarization-induced suppression of inhibition (DSI) in slices from young rats. Depolarization of the pyramidal neuron from −60 to 0 mV for 5 sec induced a fast-onset, transient (15–20 sec post-depolarization duration) decrease in sIPSC frequency (25.7 ± 2.7% below baseline, p < 0.01 paired t test, n = 45) (Fig. 5A, B), as observed in previous studies (Patel et al., 2009; Zhu & Lovinger, 2006). Application of 50- or 80-mM ethanol for 3 min increased sIPSC frequency as previously observed (% increase = 28 ± 5.1 at 50 mM, 33 ± 13.6 at 80 mM), while 25-mM ethanol had no effect. In the pre-ethanol baseline condition, postsynaptic depolarization produced significant sIPSC frequency depression (Fig. 5B, C) (*p < 0.05 vs. baseline, t test). As shown in Fig. 5B, C, application of different concentrations of ethanol reduced the depolarization-induced decrease in sIPSC frequency (repeated-measures two-way ANOVA, significant treatment effect F[1,30] = 23.1, p < 0.05), with no significant effect of ethanol concentration or significant interaction. No significant decrease in sIPSC frequency was observed following depolarization in the presence of the 50- or 80-mM concentrations (Fig. 5C). This decrease in DSI was reversed when ethanol was removed from the preparation (Fig. 5C), indicating that the loss of sIPSC depression was not due to a time-dependent loss of DSI. The depolarization protocol did not alter sIPSC amplitude in the absence or presence of ethanol (Fig. 5D) (p > 0.05, two-way ANOVA).
Fig. 5. Depolarization-induced suppression of inhibition in BLA is prevented by ethanol.
A) Representative traces showing that a depolarizing step from −60 mV to 0 mV, applied for 5 sec to a single BLA pyramidal neuron, produced a rapid, short-lasting decrease in sIPSC frequency vs. the baseline-recording period, without any significant modification in amplitude (scale bar 50 pA, 5 sec). B) Graph showing normalized sIPSC frequency over time before, during, and after depolarization using our standard CsCl-containing intracellular solution (open circles) and in the presence of 80-mM ethanol (closed circles) (n =28, 11). C, D) Bar graphs show the average normalized sIPSC frequency (C) and amplitude (D) measured over the first 15 sec after depolarization in control conditions (ACSF only), in the presence of different concentrations of ethanol (25, 50, 80 mM), and after 5 min of ethanol washout (n = 11 for all groups) (*p < 0.05 vs. baseline, paired t test). E) Time course of normalized sIPSC frequency before, during, and after depolarization in recordings made from BLA projection neurons after loading the patch electrode with AEA (10 μM). Recordings were made either in standard aCSF (white circles), aCSF containing SR141716A (1 μM) (gray circles), or aCSF containing 80-mM ethanol (black triangles). (n = 12, 6, 3 cells). F) Summary graph showing DSI magnitude in all CsCl-filled, AEA-filled, AEA + SR and AEA + ethanol cells (n = 28, 12, 6, 3 cells) (*p < 0.05 vs. baseline, paired t test).
To try to enhance DSI magnitude, and perhaps bypass ethanol effects on eCB synthesis, we filled the patch electrode with AEA (10 μM) or 2-AG (10 μM) and applied the depolarization protocol in these eCB-loaded cells. The magnitude and time course of DSI were unchanged in neurons filled with AEA relative to neurons filled with the standard internal solution containing CsCl (Fig. 5B, E, F). We obtained the same result filling the recording electrode with 2-AG (decrease in sIPSC freq. following DSI = 36.8 ± 6.6 below baseline). Bath application of the CB1 antagonist/inverse agonist SR (1 μM) prevented DSI in AEA-loaded cells (Fig. 5E, F). Bath application of 80-mM ethanol prevented DSI in the AEA-loaded cells, as observed in the non-loaded neurons (Fig. 5E, F), (F[3,45] = 11.43, p < 0.05). This finding suggests that the effects of ethanol are not via inhibition of eCB synthesis.
Effect of ethanol and CB1 agonist in adult rats
The effects of ethanol and CB1 agonists on GABAergic synapses might be dependent on the developmental stage of animals tested, as previous studies have indicated such changes over development in other brain regions and in behavioral tests (Little et al., 1996; Silveri & Spear, 1998; Van Skike et al., 2010). As observed in young rats, GABAergic sIPSCs occur with reliable frequency and amplitude in BLA principal neurons from adults (Ampl. 38.86 ± 3.14 pA; Freq. 3.31 ± 0.38 Hz; n = 26). Application of 80-mM ethanol induced a significant increase in sIPSC amplitude and frequency recorded in BLA slices from adult animals, similar to what was observed in young rats (Amp: 27.8 ± 3.38% increase; Freq: 37.8 ± 9.6% increase; p < 0.05 one-sample t test vs. baseline) (Fig. 6A–D). As previously observed, the potentiation reversed within 5 min after cessation of ethanol application. To evaluate the interaction between ethanol and eCB, we first examined ethanol actions in the presence of the CB1 agonist. As observed in young rats, WIN 55,212 (5 μM) decreased sIPSC frequency (65.4 ± 6.2% below baseline, p < 0.001) (Fig. 7A) without any change in sIPSC amplitude (data not shown). After sIPSC frequency had reached a stable level in the presence of WIN, we applied 80-mM ethanol in the continued presence of CB1 agonist. Even in adult animals, no significant change in sIPSC frequency was observed during ethanol application in the presence of WIN (Fig. 7A, B) (*p < 0.05 vs. baseline, t test). To determine whether ethanol alters eCB actions at GABAergic synapses in neurons from adult animals, we used the DSI protocol. DSI induced a decrease in sIPSC frequency (40.61 ± 6.42% below baseline, p < 0.01 paired t test, n = 4) (Fig. 7C) during the first 15 sec after cessation of the depolarization step. Application of 80-mM ethanol significantly reduced the magnitude of the decrease in sIPSC frequency induced by the DSI protocol, and the effect of DSI recovered completely after a 5–10-min ethanol washout (Fig. 7C, D) (*p < 0.05 vs. baseline, t test), indicating that the loss of sIPSC depression was not due to a time-dependent loss of DSI.
Fig. 6. Ethanol increases GABAergic transmission onto BLA principal neurons in adult rats (PND 50–60).
A, B) Time course plots showing the effect of 5-min 80-mM ethanol perfusion on both sIPSC amplitude (A) and frequency (B). C) Representative current traces obtained from a single neuron before, during, and 5 min after ethanol perfusion (scale bar 100 pA, 10 sec). D) Bar graph showing the average effect of 80-mM ethanol on sIPSCs amplitude and frequency. The extent of the ethanol effect was calculated during the 2 min in which the drug showed its maximal effect. Data are expressed as mean ± SEM (n = 8 cells), (*p < 0.05 vs. baseline, paired t test).
Fig. 7. CB1 agonists modulate ethanol effects on sIPSCs in BLA slices from adult rats.
A) sIPSC frequency plotted as a function of time during an experiment in which the CB1 agonist WIN 55,212 (5 μM) was applied to BLA slices prior to application of 80-mM ethanol. The agonist reduced sIPSC frequency and abolished potentiation by ethanol. B) Bar graph showing the effect of ethanol alone as well as ethanol + WIN in the modulation of sIPSC frequency. (*p < 0.05 vs. baseline, paired t test). C) Graph showing normalized sIPSC frequency over time before, during, and after depolarization using our standard CsCl (open circles), in the presence of 80-mM ethanol (closed squares) and after ethanol washout (open triangles). D) Bar graphs show the DSI effect on sIPSC frequency during the first 15 sec after DSI, using our standard CsCl, in the presence of ethanol and after ethanol washout (*p < 0.05 vs. baseline, paired t test).
Ethanol prevents eCB-mediated synaptic depression induced by low-frequency afferent activation
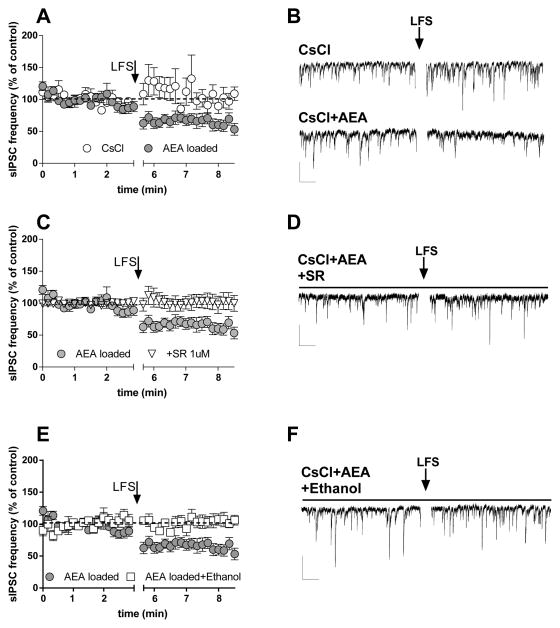
We combined afferent stimulation with postsynaptic loading of AEA in neurons from young animals, in the presence of NBQX (5 μM) and APV (50 μM), to determine whether we could produce CB1-dependent synaptic depression while bypassing mechanisms involved in eCB production. Afferent stimulation at 0.1 Hz (LFS) for 3 min induced a significant decrease in sIPSC frequency that persisted for over 3 min following cessation of stimulation in recordings from AEA-filled BLA pyramidal neurons (Fig. 8A, B). In cells filled only with the CsCl-based pipette solution, LFS did not alter sIPSC frequency (Fig. 8A, B). The decrease in sIPSC frequency observed with LFS plus AEA loading (10 μM) was prevented by SR (Fig. 8C, D), consistent with previous observations in striatum that combined synaptic activation and postsynaptic eCB loading induce CB1-dependent synaptic depression (Adermark & Lovinger, 2009; Ronesi & Lovinger, 2005). LFS failed to alter sIPSC frequency in AEA-loaded neurons in the presence of 80-mM ethanol (Fig. 8E, F). One-way ANOVA indicated significant differences across conditions (F[3,140] = 26.6, p < 0.05), and post hoc analyses with the Neuman-Keuls Multiple Comparison Test revealed significant differences (p < 0.05) in the AEA vs. CsCl alone-loaded comparison, the AEA vs. AEA + SR comparison, and the AEA vs. AEA + ethanol comparison, but not in other comparisons. These findings indicate that ethanol inhibits eCB-mediated synaptic depression and likely does so at a step that occurs after eCB synthesis, as the depression we observed only occurred at synapses onto eCB-loaded neurons.
Fig. 8. Ethanol antagonized the decreased sIPSC frequency induced by low-frequency afferent stimulation (LFS) in BLA slices.
A, B) Low frequency electrical afferent stimulation (0.1 Hz) in BLA induced a decrease in sIPSC frequency only when the patch electrode was loaded with the standard CsCl-based intracellular solution plus the eCB AEA (AEA loaded), but not in the absence of the eCB (CsCl) (n = 3, 9). C, D) The decrease in sIPSC frequency induced by LFS in AEA-loaded neurons is antagonized by SR 1-μM bath application throughout the experiment (n = 7). E, F) The decrease in sIPSC frequency induced by LFS in AEA-loaded neurons is also prevented by bath application of 80-mM ethanol (n = 5) (all scale bars 50 pA, 5 sec).
Discussion
The present study indicates that ethanol interacts with the BLA eCB/CB1 signaling system in several ways. Strong activation of the CB1 receptor prevents ethanol potentiation of GABA release, consistent with previous studies (Perra et al., 2008; Roberto et al., 2010), and consistent with other studies indicating that several Gi/o-coupled GPCRs prevent presynaptic ethanol actions in BLA and elsewhere (Ariwodola & Weiner, 2004; Kang-Park et al., 2007; Kelm et al., 2008; Roberto et al., 2010; Silberman et al., 2009; Wan et al., 1996; Zhu & Lovinger, 2006). Importantly, we observed that ethanol interactions with eCB/CB1 signaling are not only confined to young animals but are also present in the adult. Thus, developmental changes in ethanol sensitivity that have been noted in other brain regions and in behavioral tests (Little et al., 1996; Silveri & Spear, 1998; Van Skike et al., 2010) do not appear to be occurring at BLA GABAergic synapses. The opposing ethanol and GPCR actions may involve AC, as ethanol is known to potentiate the function of certain ACs (Rabin & Molinoff, 1981; Tabakoff et al., 2001), while Gi/oα subunits liberated by receptor activation are AC inhibitors (Davis, Ronesi, & Lovinger, 2003; Freund et al., 2003; Wartmann, Campbell, Subramanian, Burstein, & Davis, 1995). Our findings indicate that AC inhibition interferes with ethanol potentiation of GABA release, supporting a role for this signaling system in ethanol actions. Interestingly, PKA inhibition did not appear to alter ethanol actions as effectively as did AC inhibition. This might indicate a role for increased cAMP independent of PKA activation. It is not yet known how these signaling pathways are recruited in vivo, and how they may alter ethanol-related behaviors. The ability of CB1 agonists to prevent ethanol potentiation of GABAergic transmission has implications for the effect of combined ethanol and synthetic or phytocannabinoid use. The cannabinoid drugs appear to be able to suppress this ethanol action, adding another layer of complexity to the interactions of these often co-abused substances that must be kept in mind when examining interactions in vivo.
Surprisingly, CB1 antagonists/inverse agonists also reduced ethanol potentiation in both brain slices and isolated neurons. It is odd that both agonists and antagonists/inverse agonists have the same action. Perhaps CB1 receptors are constantly activated by tonic eCB signaling that would produce a tone of AC inhibition. The antagonist/inverse agonist could relieve this tone, and might then occlude ethanol potentiation. This scenario seems unlikely, given that no increase in sIPSC frequency, indicative of relief of the tone, was observed during CB1 antagonist/inverse agonist application in BLA slices. Another possibility is that ethanol potentiation involves, at least in part, actions on the CB1 receptor or proximal signaling molecules such as the G protein itself. In this case, strong receptor activation would counteract ethanol actions while receptor occupancy with an inverse agonist might also render the receptor or G protein insensitive to other modulators. The SR prevention of ethanol actions in the neuron/synaptic bouton preparation supports the idea that the site of interaction is located at the synaptic terminal itself. A high ethanol concentration was effective in potentiating GABA release in the presence of SR, indicating that the antagonist/inverse agonist effect can be overcome if the alcohol effect is sufficiently strong. This finding may provide a clue as to how prevention of ethanol effects differs when CB1 is occupied by different ligands. Our findings are similar to the observation in hippocampus by Basavarajappa et al. (2008), who showed antagonism between SR and ethanol effects on glutamate release as well as in BLA by Perra et al. (2008), but differ from those in previous studies that examined effects of GPCR blockade on ethanol potentiation of GABA release (Silberman et al., 2009), including a study in which CB1 antagonist/inverse agonist treatment did not interfere with ethanol potentiation in central amygdala (Roberto et al., 2010). It is likely that the interactions between ethanol and eCB/CB1 signaling are more complex in BLA than in other brain regions, as our findings with DSI- and LFS-induced synaptic depression (discussed below) suggest.
While the observation that ethanol interacts with CB1 receptor function is not new when considering findings from other brain regions, acute ethanol interactions with synaptic signaling by endocannabinoids has not been explored. This set of experiments is crucial for understanding the full range of effects that ethanol will have on this neuromodulatory system in the intact nervous system. Thus, we examined ethanol effects on DSI, and observed a reversible prevention of the decrease in GABA release normally induced by postsynaptic depolarization in the DSI protocol. This action may actually override any effects of CB1 activation on ethanol potentiation of GABAergic synaptic transmission (i.e., those observed with exogenous agonist application as in Fig. 3, 4, & 7). It appears that, in the presence of ethanol, the endocannabinoid is either not released or cannot activate CB1 receptors, and this action would have the net effect of maintaining strong GABAergic transmission under conditions when it would normally be suppressed. The combined potentiation of GABAergic transmission and DSI suppression by ethanol has the potential to suppress BLA output quite strongly. There are several mechanisms through which ethanol might alter endocannabinoid modulation of GABA release, and we explored some of these possibilities with additional experiments. Ethanol prevented DSI even when the postsynaptic neuron was loaded with AEA during the experiment, suggesting that increasing the eCB available for release could not overcome the ethanol effect. It must be noted, however, that postsynaptic AEA loading did not alter DSI magnitude, and thus we cannot be confident that this treatment enhances eCB signaling under this experimental condition. Application of LFS to activate transmission onto AEA-loaded BLA neurons induced depression of sIPSC frequency. This synaptic depression resembles effects of postsynaptically loaded eCBs previously observed in striatum and hypothalamus (Adermark & Lovinger, 2009; Gerdeman, Ronesi, & Lovinger, 2002; Jo, Chen, Chua, Talmage, & Role, 2005; Ronesi & Lovinger, 2005). As observed with DSI, ethanol prevented the LFS/loading-induced synaptic depression. The ability of ethanol to prevent eCB/CB1-mediated synaptic plasticity at GABAergic BLA synapses is likely due to interference with eCB release or presynaptic signaling. It seems unlikely that interference with eCB synthesis accounts for these actions, as there is ample AEA available in the LFS/AEA-loading experiment. Given the ethanol potentiation of GABA release, the interference with eCB-retrograde signaling by this drug might be due to increased probability of GABA release that counteracts the ability of CB1 to inhibit this process in both young and adult rats. However, the experiments examining CB1 agonist interactions with ethanol suggest that the interpretation is not so simple, as agonist-induced synaptic depression is clearly intact during ethanol exposure (Fig. 3B).
Ethanol blockade of eCB retrograde signaling and antagonist/inverse agonist prevention of ethanol potentiation may be related. Ethanol may interfere with CB1/G protein signaling when receptors are occupied by a partial agonist like AEA, but this effect might be reduced in the presence of an antagonist/inverse agonist and eliminated altogether when a full agonist acts on the receptor. Regardless of the molecular mechanisms involved, our findings indicate complex antagonistic interactions between ethanol and eCB/CB1 signaling that may differ when ethanol is the only drug ingested or when a CB1 agonist is also present.
Consistent with previous studies, we observed that ethanol increases GABAergic synaptic transmission via apparent presynaptic mechanisms in brain slices and the “neuron/synaptic bouton” preparation from BLA, supporting the idea that ethanol actions take place at the axon terminal itself (Lovinger & Roberto, 2013; Roberto, Madamba, Moore, Tallent, & Siggins, 2003; Silberman et al., 2009; Zhu & Lovinger, 2006). It is tempting to speculate that this may occur mainly at synapses made by local, as opposed to intercalated, BLA interneurons, given the finding that presynaptic ethanol potentiation mainly occurs at local-circuit synapses (Silberman et al., 2009). It is known that CB1 receptors are expressed by CCK-positive local interneurons in the basal and lateral amygdala, most likely on synaptic terminals (Katona et al., 2001). This study and physiological evidence from Geracitano, Kaufmann, Szabo, Ferraguti, & Capogna (2007) indicate that CB1 receptors are not present or functional on paracapsular synapses onto BLA principal neurons. These findings support the idea that the ethanol-eCB/CB1 interactions we have observed mainly involve local interneuron synapses. However, we did not directly evaluate the cellular origin of the affected synapses in this study. Similar acute ethanol effects have now been observed repeatedly at GABAergic synapses in many brain regions (Kelm, Criswell, & Breese, 2011; Roberto et al., 2010; Zhu & Lovinger, 2006). Thus, the presynaptic effects of ethanol appear to strongly contribute to the pro-GABAergic effects observed in vivo (Criswell & Breese, 2005; Perra et al., 2008).
The potentiation of GABA release by forskolin, and the reduction in ethanol potentiation by an AC inhibitor, also fit with a growing literature implicating this signaling molecule in presynaptic ethanol actions at GABAergic synapses (Das, Pany, Rahman, & Slater, 2009; Kelm et al., 2008; Yoshimura, Pearson, Kadota, & Gonzalez, 2006). Pharmacological and gene knockout data indicate that intact AC/PKA signaling is necessary for acute ethanol-induced increases in GABA release (Kelm et al., 2008; Roberto et al., 2010). This may be related to the ability of ethanol to stimulate some AC subtypes (Luthin & Tabakoff, 1984; Rabin & Molinoff, 1981; Tabakoff et al., 2001). It will be important to determine the pattern of AC subtype expression at ethanol-sensitive GABAergic synapses.
Considerable evidence suggests involvement of the eCB/CB1 signaling system in the pharmacological (Perra et al., 2008; Roberto et al., 2010) and behavioral actions of ethanol, including reward, preference, and self-administration (Arnone et al., 1997; Colombo et al., 2005, 2007; Hungund & Basavarajappa, 2004; Manzanares et al., 2005; Thanos et al., 2005). The specific role in alcohol-related behaviors of eCBs and CB1 in the BLA has not been investigated. However, the BLA has important roles in value coding, and evidence indicates important roles for BLA GABAergic transmission in control of anxiety (Bueno, Zangrossi, & Viana, 2005; Diaz, Chappell, et al., 2011; Gonzalez, Andrews, & File, 1996; Pesold & Treit, 1995; Sanders & Shekhar, 1995; Silberman, Ariwodola, Chappell, Yorgason, & Weiner, 2010). Acute ethanol intoxication is generally associated with decreased anxiety, while withdrawal after chronic exposure can be anxiogenic (Heilig, Egli, Crabbe, & Becker, 2010; Kliethermes, 2005). Treatments that reduce activation of BLA projection neurons reduce withdrawal-induced increases in anxiety (Läck et al., 2007, 2009). Furthermore, CB1 receptors regulate affective behavior and responses to stressful stimuli via mechanisms involving the BLA (Brzózka, Fischer, Falkai, & Havemann-Reinecke, 2011; Martin, Ledent, Parmentier, Maldonado, & Valverde, 2002), and these neural actions are likely to be altered by ethanol. Altering inhibitory input to BLA projection neurons will influence output from this important nucleus, and thus it will be interesting to see whether manipulation of BLA GABAergic inhibition by eCBs or CB1 alters withdrawal-induced anxiety.
Highlights.
Ethanol potentiates GABA release at synapses onto principal neurons of the rat basolateral amygdala in brain slices and an isolated neuron/synaptic bouton preparation.
Potentiation by ethanol is prevented by cannabinoid receptor type 1 agonists and antagonists.
The ethanol actions and ethanol-cannabinoid interactions are observed in brain slices from young and adult rats.
Ethanol prevents endocannabinoid-mediated suppression of GABA release.
These ethanol-cannabinoid interactions may contribute to acute intoxication.
Acknowledgments
Support: Division of Intramural Clinical and Biological Research of the National Institute on Alcohol Abuse and Alcoholism
We acknowledge members of the Section on Synaptic Pharmacology, Laboratory for Integrative Neuroscience for helping us with the research and preparation of this manuscript, with a special thanks to Dr. Anton Sheinin for training in the vibrodissociation technique. This work was supported by the Division of Intramural Clinical and Biological Research of the National Institute on Alcohol Abuse and Alcoholism.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adermark L, Lovinger DM. Frequency-dependent inversion of net striatal output by endocannabinoid-dependent plasticity at different synaptic inputs. The Journal of Neuroscience. 2009;29:1375–1380. doi: 10.1523/JNEUROSCI.3842-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alén F, Santos A, Moreno-Sanz G, González-Cuevas G, Giné E, Franco-Ruiz L, et al. Cannabinoid-induced increase in relapse-like drinking is prevented by the blockade of the glycine-binding site of N-methyl-D-aspartate receptors. Neuroscience. 2009;158:465–473. doi: 10.1016/j.neuroscience.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Alger BE. Endocannabinoid signaling in neural plasticity. Current Topics in Behavioral Neurosciences. 2009;1:141–172. doi: 10.1007/978-3-540-88955-7_6. [DOI] [PubMed] [Google Scholar]
- Alger BE, Pitler TA, Wagner JJ, Martin LA, Morishita W, Kirov SA, et al. Retrograde signalling in depolarization-induced suppression of inhibition in rat hippocampal CA1 cells. The Journal of Physiology. 1996;496(Pt 1):197–209. doi: 10.1113/jphysiol.1996.sp021677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariwodola OJ, Weiner JL. Ethanol potentiation of GABAergic synaptic transmission may be self-limiting: role of presynaptic GABA(B) receptors. The Journal of Neuroscience. 2004;24:10679–10686. doi: 10.1523/JNEUROSCI.1768-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnone M, Maruani J, Chaperon F, Thiébot MH, Poncelet M, Soubrié P, et al. Selective inhibition of sucrose and ethanol intake by SR 141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology. 1997;132:104–106. doi: 10.1007/s002130050326. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Ninan I, Arancio O. Acute ethanol suppresses glutamatergic neurotransmission through endocannabinoids in hippocampal neurons. Journal of Neurochemistry. 2008;107:1001–1013. doi: 10.1111/j.1471-4159.2008.05685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basavarajappa BS, Yalamanchili R, Cravatt BF, Cooper TB, Hungund BL. Increased ethanol consumption and preference and decreased ethanol sensitivity in female FAAH knockout mice. Neuropharmacology. 2006;50:834–844. doi: 10.1016/j.neuropharm.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Cravatt BF, Boehm SL, 2nd, Walker D, Harris RA. Role of endocannabinoids in alcohol consumption and intoxication: studies of mice lacking fatty acid amide hydrolase. Neuropsychopharmacology. 2007;32:1570–1582. doi: 10.1038/sj.npp.1301274. [DOI] [PubMed] [Google Scholar]
- Brown EE, Fibiger HC. Differential effects of excitotoxic lesions of the amygdala on cocaine-induced conditioned locomotion and conditioned place preference. Psychopharmacology. 1993;113:123–130. doi: 10.1007/BF02244344. [DOI] [PubMed] [Google Scholar]
- Brzózka MM, Fischer A, Falkai P, Havemann-Reinecke U. Acute treatment with cannabinoid receptor agonist WIN55212.2 improves prepulse inhibition in psychosocially stressed mice. Behavioural Brain Research. 2011;218:280–287. doi: 10.1016/j.bbr.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Bueno CH, Zangrossi H, Jr, Viana MB. The inactivation of the basolateral nucleus of the rat amygdala has an anxiolytic effect in the elevated T-maze and light/dark transition tests. Brazilian Journal of Medical and Biological Research. 2005;38:1697–1701. doi: 10.1590/s0100-879x2005001100019. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Heifets BD, Kaeser PS, Südhof TC, Castillo PE. Endocannabinoid-mediated long-term plasticity requires cAMP/PKA signaling and RIM1alpha. Neuron. 2007;54:801–812. doi: 10.1016/j.neuron.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CQ, Puente N, Grandes P, Castillo PE. Dopaminergic modulation of endocannabinoid-mediated plasticity at GABAergic synapses in the prefrontal cortex. The Journal of Neuroscience. 2010;30:7236–7248. doi: 10.1523/JNEUROSCI.0736-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cippitelli A, Bilbao A, Gorriti MA, Navarro M, Massi M, Piomelli D, et al. The anandamide transport inhibitor AM404 reduces ethanol self administration. The European Journal of Neuroscience. 2007;26:476–486. doi: 10.1111/j.1460-9568.2007.05665.x. [DOI] [PubMed] [Google Scholar]
- Cippitelli A, Bilbao A, Hansson AC, del Arco I, Sommer W, Heilig M, et al. Cannabinoid CB1 receptor antagonism reduces conditioned reinstatement of ethanol-seeking behavior in rats. The European Journal of Neuroscience. 2005;21:2243–2251. doi: 10.1111/j.1460-9568.2005.04056.x. [DOI] [PubMed] [Google Scholar]
- Colombo G, Orrù A, Lai P, Cabras C, Maccioni P, Rubio M, et al. The cannabinoid CB1 receptor antagonist, rimonabant, as a promising pharmacotherapy for alcohol dependence: preclinical evidence. Molecular Neurobiology. 2007;36:102–112. doi: 10.1007/s12035-007-0017-y. [DOI] [PubMed] [Google Scholar]
- Colombo G, Serra S, Vacca G, Carai MA, Gessa GL. Endocannabinoid system and alcohol addiction: pharmacological studies. Pharmacology, Biochemistry, and Behavior. 2005;81:369–380. doi: 10.1016/j.pbb.2005.01.022. [DOI] [PubMed] [Google Scholar]
- Criswell HE, Breese GR. A conceptualization of integrated actions of ethanol contributing to its GABAmimetic profile: a commentary. Neuropsychopharmacology. 2005;30:1407–1425. doi: 10.1038/sj.npp.1300750. [DOI] [PubMed] [Google Scholar]
- Das J, Pany S, Rahman GM, Slater SJ. PKC epsilon has an alcohol-binding site in its second cysteine-rich regulatory domain. The Biochemical Journal. 2009;421:405–413. doi: 10.1042/BJ20082271. [DOI] [PubMed] [Google Scholar]
- Davis M, Rainnie D, Cassell M. Neurotransmission in the rat amygdala related to fear and anxiety. Trends in Neurosciences. 1994;17:208–214. doi: 10.1016/0166-2236(94)90106-6. [DOI] [PubMed] [Google Scholar]
- Davis MI, Ronesi J, Lovinger DM. A predominant role for inhibition of the adenylate cyclase/protein kinase A pathway in ERK activation by cannabinoid receptor 1 in N1E-115 neuroblastoma cells. The Journal of Biological Chemistry. 2003;278:48973–48980. doi: 10.1074/jbc.M305697200. [DOI] [PubMed] [Google Scholar]
- Diaz MR, Chappell AM, Christian DT, Anderson NJ, McCool BA. Dopamine D3-like receptors modulate anxiety-like behavior and regulate GABAergic transmission in the rat lateral/basolateral amygdala. Neuropsychopharmacology. 2011;36:1090–1103. doi: 10.1038/npp.2010.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz MR, Christian DT, Anderson NJ, McCool BA. Chronic ethanol and withdrawal differentially modulate lateral/basolateral amygdala paracapsular and local GABAergic synapses. The Journal of Pharmacology and Experimental Therapeutics. 2011;337:162–170. doi: 10.1124/jpet.110.177121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economidou D, Mattioli L, Cifani C, Perfumi M, Massi M, Cuomo V, et al. Effect of the cannabinoid CB1 receptor antagonist SR-141716A on ethanol self-administration and ethanol-seeking behaviour in rats. Psychopharmacology. 2006;183:394–403. doi: 10.1007/s00213-005-0199-9. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Morris KA, O’Brien A, Robbins TW. The basolateral amygdala-ventral striatal system and conditioned place preference: further evidence of limbic-striatal interactions underlying reward-related processes. Neuroscience. 1991;42:1–18. doi: 10.1016/0306-4522(91)90145-e. [DOI] [PubMed] [Google Scholar]
- Freedland CS, Sharpe AL, Samson HH, Porrino LJ. Effects of SR141716A on ethanol and sucrose self-administration. Alcoholism: Clinical and Experimental Research. 2001;25:277–282. [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiological Reviews. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Geracitano R, Kaufmann WA, Szabo G, Ferrraguti F, Capogna M. Synaptic heterogeneity between mouse paracapsular intercalated neurons of the amygdala. The Journal of Physiology. 2007;585(Pt 1):117–134. doi: 10.1113/jphysiol.2007.142570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdeman GL, Ronesi J, Lovinger DM. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nature Neuroscience. 2002;5:446–451. doi: 10.1038/nn832. [DOI] [PubMed] [Google Scholar]
- Gonzalez LE, Andrews N, File SE. 5-HT1A and benzodiazepine receptors in the basolateral amygdala modulate anxiety in the social interaction test, but not in the elevated plus-maze. Brain Research. 1996;732:145–153. doi: 10.1016/0006-8993(96)00517-3. [DOI] [PubMed] [Google Scholar]
- Gremel CM, Cunningham CL. Involvement of amygdala dopamine and nucleus accumbens NMDA receptors in ethanol-seeking behavior in mice. Neuropsychopharmacology. 2009;34:1443–1453. doi: 10.1038/npp.2008.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heifets BD, Castillo PE. Endocannabinoid signaling and long-term synaptic plasticity. Annual Review of Physiology. 2009;71:283–306. doi: 10.1146/annurev.physiol.010908.163149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Egli M, Crabbe JC, Becker HC. Acute withdrawal, protracted abstinence and negative effect in alcoholism: are they linked? Addiction Biology. 2010;15:169–184. doi: 10.1111/j.1369-1600.2009.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroi N, White NM. The lateral nucleus of the amygdala mediates expression of the amphetamine-produced conditioned place preference. The Journal of Neuroscience. 1991;11:2107–2116. doi: 10.1523/JNEUROSCI.11-07-02107.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houchi H, Babovic D, Pierrefiche O, Ledent C, Daoust M, Naassila M. CB1 receptor knockout mice display reduced ethanol-induced conditioned place preference and increased striatal dopamine D2 receptors. Neuropsychopharmacology. 2005;30:339–349. doi: 10.1038/sj.npp.1300568. [DOI] [PubMed] [Google Scholar]
- Hungund BL, Basavarajappa BS. Distinct differences in the cannabinoid receptor binding in the brain of C57BL/6 and DBA/2 mice, selected for their differences in voluntary ethanol consumption. Journal of Neuroscience Research. 2000;60:122–128. doi: 10.1002/(SICI)1097-4547(20000401)60:1<122::AID-JNR13>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Hungund BL, Basavarajappa BS. Role of endocannabinoids and cannabinoid CB1 receptors in alcohol-related behaviors. Annals of the New York Academy of Sciences. 2004;1025:515–527. doi: 10.1196/annals.1316.064. [DOI] [PubMed] [Google Scholar]
- Jo YH, Chen YJ, Chua SC, Jr, Talmage DA, Role LW. Integration of endocannabinoid and leptin signaling in an appetite-related neural circuit. Neuron. 2005;48:1055–1066. doi: 10.1016/j.neuron.2005.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun SB, Cuzon Carlson V, Ikeda S, Lovinger D. Vibrodissociation of neurons from rodent brain slices to study synaptic transmission and image presynaptic terminals. Journal of Visualized Experiments. 2011;51 doi: 10.3791/2752. pii 2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang-Park MH, Kieffer BL, Roberts AJ, Siggins GR, Moore SD. Presynaptic delta opioid receptors regulate ethanol actions in central amygdala. The Journal of Pharmacology and Experimental Therapeutics. 2007;320:917–925. doi: 10.1124/jpet.106.112722. [DOI] [PubMed] [Google Scholar]
- Katona I, Rancz EA, Acsady L, Ledent C, Mackie K, Hajos N, et al. Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. The Journal of Neuroscience. 2001;21:9506–9518. doi: 10.1523/JNEUROSCI.21-23-09506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelm MK, Criswell HE, Breese GR. The role of protein kinase A in the ethanol-induced increase in spontaneous GABA release onto cerebellar Purkinje neurons. Journal of Neurophysiology. 2008;100:3417–3428. doi: 10.1152/jn.90970.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelm MK, Criswell HE, Breese GR. Ethanol-enhanced GABA release: a focus on G protein-coupled receptors. Brain Research Reviews. 2011;65:113–123. doi: 10.1016/j.brainresrev.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliethermes CL. Anxiety-like behaviors following chronic ethanol exposure. Neuroscience & Biobehavioral Reviews. 2005;28:837–850. doi: 10.1016/j.neubiorev.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Läck AK, Christian DT, Diaz MR, McCool BA. Chronic ethanol and withdrawal effects on kainate receptor-mediated excitatory neurotransmission in the rat basolateral amygdala. Alcohol. 2009;43:25–33. doi: 10.1016/j.alcohol.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Läck AK, Diaz MR, Chappell A, DuBois DW, McCool BA. Chronic ethanol and withdrawal differentially modulate pre- and postsynaptic function at glutamatergic synapses in rat basolateral amygdala. Journal of Neurophysiology. 2007;98:3185–3196. doi: 10.1152/jn.00189.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotional memory: in search of systems and synapses. Annals of the New York Academy of Sciences. 1993;702:149–157. doi: 10.1111/j.1749-6632.1993.tb17246.x. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Little PJ, Kuhn CM, Wilson WA, Swartzwelder HS. Differential effects of ethanol in adolescent and adult rats. Alcoholism: Clinical and Experimental Research. 1996;20:1346–1351. doi: 10.1111/j.1530-0277.1996.tb01133.x. [DOI] [PubMed] [Google Scholar]
- López-Moreno JA, González-Cuevas G, Moreno G, Navarro M. The pharmacology of the endocannabinoid system: functional and structural interactions with other neurotransmitter systems and their repercussions in behavioral addiction. Addiction Biology. 2008;13:160–187. doi: 10.1111/j.1369-1600.2008.00105.x. [DOI] [PubMed] [Google Scholar]
- Lovinger DM. Endocannabinoid liberation from neurons in transsynaptic signaling. Journal of Molecular Neuroscience. 2007;33:87–93. doi: 10.1007/s12031-007-0043-2. [DOI] [PubMed] [Google Scholar]
- Lovinger DM. Presynaptic modulation by endocannabinoids. Handbook of Experimental Pharmacology. 2008;184:435–477. doi: 10.1007/978-3-540-74805-2_14. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, Roberto M. Synaptic effects induced by alcohol. Current Topics in Behavioral Neurosciences. 2013;13:31–86. doi: 10.1007/7854_2011_143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthin GR, Tabakoff B. Activation of adenylate cyclase by alcohols requires the nucleotide-binding protein. The Journal of Pharmacology and Experimental Therapeutics. 1984;228:579–587. [PubMed] [Google Scholar]
- Mackie K. Cannabinoid receptors: where they are and what they do. Journal of Neuroendocrinology. 2008;20(Suppl 1):10–14. doi: 10.1111/j.1365-2826.2008.01671.x. [DOI] [PubMed] [Google Scholar]
- Manzanares J, Ortiz S, Oliva JM, Pérez-Rial S, Palomo T. Interactions between cannabinoid and opioid receptor systems in the mediation of ethanol effects. Alcohol and Alcoholism. 2005;40:25–34. doi: 10.1093/alcalc/agh112. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Martin M, Ledent C, Parmentier P, Maldonado R, Valverde O. Involvement of CB1 cannabinoid receptors in emotional behaviour. Psychopharmacology. 2002;159:379–387. doi: 10.1007/s00213-001-0946-5. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Bonner TI, Lolait SJ. Localization of cannabinoid receptor mRNA in rat brain. The Journal of Comparative Neurology. 1993;327:535–550. doi: 10.1002/cne.903270406. [DOI] [PubMed] [Google Scholar]
- Offermanns S. G-proteins as transducers in transmembrane signalling. Progress in Biophysics and Molecular Biology. 2003;83:101–130. doi: 10.1016/s0079-6107(03)00052-x. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Maejima T, Kano M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron. 2001;29:729–738. doi: 10.1016/s0896-6273(01)00247-1. [DOI] [PubMed] [Google Scholar]
- Olmstead MC, Franklin KB. The development of a conditioned place preference to morphine: effects of lesions of various CNS sites. Behavioral Neuroscience. 1997;111:1313–1323. doi: 10.1037//0735-7044.111.6.1313. [DOI] [PubMed] [Google Scholar]
- Patel S, Kingsley PJ, Mackie K, Marnett LJ, Winder DG. Repeated homotypic stress elevates 2-arachidonoylglycerol levels and enhances short-term endocannabinoid signaling at inhibitory synapses in basolateral amygdala. Neuropsychopharmacology. 2009;34:2699–2709. doi: 10.1038/npp.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pava MJ, Blake EM, Green ST, Mizroch BJ, Mulholland PJ, Woodward JJ. Tolerance to cannabinoid-induced behaviors in mice treated chronically with ethanol. Psychopharmacology. 2012;219:137–147. doi: 10.1007/s00213-011-2387-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perra S, Pillolla G, Luchicchi A, Pistis M. Alcohol inhibits spontaneous activity of basolateral amygdala projection neurons in the rat: involvement of the endocannabinoid system. Alcoholism: Clinical and Experimental Research. 2008;32:443–449. doi: 10.1111/j.1530-0277.2007.00588.x. [DOI] [PubMed] [Google Scholar]
- Pesold C, Treit D. The central and basolateral amygdala differentially mediate the anxiolytic effects of benzodiazepines. Brain Research. 1995;671:213–221. doi: 10.1016/0006-8993(94)01318-c. [DOI] [PubMed] [Google Scholar]
- Rabin RA, Molinoff PB. Activation of adenylate cyclase by ethanol in mouse striatal tissue. The Journal of Pharmacology and Experimental Therapeutics. 1981;216:129–134. [PubMed] [Google Scholar]
- Roberto M, Cruz M, Bajo M, Siggins GR, Parsons LH, Schweitzer P. The endocannabinoid system tonically regulates inhibitory transmission and depresses the effect of ethanol in central amygdala. Neuropsychopharmacology. 2010;35:1962–1972. doi: 10.1038/npp.2010.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Moore SD, Tallent MK, Siggins GR. Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:2053–2058. doi: 10.1073/pnas.0437926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronesi J, Lovinger DM. Induction of striatal long-term synaptic depression by moderate frequency activation of cortical afferents in rat. The Journal of Physiology. 2005;562(Pt 1):245–256. doi: 10.1113/jphysiol.2004.068460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders SK, Shekhar A. Regulation of anxiety by GABAA receptors in the rat amygdala. Pharmacology, Biochemistry, and Behavior. 1995;52:701–706. doi: 10.1016/0091-3057(95)00153-n. [DOI] [PubMed] [Google Scholar]
- Sarter M, Markowitsch HJ. Involvement of the amygdala in learning and memory: a critical review, with emphasis on anatomical relations. Behavioral Neuroscience. 1985;99:342–380. doi: 10.1037//0735-7044.99.2.342. [DOI] [PubMed] [Google Scholar]
- See RE, Fuchs RA, Ledford CC, McLaughlin J. Drug addiction, relapse, and the amygdala. Annals of the New York Academy of Sciences. 2003;985:294–307. doi: 10.1111/j.1749-6632.2003.tb07089.x. [DOI] [PubMed] [Google Scholar]
- Silberman Y, Ariwodola OJ, Chappell AM, Yorgason JT, Weiner JL. Lateral paracapsular GABAergic synapses in the basolateral amygdala contribute to the anxiolytic effects of beta 3 adrenoceptor activation. Neuropsychopharmacology. 2010;35:1886–1896. doi: 10.1038/npp.2010.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberman Y, Ariwodola OJ, Weiner JL. Differential effects of GABAB autoreceptor activation on ethanol potentiation of local and lateral paracapsular GABAergic synapses in the rat basolateral amygdala. Neuropharmacology. 2009;56:886–895. doi: 10.1016/j.neuropharm.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberman Y, Shi L, Brunso-Bechtold JK, Weiner JL. Distinct mechanisms of ethanol potentiation of local and paracapsular GABAergic synapses in the rat basolateral amygdala. The Journal of Pharmacology and Experimental Therapeutics. 2008;324:251–260. doi: 10.1124/jpet.107.128728. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcoholism: Clinical and Experimental Research. 1998;22:670–676. doi: 10.1111/j.1530-0277.1998.tb04310.x. [DOI] [PubMed] [Google Scholar]
- Spear LP, Varlinskaya EI. Adolescence. Alcohol sensitivity, tolerance, and intake. Recent Developments in Alcoholism. 2005;17:143–159. [PubMed] [Google Scholar]
- Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, et al. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475:377–380. doi: 10.1038/nature10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo B, Schlicker E. Effects of cannabinoids on neurotransmission. Handbook of Experimental Pharmacology. 2005;168:327–365. doi: 10.1007/3-540-26573-2_11. [DOI] [PubMed] [Google Scholar]
- Tabakoff B, Nelson E, Yoshimura M, Hellevuo K, Hoffman PL. Phosphorylation cascades control the actions of ethanol on cell cAMP signalling. Journal of Biomedical Science. 2001;8:44–51. doi: 10.1007/BF02255970. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Dimitrakakis ES, Rice O, Gifford A, Volkow ND. Ethanol self-administration and ethanol conditioned place preference are reduced in mice lacking cannabinoid CB1 receptors. Behavioural Brain Research. 2005;164:206–213. doi: 10.1016/j.bbr.2005.06.021. [DOI] [PubMed] [Google Scholar]
- Theile JW, Morikawa H, Gonzales RA, Morrisett RA. Ethanol enhances GABAergic transmission onto dopamine neurons in the ventral tegmental area of the rat. Alcoholism: Clinical and Experimental Resesearch. 2008;32:1040–1048. doi: 10.1111/j.1530-0277.2008.00665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Skike CE, Botta P, Chin VS, Tokunaga S, McDaniel JM, Venard J, et al. Behavioral effects of ethanol in cerebellum are age dependent: potential system and molecular mechanisms. Alcoholism: Clinical and Experimental Research. 2010;34:2070–2080. doi: 10.1111/j.1530-0277.2010.01303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Acute effects of ethanol on social behavior of adolescent and adult rats: role of familiarity of the test situation. Alcoholism: Clinical and Experimental Research. 2002;26:1502–1511. doi: 10.1097/01.ALC.0000034033.95701.E3. [DOI] [PubMed] [Google Scholar]
- Vinod KY, Sanguino E, Yalamanchili R, Manzanares J, Hungund BL. Manipulation of fatty acid amide hydrolase functional activity alters sensitivity and dependence to ethanol. Journal of Neurochemistry. 2008;104:233–243. doi: 10.1111/j.1471-4159.2007.04956.x. [DOI] [PubMed] [Google Scholar]
- Vinod KY, Yalamanchili R, Thanos PK, Vadasz C, Cooper TB, Volkow ND, et al. Genetic and pharmacological manipulations of the CB(1) receptor alter ethanol preference and dependence in ethanol preferring and nonpreferring mice. Synapse. 2008;62:574–581. doi: 10.1002/syn.20533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan FJ, Berton F, Madamba SG, Francesconi W, Siggins GR. Low ethanol concentrations enhance GABAergic inhibitory postsynaptic potentials in hippocampal pyramidal neurons only after block of GABAB receptors. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:5049–5054. doi: 10.1073/pnas.93.10.5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wartmann M, Campbell D, Subramanian A, Burstein SH, Davis RJ. The MAP kinase signal transduction pathway is activated by the endogenous cannabinoid anandamide. FEBS Letters. 1995;359:133–136. doi: 10.1016/0014-5793(95)00027-7. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Pearson S, Kadota Y, Gonzalez CE. Identification of ethanol responsive domains of adenylyl cyclase. Alcoholism: Clinical and Experimental Resesarch. 2006;30:1824–1832. doi: 10.1111/j.1530-0277.2006.00219.x. [DOI] [PubMed] [Google Scholar]
- Zhu PJ, Lovinger DM. Retrograde endocannabinoid signaling in a postsynaptic neuron/synaptic bouton preparation from basolateral amygdala. The Journal of Neuroscience. 2005;25:6199–6207. doi: 10.1523/JNEUROSCI.1148-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu PJ, Lovinger DM. Ethanol potentiates GABAergic synaptic transmission in a postsynaptic neuron/synaptic bouton preparation from basolateral amygdala. Journal of Neurophysiology. 2006;96:433–441. doi: 10.1152/jn.01380.2005. [DOI] [PubMed] [Google Scholar]