Abstract
Background
Depression after cardiac surgery (CS) is associated with increased pain and decreased sleep quality. While cognitive behavioral therapy (CBT) aimed at depression is effective in relieving depressive symptoms after cardiac surgery, little is known about its ability to ameliorate other common postoperative problems that affect recovery and quality of life.
Aims
The purpose of this study was to evaluate the effects of CBT for depression on pain severity, pain interference, sleep, and perceived control in patients recovering from CS.
Methods
Depressed patients recovering from CS were randomized to receive either eight weeks of CBT or usual care. At baseline and post-intervention, patients completed questionnaires for depressive symptoms, pain, sleep, and perceived control. Group comparisons were conducted using t-tests or chi square analysis. Repeated measures analysis was used to assess the effect of the intervention in changes over time.
Results
The sample (n=53) included 16.9% women and had a mean age of 67.8±9.2 years. CBT for depression increased perceived control (p<0.001) and decreased pain interference (p=0.02) and pain severity (p=0.03). Group effects remained significant (p<0.05) for perceived control and pain interference and a trend was observed for pain severity (p<0.10) after controlling for variables that differed at baseline. There were no group differences in sleep disturbance over time.
Conclusions
A depression-focused CBT intervention yields benefits in other common postoperative problems, specifically improved perceived control and decreased pain in depressed cardiac surgery patients.
Keywords: Cardiovascular disease, depression, pain, cognitive behavioral therapy
Introduction
Depression after cardiac surgery is common, with 23%– 45% of patients believed to suffer postoperatively from elevated depressive symptoms or clinical depression.1–4 Among effective treatments for depression, cognitive behavioral therapy (CBT) is considered to be as effective for moderate depression as antidepressants.5 In cardiac patients, CBT alone or in combination with antidepressants has been effective in relieving depressive symptoms and clinical depression.6–9 CBT is a form of psychotherapy based on the theory that emotions and behaviors are influenced by one's perceptions of events.10 A major component of CBT is the process of cognitive restructuring in which patients learn to identify and challenge dysfunctional beliefs (e.g. catastrophizing, overgeneralizing) and alter maladaptive behaviors caused by those dysfunctional beliefs.11 Cognitive restructuring leads to changes in depressive symptoms by altering thinking, emotions, and behavior. Other components of CBT may include behavioral activation (increase engagement in previously enjoyed activities), social skills training, or coping skills.
Cognitive behavioral interventions designed specifically to address other symptoms common after cardiac surgery, such as sleep disturbance and pain, are known to be effective in older adults. For example, for chronic insomnia, specialized CBT (including modules on sleep restriction, stimulus control, sleep hygiene, and cognitive reframing related to sleep) is considered the standard of care for older adults.12,13 Likewise, CBT designed specifically for chronic pain is also well established as a treatment for adults with back pain and other non-cardiac conditions.14,15 Another problem common after cardiac surgery, a low sense of perceived control over one's health, has yet to be treated with CBT. Perceived control, which is the degree to which an individual believes that he/she controls key processes related to his/her heart condition,16 has been associated with health outcomes in cardiac patients, with lower levels indicating higher levels of anxiety and decreased levels of quality of life in coronary artery disease and heart failure patients.17–20
While CBT aimed at depression is effective in relieving depressive symptoms after cardiac surgery, little is known about its ability to ameliorate other common postoperative problems that affect recovery and quality of life after cardiac surgery. The purpose of this study was to evaluate the effects of CBT for depression (CBT-D) on pain severity, pain interference, sleep and perceived control in patients recovering from cardiac surgery.
Methods
Sample and setting
This report is a secondary analysis from a randomized controlled trial testing the effect of CBT on depressive symptoms in patients early after cardiac surgery.8 Institutional Review Board (IRB) approvals were obtained from all participating sites, and the study was registered on clinicaltrials.gov (Identifier: NCT00522717). The investigation conforms with the principles outlined in the Declaration of Helsinki.21 Participants who underwent cardiac surgery were recruited prior to hospital discharge. Exclusion criteria, which included age <30 years, residing outside the greater Los Angeles area, evidence of cognitive impairment (Mini-Mental State Examination22 score ≥24), major psychiatric condition (i.e. schizophrenia, bipolar disorder, substance abuse), and autoimmune disorder or malignancy, were evaluated after informed consent and prior to hospital discharge. After an initial screening for depression while still hospitalized post-operatively, a second screening was conducted within one month after hospital discharge, followed by a structured interview conducted by trained researchers to diagnose depression using the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) (SCID-I).23 Eighty-one participants met inclusion criteria for major or minor depression and were randomized to receive either CBT or usual care (UC). This report includes 53 participants (CBT=33; UC=20) who completed measures for pain, sleep, and perceived control at baseline and after eight weeks.
Intervention
CBT consisted of eight one-hour sessions with an advanced practice nurse, who had received standardized training (Beck Institute, Philadelphia, Pennsylvania, USA). All sessions were conducted face-to-face in the participant's home and included only the nurse and the participant. To ensure fidelity and consistency of the intervention, therapy nurses used a weekly manual, which included agenda templates and commonly used analysis forms. Every session included a mood check using the Beck Depression Inventory (BDI), collaborative agenda setting by the nurse and the participant, discussion of events or problems important to the participant, and some mutually agreed upon behavioral or cognitive work (“homework”) for the participant to complete between sessions.24 A more detailed description of the intervention has been previously published.8
Outcomes
Primary outcomes assessed depressive symptoms (BDI), which have been previously published.8 Secondary outcomes assessed pain (Brief Pain Inventory Short Form (BPI)), sleep (Pittsburg Sleep Quality Index (PSQI)), and perceived control (Cardiac Attitude Scale-Revised (CAS-R)) at two time points: baseline and post-intervention.
The BDI is a 21-item self-report measure used widely to measure depressive symptoms in cardiac patients.25,26 Scores range from 0–63, with higher scores indicative of more depressive symptoms. Its internal consistency (mean Cronbach alpha=0.82) and concurrent validity (with the Hamilton Rating Scale for Depression, r=0.075) have been supported by numerous reports.27 For this study, the BDI yielded an internal consistency of 0.87 for the baseline administration.8 The BPI is an 11-item self-report measure that assesses both a respondent's current pain state (pain severity) and the degree to which pain interferes with his/her daily living (pain interference).28 Pain severity is a composite of four pain questions (a mean severity score, ranging from 0–10). Pain interference is scored as the mean of seven interference items (range 0–10). Its reliability has been established in both medical and surgical populations, with alpha coefficients ranging from 0.94–0.97.29,30 The PSQI is a 19-item self-report questionnaire designed to measure seven components of sleep (subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medications, and daytime dysfunction). Scores range from 0–21, with higher scores indicative of poorer sleep quality. The reliability coefficient for the PSQI has been reported at 0.83 for both healthy and depressed subjects, and the sensitivity and specificity are 89.6% and 86.5% respectively.31 The CAS-R is an eight-item scale designed to measure the perception of control felt by individuals with cardiac disease. Scores ranges from 8–40; higher scores indicate higher feelings of control.32 Perceived control is believed to play a vital role in determining adaptation to cardiac disease and plays a more prominent role in determining psychosocial recovery.33 Cronbach's alpha values for the CAS-R in the coronary heart disease (CHD), acute myocardial infarction (AMI) and heart failure (HF) patient sample were 0.73, 0.72, and 0.76, respectively.32
Analysis
All data analyses were performed using IBM SPSS 22.34 First, baseline values were carried forward for missing follow-up data. Group comparisons were conducted using independent t-tests or chi square analysis. Repeated measures analysis of variance was used to measure the effect of the intervention in changes over time in perceived control, pain interference and severity, and sleep disturbance. In each analysis, we controlled for baseline values and gender, since despite randomization the prevalence of women was not equal across groups. To assess the effect of baseline group differences, we used linear regression with change scores for each variable as the dependent variable. Independent variables that differed at baseline (gender and weight) were entered into the equation first, with group assignment (CBT or UC) entered separately into a second block. Significance was set at 0.05 for all analyses.
Results
Sample characteristics
For this report, the sample was characteristic of the adult cardiac surgery population.35,36 The sample included 16.9% women and the mean age was 67.8±9.2 years. The most common procedure was coronary artery bypass (71.7%), followed by aortic or mitral valve replacement (18.8%) or combined bypass/valve replacement (9.4%). Most procedures were elective (60.4%) (Table 1). There were no group differences (CBT vs UC) by marital status, ethnicity, procedure type, or urgency of surgery. Compared to UC, fewer women were allocated to CBT (p=0.05) and patients in the CBT group tended to be younger than those in UC (p=0.06) (Table 1).
Table 1.
Sample characteristics.
| Variable | CBT n=33 | Usual care n=20 | p |
|---|---|---|---|
|
|
|
||
| Mean±SD | Mean±SD | ||
| Age (years) | 63.9±7.8 | 68.4±9.0 | 0.06 |
| Time from surgery to enrollment (days) | 16.8±20.6 | 9.50±7.6 | 0.13 |
| Weight (kg) | 87.7±16.2 | 77.0±16.8 | 0.03 |
| BMI (kg/m2) | 29.8±7.7 | 26.2±5.0 | 0.07 |
| No (%) | No (%) | ||
| Female | 3 (9.1) | 6 (30) | 0.05 |
| Single | 8 (24.2) | 5 (25.0) | 0.95 |
| Minority | 11 (33.3) | 6 (30) | 0.80 |
| Non-elective surgery | 11 (33.3) | 10 (50) | 0.23 |
| On antidepressants | 12 (36.4) | 7 (35.0) | 0.92 |
| History of depression | 11 (33.3) | 6 (30.0) | 0.80 |
| Major depression | 23 (69.7) | 16 (80.0) | 0.27 |
| BDIa,b | |||
| Pre | 17.4±9.8 | 14.9±10.5 | 0.39 |
| Post | 8.6±9.6 | 17.6±10.8 | 0.003 |
| BPI–Interferencea,b | |||
| Pre | 5.3±3.1 | 3.8±3.2 | 0.10 |
| Post | 1.9±2.3 | 3.4±3.4 | 0.08 |
| BPI–Severitya,b | |||
| Pre | 4.0±2.2 | 3.4±2.7 | 0.37 |
| Post | 2.1±2.1 | 2.5±2.4 | 0.49 |
| CAS-Ra,c | |||
| Pre | 26.6±5.6 | 24.5±7.7 | 0.26 |
| Post | 34.4±5.6 | 27.6±6.5 | <.001 |
| PSQIa,b | |||
| Pre | 10.6±4.4 | 10.4±4.8 | 0.88 |
| Post | 7.7±4.3 | 9.2±5.8 | 0.30 |
Ranges of possible scores: 0–63 (BDI), 0–10 (BPI), 8–40 (CAS-R), 0–21 (PSQI).
Higher scores indicate greater symptom severity.
Higher scores indicate greater control.
BDI: Beck Depression Inventory; BMI: body mass index; BPI: Brief Pain Inventory; CAS-R: Control Attitudes Scale-Revised; PSQI: Pittsburgh Sleep Quality Index; CBT: cognitive behavioral therapy; PSQI: Pittsburg Sleep Quality Index; SD: standard deviation.
We excluded patients with missing questionnaires at both time points. There were no baseline differences in non-responders by group assignment (CBT vs UC). Compared to responders, the non-responders were younger (mean age 60 vs 68 years, p=0.02), female (57% vs 17%, p<0.001), and had a higher body mass index (BMI) on average (35 vs 28 kg/m2, p<0.001).
Effect of CBT on postoperative recovery
Compared to those in the UC group, those in the CBT group experienced increased perceived control and decreased pain interference and pain severity. The repeated measurement analysis with group (CBT and UC) and follow-up (baseline and eight weeks) showed significant effects (i.e. group×follow-up interaction) in perceived control (F(1,50)=14.3, p<0.001) decreased pain interference (F(1,45)=5.4, p=0.02), and pain severity (F(1,46)=5.1, p=0.03), in favor for the CBT group (Figures 1 and 2). No effect was found in sleep disturbance (F(1,42)=1.3, p=0.26) (Figure 1). In linear regression models, group effects remained significant (p<0.05) for perceived control (Table 2) and pain interference (Table 3) and a trend was observed for pain severity (p<0.10) after controlling for baseline differences in weight and gender.
Figure 1. Changes in perceived control and sleep disturbance over time.
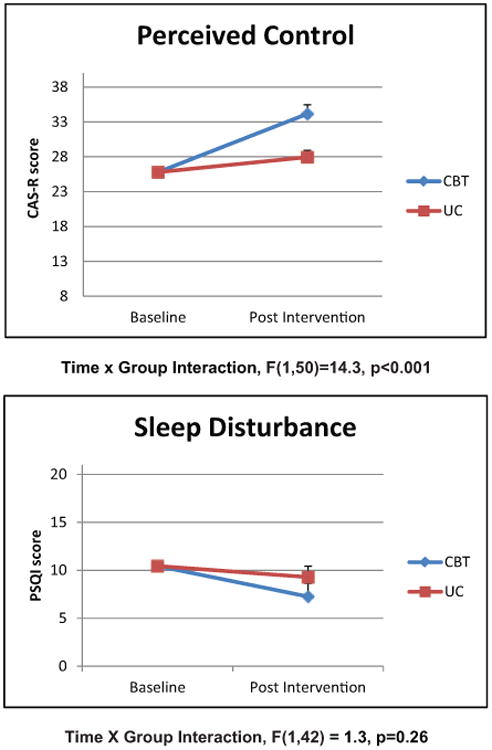
Scales show estimated marginal means controlling for baseline score and gender. CAS-R: Cardiac Attitude Scale-Revised; CBT: cognitive behavioral therapy; PSQI: Pittsburg Sleep Quality Index; UC: usual care.
Figure 2. Changes in pain over time.
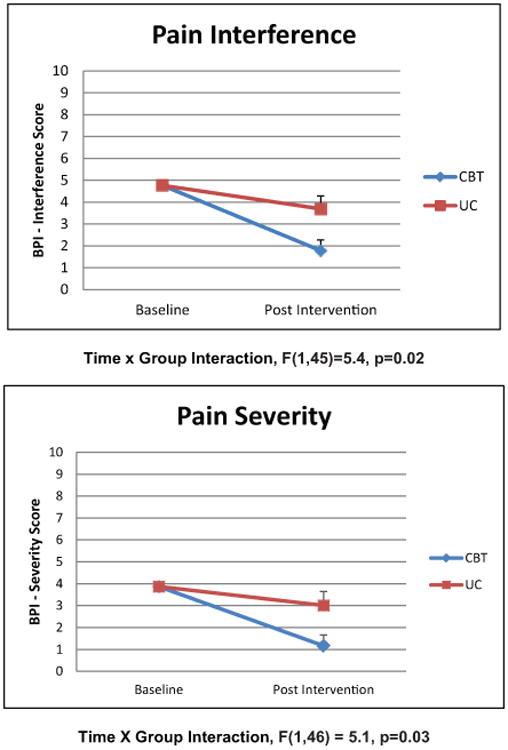
Scales show estimated marginal means controlling for baseline score and gender. BPI: Brief Pain Inventory Short Form; CBT: cognitive behavioral therapy; UC: usual care.
Table 2.
Effect of cognitive behavioral therapy (CBT) on perceived control controlling for potentially confounding variables.
| Model | R2 | Adjusted R2 | R square change | F change | Sig F change | ||
|---|---|---|---|---|---|---|---|
| 0.13 | 0.08 | 0.13 | 2.47 | 0.07 | |||
| Beta | Std error | Standardized beta | T | Sig | 95% confidence interval | ||
| Constant | −4.34 | 5.79 | −0.75 | 0.46 | −15.99 | 7.31 | |
| Female | 3.94 | 3.00 | 0.20 | 1.31 | 0.20 | −2.09 | 9.97 |
| Weight (kg) | 0.08 | 0.07 | 0.18 | 1.21 | 0.23 | −0.05 | 0.22 |
| CBT group | 4.64 | 2.22 | 0.30 | 2.09 | 0.04 | 0.19 | 9.09 |
Table 3.
Effect of cognitive behavioral therapy (CBT) on pain interference controlling for potentially confounding variables.
| Model | R2 | Adjusted R2 | R square change | F change | Sig F change | ||
|---|---|---|---|---|---|---|---|
| 0.17 | 0.12 | 0.17 | 3.15 | 0.03 | |||
| Beta | Std error | Standardized beta | T | Sig | 95% confidence interval | ||
| Constant | 0.34 | 2.69 | 0.13 | 0.90 | −5.06 | 5.75 | |
| Female | 0.39 | 1.44 | 0.04 | 0.27 | 0.79 | −2.51 | 3.30 |
| Weight (kg) | −0.01 | 0.03 | −0.06 | −0.38 | 0.71 | −0.08 | 0.05 |
| CBT group | −2.7 | 1.06 | −0.37 | −2.56 | 0.01 | −4.84 | −0.58 |
Discussion
Perceived control
Significant improvements in perceived control occurred in the CBT group compared to the UC group, even after controlling for group differences (Figure 1). It is likely that cognitive reframing of negative thoughts, an integral part of CBT, helped participants to accept more balanced assessments of their control over their cardiac health. Only two studies have investigated the role of perceived control in the context of CBT for depression. A recent report of a randomized trial of CBT in individuals with non-cardiac chest pain found that illness perceptions and personal control accounted up to 50% of the change in post-CBT depressive symptoms.37 In a study on online self-help CBT for individuals with depressive symptoms, investigators found that perceived control mediated the outcomes of the program on depressive symptoms.38 Further study is needed to determine what elements or activities in CBT are associated with positive changes in perceived control and to investigate further the relationship of perceived control and depressive symptoms in CBT for cardiac patients.
Pain
We found that a CBT intervention directed at reducing depressive symptoms also reduced pain interference and pain severity. With respect to pain, CBT has been studied primarily as an intervention for chronic pain in such conditions as back pain and fibromyalgia.39–43 Our study differed from these reports in two ways. First, the focus of CBT in this report was depressive symptoms, rather than pain. Second, our post cardiac surgery cohort, recruited at hospital discharge, was likely to experience acute or sub-acute postoperative pain. However, chronic pain is relevant for this population because chronic postoperative pain occurs in up to 55% of cardiac surgery patients.44 In some studies of CBT for chronic pain, investigators reported that CBT decreased catastrophizing and increased coping with pain and that these changes mediated improvements in functioning and daily pain intensity.39,43 These mechanisms are consistent with our findings that CBT for depression decreased pain interference and severity levels. A recent study suggests that CBT may act to reduce acute postoperative pain by reducing catastrophizing.45
There have been few studies of CBT for acute or postoperative pain. An experimental study of healthy young adult volunteers showed that cognitive reappraisal training produced increased anticipatory psychological appraisals of self-efficacy and control in response to a physical pain cold pressor task, but did not change ratings of pain intensity.46 This is consistent with our finding that CBT reduced pain interference and supports the hypothesis that cognitive reframing may be a mechanism of this change. Cognitive reframing in CBT may have helped participants to cope better with acute and sub-acute pain, so that they were able to adhere to postoperative recommendations, such as taking analgesics or attending cardiac rehabilitation.47 More study with at least three months of follow-up is needed to further explore the mechanisms by which CBT may have acted to reduce postoperative pain interference.
Sleep disturbance
We did not find any differences in overall sleep quality in CBT vs UC groups. Few studies have evaluated directly the effect of CBT for depression on sleep outcomes. However, pain and sleep disturbance are related,48 so it is noteworthy that CBT for depression improved pain, but not sleep disturbance. Several factors may contribute to this finding. First, we used self-report measures for sleep. Some reports show that differences between subjective and objective sleep reports may be influenced by psychosocial factors and affect.49 Second, the postoperative period is associated with changes in Circadian rhythms, which could have influenced sleep disturbance and pain in different ways.50 Third, other forms of pain, such as chronic pain from preexisting comorbidities, may contribute disproportionately to sleep quality after surgery.51 All of these factors could have influenced patients' responses to CBT for depression. In addition, previous reports found that patients who experienced remission of major depression after either CBT or antidepressant therapy continued to have ongoing sleep disturbances, including sleep-onset insomnia (22%), sleep-maintenance insomnia (26%), and early awakening (17%).52 These findings suggest that therapists need to be more attuned to sleep disturbances in the context of depression, especially because sleep disturbance has been associated with depression relapse.53,54 Conversely, relatively new reports suggest that CBT for insomnia may have positive effects on depressive symptoms.55
Another explanation for our findings related to both pain and sleep may be in the concept of symptom clusters. Symptom clusters are defined as two or more symptoms that are related to each other and occur together and are relatively independent of other clusters.56 Recent studies in cancer patients using latent class analysis have identified symptom clusters that include pain, fatigue, sleep disturbance and depression.57,58 Further, these clusters have been associated with pro-inflammatory genetic variations, which suggests that there may be a biological pathway common to the symptoms.57,59 However, symptom cluster analysis has not been extended to postoperative cardiac patients. Studies of symptoms clusters after coronary artery bypass graft (CABG) are warranted to understand relations among symptoms, identify possible biological pathways, and develop multimodal interventions to relieve symptoms.
Limitations
Our study has several limitations. First, we measured pain with a single instrument and did not distinguish postoperative pain from other chronic pain. Given the age of our sample, it is likely that some participants may have experienced chronic pain, which could have influenced their responses to the intervention. Distinguishing non-surgical chronic pain (i.e. neuromuscular or skeletal pain) from acute or sub-acute postoperative pain may have provided insight into the effect of CBT on both pain severity and pain interference. Second, we had a relatively small sample. Consequently, our study may have been underpowered to find effects of CBT on sleep. Also, we included only participants who met diagnostic criteria for clinical depression. Thus, these findings should be applied cautiously to cardiac surgery patients who have mild to moderate depressive symptoms, but do not meet diagnostic criteria. In our design, we were not able to control for the effect of home visits, which may have contributed to the impact of our intervention. Finally, we conducted pre- and post-test evaluations only. Further study is needed to elucidate relationships of CBT to perceived control, pain, and depressive symptoms, to evaluate the feasibility of visiting nurse CBT as a part of standard treatment, and to study the long-term effect of CBT for depression on quality of life and overall recovery after cardiac surgery.
Conclusions and clinical implications
Our study has important clinical and research implications for postoperative nursing and medical management of depressed cardiac surgery patients. Importantly, our study shows that CBT for depression has multiple benefits in this population. Although the intervention was intended to treat depression and its symptoms, it also yielded positive results for perceived control, which has been linked to quality of life. Improvements in pain interference were also noted, which suggests that CBT for depression may have improved coping skills related to pain. Further study is needed to elucidate relationships of CBT to perceived control, pain, and depressive symptoms, to evaluate the clinical importance of CBT for depression on pain and perceived control in specific settings, and to study the long-term effect of CBT for depression on quality of life and overall recovery after cardiac surgery.
Implications for practice.
Cognitive behavioral therapy for depression has multiple benefits for depressed cardiac surgery patients.
Cognitive behavioral therapy for depression improves perceived control, which has been linked to quality of life.
Cognitive behavioral therapy for depression may improve coping skills related to pain.
Acknowledgments
Funding: The study was supported by NIH grant 5R01NR009228-02. This study also received support from the Inflammatory Biology Core of the UCLA Older Americans Independence Center, NIH/NIA Grant P30-AG028748.
Footnotes
Conflict of interest: The authors declare that they have no conflicts of interest.
References
- 1.Korbmacher B, Ulbrich S, Dalyanoglu H, et al. Perioperative and long-term development of anxiety and depression in CABG patients. Thorac Cardiovasc Surg. 2013;61:676–681. doi: 10.1055/s-0032-1333326. [DOI] [PubMed] [Google Scholar]
- 2.Gallagher R, McKinley S. Anxiety, depression and perceived control in patients having coronary artery bypass grafts. J Adv Nurs. 2009;65:2386–2396. doi: 10.1111/j.1365-2648.2009.05101.x. [DOI] [PubMed] [Google Scholar]
- 3.Murphy BM, Elliott PC, Higgins RO, et al. Anxiety and depression after coronary artery bypass graft surgery: Most get better, some get worse. Eur J Cardiovasc Prev Rehabil. 2008;15:434–440. doi: 10.1097/HJR.0b013e3282fbc945. [DOI] [PubMed] [Google Scholar]
- 4.Tully PJ, Baker RA. Depression, anxiety, and cardiac morbidity outcomes after coronary artery bypass surgery: a contemporary and practical review. J Geriatr Cardiol. 2012;9:197–208. doi: 10.3724/SP.J.1263.2011.12221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bortolotti B, Menchetti M, Bellini F, et al. Psychological interventions for major depression in primary care: A meta-analytic review of randomized controlled trials. Gen Hosp Psychiatry. 2008;30:293–302. doi: 10.1016/j.genhosppsych.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Berkman LF, Blumenthal J, Burg M, et al. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: The enhancing recovery in coronary heart disease patients (enrichd) randomized trial. JAMA. 2003;289:3106–3116. doi: 10.1001/jama.289.23.3106. [DOI] [PubMed] [Google Scholar]
- 7.Freedland KE, Skala JA, Carney RM, et al. Treatment of depression after coronary artery bypass surgery: A randomized controlled trial. Arch Gen Psychiatry. 2009;66:387–396. doi: 10.1001/archgenpsychiatry.2009.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doering LV, Chen B, Cross Bodan R, et al. Early cognitive behavioral therapy for depression after cardiac surgery. J Cardiovasc Nur. 2013;28:370–379. doi: 10.1097/JCN.0b013e31824d967d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dickens C, Cherrington A, Adeyemi I, et al. Characteristics of psychological interventions that improve depression in people with coronary heart disease: A systematic review and meta-regression. Psychosom Med. 2013;75:211–221. doi: 10.1097/PSY.0b013e31827ac009. [DOI] [PubMed] [Google Scholar]
- 10.Beck AT, Haigh EA. Advances in cognitive theory and therapy: The generic cognitive model. Annu Rev Clin Psychol. 2014;10:1–24. doi: 10.1146/annurev-clinpsy-032813-153734. [DOI] [PubMed] [Google Scholar]
- 11.Cuijpers P, van Straten A, Andersson G, et al. Psychotherapy for depression in adults: A meta-analysis of comparative outcome studies. J Consult Clin Psychol. 2008;76:909–922. doi: 10.1037/a0013075. [DOI] [PubMed] [Google Scholar]
- 12.Morin CM, Bootzin RR, Buysse DJ, et al. Psychological and behavioral treatment of insomnia: Update of the recent evidence (1998–2004) Sleep. 2006;29:1398–1414. doi: 10.1093/sleep/29.11.1398. [DOI] [PubMed] [Google Scholar]
- 13.Rybarczyk B, Lund HG, Garroway AM, et al. Cognitive behavioral therapy for insomnia in older adults: Background, evidence, and overview of treatment protocol. Clin Gerontol. 2012;36:70–93. [Google Scholar]
- 14.Williams AC, Eccleston C, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev. 2012;11:CD007407. doi: 10.1002/14651858.CD007407.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ehde DM, Dillworth TM, Turner JA. Cognitive-behavioral therapy for individuals with chronic pain: Efficacy, innovations, and directions for research. Am Psychol. 2014;69:153–166. doi: 10.1037/a0035747. [DOI] [PubMed] [Google Scholar]
- 16.Johnston M, Morrison V, Macwalter R, et al. Perceived control, coping and recovery from disability following stroke. Psychol Health. 1999;14:181–192. [Google Scholar]
- 17.Eastwood JA, Moser DK, Riegel BJ, et al. Commonalities and differences in correlates of depressive symptoms in men and women with heart failure. Eur J Cardiovasc Nurs. 2012;11:356–365. doi: 10.1177/1474515112438010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heo S, Lennie TA, Pressler SJ, et al. Factors associated with perceived control and the relationship to quality of life in patients with heart failure. Eur J Cardiovasc Nurs. 2014;14:137–144. doi: 10.1177/1474515113519931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doering LV, McKinley S, Riegel B, et al. Gender-specific characteristics of individuals with depressive symptoms and coronary heart disease. Heart Lung. 2011;40:e4–e14. doi: 10.1016/j.hrtlng.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moser DK, Riegel B, McKinley S, et al. Impact of anxiety and perceived control on in-hospital complications after acute myocardial infarction. Psychosom Med. 2007;69:10–16. doi: 10.1097/01.psy.0000245868.43447.d8. [DOI] [PubMed] [Google Scholar]
- 21.Rickham PP. Human experimentation. Code of Ethics of the World Medical Association. Declaration of Helsinki. Br Med J. 1964;2:177. doi: 10.1136/bmj.2.5402.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatric Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 23.Riskind JH, Beck AT, Berchick RJ, et al. Reliability of DSM-III diagnoses for major depression and generalized anxiety disorder using the structured clinical interview for DSM-III. Arch Gen Psychiatry. 1987;44:817–820. doi: 10.1001/archpsyc.1987.01800210065010. [DOI] [PubMed] [Google Scholar]
- 24.Beck AT. Cognitive therapy of depression. New York: Guilford Press; 1979. p. 425. [Google Scholar]
- 25.Beck AT, Steer RA. Psychometric properties of the Beck Depression inventory: Twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- 26.Frasure-Smith N, Lesperance F, Juneau M, et al. Gender, depression, and one-year prognosis after myocardial infarction. Psychosom Med. 1999;61:26–37. doi: 10.1097/00006842-199901000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Richter P, Werner J, Heerlein A, et al. On the validity of the Beck Depression Inventory. A review Psychopathology. 1998;31:160–168. doi: 10.1159/000066239. [DOI] [PubMed] [Google Scholar]
- 28.Cleeland CS. The Brief Pain Inventory User Guide. Houston, TX: University of Texas M.D. Anderson Cancer Center; 2009. [Google Scholar]
- 29.Tittle MB, McMillan SC, Hagan S. Validating the brief pain inventory for use with surgical patients with cancer. Oncol Nurs Forum. 2003;30:325–330. doi: 10.1188/03.ONF.325-330. [DOI] [PubMed] [Google Scholar]
- 30.Zelman DC, Gore M, Dukes E, et al. Validation of a modified version of the brief pain inventory for painful diabetic peripheral neuropathy. J Pain Symptom Manage. 2005;29:401–410. doi: 10.1016/j.jpainsymman.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 31.Buysse DJ, Reynolds CF, III, Monk TH, et al. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 32.Moser DK, Riegel B, McKinley S, et al. The Control Attitudes Scale-Revised: Psychometric evaluation in three groups of patients with cardiac illness. Nurs Res. 2009;58:42–51. doi: 10.1097/NNR.0b013e3181900ca0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moser DK, Dracup K. Psychosocial recovery from a cardiac event: The influence of perceived control. Heart Lung. 1995;24:273–280. doi: 10.1016/s0147-9563(05)80070-6. [DOI] [PubMed] [Google Scholar]
- 34.Corp I. IBM SPSS Statistics for Windows, version 22.0. Armonk, NY: IBM Corp; 2013. [Google Scholar]
- 35.ElBardissi AW, Aranki SF, Sheng S, et al. Trends in isolated coronary artery bypass grafting: An analysis of the Society of Thoracic Surgeons adult cardiac surgery database. J Thorac Cardiovasc Surg. 2012;143:273–281. doi: 10.1016/j.jtcvs.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 36.Varma P, Kundan S, Ananthanarayanan C, et al. Demographic profile, clinical characteristics and outcomes of patients undergoing coronary artery bypass grafting– retrospective analysis of 4,024 patients. Indian J Thorac Cardiovasc Surg. 2014:1–6. [Google Scholar]
- 37.Jonsbu E, Martinsen EW, Morken G, et al. Change and impact of illness perceptions among patients with non-cardiac chest pain or benign palpitations following three sessions of CBT. Behav Cogn Psychother. 2013;41:398–407. doi: 10.1017/S1352465813000179. [DOI] [PubMed] [Google Scholar]
- 38.Warmerdam L, van Straten A, Jongsma J, et al. Online cognitive behavioral therapy and problem-solving therapy for depressive symptoms: Exploring mechanisms of change. J Behav Ther Exp Psychiatry. 2010;41:64–70. doi: 10.1016/j.jbtep.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 39.Kashikar-Zuck S, Sil S, Lynch-Jordan AM, et al. Changes in pain coping, catastrophizing, and coping efficacy after cognitive-behavioral therapy in children and adolescents with juvenile fibromyalgia. J Pain. 2013;14:492–501. doi: 10.1016/j.jpain.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smeets RJ, Vlaeyen JW, Kester AD, et al. Reduction of pain catastrophizing mediates the outcome of both physical and cognitive-behavioral treatment in chronic low back pain. J Pain. 2006;7:261–271. doi: 10.1016/j.jpain.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 41.Burns JW, Day MA, Thorn BE. Is reduction in pain catastrophizing a therapeutic mechanism specific to cognitive-behavioral therapy for chronic pain? Transl Behav Med. 2012;2:22–29. doi: 10.1007/s13142-011-0086-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sveinsdottir V, Eriksen HR, Reme SE. Assessing the role of cognitive behavioral therapy in the management of chronic nonspecific back pain. J Pain Res. 2012;5:371–380. doi: 10.2147/JPR.S25330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Litt MD, Shafer DM, Ibanez CR, et al. Momentary pain and coping in temporomandibular disorder pain: Exploring mechanisms of cognitive behavioral treatment for chronic pain. Pain. 2009;145:160–168. doi: 10.1016/j.pain.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Macrae WA. Chronic post-surgical pain: 10 Years on. Br J Anaesth. 2008;101:77–86. doi: 10.1093/bja/aen099. [DOI] [PubMed] [Google Scholar]
- 45.Khan RS, Ahmed K, Blakeway E, et al. Catastrophizing: A predictive factor for postoperative pain. Am J Surg. 2011;201:122–131. doi: 10.1016/j.amjsurg.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 46.Denson TF, Creswell JD, Terides MD, et al. Cognitive reappraisal increases neuroendocrine reactivity to acute social stress and physical pain. Psychoneuroendocrinology. 2014;49:69–78. doi: 10.1016/j.psyneuen.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 47.Gjeilo KH, Stenseth R, Klepstad P. Risk factors and early pharmacological interventions to prevent chronic postsurgical pain following cardiac surgery. Am J Cardiovasc Drugs. 2014;14:335–342. doi: 10.1007/s40256-014-0083-2. [DOI] [PubMed] [Google Scholar]
- 48.Cronin AJ, Keifer JC, Davies MF, et al. Postoperative sleep disturbance: Influences of opioids and pain in humans. Sleep. 2001;24:39–44. doi: 10.1093/sleep/24.1.39. [DOI] [PubMed] [Google Scholar]
- 49.Jackowska M, Dockray S, Hendrickx H, et al. Psychosocial factors and sleep efficiency: Discrepancies between subjective and objective evaluations of sleep. Psychosom Med. 2011;73:810–816. doi: 10.1097/PSY.0b013e3182359e77. [DOI] [PubMed] [Google Scholar]
- 50.Gogenur I. Postoperative circadian disturbances. Dan Med J. 2010;57:B4205. [PubMed] [Google Scholar]
- 51.Myoji Y, Fujita K, Mawatari M, et al. Changes in sleep-wake rhythms, subjective sleep quality and pain among patients undergoing total hip arthroplasty. Intl J Nurs Pract. doi: 10.1111/ijn.12345. Epub ahead of print 30 April 2014. [DOI] [PubMed] [Google Scholar]
- 52.Carney CE, Segal ZV, Edinger JD, et al. A comparison of rates of residual insomnia symptoms following pharmacotherapy or cognitive-behavioral therapy for major depressive disorder. The J Clin Psychiatry. 2007;68:254–260. doi: 10.4088/jcp.v68n0211. [DOI] [PubMed] [Google Scholar]
- 53.Perlis ML, Giles DE, Buysse DJ, et al. Self-reported sleep disturbance as a prodromal symptom in recurrent depression. J Affect Disord. 1997;42:209–212. doi: 10.1016/s0165-0327(96)01411-5. [DOI] [PubMed] [Google Scholar]
- 54.Cho HJ, Lavretsky H, Olmstead R, et al. Sleep disturbance and depression recurrence in community-dwelling older adults: A prospective study. Am J Psychiatry. 2008;165:1543–1550. doi: 10.1176/appi.ajp.2008.07121882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Irwin MR, Olmstead R, Carrillo C, et al. Cognitive behavioral therapy vs. Tai Chi for late life insomnia and inflammatory risk: A randomized controlled comparative efficacy trial. Sleep. 2014;37:1543–1552. doi: 10.5665/sleep.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim HJ, McGuire DB, Tulman L, et al. Symptom clusters: Concept analysis and clinical implications for cancer nursing. Cancer Nurs. 2005;28:270–282. doi: 10.1097/00002820-200507000-00005. quiz 83–84. [DOI] [PubMed] [Google Scholar]
- 57.Doong SH, Dhruva A, Dunn LB, et al. Associations between cytokine genes and a symptom cluster of pain, fatigue, sleep disturbance, and depression in patients prior to breast cancer surgery. Biol Res Nurs. 2015;17:237–247. doi: 10.1177/1099800414550394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miaskowski C, Dunn L, Ritchie C, et al. Latent class analysis reveals distinct subgroups of patients based on symptom occurrence and demographic and clinical characteristics. J Pain Symptom Manage. doi: 10.1016/j.jpainsymman.2014.12.011. Epub ahead of print 31 January 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim HJ, Barsevick AM, Fang CY, et al. Common biological pathways underlying the psychoneurological symptom cluster in cancer patients. Cancer Nurs. 2012;35:E1–E20. doi: 10.1097/NCC.0b013e318233a811. [DOI] [PubMed] [Google Scholar]


