Abstract
The cerebellum is essential for error-driven motor learning and is strongly implicated in detecting and correcting for motor errors. Therefore, elucidating how motor errors are represented in the cerebellum is essential in understanding cerebellar function, in general, and its role in motor learning, in particular. This review examines how motor errors are encoded in the cerebellar cortex in the context of a forward internal model that generates predictions about the upcoming movement and drives learning and adaptation. In this framework, sensory prediction errors, defined as the discrepancy between the predicted consequences of motor commands and the sensory feedback, are crucial for both on-line movement control and motor learning. While many studies support the dominant view that motor errors are encoded in the complex spike discharge of Purkinje cells, others have failed to relate complex spike activity with errors. Given these limitations, we review recent findings in the monkey showing that complex spike modulation is not necessarily required for motor learning or for simple spike adaptation. Also, new results demonstrate that the simple spike discharge provides continuous error signals that both lead and lag the actual movements in time, suggesting errors are encoded as both an internal prediction of motor commands and the actual sensory feedback. These dual error representations have opposing effects on simple spike discharge, consistent with the signals needed to generate sensory prediction errors used to update a forward internal model.
Keywords: Cerebellum, Purkinje cells, Motor learning, Internal model, Complex spikes, Simple spikes
Introduction
Errors in motor performance, defined as the divergence between the desired goal and the actual motor behavior, are critical to how we control ongoing movements and motor learning. Numerous studies over the last 40 years document that the cerebellum plays a key role in many types of motor learning including being a site of plasticity. The cerebellum’s involvement in motor adaptation is thought to utilize a supervised learning scheme instructed by error signals. Therefore, understanding cerebellar-dependent motor learning is intimately tied to understanding cerebellar processing of motor errors. One of the earliest and most investigated mechanisms of cerebellar learning, the Marr-Albus-Ito hypothesis, is based on the architecture of the cerebellar cortex and assigns specific functions to the climbing fiber-Purkinje cell and the mossy fiber-granule cell-parallel fiber-Purkinje cell circuits [1–3]. The hypothesis stipulates that climbing fiber inputs from the inferior olive are activated by the feedback of motor errors. In turn, the evoked complex spikes in Purkinje cells induce changes at parallel fiber-Purkinje cell synapses through plasticity mechanisms that alter the simple spike responses to mossy fiber inputs. The resulting learned simple spike responses of Purkinje cells, which provide the only output of the cerebellar cortex, are then reflected in the behavioral modifications.
An alternate approach to understanding cerebellar function and its role in learning has emerged from computational theories of motor control. In this perspective, the cerebellum functions as a forward internal model that predicts the consequences of the motor commands. A corollary of this hypothesis is that motor learning involves updating the forward internal model to minimize systematic errors. In forward internal model theories, however, errors are not simply due to sensory feedback. Instead, errors are defined as the discrepancy between the predicted consequences of motor commands and the sensory feedback. Referred to as sensory prediction error, this discrepancy is thought to be the primary signal for both on-line motor control and motor learning.
Given the importance of errors in both motor control and our understanding of cerebellar function, this review focuses on error signaling in the cerebellar cortex in the context of motor learning. Error processing in the cerebellar cortex is examined from the perspectives of both the Marr-Albus-Ito hypothesis and the computational view that the cerebellum functions as a forward internal model. The review evaluates whether the complex spike and/or simple spike discharge encodes motor errors and examines if the discharge of Purkinje cells provides both the internal prediction and sensory feedback signals required for generating sensory prediction errors.
Errors are Critical to the Control of Movements and Motor Learning
Performance errors are used to maintain the accuracy of current movements and to adapt to changes in the environment or body’s response to motor commands [4–7]. Early theories relied on closed-loop control in which ongoing movements are continuously updated by sensory feedback [6–9]. However, sensory feedback is subject to significant delays, rendering a simple closed-loop control scheme inadequate or unstable [6–9]. The sensory delay constraint can be resolved by assuming that the central nervous system (CNS) implements forward internal models that predict the sensory consequences of motor commands. Extensive evidence supports the hypothesis that the CNS uses and acquires forward internal models [6, 10–17]. Typically, the output of a forward internal has been conceptualized as predicting the kinematics of an effector, for example limb position and velocity [7–9]. However, predicting the sensory consequences of motor commands is certainly not restricted to effector kinematics, and the CNS is likely to acquire forward internal models that estimate task-specific performance measures. Furthermore, optimal feedback control theory requires on-line monitoring of task-relevant performance errors in addition to signals encoding the prediction and feedback of effector states [4].
The predictions made by a forward internal model have many potential functions including filtering sensory signals, highlighting or attenuating information as needed for controlling movements, and cancelling the sensory effects of self-generated movements to enhance more pertinent sensory inputs [8, 18, 19]. One of the more important uses of motor predictions is in computing sensory prediction errors that are used to guide present and future actions [6, 7]. Many investigators have proposed that sensory prediction errors are the critical error signals for both online control and motor learning [6, 7, 20–24].
Other types of errors have been postulated to induce adaptation including the discrepancy between vision and proprioception [25–27], the actual corrective movements [28, 29], sensory feedback at the end of a movement [30, 31], and rewards/reward prediction errors [32, 33]. Several of these alternative performance measures have been shown, at least to some degree, to contribute to the control of movement and induce adaptation [32–35]. However, this review focuses on sensory prediction errors because extensive evidence consistently supports their role in motor adaptation and dominance over other types of errors in updating a forward internal model [6, 7, 21, 22, 24, 32, 36].
A widely accepted view is that the cerebellum implements and acquires forward internal models [6, 9, 37–39]. Numerous studies have shown that cerebellum plays a central role in motor learning. Adaptation to novel visuomotor transformations and force fields results in increased cerebellar activation in healthy subjects [15, 40–42]. In patients suffering from cerebellar disorders or lesions, eye movement adaptation is reduced [13, 43, 44] and learning predictable perturbations of limb movements is degraded [11, 14, 45–47]. Cerebellar-dependent learning has also been described for different effectors and across a range of motor behaviors in numerous animal studies, including vestibulo-ocular reflex (VOR) and smooth pursuit adaptation, classical eye-blink conditioning, and reach adaptation (for reviews see [48–52]).
More recently, it has been suggested that the cerebellum is involved in processing sensory prediction errors [6, 8, 16, 53]. For example, increased cerebellar activation is associated with the divergence between movement goal and the actual consequences induced by an unexpected force field or omission of a sensory stimulus [54]. Patients with cerebellar disorders exhibit deficits in sensory prediction error-dependent motor learning during visuomotor transformations in reaching [53] and saccade adaptation [13]. In a reaching experiment, healthy subjects given an explicit instruction on how to compensate for a visuomotor rotation showed a gradual decay in performance consistent with an implicit motor adaptation process driven by sensory prediction errors [21]. In a similar experiment, patients suffering from spinocerebellar ataxia exhibited a very attenuated reduction in performance compared to the controls [55], suggesting the cerebellum is required for processing of the sensory prediction errors. Together, these results strongly implicate the cerebellum in the generation and use of sensory prediction errors in motor adaption. Clearly, there is a need to understand how sensory prediction errors are represented in the firing of cerebellar neurons.
Classical Views of Error Encoding Based on Complex Spikes
Error monitoring and correction has been a long-standing hypothesis of cerebellar function, and there is a long history of studies focused on identifying error-related signals in the cerebellum [56–60]. The principal hypothesis is that motor error signals are encoded in the low-frequency, complex spike discharge of Purkinje cells [57–62]. This view is a central element of the Marr-Albus-Ito hypothesis in which long-term depression (LTD) of parallel fiber-Purkinje cell synapses results from co-activation of parallel fiber and climbing fiber inputs [1–3]. The concept that climbing fibers provide error signals offered a circuitry framework for LTD at parallel fiber-Purkinje cell synapses, in which the two major inputs to the cerebellum, the climbing fiber and mossy fiber afferents, were assigned unique functional roles.
Many studies have observed that complex spike discharge modulates with motor errors. In the floccular complex, complex spikes are driven by retinal slip during smooth pursuit, VOR adaptation, and ocular following [63–66]. In limb movements, complex spikes are modulated by unexpected loads [57], redirection of a reach [67], and reach end point errors [61]. Complex spike discharge is also associated with various perturbations applied during locomotion [67–69]. However, the degree to which this modulation can accurately represent different aspects of motor errors has received less attention. For example, while complex spike firing can be used to decode the quadrant in which an error occurred during reaching, the specificity of the spatial encoding was limited [61]. In the oculomotor vermis, complex spikes signal the direction of eye-position errors during saccadic adaptation; however, whether they encode error magnitude is questionable [70]. Additionally, the probability of evoking a complex spike was not correlated with the reduction in error. A subsequent study that explicitly examined for both amplitude and direction error sensitivity found that in one group of Purkinje cells, the increase in complex spikes is tuned to small errors in eye position, and in another group of neurons, the complex spike discharge did not encode error magnitude [71]. The size of motor errors has differential effects on learning and adaptation [72–74] and would necessitate some level of encoding of error magnitude by the cerebellum. Therefore, while in many behaviors complex spike discharge is strongly coupled to motor errors, the precision of the error signals provided by the climbing fiber input remains to be determined.
Complex Spikes and Adaptation
A consequence of the Marr-Albus-Ito hypothesis is the view that climbing fiber inputs serve as teaching signals that drive motor adaptation. Support for this hypothesis includes complex spike discharge associated with learning a predictable target redirection during VOR [75–77] and smooth pursuit adaptation [78, 79] as well as adaptation of reach movements to unexpected loads [57] and visuomotor transformations [80, 81]. Both the degree of Purkinje cell plasticity and the motor adaptation are graded by the duration of the complex spikes during smooth pursuit adaptation [79] and eye-blink conditioning [82]. In cerebellar-dependent classical conditioning paradigms such as the adaptation of an eye-blink in response to a tone (i.e., conditioned stimulus) after repeated pairing with an air puff (i.e., unconditioned stimulus, US), a longstanding view is that information about the US is carried by climbing fiber input [83, 84]. Indeed, the US can be substituted by climbing fiber stimulation to successfully evoke the conditioned eye-blink response [85]. Additionally, inactivation of the inferior olive and blockade of cerebellar LTD prevents acquisition of the response to the conditioned stimulus (for review, see [83]) [86–88]. In classical conditioning, the unconditioned stimulus can be viewed as an error signal [88] that represents the failure to avoid the potentially damaging stimulus of an air puff to the eye. The Marr-Albus-Ito hypothesis provides a coherent view of cerebellar function that elegantly overlaps with the cortex architecture and physiology. However, this hypothesis has yet to be cohesively integrated with the computational views on cerebellar function as described by the forward internal model theory.
Potential Issues with Error Encoding by Complex Spikes
Despite the evidence described above, a growing body of research suggests that complex spike discharge may not serve as a pure error signal. For example, although complex spikes are activated during center-out reaching, their modulation could not be related to direction or speed errors [89, 90]. Perturbations and performance errors during reaching in cats failed to evoke responses in inferior olive neurons, the origin of the climbing fiber projection [91]. In a saccadic adaptation task, complex spike discharge in the oculomotor vermis increases late in adaptation and persists after learning has stabilized, when the errors are minimal [92]. A similar build-up of complex spike discharge occurs as performance errors decrease during smooth pursuit adaptation [50, 93]. Both observations strongly deviate from the classical error encoding hypothesis that would predict inhibition of complex spike discharge as learning progresses. Additionally, there is no evidence to date that complex spikes encode the prediction of motor errors that are essential to generating sensory prediction errors [72, 94].
It has been suggested that the low frequency of the complex spike discharge provides only a limited computational bandwidth to encode errors, particularly errors during fast or continuous movements. For example, in reaching tasks, complex spike error signals occur in a small fraction of trials and are evident only after extensive averaging [61, 81]. Graded changes in intracellular Ca++ as well the distribution of response latencies in Purkinje cell dendrites to sensory events provide possible mechanisms for encoding more than binary information in the complex spike discharge [95, 96]. Also, mitigation of the bandwidth problem has been proposed based on the synchrony of the complex spike discharge at the population level. A large number of studies have shown that complex spikes can become synchronized, particularly in response to sensory inputs or motor behaviors [95–101]. Several solutions have been proposed on how the population complex spike activity can provide a much finer temporal resolution than individual Purkinje cells. One solution relies on the observation of non-zero phase lags among the complex spike discharge of Purkinje cells in different regions of the cerebellar cortex to generate time intervals shorter than the normal periodicity of inferior olivary neurons [102]. However, support for this concept has only been obtained after administration of harmaline. A second proposal relies on chaotic resonance in the population response to encode finer temporal representation of errors [103]. However, this proposal is based only on modeling and simulations. Therefore, while the view that increased temporal resolution in the complex spike firing can be found in the population activity is intriguing, experimental support is needed.
There are similar inconsistencies with the hypothesis that complex spikes are essential for adaptation. For example, although similar climbing fiber activation occurs in paradigms that drive both increase and decrease VOR gain, complex spike-driven adaptation occurs only in gain increase paradigms [104]. Moreover, while optogenetic activation of climbing fibers can induce VOR gain-increase adaptation [104], similar findings result from optogenetically driven increases in simple spike discharge [105]. During VOR adaptation in which monkeys tracked a target against a moving background, simple spike firing was modulated by target motion across all conditions while complex spike modulation could be reduced, negated, or dominated by background movement [106]. These changes in complex spike modulation occurred even though the background movement was irrelevant to behavior and did not contribute to the learning, suggesting a potential contribution of attention or other processes to complex spike firing not directly related to motor adaptation. Additionally, VOR adaptation can occur in the absence of complex spike modulation [107]. It is also worth noting that retinal slip errors, which are perhaps the most reliable stimuli to evoke complex spikes, are not the best predictors of VOR gain adaptation or phase learning [108]. Therefore, the complex spikes’ roles in signaling motor errors and in cerebellar adaptation appear highly dependent on the experimental paradigm.
Simple Spike Error Encoding
Given the many unresolved issues regarding the role of the complex spikes as the primary source of error information and driver of cerebellar cortical plasticity, it is necessary to consider the possibility that error signals are also encoded in the simple spike discharge. Until recently, there was minimal information regarding the presence of error signals encoded in the simple spike activity. Simple spike discharge modulates with trial success or failure in a reaching task [109] and also with direction and speed errors during manual circular tracking [110]. However, in the circular tracking task, interpretation of the results is confounded because the error parameters were not independent of the kinematics. Adaptation studies also provide evidence for simple spike error encoding. Cerebellar-dependent VOR adaptation can be driven by instructive signals in the simple spike firing [107] such as the phase of eye movement relative to head motion that is encoded by mossy fibers [108]. Gain increase of the VOR appears dependent on complex spike-driven LTD and gain decrease on non-complex spike-driven LTP mechanisms [76, 77]. At the population level, changes in simple spike output after smooth pursuit adaptation are sufficient to drive learning [111]. Together, these observations suggest the presence of error signals in the simple spike discharge and challenge the assumption that cerebellar learning is completely climbing fiber-dependent.
More recently, studies based on a manual random tracking paradigm permitted a more rigorous description of the signals carried by simple spike firing and eliminated some of the previous limitations and confounds. Random tracking provides an exhaustive coverage of the work space that facilitates a more robust analysis of the relationship between neuronal activity and parameters describing motor behavior while maintaining statistical independence between movement parameters [112–114]. Other advantages of random tracking capitalize upon the task difficulty and flexibility of the paradigm. Tracking a randomly moving target over relatively long time intervals (6 to 10 s) is challenging and results in frequent excursions outside the target. However, the monkeys are allowed 500 ms to correct the cursor position and avoid trial abort. As a result, the task depends on continuous evaluation of motor performance and implementation of corrective movements to compensate for errors.
In random tracking, performance error is defined as the divergence between the current movement goal, approximated by the target center, and the consequences of the motor commands, indicated by cursor movement. The performance errors evaluated included the cursor position relative to the target center, the distance between cursor and target center, and angular distance from the direction necessary to move from the current position to the target center [113]. Although related to arm movement, an important difference between the kinematic and error variables is that the former describe arm movements irrespective of the movement goal [112] while the latter are task-specific, provide a continuous measure of motor performance, and depend explicitly on the behavioral goal. Decoding analysis demonstrates remarkable accuracy in predicting upcoming errors [113]. In contrast with the classical view that error signals are confined to the complex spike discharge, these findings demonstrate a rich and accurate representation of performance errors in the simple spike firing.
A notable aspect of the error encoding by the simple spike discharge is its temporal properties. In a majority of the Purkinje cells, at least one error parameter is represented by a pair of signals predicting upcoming errors and conveying feedback information of past errors. Moreover, the dual representations of error parameters have opposing effects on simple spike discharge, consistent with the predictive and feedback signals necessary for computing a sensory prediction error [113, 115]. Dual encoding has also been observed in different brain regions; therefore, signaling both predictive and feedback information may be a general aspect of neural representations in the CNS. During a similar random tracking task, the temporal tuning curves of the information content for many neurons in the primary motor cortex both lead and lag hand position and velocity [114]. Interestingly, during a monkey rule processing task, signals between prefrontal and parietal cortices show bi-modal properties with anti-correlated modulations temporally segregated at 50 and 150 ms [116]. The predictive signal in the dual representation of an error parameter can be understood as predicting the sensory consequences of a motor command in the error domain and is consistent with the hypothesis that the cerebellum acts as a forward internal model [6–8, 12, 38].
The instructive signal that drives adaptation of a forward internal model is the sensory prediction error. As reviewed above, psychophysical, imaging, and patient studies demonstrate that sensory prediction errors are critical in driving adaptation of both eye and limb movements [6, 21, 117, 118] and that the cerebellum is involved in processing error signals consistent with sensory prediction errors [11, 13, 16, 23, 53]. Interestingly, most discussions of forward internal models have focused on the effectors and the mismatch between internal predictions and feedback of the effector. We interpret the task-specific performance error signals in the simple spike firing as evidence that the cerebellum acquires a forward internal model of the task that explicitly predicts the sensory consequences of motor commands relative to the current self-directed movement goal.
We hypothesize that the predictive and feedback signals with opposing modulations of simple spike activity provide the neural substrates needed to approximate sensory prediction errors. A consequence of this hypothesis is that a well-adapted internal model will reduce the simple spike sensitivity to self-generated sensory information, in which the signals encoding consequences of motor commands and those encoding sensory feedback cancel each other. Consistent with this interpretation, rostral fastigial nuclear projection targets of Purkinje cells show greater sensitivity to passive self-motion driven by sensory feedback than to active, self-generated motion driven by both sensory and internal feedback [19]. Also, the long-term decrease in cerebellar sensitivity to motor errors observed with PET imaging during adaptation to constant force fields could be due to an improved match between internally generated predictions and sensory feedback [119]. A similar reduction in the cerebellar BOLD response occurs during a cognitive task in which subjects learn first-order rules [120], and a study of fear conditioning found that cerebellar activation decreased during the conditioning phase while either unexpected application or omission of the noxious stimuli resulted in increased cerebellar activation [121]. Although the hypothesis that individual Purkinje cells directly compute sensory prediction errors by comparing the predictive and feedback signals is very seductive, the fact that the two signals are separated in time requires further investigation. It is possible that this computation is performed downstream, in the cerebellar nuclei.
Simple Spike and Complex Spike Changes During Reach Adaptation
Single cell investigations in the monkey into the changes in cerebellar neuron firing during motor adaptation have primarily focused on eye movements including VOR, saccades and smooth pursuit [75, 78, 79, 92, 93, 104]. As reviewed above, in many of these studies, the complex spikes are strongly activated and correlated with changes in simple spike firing [57, 76, 78, 79]; however, the role of the climbing fibers in the adaptation or signaling errors is less evident in others [70, 81, 92, 93]. Few studies have examined the changes in simple spike and complex spike discharge during limb movement adaptation [57, 80, 81].
Although simple spike firing during learning has been documented in many behaviors and cerebellar cortical regions [57, 78, 80, 122–125], few studies have quantified changes in the encoding of movement parameters. Simple spike encoding of eye velocity and acceleration progressively changes in the floccular complex during smooth pursuit adaptation [126]. In the oculomotor vermis, increases in smooth pursuit gain involve increases in velocity coding in the summed Purkinje cell simple spike activity [50, 127]. Therefore, we recently investigated how the representation of kinematics in the simple spike discharge changes during reach adaptation [128].
Using an error clamp design that restricted movements along the direction of the reach, monkeys rapidly adapted to assistive or resistive perturbations. Perturbation magnitude, sign (assistive or resistive), duration, and start position were varied to require that the animal adapted in each experiment by minimizing the perturbation’s effect on the reach kinematics. As a result, the position, velocity, and acceleration profiles late in adaptation are smooth and continuous. Randomly introduced catch trials without a perturbation produced aftereffects in the movement kinematics approximately equal to those produced during early learning, but opposite in sign. The prominent after-effects demonstrate that the motor system is generating feedforward commands that effectively counteract the kinematic disturbances produced by the perturbation, consistent with modifying a forward internal model [17, 78, 79, 129–134].
One of the more important behavioral aspects of the learning is that the reach kinematics before and after adaptation are highly similar. In most motor learning experiments, movements in the adapted state differ from the baseline including paradigms involving eye movements [78, 123, 125, 126, 135–137], reaching [80], and classical conditioning [124, 138]. In our study, the high degree of similarity before and after adaptation allowed a direct evaluation of changes in simple spike kinematic encoding not confounded by changes in the movement.
The first major observation is that the adaption to mechanical perturbation involved widespread modifications in the simple spike discharge. The changes were multifaceted, with both increases and decreases of existing responses as well as addition of new components. Therefore, the changes do not correspond to the Marr-Albus-Ito model of decreased simple spike firing. Instead, a much more varied set of firing alterations occurred, suggesting that different plasticity mechanisms were engaged, either within or outside the cerebellum (for reviews see [139–141]).
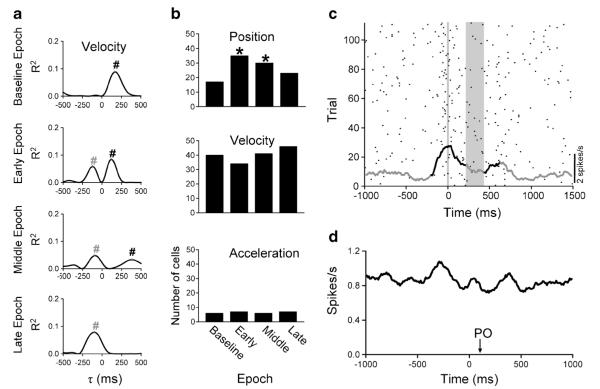
The sensitivity and the timing of the simple spike encoding of position and velocity also progressively changed during the learning with a shift toward position encoding early in adaptation followed by increased velocity encoding later in adaptation. The timing of the simple spike kinematic representations changed within individual cells, including shifts in predictive versus feedback signals (Fig. 1a, b). Feedback-based encoding of position increased early in learning, while velocity encoding decreased, consistent with increased reliance on position sensory feedback when initially confronted with the perturbation. As learning occurred, the timing shifts reversed. The shifts in the timing of position and velocity encoding may be neural correlates of the psychophysical observation that in adaptation to novel force fields, the motor system utilizes a combination of position and velocity primitives that shifts over the course of learning [142].
Fig. 1.
Simple spike and complex spike modulation during adaptation to a mechanical perturbation. a Example of velocity encoding from a Purkinje cell. R2 profiles are plotted as functions of time between neural activity and behavior. At negative τ values (leads), neural activity leads behavior. At positive τ values (lags), neural activity lags behavior. This example demonstrates changes in timing with learning epochs, from significant simple spike velocity encoding at feedback times during baseline to feedforward only encoding in late adaptation. Graphs depict the four task epochs of baseline, early, middle, and late adaptation and number sign (#) indicates the timing of feedforward (gray) or feedback (black) signals. b Histograms of the dominant kinematic parameter (i.e., greatest R2 value) before and during adaptation to the perturbation. Early learning involves an increase in the position encoding that gradually decreases by late learning. Conversely, velocity encoding decreases in early learning and reverts in late learning. Asterisk (*) indicates significant difference (p<0.05). c Raster and average firing plots of the complex spike discharge of a single Purkinje cell throughout the adapt epoch. Complex spike discharge significantly increased around movement onset and after the perturbation. Significant change in complex spike firing compared to baseline is denoted by black and periods of no change by gray. Vertical gray line denotes movement onset and the time of the mechanical perturbation is denoted by the gray vertical bar. d Complex spike average firing from 49 Purkinje cells aligned on perturbation onset (PO). The overall weak effect of the perturbation on the complex spike firing can also be appreciated from the population averages. All data are from Hewitt et al. [128], with permission
The second major observation is that the behavioral and simple spike changes are primarily independent of climbing fiber input. The perturbation resulted in modulation of the complex spike discharge in a small subset of Purkinje cells, suggesting that climbing fiber input is not necessary for motor learning or simple spike adaptation (Fig. 1c, d). Complex spike firing modulation was more pronounced around reach onset, as is commonly observed for reaching movements [81, 143, 144].
The final major observation is that the kinematic adaptations preceded the changes in simple spike firing. On average, the time constant for the simple spike firing adaptation was ~30 trials and ~15 trials for the kinematic adaptation, implying that Purkinje cell simple spike discharge does not encode the motor command and that as such, the cerebellar cortex does not function as an inverse dynamics internal model. Consistent with this conclusion, Purkinje cell discharge does not encode loads and/or muscle activity [37, 127, 145]. Alternatively, the findings support the hypothesis that the cerebellar cortex functions as a forward internal model [37, 113]. In this view, the forward internal model remains compromised until the inverse dynamics model, located elsewhere in the CNS, computes the dynamics necessary to adapt to the mechanical perturbation, possibly using an explicit, conscious strategy. The later changes in simple spike activity reflect updates to the forward internal model that allow for more automated control [120, 146]. However, it needs to be acknowledged that there are differing views on the exact order for updating inverse and forward internal models during adaptation [39, 147, 148].
These new results are divergent from the Marr-Albus-Ito hypothesis of motor learning in which complex spikes provide the teaching signals that drive adaptation. Instead, the results are in agreement with extensive changes in the simple spike modulation observed during smooth pursuit and VOR adaptation [50, 92, 93]. The new findings also provide further evidence that Purkinje cell simple spike firing is compatible with the output of a forward internal model [37, 112, 149, 150]. The changes in the predictive and feedback kinematic simple spike signals thought to be involved in the computation of sensory prediction errors [113, 115] suggest that the teaching signals driving adaptation are conveyed by the simple spike activity.
Conclusions
The history of cerebellar studies shows an intriguing yet frustrating mixture of experimental findings. The cerebellum is essential for accurate behavioral predictions and learns from motor errors, especially from sensory prediction errors. However, a theory of cerebellar processing encompassing all aspects of its circuitry and function in a single, unified model remains elusive. Understanding cerebellar contribution to motor adaptation illustrates the challenges in providing a general framework of cerebellar function. While the classical Marr-Albus-Ito hypothesis that error signals are encoded by climbing fibers and supervise learning at the parallel fiber-Purkinje cell synapse provides an elegant view of the roles of specific cerebellar circuits, it fails to generalize across all experimental conditions and its confirmation remains conspicuously paradigm specific. Alternatively, forward internal model theories of the cerebellum arising from computational motor control impose specific requirements on the error signals but remain agnostic as to the implementation at the circuitry level. Although these two theories are not mutually exclusive, attempting to integrate them exposes the limitations of the Marr-Albus-Ito hypothesis, as complex spikes likely lack the computational bandwidth necessary to carry the required error information, and there exists no evidence to date that they encode motor predictions.
If the classical hypothesis that motor errors are encoded by the complex spikes cannot be generalized across all experimental paradigms, and if the Marr-Albus-Ito hypothesis accounts for only a part of the cerebellar-dependent motor learning, different frameworks need to be considered. One important consideration is that cerebellar cortical output modulates complex spike activity. Optogenetic activation of Purkinje cells induces a complex spike response [151, 152], demonstrating that simple spike activity can modulate complex spike discharge. At the population level, complex spike synchrony is modulated by simple spike activity [153]. During smooth pursuit learning, simple spike firing increase also increases the probability of complex spike discharge, which in turn decreases the simple spike response on the subsequent trial [154]. The finding that simple spike firing modulates complex spike discharge suggests that rather than providing an error signal driven by sensory feedback, climbing fiber input may reflect the appropriateness of the cerebellar cortex’s response to specific behaviors. Based on the observations that the probability of complex spike discharge is greater for small errors during saccades as is error sensitivity in humans during reaching, another refinement to the classical Marr-Albus-Ito hypothesis postulated that complex spikes encode the sensitivity to errors rather than the actual errors [72].
The findings that robust performance error signals are encoded in the simple spike discharge and that motor adaptation occurs in the absence of climbing fiber modulation demonstrate that there is no strict delegation or segregation of error signals to the climbing fiber discharge. Because the simple spike discharge represents many aspects of motor behavior including effector states and task performance, we propose that the simple spikes play a major role providing the instructive signals used for motor adaptation. Next steps include demonstrating how motor adaptation can be driven by instructive signals in the simple spike discharge, under what conditions cerebellar plasticity is driven by simple spike versus complex spike signaling, and how these two classes of Purkinje cell discharge work in concert to shape the cerebellar function.
Acknowledgments
We wish to thank Lijuan Zhou for technical support and Kris Bettin for manuscript preparation. Supported in part by NIH grants R01 NS18338 and T32 GM008471 and NSF grant IGERT DGE-1069104.
Footnotes
Conflict of Interest Statement There are no current or potential conflicts of interest for the four authors, Laurentiu S. Popa, Martha L. Streng, Angela L. Hewitt, and Timothy J. Ebner
References
- 1.Marr D. A theory of cerebellar cortex. J Physiol. 1969;202:437–70. doi: 10.1113/jphysiol.1969.sp008820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albus JS. A theory of cerebellar function. Math Biosci. 1971;10:25–61. [Google Scholar]
- 3.Ito M, Kano M. Long-lasting depression of parallel fiber-Purkinje cell transmission induced by conjunctive stimulation of parallel fibers and climbing fibers in the cerebellar cortex. Neurosci Lett. 1982;33:253–8. doi: 10.1016/0304-3940(82)90380-9. [DOI] [PubMed] [Google Scholar]
- 4.Todorov E, Jordan MI. Optimal feedback control as a theory of motor coordination. Nat Neurosci. 2002;5:1226–35. doi: 10.1038/nn963. [DOI] [PubMed] [Google Scholar]
- 5.Berniker M, Kording K. Estimating the sources of motor errors for adaptation and generalization. Nat Neurosci. 2008;11:1454–61. doi: 10.1038/nn.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shadmehr R, Smith MA, Krakauer JW. Error correction, sensory prediction, and adaptation in motor control. Annu Rev Neurosci. 2010;33:89–108. doi: 10.1146/annurev-neuro-060909-153135. [DOI] [PubMed] [Google Scholar]
- 7.Wolpert DM, Ghahramani Z. Computational principles of movement neuroscience. Nat Neurosci. 2000;3(Suppl):1212–7. doi: 10.1038/81497. [DOI] [PubMed] [Google Scholar]
- 8.Miall RC, Wolpert DM. Forward models for physiological motor control. Neural Netw. 1996;9:1265–79. doi: 10.1016/s0893-6080(96)00035-4. [DOI] [PubMed] [Google Scholar]
- 9.Kawato M. Internal models for motor control and trajectory planning. Curr Opin Neurobiol. 1999;9:718–27. doi: 10.1016/s0959-4388(99)00028-8. [DOI] [PubMed] [Google Scholar]
- 10.Wolpert DM, Ghahramani Z, Jordan MI. An internal model for sensorimotor integration. Science. 1995;269:1880–2. doi: 10.1126/science.7569931. [DOI] [PubMed] [Google Scholar]
- 11.Morton SM, Bastian AJ. Cerebellar contributions to locomotor adaptations during splitbelt treadmill walking. J Neurosci. 2006;26:9107–16. doi: 10.1523/JNEUROSCI.2622-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robinson DA. Oculomotor control signals. In: Bachyrita P, Lennerstrand G, editors. Basic mechanisms of ocular motility and their clinical implications. Pergamon; Oxford: 1975. pp. 337–74. [Google Scholar]
- 13.Xu-Wilson M, Chen-Harris H, Zee DS, Shadmehr R. Cerebellar contributions to adaptive control of saccades in humans. J Neurosci. 2009;29:12930–9. doi: 10.1523/JNEUROSCI.3115-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maschke M, Gomez CM, Ebner TJ, Konczak J. Hereditary cerebellar ataxia progressively impairs force adaptation during goal-directed arm movements. J Neurophysiol. 2004;91:230–8. doi: 10.1152/jn.00557.2003. [DOI] [PubMed] [Google Scholar]
- 15.Imamizu H, Miyauchi S, Tamada T, et al. Human cerebellar activity reflecting an acquired internal model of a new tool. Nature. 2000;403:192–5. doi: 10.1038/35003194. [DOI] [PubMed] [Google Scholar]
- 16.Diedrichsen J, Hashambhoy Y, Rane T, Shadmehr R. Neural correlates of reach errors. J Neurosci. 2005;25:9919–31. doi: 10.1523/JNEUROSCI.1874-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flanagan JR, Wing AM. The role of internal models in motion planning and control: evidence from grip force adjustments during movements of hand-held loads. J Neurosci. 1997;17:1519–28. doi: 10.1523/JNEUROSCI.17-04-01519.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bell CC, Han V, Sawtell NB. Cerebellum-like structures and their implications for cerebellar function. Annu Rev Neurosci. 2008;31:1–24. doi: 10.1146/annurev.neuro.30.051606.094225. [DOI] [PubMed] [Google Scholar]
- 19.Brooks JX, Cullen KE. The primate cerebellum selectively encodes unexpected self-motion. Curr Biol. 2013;23:947–55. doi: 10.1016/j.cub.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong AL, Shelhamer M. Sensorimotor adaptation error signals are derived from realistic predictions of movement outcomes. J Neurophysiol. 2011;105:1130–40. doi: 10.1152/jn.00394.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mazzoni P, Krakauer JW. An implicit plan overrides an explicit strategy during visuomotor adaptation. J Neurosci. 2006;26:3642–5. doi: 10.1523/JNEUROSCI.5317-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taylor JA, Ivry RB. The role of strategies in motor learning. Ann N Y Acad Sci. 2012;1241:1–12. doi: 10.1111/j.1749-6632.2011.06430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Izawa J, Criscimagna-Hemminger SE, Shadmehr R. Cerebellar contributions to reach adaptation and learning sensory consequences of action. J Neurosci. 2012;32:4230–9. doi: 10.1523/JNEUROSCI.6353-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaveau V, Prablanc C, Laurent D, Rossetti Y, Priot AE. Visuomotor adaptation needs a validation of prediction error by feedback error. Front Hum Neurosci. 2014;8:880. doi: 10.3389/fnhum.2014.00880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henriques DYP, Cressman EK. Visuomotor adaptation and proprioceptive recalibration. J Mot Behav. 2012;44:435–44. doi: 10.1080/00222895.2012.659232. [DOI] [PubMed] [Google Scholar]
- 26.Simani MC, McGuire LM, Sabes PN. Visual-shift adaptation is composed of separable sensory and task-dependent effects. J Neurophysiol. 2007;98:2827–41. doi: 10.1152/jn.00290.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Beers RJ, Wolpert DM, Haggard P. When feeling is more important than seeing in sensorimotor adaptation. Curr Biol. 2002;12:834–7. doi: 10.1016/s0960-9822(02)00836-9. [DOI] [PubMed] [Google Scholar]
- 28.Kawato M. Learning internal models of the motor apparatus. In: Bloedel JR, Ebner TJ, Wise SP, editors. The acquisition of motor behavior in vertebrates. MIT Press; Cambridge: 1996. pp. 409–30. [Google Scholar]
- 29.Miles FA, Lisberger SG. The “error” signals subserving adaptive gain control in the primate vestibulo-ocular reflex. Ann N Y Acad Sci. 1981;374:513–25. doi: 10.1111/j.1749-6632.1981.tb30896.x. [DOI] [PubMed] [Google Scholar]
- 30.Magescas F, Prablanc C. Automatic drive of limb motor plasticity. J Cogn Neurosci. 2006;18:75–83. doi: 10.1162/089892906775250058. [DOI] [PubMed] [Google Scholar]
- 31.Cameron BD, Franks IM, Inglis JT, Chua R. Reach adaptation to explicit vs. implicit target error. Exp Brain Res. 2010;203:367–80. doi: 10.1007/s00221-010-2239-x. [DOI] [PubMed] [Google Scholar]
- 32.Izawa J, Shadmehr R. Learning from sensory and reward prediction errors during motor adaptation. PLoS Comput Biol. 2011;7:e1002012. doi: 10.1371/journal.pcbi.1002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schonberg T, Daw ND, Joel D, O'Doherty JP. Reinforcement learning signals in the human striatum distinguish learners from nonlearners during reward-based decision making. J Neurosci. 2007;27:12860–7. doi: 10.1523/JNEUROSCI.2496-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Redding GM, Wallace B. Adaptive spatial alignment and strategic perceptual-motor control. J Exp Psychol Hum Percept Perform. 1996;22:379–94. doi: 10.1037//0096-1523.22.2.379. [DOI] [PubMed] [Google Scholar]
- 35.Cressman EK, Henriques DY. Reach adaptation and proprioceptive recalibration following exposure to misaligned sensory input. J Neurophysiol. 2010;103:1888–95. doi: 10.1152/jn.01002.2009. [DOI] [PubMed] [Google Scholar]
- 36.Held R, Freedman SJ. Plasticity in human sensorimotor control. Science. 1963;142:455–62. doi: 10.1126/science.142.3591.455. [DOI] [PubMed] [Google Scholar]
- 37.Pasalar S, Roitman AV, Durfee WK, Ebner TJ. Force field effects on cerebellar Purkinje cell discharge with implications for internal models. Nat Neurosci. 2006;9:1404–11. doi: 10.1038/nn1783. [DOI] [PubMed] [Google Scholar]
- 38.Bastian AJ. Learning to predict the future: the cerebellum adapts feedforward movement control. Curr Opin Neurobiol. 2006;16:645–9. doi: 10.1016/j.conb.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 39.Wolpert DM, Miall RC, Kawato M. Internal models in the cerebellum. Trends Cogn Sci. 1998;2:338–47. doi: 10.1016/s1364-6613(98)01221-2. [DOI] [PubMed] [Google Scholar]
- 40.Shadmehr R, Holcomb HH. Neural correlates of motor memory consolidation. Science. 1997;277:821–5. doi: 10.1126/science.277.5327.821. [DOI] [PubMed] [Google Scholar]
- 41.Kawato M, Kuroda T, Imamizu H, Nakano E, Miyauchi S, Yoshioka T. Internal forward models in the cerebellum: fMRI study on grip force and load force coupling. Prog Brain Res. 2003;142:171–88. doi: 10.1016/S0079-6123(03)42013-X. [DOI] [PubMed] [Google Scholar]
- 42.Krakauer JW, Ghilardi MF, Mentis M, et al. Differential cortical and subcortical activations in learning rotations and gains for reaching: a PET study. J Neurophysiol. 2004;91:924–33. doi: 10.1152/jn.00675.2003. [DOI] [PubMed] [Google Scholar]
- 43.Takagi M, Zee DS, Tamargo RJ. Effects of lesions of the oculomotor cerebellar vermis on eye movements in primate: smooth pursuit. J Neurophysiol. 2000;83:2047–62. doi: 10.1152/jn.2000.83.4.2047. [DOI] [PubMed] [Google Scholar]
- 44.Golla H, Tziridis K, Haarmeier T, Catz N, Barash S, Thier P. Reduced saccadic resilience and impaired saccadic adaptation due to cerebellar disease. Eur J Neurosci. 2008;27:132–44. doi: 10.1111/j.1460-9568.2007.05996.x. [DOI] [PubMed] [Google Scholar]
- 45.Muller F, Dichgans J. Impairments of precision grip in two patients with acute unilateral cerebellar lesions: a simple parametric test for clinical use. Neuropsychologia. 1994;32:265–9. doi: 10.1016/0028-3932(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 46.Nowak DA, Hermsdorfer J, Rost K, Timmann D, Topka H. Predictive and reactive finger force control during catching in cerebellar degeneration. Cerebellum. 2004;3:227–35. doi: 10.1080/14734220410019057. [DOI] [PubMed] [Google Scholar]
- 47.Smith MA, Shadmehr R. Intact ability to learn internal models of arm dynamics in Huntington’s disease but not cerebellar degeneration. J Neurophysiol. 2005;93:2809–21. doi: 10.1152/jn.00943.2004. [DOI] [PubMed] [Google Scholar]
- 48.Ito M. Cerebellar learning in the vestibulo-ocular reflex. Trends Cogn Sci. 1998;2:313–21. doi: 10.1016/s1364-6613(98)01222-4. [DOI] [PubMed] [Google Scholar]
- 49.Ito M. Cerebellar long-term depression: characterization, signal transduction, and functional roles. Physiol Rev. 2001;81:1143–95. doi: 10.1152/physrev.2001.81.3.1143. [DOI] [PubMed] [Google Scholar]
- 50.Prsa M, Thier P. The role of the cerebellum in saccadic adaptation as a window into neural mechanisms of motor learning. Eur J Neurosci. 2011;33:2114–28. doi: 10.1111/j.1460-9568.2011.07693.x. [DOI] [PubMed] [Google Scholar]
- 51.Thompson RF, Steinmetz JE. The role of the cerebellum in classical conditioning of discrete behavioral responses. Neuroscience. 2009;162:732–55. doi: 10.1016/j.neuroscience.2009.01.041. [DOI] [PubMed] [Google Scholar]
- 52.Thier P, Ilg U. The neural basis of smooth-pursuit eye movements. Curr Opin Neurobiol. 2005;15:645–52. doi: 10.1016/j.conb.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 53.Tseng YW, Diedrichsen J, Krakauer JW, Shadmehr R, Bastian AJ. Sensory prediction errors drive cerebellum-dependent adaptation of reaching. J Neurophysiol. 2007;98:54–62. doi: 10.1152/jn.00266.2007. [DOI] [PubMed] [Google Scholar]
- 54.Schlerf JE, Ivry RB, Diedrichsen J. Encoding of sensory prediction errors in the human cerebellum. J Neurosci. 2012;32:4913–22. doi: 10.1523/JNEUROSCI.4504-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taylor JA, Klemfuss NM, Ivry RB. An explicit strategy prevails when the cerebellum fails to compute movement errors. Cerebellum. 2010;9:580–6. doi: 10.1007/s12311-010-0201-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oscarsson O. Functional organization of olivary projection to the cerebellar anterior lobe. In: Courville J, editor. The inferior olivary nucleus: anatomy and physiology. Raven; New York: 1980. pp. 279–90. [Google Scholar]
- 57.Gilbert PF, Thach WT. Purkinje cell activity during motor learning. Brain Res. 1977;128:309–28. doi: 10.1016/0006-8993(77)90997-0. [DOI] [PubMed] [Google Scholar]
- 58.Ito M. Mechanisms of motor learning in the cerebellum. Brain Res. 2000;886:237–45. doi: 10.1016/s0006-8993(00)03142-5. [DOI] [PubMed] [Google Scholar]
- 59.Stone LS, Lisberger SG. Detection of tracking errors by visual climbing fiber inputs to monkey cerebellar flocculus during pursuit eye movements. Neurosci Lett. 1986;72:163–8. doi: 10.1016/0304-3940(86)90073-x. [DOI] [PubMed] [Google Scholar]
- 60.Kawato M, Gomi H. A computational model of four regions of the cerebellum based on feedback-error learning. Biol Cybern. 1992;68:95–103. doi: 10.1007/BF00201431. [DOI] [PubMed] [Google Scholar]
- 61.Kitazawa S, Kimura T, Yin PB. Cerebellar complex spikes encode both destinations and errors in arm movements. Nature. 1998;392:494–7. doi: 10.1038/33141. [DOI] [PubMed] [Google Scholar]
- 62.Ito M. Error detection and representation in the olivo-cerebellar system. Front Neural Circuits. 2013;7:1–8. doi: 10.3389/fncir.2013.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Graf W, Simpson JI, Leonard CS. Spatial organization of visual messages of the rabbit’s cerebellar flocculus. II. Complex and simple spike responses of Purkinje cells. J Neurophysiol. 1988;60:2091–121. doi: 10.1152/jn.1988.60.6.2091. [DOI] [PubMed] [Google Scholar]
- 64.Kobayashi Y, Kawano K, Takemura A, et al. Temporal firing patterns of Purkinje cells in the cerebellar ventral paraflocculus during ocular following responses in monkeys II. Complex spikes. J Neurophysiol. 1998;80:832–48. doi: 10.1152/jn.1998.80.2.832. [DOI] [PubMed] [Google Scholar]
- 65.Barmack NH, Shojaku H. Vestibular and visual climbing fiber signals evoked in the uvula-nodulus of the rabbit cerebellum by natural stimulation. J Neurophysiol. 1995;74:2573–89. doi: 10.1152/jn.1995.74.6.2573. [DOI] [PubMed] [Google Scholar]
- 66.Stone LS, Lisberger SG. Visual responses of Purkinje cells in the cerebellar flocculus during smooth-pursuit eye movements in monkeys. II. Complex spikes. J Neurophysiol. 1990;63:1262–75. doi: 10.1152/jn.1990.63.5.1262. [DOI] [PubMed] [Google Scholar]
- 67.Kim JH, Wang JJ, Ebner TJ. Climbing fiber afferent modulation during treadmill locomotion in the cat. J Neurophysiol. 1987;57:787–802. doi: 10.1152/jn.1987.57.3.787. [DOI] [PubMed] [Google Scholar]
- 68.Lou JS, Bloedel JR. The responses of simultaneously recorded Purkinje cells to the perturbations of the step cycle in the walking ferret: a study using a new analytical method—the real-time post-synaptic response (RTPR) Brain Res. 1986;365:340–4. doi: 10.1016/0006-8993(86)91646-x. [DOI] [PubMed] [Google Scholar]
- 69.Andersson G, Armstrong DM. Complex spikes in Purkinje cells in the lateral vermis (b zone) of the cat cerebellum during locomotion. J Physiol. 1987;385:107–34. doi: 10.1113/jphysiol.1987.sp016487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Soetedjo R, Fuchs AF. Complex spike activity of Purkinje cells in the oculomotor vermis during behavioral adaptation of monkey saccades. J Neurosci. 2006;26:7741–55. doi: 10.1523/JNEUROSCI.4658-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Soetedjo R, Kojima Y, Fuchs AF. Complex spike activity in the oculomotor vermis of the cerebellum: a vectorial error signal for saccade motor learning? J Neurophysiol. 2008;100:1949–66. doi: 10.1152/jn.90526.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marko MK, Haith AM, Harran MD, Shadmehr R. Sensitivity to prediction error in reach adaptation. J Neurophysiol. 2012;108:1752–63. doi: 10.1152/jn.00177.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Robinson FR, Noto CT, Bevans SE. Effect of visual error size on saccade adaptation in monkey. J Neurophysiol. 2003;90:1235–44. doi: 10.1152/jn.00656.2002. [DOI] [PubMed] [Google Scholar]
- 74.Wei K, Kording K. Relevance of error: what drives motor adaptation? J Neurophysiol. 2009;101:655–64. doi: 10.1152/jn.90545.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miles FA, Lisberger SG. Plasticity in the vestibulo-ocular reflex: a new hypothesis. Annu Rev Neurosci. 1981;4:273–99. doi: 10.1146/annurev.ne.04.030181.001421. [DOI] [PubMed] [Google Scholar]
- 76.Boyden ES, Raymond JL. Active reversal of motor memories reveals rules governing memory encoding. Neuron. 2003;39:1031–42. doi: 10.1016/s0896-6273(03)00562-2. [DOI] [PubMed] [Google Scholar]
- 77.Boyden ES, Katoh A, Raymond JL. Cerebellum-dependent learning: the role of multiple plasticity mechanisms. Annu Rev Neurosci. 2004;27:581–609. doi: 10.1146/annurev.neuro.27.070203.144238. [DOI] [PubMed] [Google Scholar]
- 78.Medina JF, Lisberger SG. Links from complex spikes to local plasticity and motor learning in the cerebellum of awake-behaving monkeys. Nat Neurosci. 2008;11:1185–92. doi: 10.1038/nn.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang Y, Lisberger SG. Purkinje-cell plasticity and cerebellar motor learning are graded by complex-spike duration. Nature. 2014;510:529–32. doi: 10.1038/nature13282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ojakangas CL, Ebner TJ. Purkinje cell complex and simple spike changes during a voluntary arm movement learning task in the monkey. J Neurophysiol. 1992;68:2222–36. doi: 10.1152/jn.1992.68.6.2222. [DOI] [PubMed] [Google Scholar]
- 81.Ojakangas CL, Ebner TJ. Purkinje cell complex spike activity during voluntary motor learning: relationship to kinematics. J Neurophysiol. 1994;72:2617–30. doi: 10.1152/jn.1994.72.6.2617. [DOI] [PubMed] [Google Scholar]
- 82.Rasmussen A, Jirenhed DA, Zucca R, Johansson F, Svensson P, Hesslow G. Number of spikes in climbing fibers determines the direction of cerebellar learning. J Neurosci. 2013;33:13436–40. doi: 10.1523/JNEUROSCI.1527-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.De Zeeuw CI, Yeo CH. Time and tide in cerebellar memory formation. Curr Opin Neurobiol. 2005;15:667–74. doi: 10.1016/j.conb.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 84.Yeo CH, Hardiman MJ, Glickstein M. Classical conditioning of the nictitating membrane response of the rabbit. II. Lesions of the cerebellar cortex. Exp Brain Res. 1985;60:99–113. doi: 10.1007/BF00237023. [DOI] [PubMed] [Google Scholar]
- 85.Mauk MD, Steinmetz JE, Thompson RF. Classical conditioning using stimulation of the inferior olive as the unconditioned stimulus. Proc Natl Acad Sci U S A. 1986;83:5349–53. doi: 10.1073/pnas.83.14.5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Welsh JP. Systemic harmaline blocks associative and motor learning by the actions of the inferior olive. Eur J Neurosci. 1998;10:3307–20. doi: 10.1046/j.1460-9568.1998.00334.x. [DOI] [PubMed] [Google Scholar]
- 87.Ghelarducci B, Ito M, Yagi N. Impulse discharges from flocculus Purkinje cells of alert rabbits during visual stimulation combined with horizontal head rotation. Brain Res. 1975;87:66–72. doi: 10.1016/0006-8993(75)90780-5. [DOI] [PubMed] [Google Scholar]
- 88.Ohyama T, Nores WL, Murphy M, Mauk MD. What the cerebellum computes. Trends Neurosci. 2003;26:222–7. doi: 10.1016/S0166-2236(03)00054-7. [DOI] [PubMed] [Google Scholar]
- 89.Ebner TJ, Johnson MT, Roitman A, Fu Q. What do complex spikes signal about limb movements? Ann NY Acad Sci. 2002;978:205–18. doi: 10.1111/j.1749-6632.2002.tb07568.x. [DOI] [PubMed] [Google Scholar]
- 90.Fu QG, Mason CR, Flament D, Coltz JD, Ebner TJ. Movement kinematics encoded in complex spike discharge of primate cerebellar Purkinje cells. NeuroReport. 1997;8:523–9. doi: 10.1097/00001756-199701200-00029. [DOI] [PubMed] [Google Scholar]
- 91.Horn KM, van Kan PL, Gibson AR. Reduction of rostral dorsal accessory olive responses during reaching. J Neurophysiol. 1996;76:4140–51. doi: 10.1152/jn.1996.76.6.4140. [DOI] [PubMed] [Google Scholar]
- 92.Catz N, Dicke PW, Thier P. Cerebellar complex spike firing is suitable to induce as well as to stabilize motor learning. Curr Biol. 2005;15:2179–89. doi: 10.1016/j.cub.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 93.Dash S, Catz N, Dicke PW, Thier P. Specific vermal complex spike responses build up during the course of smooth-pursuit adaptation, paralleling the decrease of performance error. Exp Brain Res. 2010;205:41–55. doi: 10.1007/s00221-010-2331-2. [DOI] [PubMed] [Google Scholar]
- 94.Ebner TJ, Hewitt A, Popa LS. What features of movements are encoded in the discharge of cerebellar neurons during limb movements? Cerebellum. 2011;10:683–93. doi: 10.1007/s12311-010-0243-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Najafi F, Giovannucci A, Wang SS, Medina JF. Sensory-driven enhancement of calcium signals in individual Purkinje cell dendrites of awake mice. Cell Rep. 2014;6:792–8. doi: 10.1016/j.celrep.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Najafi F, Giovannucci A, Wang SS, Medina JF. Coding of stimulus strength via analog calcium signals in Purkinje cell dendrites of awake mice. Elife. 2014;3:e03663. doi: 10.7554/eLife.03663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Welsh JP, Lang EJ, Suglhara I, Llinas R. Dynamic organization of motor control within the olivocerebellar system. Nature. 1995;374:453–7. doi: 10.1038/374453a0. [DOI] [PubMed] [Google Scholar]
- 98.Lang EJ. Excitatory afferent modulation of complex spike synchrony. Cerebellum. 2003;2:165–70. doi: 10.1080/14734220310002542. [DOI] [PubMed] [Google Scholar]
- 99.Ozden I, Dombeck DA, Hoogland TM, Tank DW, Wang SS. Widespread state-dependent shifts in cerebellar activity in locomoting mice. PLoS ONE. 2012;7:e42650. doi: 10.1371/journal.pone.0042650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sasaki K, Bower JM, Llinas R. Multiple Purkinje cell recording in rodent cerebellar cortex. Eur J Neurosci. 1989;1:572–86. doi: 10.1111/j.1460-9568.1989.tb00364.x. [DOI] [PubMed] [Google Scholar]
- 101.Lou JS, Bloedel JR. Responses of sagittally aligned Purkinje cells during perturbed locomotion: synchronous activation of climbing fiber inputs. J Neurophysiol. 1992;68:570–80. doi: 10.1152/jn.1992.68.2.570. [DOI] [PubMed] [Google Scholar]
- 102.Jacobson GA, Lev I, Yarom Y, Cohen D. Invariant phase structure of olivo-cerebellar oscillations and its putative role in temporal pattern generation. Proc Natl Acad Sci U S A. 2009;106:3579–84. doi: 10.1073/pnas.0806661106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schweighofer N, Doya K, Fukai H, Chiron JV, Furukawa T, Kawato M. Chaos may enhance information transmission in the inferior olive. Proc Natl Acad Sci U S A. 2004;101:4655–60. doi: 10.1073/pnas.0305966101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kimpo RR, Rinaldi JM, Kim CK, Payne HL, Raymond JL. Gating of neural error signals during motor learning. Elife. 2014;3:e02076. doi: 10.7554/eLife.02076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nguyen-Vu TD, Kimpo RR, Rinaldi JM, et al. Cerebellar Purkinje cell activity drives motor learning. Nat Neurosci. 2013;16:1734–6. doi: 10.1038/nn.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Guo CC, Ke MC, Raymond JL. Cerebellar encoding of multiple candidate error cues in the service of motor learning. J Neurosci. 2014;34:9880–90. doi: 10.1523/JNEUROSCI.5114-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ke MC, Guo CC, Raymond JL. Elimination of climbing fiber instructive signals during motor learning. Nat Neurosci. 2009;12:1171–9. doi: 10.1038/nn.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shin SL, Zhao GQ, Raymond JL. Signals and learning rules guiding oculomotor plasticity. J Neurosci. 2014;34:10635–44. doi: 10.1523/JNEUROSCI.4510-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Greger B, Norris S. Simple spike firing in the posterior lateral cerebellar cortex of Macaque Mulatta was correlated with success-failure during a visually guided reaching task. Exp Brain Res. 2005;167:660–5. doi: 10.1007/s00221-005-0155-2. [DOI] [PubMed] [Google Scholar]
- 110.Roitman AV, Pasalar S, Ebner TJ. Single trial coupling of Purkinje cell activity to speed and error signals during circular manual tracking. Exp Brain Res. 2009;192:241–51. doi: 10.1007/s00221-008-1580-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kahlon M, Lisberger SG. Changes in the responses of Purkinje cells in the floccular complex of monkeys after motor learning in smooth pursuit eye movements. J Neurophysiol. 2000;84:2945–60. doi: 10.1152/jn.2000.84.6.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hewitt A, Popa LS, Pasalar S, Hendrix CM, Ebner TJ. Representation of limb kinematics in Purkinje cell simple spike discharge is conserved across multiple tasks. J Neurophysiol. 2011;106:2232–47. doi: 10.1152/jn.00886.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Popa LS, Hewitt AL, Ebner TJ. Predictive and feedback performance errors are signaled in the simple spike discharge of individual Purkinje cells. J Neurosci. 2012;32:15345–58. doi: 10.1523/JNEUROSCI.2151-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Paninski L, Fellows MR, Hatsopoulos NG, Donoghue JP. Spatiotemporal tuning of motor cortical neurons for hand position and velocity. J Neurophysiol. 2004;91:515–32. doi: 10.1152/jn.00587.2002. [DOI] [PubMed] [Google Scholar]
- 115.Popa LS, Hewitt AL, Ebner TJ. The cerebellum for jocks and nerds alike. Front Syst Neurosci. 2014;8:1–13. doi: 10.3389/fnsys.2014.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Crowe DA, Goodwin SJ, Blackman RK, et al. Prefrontal neurons transmit signals to parietal neurons that reflect executive control of cognition. Nat Neurosci. 2013;16:1484–91. doi: 10.1038/nn.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wallman J, Fuchs AF. Saccadic gain modification: visual error drives motor adaptation. J Neurophysiol. 1998;80:2405–16. doi: 10.1152/jn.1998.80.5.2405. [DOI] [PubMed] [Google Scholar]
- 118.Noto CT, Robinson FR. Visual error is the stimulus for saccade gain adaptation. Brain Res Cogn Brain Res. 2001;12:301–5. doi: 10.1016/s0926-6410(01)00062-3. [DOI] [PubMed] [Google Scholar]
- 119.Nezafat R, Shadmehr R, Holcomb HH. Long-term adaptation to dynamics of reaching movements: a PET study. Exp Brain Res. 2001;140:66–76. doi: 10.1007/s002210100787. [DOI] [PubMed] [Google Scholar]
- 120.Balsters JH, Ramnani N. Cerebellar plasticity and the automation of first-order rules. J Neurosci. 2011;31:2305–12. doi: 10.1523/JNEUROSCI.4358-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ploghaus A, Tracey I, Clare S, Gati JS, Rawlins JN, Matthews PM. Learning about pain: the neural substrate of the prediction error for aversive events. Proc Natl Acad Sci U S A. 2000;97:9281–6. doi: 10.1073/pnas.160266497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Meyer-Lohmann J, Hore J, Brooks VB. Cerebellar participation in generation of prompt arm movements. J Neurophysiol. 1977;40:1038–50. doi: 10.1152/jn.1977.40.5.1038. [DOI] [PubMed] [Google Scholar]
- 123.Lisberger SG, Fuchs AF. Role of primate flocculus during rapid behavioral modification of vestibuloocular reflex. I. Purkinje cell activity during visually guided horizontal smooth-pursuit eye movements and passive head rotation. J Neurophysiol. 1978;41:733–63. doi: 10.1152/jn.1978.41.3.733. [DOI] [PubMed] [Google Scholar]
- 124.Jirenhed DA, Bengtsson F, Hesslow G. Acquisition, extinction, and reacquisition of a cerebellar cortical memory trace. J Neurosci. 2007;27:2493–502. doi: 10.1523/JNEUROSCI.4202-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Catz N, Dicke PW, Thier P. Cerebellar-dependent motor learning is based on pruning a Purkinje cell population response. Proc Natl Acad Sci U S A. 2008;105:7309–14. doi: 10.1073/pnas.0706032105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Medina JF, Lisberger SG. Encoding and decoding of learned smooth pursuit eye movements in the floccular complex of the monkey cerebellum. J Neurophysiol. 2009;102:2039–54. doi: 10.1152/jn.00075.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dash S, Dicke PW, Thier P. A vermal Purkinje cell simple spike population response encodes the changes in eye movement kinematics due to smooth pursuit adaptation. Front Syst Neurosci. 2013;7:3. doi: 10.3389/fnsys.2013.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hewitt AL, Popa LS, Ebner TJ. Changes in Purkinje cell simple spike encoding of reach kinematics during adaptation to a mechanical perturbation. J Neurosci. 2015;35:1106–24. doi: 10.1523/JNEUROSCI.2579-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dugas C, Smith AM. Responses of cerebellar Purkinje cells to slip of a hand-held object. J Neurophysiol. 1992;67:483–95. doi: 10.1152/jn.1992.67.3.483. [DOI] [PubMed] [Google Scholar]
- 130.Nowak DA, Topka H, Timmann D, Boecker H, Hermsdorfer J. The role of the cerebellum for predictive control of grasping. Cerebellum. 2007;6:7–17. doi: 10.1080/14734220600776379. [DOI] [PubMed] [Google Scholar]
- 131.Shadmehr R, Mussa-Ivaldi FA. Adaptive representation of dynamics during learning of a motor task. J Neurosci. 1994;14:3208–24. doi: 10.1523/JNEUROSCI.14-05-03208.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Krakauer JW, Pine ZM, Ghilardi MF, Ghez C. Learning of visuomotor transformations for vectorial planning of reaching trajectories. J Neurosci. 2000;20:8916–24. doi: 10.1523/JNEUROSCI.20-23-08916.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Scheidt RA, Reinkensmeyer DJ, Conditt MA, Rymer WZ, Mussa-Ivaldi FA. Persistence of motor adaptation during constrained, multi-joint, arm movements. J Neurophysiol. 2000;84:853–62. doi: 10.1152/jn.2000.84.2.853. [DOI] [PubMed] [Google Scholar]
- 134.Richter S, Maschke M, Timmann D, et al. Adaptive motor behavior of cerebellar patients during exposure to unfamiliar external forces. J Mot Behav. 2004;36:28–38. doi: 10.3200/JMBR.36.1.28-38. [DOI] [PubMed] [Google Scholar]
- 135.Kojima Y, Soetedjo R, Fuchs AF. Changes in simple spike activity of some Purkinje cells in the oculomotor vermis during saccade adaptation are appropriate to participate in motor learning. J Neurosci. 2010;30:3715–27. doi: 10.1523/JNEUROSCI.4953-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Miles FA, Braitman DJ, Dow BM. Long-term adaptive changes in primate vestibuloocular reflex. IV. Electrophysiological observations in flocculus of adapted monkeys. J Neurophysiol. 1980;43:1477–93. doi: 10.1152/jn.1980.43.5.1477. [DOI] [PubMed] [Google Scholar]
- 137.Raymond JL, Lisberger SG. Multiple subclasses of Purkinje cells in the primate floccular complex provide similar signals to guide learning in the vestibulo-ocular reflex. Learn Mem. 1997;3:503–18. doi: 10.1101/lm.3.6.503. [DOI] [PubMed] [Google Scholar]
- 138.Koekkoek SK, Hulscher HC, Dortland BR, et al. Cerebellar LTD and learning-dependent timing of conditioned eyelid responses. Science. 2003;301:1736–9. doi: 10.1126/science.1088383. [DOI] [PubMed] [Google Scholar]
- 139.Gao Z, van Beugen BJ, De Zeeuw CI. Distributed synergistic plasticity and cerebellar learning. Nat Rev Neurosci. 2012;13:619–35. doi: 10.1038/nrn3312. [DOI] [PubMed] [Google Scholar]
- 140.Hansel C, Linden DJ, D'Angelo E. Beyond parallel fiber LTD: the diversity of synaptic and non-synaptic plasticity in the cerebellum. Nat Neurosci. 2001;4:467–75. doi: 10.1038/87419. [DOI] [PubMed] [Google Scholar]
- 141.Jorntell H, Hansel C. Synaptic memories upside down: bidirectional plasticity at cerebellar parallel fiber-Purkinje cell synapses. Neuron. 2006;52:227–38. doi: 10.1016/j.neuron.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 142.Sing GC, Joiner WM, Nanayakkara T, Brayanov JB, Smith MA. Primitives for motor adaptation reflect correlated neural tuning to position and velocity. Neuron. 2009;64:575–89. doi: 10.1016/j.neuron.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 143.Bauswein E, Kolb FP, Leimbeck B, Rubia FJ. Simple and complex spike activity of cerebellar Purkinje cells during active and passive movements in the awake monkey. J Physiol. 1983;339:379–94. doi: 10.1113/jphysiol.1983.sp014722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mano N, Kanazawa I, Yamamoto K. Complex-spike activity of cerebellar Purkinje cells related to wrist tracking movement in monkey. J Neurophysiol. 1986;56:137–58. doi: 10.1152/jn.1986.56.1.137. [DOI] [PubMed] [Google Scholar]
- 145.Roitman AV, Pasalar S, Johnson MT, Ebner TJ. Position, direction of movement, and speed tuning of cerebellar Purkinje cells during circular manual tracking in monkey. J Neurosci. 2005;25:9244–57. doi: 10.1523/JNEUROSCI.1886-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ramnani N. Automatic and controlled processing in the corticocerebellar system. Prog Brain Res. 2014;210:255–85. doi: 10.1016/B978-0-444-63356-9.00010-8. [DOI] [PubMed] [Google Scholar]
- 147.Wolpert DM, Kawato M. Multiple paired forward and inverse models for motor control. Neural Netw. 1998;11:1317–29. doi: 10.1016/s0893-6080(98)00066-5. [DOI] [PubMed] [Google Scholar]
- 148.Krakauer JW, Ghilardi MF, Ghez C. Independent learning of internal models for kinematic and dynamic control of reaching. Nat Neurosci. 1999;2:1026–31. doi: 10.1038/14826. [DOI] [PubMed] [Google Scholar]
- 149.Ebner TJ, Pasalar S. Cerebellum predicts the future motor state. Cerebellum. 2008;7:583–8. doi: 10.1007/s12311-008-0059-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cerminara NL, Apps R, Marple-Horvat DE. An internal model of a moving visual target in the lateral cerebellum. J Physiol. 2009;587:429–42. doi: 10.1113/jphysiol.2008.163337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Witter L, Canto CB, Hoogland TM, de Gruijl JR, De Zeeuw CI. Strength and timing of motor responses mediated by rebound firing in the cerebellar nuclei after Purkinje cell activation. Front Neural Circuits. 2013;7:133. doi: 10.3389/fncir.2013.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chaumont J, Guyon N, Valera AM, et al. Clusters of cerebellar Purkinje cells control their afferent climbing fiber discharge. Proc Natl Acad Sci U S A. 2013;110:16223–8. doi: 10.1073/pnas.1302310110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Marshall SP, Lang EJ. Local changes in the excitability of the cerebellar cortex produce spatially restricted changes in complex spike synchrony. J Neurosci. 2009;29:14352–62. doi: 10.1523/JNEUROSCI.3498-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yang Y, Lisberger SG. Interaction of plasticity and circuit organization during the acquisition of cerebellum-dependent motor learning. Elife. 2013;2:e01574. doi: 10.7554/eLife.01574. [DOI] [PMC free article] [PubMed] [Google Scholar]