Abstract
CD4+ memory T cells play an important role in induction of autoimmunity and chronic inflammatory responses; however, regulatory mechanisms of CD4+ memory T cell-mediated inflammatory responses are poorly understood. Here we show that apoptotic cell-treated dendritic cells inhibit development and differentiation of CD4+ effector memory T cells in vitro and in vivo. Simultaneously, i.v. transfer of apoptotic T cell-induced tolerogenic dendritic cells can block development of experimental autoimmune encephalomyelitis (EAE), an inflammatory disease of the central nervous system in C57 BL/6J mouse. Our results imply that it is effector memory CD4+ T cells, not central memory CD4+ T cells, which play a major role in chronic inflammatory responses in mice with EAE. I.V. transfer of tolerogenic dendritic cells induced by apoptotic T cells leads to immune tolerance by specifically blocking development of CD4+ effector memory T cells compared with results of EAE control mice. These results reveal a new mechanism of apoptotic cell-treated dendritic cell-mediated immune tolerance in vivo
Keywords: Dendritic cell, Immune tolerance, Immunotherapy, Memory T cell, Autoimmunity, Inflammation, Apoptosis
Introduction
Memory T cells play a central role in immune surveillance and chronic inflammation [1]. Memory T cells can be divided by two groups including CD4+ and CD8+ memory T cells according to subpopulation of T cells [2]. However, memory T cells are also composed of central memory T cells (TCMs), effector memory T cells (TEMs) and tissue-resident memory T cells (TRMs) according to distribution in vivo [3, 4]. It is known that the phenotypes of the three subsets of memory T cells are different. For example, TCMs are CD44hiCD62L+ T cells and are mainly located in spleen, lymph nodes and blood. TEMs are CD44hiCD62L− T cells and are mainly distributed in spleen. There are rare TEMs in lymph nodes and blood. TRMs are also CD44hiCD62L− T cells [5], but CD103 molecules are expressed on TRMs [6]. In contrast, there are no CD103 molecules expressed on TCMs and TEMs [5]. TRMs are mainly located in peripheral tissue, for example, CD8+ TRMs are in the epithelium of skin, brain and ganglia; there are CD4+ TRMs in lung parenchyma. Immune function of CD4+ TRMs in the central nervous system are still obscure [5]. To date, cellular regulatory mechanisms of memory T cell development and differentiation in vivo have not been fully elucidated. Our research is focused on dendritic cell-mediated memory T cell development and differentiation. Our results imply that apoptotic cell-treated dendritic cells inhibit chronic inflammatory responses by specifically blocking development of CD4+ effector memory T cells in vivo.
Dendritic cells (DCs) are important regulatory cells in the immune system and play a critical role in induction of immune tolerance and autoimmunity [7-9]. There are at least four subpopulations of DCs, including conventional DCs (cDCs), plasmacytoid DCs (pDCs), monocyte-derived DCs and myeloid DCs (mDCs) [10-12]. Moreover, DCs are also divided into inflammatory and tolerogenic DCs depending on their different functions [13, 14]. Production of inflammatory and tolerogenic DCs can be induced in several ways. For example, tolerogenic DCs can be induced by anti-inflammatory cytokines such as IL-10 and TGF-β and by some biological molecules such as vitamin D3 and apoptotic cells [15-17]. Our project is focused on tolerogenic DCs induced by apoptotic T cells [18]. Our results suggest that apoptotic T cell-induced tolerogenic DCs inhibit development of CD4+ memory T cells, leading to immune tolerance in vivo.
Experimental autoimmune encephalomyelitis (EAE) is an animal model of multiple sclerosis (MS) [19]. EAE is also an inflammatory disease induced by CD4+ T cells [20]. Regulatory mechanisms of chronic inflammatory responses mediated by CD4+ memory T cells in EAE have not yet been elucidated. Our project is focused on how tolerogenic DCs induced by apoptotic T cells inhibit chronic inflammatory responses mediated by CD4+ memory T cells in EAE. Our results suggest that apoptotic T cell-induced tolerogenic DCs inhibit development of EAE by specifically suppressing chronic inflammatory responses mediated by CD4+ effector memory T cells.
Materials and Methods
Peptide
Mouse MOG35-55 peptide (MEVGWYRSPFSRVVHLYRNGK), a component of myelin oligodendrocyte glycoprotein (MOG), was purchased from Invitrogen (Invitrogen, Carlsbad, California, USA).
Isolation of Spleen DCs
Splenocytes were isolated from naïve C57 BL/6J mice (Jackson Laboratory, Bar Harbor, ME, USA). Cells were collected and erythrocytes were depleted using red cell lysis buffer (Biolegend, San Diego, CA, USA). Lymphocytes were then stained using anti-mouse cluster of differentiation (CD)11c antibody (Biolegend), and CD11c+ cells (DCs) were sorted using fluorescence-activated cell sorting (FACS; BD Biosciences, San Jose, CA, USA) for co-culture assay [21].
Induction of apoptotic thymocyte-induced tolerogenic DCs
As described previously, thymocytes were isolated from C57 BL/6J mice, and then irradiated at 1500 Rad to induce apoptosis. Fresh thymocytes without irradiation were collected as a control. Irradiated and fresh thymocytes were co-cultured with splenic CD11c+ DCs (thymocytes: DCs = 10:1) for 24 hrs at 37°C. These DCs were also primed in the presence of MOG35-55 peptide (0.1 μM) in the medium. Cells were then harvested for conducting co-culture experiments [21].
Isolation and cell culture of MOG peptide-specific CD4+T cells
Splenocytes were isolated from TCR specific to MOG/MHC class II complex transgenic mice (2D2, Jackson Laboratory) or mice immunized with MOG peptide/ complete Freund's adjuvant (CFA, Sigma, St. Louis, MO, USA). T cells were collected and co-cultured at 37 °C for 3 days with CD11c+ DCs that had been pre-treated with apoptotic or fresh thymocytes, in the presence of mouse IL-2 (1 ng/ml) and MOG peptide (0.1μM). Cells were washed at 300 g × 5 min twice using phosphate buffered saline (PBS) for flow cytometry [21].
Flow Cytometry
MOG-specific T cells were isolated from 2D2 mice or mice immunized with MOG peptide/CFA and co-cultured with 0.1 μM MOG peptide-pulsed DCs treated with apoptotic or fresh thymocytes for 72 hrs at 37 °C. Cells were washed at 300 g × 5 min twice with 5% fetal calf serum (FCS) in PBS, then incubated with anti-mouse CD4, CD25, CD44 and L-Selectin (CD62L) antibodies (Biolegend) for 24 hrs at 4 °C. These cells were washed using FACS sorting buffer (5% FCS in PBS) at 300 g × 5 min twice and collected for intracellular staining [21].
Intracellular Staining
As described previously, MOG-specific T cells were stimulated by leukocyte activator (BD) at 37 °C for 6 hrs before conducting intracellular staining. Cells were then washed twice with 5% FCS in PBS at 300 g for 5 min. Cells were fixed with 5% formalin (Biolegend) in PBS for 30 min at room temperature after completion of surface staining as described above, then washed by permeabilization buffer (Biolegend) twice at 300 g × 10 min [21-23].
MOG-primed T cells were stained by anti-mouse Forkhead box P3 (FoxP3) and IFN-gamma (IFN-γ) antibodies (Biolegend) for 24 hrs at 4 °C. Cells were washed with 5% FCS in PBS twice at 300 g × 5 min and harvested for flow cytometry [21-23].
Fixed cells were run on a FACSAria (BD) and data were analyzed with FlowJo software (Treestar, Ashland, OR, USA) [21-23].
EAE induction and treatment
C57BL/6J mice (female, 8-12 week) were immunized with MOG35-55 peptide/complete Freund's adjuvant (CFA, Sigma) at 200μg/200μl/per mouse (subcutaneous injection, s.c.). Pertussis toxin (PT, Sigma) was simultaneously injected at 200ng/per mouse (intraperitoneal injection, i.p.) and the second PT injection was conducted after 48 hrs. EAE was assessed by standard clinical scores: 0.5: paralysis of half the tail, 1: paralysis of whole tail, 2: paralysis of tail and one leg, 3: paralysis of tail and two legs, 4: moribund, 5: death [21, 22, 24].
Mice were divided into three groups. DCs were washed with PBS twice and were immediately injected via tail vein (3 X 105 cells/per mouse/per time) on days 11, 14 and 17 post-immunization (p.i): 1) injected with un-pulsed DCs incubated with apoptotic cells; 2) injected with fresh cell-treated DCs pulsed with MOG peptide; 3) injected with apoptotic cell-treated DCs pulsed with MOG peptide.
At day 50 p.i., splenocytes were isolated from immunized-mice and stimulated with MOG peptide (0.1μM) and mouse IL-2 (1ng/ml) for 3 days at 37 °C. Cells were then harvested for flow cytometry and the supernatant was assayed by enzyme-linked immunosorbent assay (ELISA)
ELISA
Anti-mouse ELISA kit to target IFN-γ was purchased from R&D Systems (Minneapolis, MN, USA). Assays were conducted according to the manufacturer's instructions. Plates were read out in Labsystems Multiskan MCC/340 (Fisher Scientific, Suwanee, GA, USA) and data were analyzed using DSJV ELISA software (Fisher Scientific) [21-24].
Statistical Analysis
Experimental data were analyzed using Prism software (GraphPad, La Jolla, CA, USA). Clinical scores of EAE were analyzed using two-way ANOVA test. A t test was conducted for analysis of flow cytometry and ELISA data. Error bars shown in this paper represent the mean and standard deviation (SD). Results were regarded as showing a significant difference if the P value was less than 0.05 [21-24].
Results
1. Apoptotic cell-treated DCs block development of central and memory CD4+ T cells in vitro
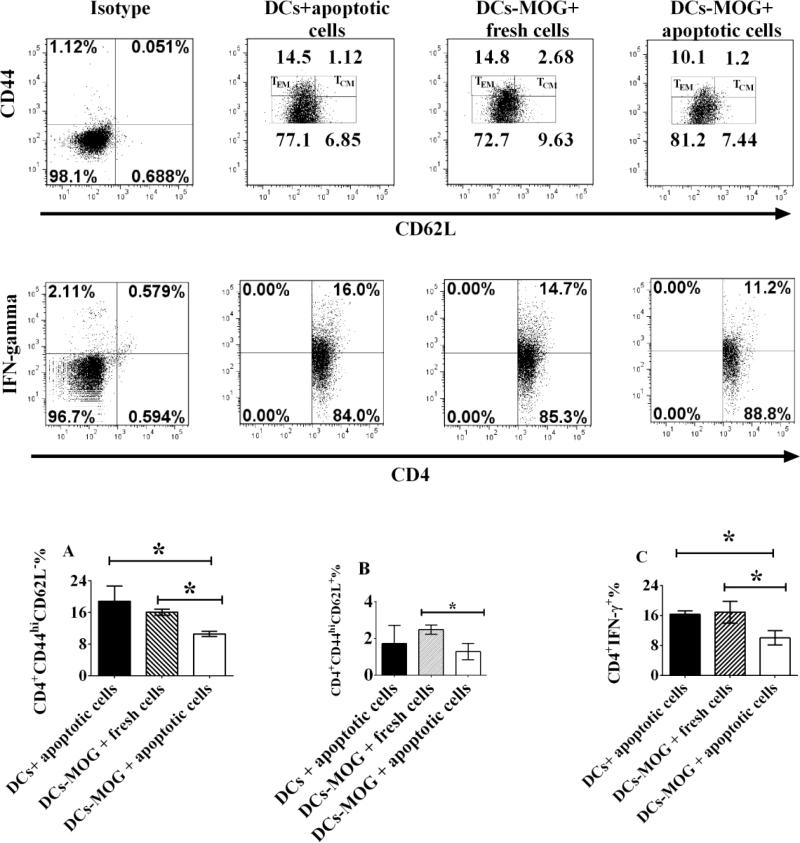
To investigate whether or not apoptotic cell-treated DCs can modulate differentiation of memory CD4+ T cells in vitro, DCs were incubated with apoptotic or fresh T cells as a control. MOG-primed CD4+ T cells were isolated from 2D2 transgenic mice and co-cultured with apoptotic or fresh T cell-treated DCs loaded with MOG peptide. Experimental data showed that apoptotic T cell-treated DCs block development of both effector and central memory CD4+ T cells (Figs.1-2). Our results imply that co-culture with apoptotic T cells leads to generation of tolerogenic DCs, which can inhibit development of central and effector memory CD4+ T cells.
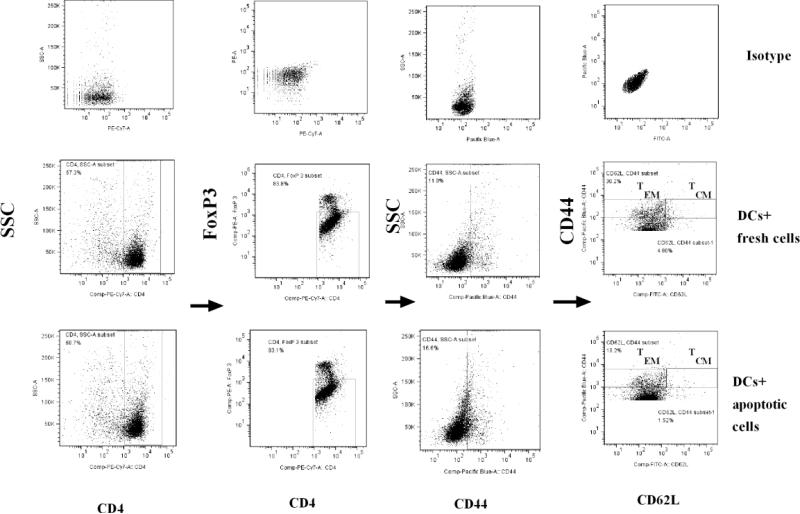
Figure 1.
Gate scheme of TEMs and TCMs. Spleen cells were isolated from 2D2 transgenic mice. CD4+CD25− T cells and CD11c+ DCs were respectively sorted using flow cytometry. T lymphocytes were re-stimulated by MOG peptide (0.1μM) and mouse IL-2 (1ng/ml) and co-cultured with MOG peptide (0.1μM)-pulsed DCs treated with fresh or apoptotic cells for 72 hrs at 37 °C. Cells were then stained with anti-mouse CD4, CD44, CD62L and FoxP3 antibodies. CD4+FoxP3−CD44+ cells were gated. TEMs (CD4+FoxP3−CD44hiCD62L− cells) and TCMs (CD4+FoxP3−CD44hiCD62L+ cells) are shown. Further studies and statistical analysis are indicated in Figure 2.
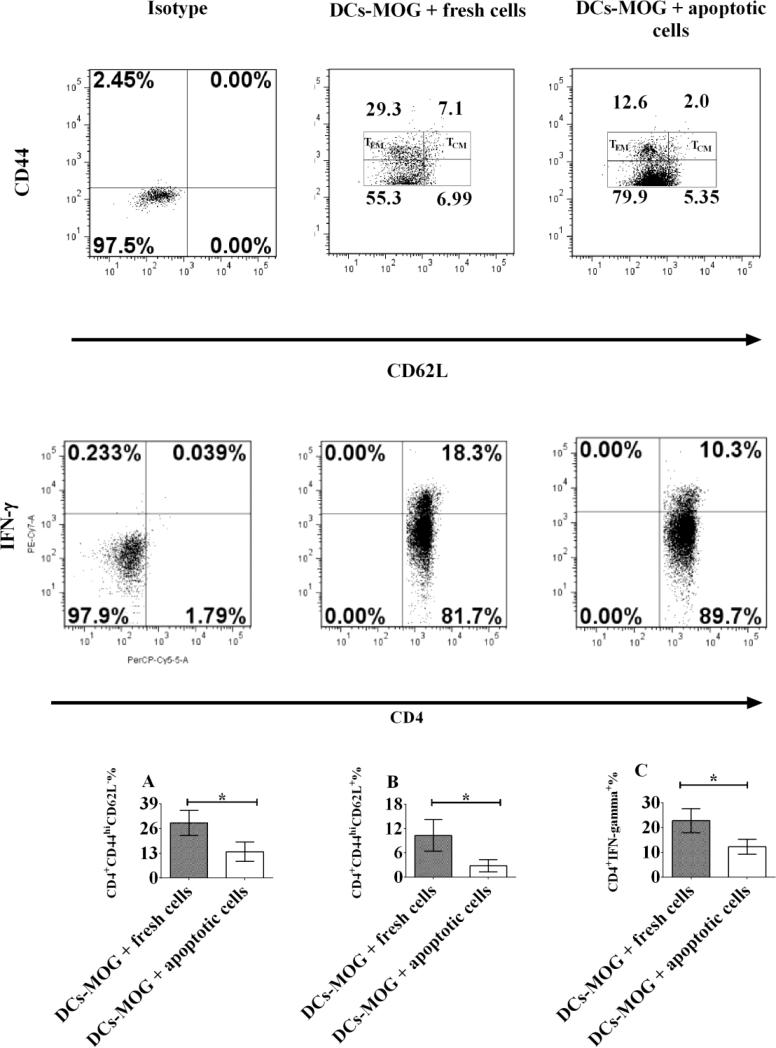
Figure 2.
Apoptotic cell-treated dendritic cells (DCs) inhibit development of effector and central memory CD4+ T cells in vitro. Splenocytes were isolated from C57BL/6J mice and stained by anti-mouse CD11c antibody. CD11c+ cells (DCs) were sorted using flow cytometry. DCs were then co-cultured with apoptotic or fresh T lymphocytes for 24 hrs at 37 °C as a control. MOG-primed T lymphocytes were isolated from 2D2 transgenic mice and stained by anti-mouse CD4 and CD25 antibodies. Only CD4+CD25− cells (effector T cells) were sorted and collected using flow cytometry. MOG-specific T lymphocytes were incubated with apoptotic or fresh T cell-treated DCs pulsed with MOG peptide (0.1μM) for 3 days at 37 °C. Cells were washed and harvested with PBS. Lymphocytes were stained with anti-mouse CD4, CD44, CD62L, IFN-γ and FoxP3 antibodies. Only CD4+CD44+FoxP3− cells were gated like those in Figure 1. Expression of CD44, CD62L on CD4+CD44+FoxP3− cells and that of IFN-γ in CD4+ T cells are shown. CD4+FoxP3−CD44hiCD62L− (TEMs) and CD4+FoxP3−CD44hiCD62L+ (TCMs) cells are gated. Error bars indicated in this figure represent mean and SD of triplicate determinations of percentage (%) of TEMs (A), TCMs (B) and IFN-γ (C) in three independent experiments (*P<0.05, n=3, t test).
2. Apoptotic cell-treated DCs inhibit development of EAE in vivo
To investigate whether or not apoptotic cell-treated DCs also inhibit autoimmunity in mice with EAE, apoptotic or fresh cell-treated DCs pulsed with MOG peptide were i.v. transferred into mice immunized with MOG peptide/CFA. Apoptotic cell-treated DCs without loading MOG peptide were also i.v. transferred into mice with EAE as a control. Experimental results indicated that i.v. transfer of apoptotic cell-treated DCs pulsed with MOG peptide can significantly inhibit EAE development in vivo compared to mice treated with DCs incubated with apoptotic cells, but without loading MOG peptide, or to fresh cell-treated DCs pulsed with MOG peptide (Fig.3A). Our results suggest that immune tolerance induced by apoptotic cell-treated DCs is specific to MOG peptide. Apoptotic cell-induced tolerogenic DCs can block autoimmune responses in vivo.
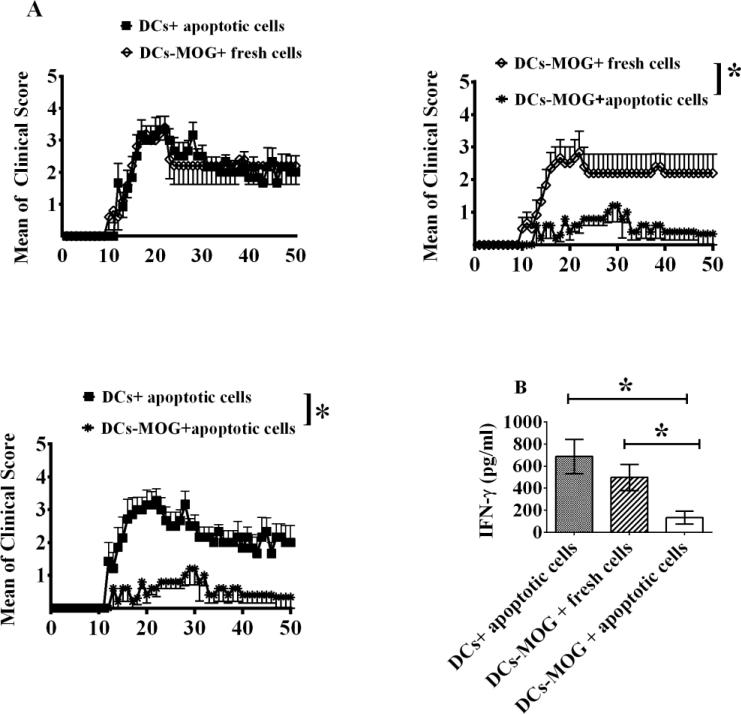
Figure 3.
i.v. transfer of apoptotic cell-treated DCs leads to inhibition of EAE development. C57 BL/6J mice were immunized with MOG/CFA on day 0 and PT was injected (i.p) at days 0 and 2. DCs were pulsed with MOG peptide (0.1μM) (DC-MOG) or without loading MOG peptide (DC). DCs were incubated with apoptotic or fresh T cells as a control for 24 hrs at 37 °C. DCs treated with apoptotic or fresh cells were i.v. transferred into mice with EAE at days 11, 14 and 17 (3×105 cells/per mouse/per time). Clinical scores of EAE are shown (A). Splenocytes were isolated from mice shown in A and re-stimulated with MOG peptide (0.1μM) and mouse IL-2 (1ng/ml) for 3 days at 37° C. Supernatant was harvested and an ELISA assay to target IFN-γ was carried out. Concentration of IFN-γ is indicated (B). Error bars shown in A represent mean and SEM of clinical score of EAE in one experiment (*P<0.05, n=6, two-way ANOVA test). Error bars shown in B represent mean and SD of triplicate determinations of concentration of IFN-γ in three independent experiments (*P<0.05, n=3, t test).
To test whether or not apoptotic cell-treated DCs can affect production of IFN-γ in lymphocytes, spleen cells were isolated from mice treated with apoptotic cell or fresh cell-treated DCs and re-stimulated respectively with MOG peptide (0.1μM) and mice IL-2 (1ng/ml). Supernatant was collected and an ELISA assay was conducted. Our results demonstrated that i.v. transfer of apoptotic cell-treated DCs pulsed with MOG peptide can significantly down-regulate production of IFN-γ in T lymphocytes compared with cells isolated from mice i.v. transferred with apoptotic cell-treated DCs without loading MOG peptide or with fresh cell-treated DCs pulsed with MOG peptide (Fig. 3B). Experimental data indicate that treatment with apoptotic cells leads to generation of suppressive DCs, which can block production of IFN-γ by T lymphocytes.
3. Apoptotic cell-treated DCs inhibit development of effector memory CD4+ T cells ex vivo
To investigate whether or not apoptotic cell-treated DCs also inhibit development of effector and central memory CD4+ T cells in vivo, splenocytes were isolated from mice treated with fresh or apoptotic cell-incubated DCs pulsed with MOG peptide or apoptotic cell-treated DCs without loading MOG peptide as a control. Frequency of effector and central memory CD4+ T cells was detected using flow cytometry (Fig.4). Our results indicated that i.v. transfer of apoptotic cell-treated DCs pulsed with MOG peptide can significantly inhibit development of effector memory CD4+ T cells compared with those in mice i.v. transferred with apoptotic cell-treated DCs without loading MOG peptide or fresh cell-treated DCs pulsed with MOG peptide (Fig. 5A). In contrast, there is no significant difference in frequency of central memory CD4+ T cells between mice i.v. transferred with apoptotic cell-treated DCs pulsed with MOG peptide and mice i.v. transferred with apoptotic cell-incubated DCs without loading MOG peptide (EAE control group) (Fig. 5A) , although there is significant difference in frequency of central memory CD4+ T cells between mice i.v. transferred with apoptotic cell-treated DCs pulsed with MOG peptide and mice i.v. transferred with fresh cell-treated DCs loaded with MOG peptide, (Fig. 5B). Our results suggest that tolerogenic DCs induced by apoptotic T cells cause immune tolerance by blocking effector memory CD4+ T cell-mediated chronic inflammatory responses in vivo.
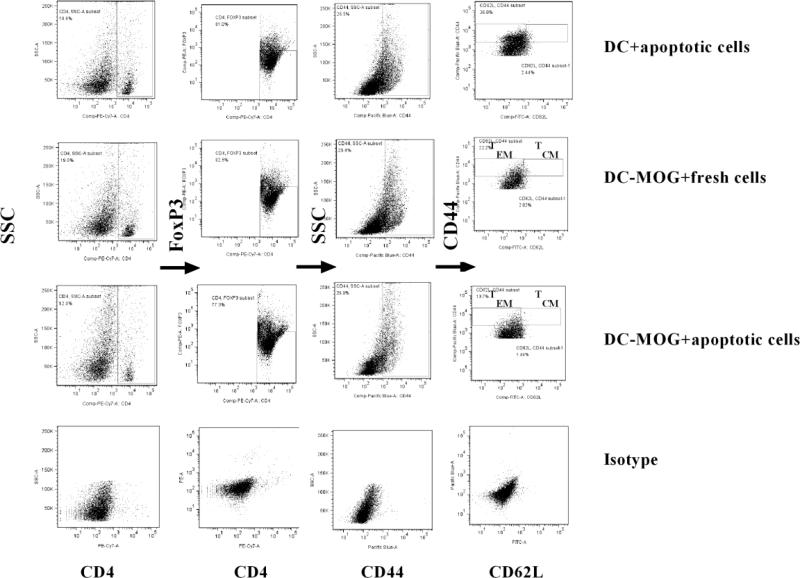
Figure 4.
Gate strategy of TEMs and TCMs ex vivo. Splenocytes were isolated from mice immunized with MOG/CFA shown in figure 3A. T lymphocytes were re-stimulated with MOG peptide (0.1μM) and mouse IL-2 (1ng/ml). Cells were collected and washed with PBS twice at 300g X 5min. Splenocytes were stained by anti-mouse CD4, CD44, CD62L and FoxP3 antibodies. CD4+FoxP3−CD44+ cells were gated. TEMs (CD4+FoxP3−CD44hiCD62L− cells) and TCMs (CD4+FoxP3−CD44hiCD62L+ cells) are indicated. Further studies and statistical analysis are demonstrated in figure 5.
Figure 5.
Apoptotic cell-treated DCs inhibit differentiation of effector memory CD4+ T cells ex vivo. Splenocytes were isolated from mice immunized with MOG/CFA shown in Fig. 3A. Cells were re-stimulated with mouse IL-2 (1ng/ml) and MOG peptide (0.1μM) for 3 days at 37 °C. Splenocytes incubated with leukocyte activator were then harvested and washed with PBS. Cells were stained by anti-mouse CD4, CD44, CD62L, FoxP3 and IFN-γ antibodies. CD4+CD44+FoxP3− cells were gated for further studies like those in figure 4. Frequencies of CD4+FoxP3−CD44hiCD62L− (TEMs) and CD4+FoxP3− CD44hiCD62L+ (TCMs) cells are demonstrated. The frequencies of TEMs (A), TCMs (B) and IFN-γ (C) in CD4+ T cells were determined using flow cytometry. Error bars indicated in this figure represent mean and SD of triplicate determinations of frequency of TEMs (A), TCMs (B) and IFN-γ (C) in CD4+ T cells in three independent experiments (*P<0.05, n=3, t test).
In addition, to test whether or not apoptotic cell-induced tolerogenic DCs can also inhibit production of IFN-γ by CD4+ T cells ex vivo, splenocytes were isolated from mice shown in Fig. 3A and re-stimulated with leukocyte activator, MOG peptide (0.1 μM) and mouse IL-2 (1ng/ml). Production of IFN-γ by CD4+ T cells was detected using flow cytometry. Experimental data demonstrated that i.v. transfer of apoptotic cell-treated DCs pulsed with MOG peptide can significantly down-regulate production of IFN-γ by CD4+ T cells compared with CD4+ T cells derived from mice i.v. transferred with fresh cell-treated DCs loaded with MOG peptide or mice i.v. transferred with apoptotic cell-treated DCs without loading MOG peptide (Fig. 5C). Our results suggest that apoptotic cell-induced tolerogenic DCs also inhibit activation of MOG-primed CD4+ T cells by affecting production of IFN-γ in vivo.
Discussion
DCs play a central role in regulating immune responses mediated by T cells and in maintaining balance between autoimmunity and tolerance. For example, tolerogenic DCs induced by apoptotic cells induce immune tolerance by suppressing activity of T helper 17 (Th17) cells, but by facilitating development of regulatory T cells [21, 22]. However, it is still unclear whether or not apoptotic cell-induced tolerogenic DCs also inhibit development of CD4+ memory T cells. Given that CD4+ memory T cells play an important role in chronic inflammatory responses and autoimmunity, in order to develop further studies, it is necessary to investigate whether or not apoptotic cell-treated DCs also block differentiation of CD4+ memory T cells.
The effect of tolerogenic DCs on activation of CD4+ memory T cells in human diseases has been widely investigated in the past few years. For example, Torres-Aguilar et al. reported that tolerogenic DCs induced by IL-10 and TGF-β block anti-phospholipid syndrome-derived effector/memory CD4+ T cell-mediated immune responses in vivo. Moreover, IL-10/TGF-β-treated DCs inhibit activation of insulin-specific CD4+ effector/memory T cells in diabetic patients. Tolerogenic DCs cause immune tolerance by inducing anergy of CD4+ effector memory T cells and facilitating development of memory regulatory T cells [15, 25, 26]. Nasreen's data also indicated that steady-state antigen-expressing DCs suppress CD4+ memory T cell-mediated immune responses [27]. These results suggest the possibility of immunotherapy using tolerogenic DCs that can inhibit CD4+ memory T cell-mediated autoimmune and inflammatory diseases such as EAE/MS. It is not known, however, whether or not apoptotic cell-induced tolerogenic DCs can block development of autoimmune diseases such as EAE by inhibiting development of CD4+ memory T cells.
Interferon gamma (IFN-γ) is an important cytokine which can modulate multiple immune functions in vivo. IFN-γ can be produced by Th1 cells [28, 29]. IFN-γ is also secreted by TEMs; it is, however, unclear whether or not apoptotic cell-induced tolerogenic DCs block TEMs activity by modulating its production of IFN-γ.
The relation between development of CD4+ memory T cells and induction of chronic inflammatory responses in human diseases has been thoroughly studied. For example, Olson found that the number of CD4+ memory T cells is increased in atherosclerosis [30]. Moreover, Egwuagu et al. reported that auto-reactive memory CD4+ T cells play an important role in chronic uveitis and their immigration to bone marrow is dependent on signal transducer and activator of transcription 3 (STAT3)-mediated pathways [31]. Moreover, Haines’ results demonstrated that autoimmune memory Th17 cell-mediated inflammatory responses in EAE mice are regulated by IL-23-mediated pathways [32]. Elyaman et al. found that development of EAE can be facilitated by pro-inflammatory memory CD4+ T cells [33]. However, it is still unclear whether or not apoptotic cell-induced tolerogenic DCs can inhibit autoimmune memory Th17 cell-mediated chronic inflammatory responses in EAE mice.
In summary, we observed the effect of apoptotic cell-treated DCs on development and differentiation of CD4+ memory T cells in vitro and in vivo. Apoptotic cell-induced tolerogenic DCs can block development of differentiation of TCMs and TEMs in vitro and in vivo. However, immune tolerance induced by apoptotic cell-treated DCs is mainly dependent on CD4+ effector memory T cells, not on CD4+ central memory T cells. Our results suggest a new mechanism of immune tolerance induced by apoptotic cell-treated DCs in vivo.
Abbreviations
- CD
Cluster of differentiation
- DC
Dendritic cell
- EAE
Experimental autoimmune encephalomyelitis
- FACS
Fluorescence-activated cell sorting
- FCS
Fetal calf serum
- FoxP3
Forkhead box P3
- GM-CSF
Granulocyte-macrophage colony-stimulating factor
- IL
Interleukin
- MOG
Myelin oligodendrocyte glycoprotein
- MS
Multiple sclerosis
- PBS
Phosphate buffered saline
- SD
Standard deviation
- SEM
Standard error of arithmetic mean
- TCM
Central memory T cell
- TCR
T cell receptor
- TEM
Effector memory T cell
- TRM
Tissue resident memory T cell
References
- 1.Sattler A, Wagner U, Rossol M, Sieper J, Wu P, Krause A, Schmidt WA, Radmer S, Kohler S, Romagnani C, Thiel A. Cytokine-induced human IFN-gamma-secreting effector-memory Th cells in chronic autoimmune inflammation. Blood. 2009;113:1948–56. doi: 10.1182/blood-2008-02-139147. [DOI] [PubMed] [Google Scholar]
- 2.Eyrich M, Croner T, Leiler C, Lang P, Bader P, Klingebiel T, Niethammer D, Schlegel PG. Distinct contributions of CD4(+) and CD8(+) naive and memory T-cell subsets to overall T-cell-receptor repertoire complexity following transplantation of T-cell-depleted CD34-selected hematopoietic progenitor cells from unrelated donors. Blood. 2002;100:1915–8. doi: 10.1182/blood-2001-11-0005. [DOI] [PubMed] [Google Scholar]
- 3.Roberts AD, Ely KH, Woodland DL. Differential contributions of central and effector memory T cells to recall responses. The Journal of experimental medicine. 2005;202:123–33. doi: 10.1084/jem.20050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Booth JS, Toapanta FR, Salerno-Goncalves R, Patil S, Kader HA, Safta AM, Czinn SJ, Greenwald BD, Sztein MB. Characterization and functional properties of gastric tissue-resident memory T cells from children, adults, and the elderly. Frontiers in immunology. 2014;5:294. doi: 10.3389/fimmu.2014.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mueller SN, Gebhardt T, Carbone FR, Heath WR. Memory T cell subsets, migration patterns, and tissue residence. Annual review of immunology. 2013;31:137–61. doi: 10.1146/annurev-immunol-032712-095954. [DOI] [PubMed] [Google Scholar]
- 6.Mackay LK, Rahimpour A, Ma JZ, Collins N, Stock AT, Hafon ML, Vega-Ramos J, Lauzurica P, Mueller SN, Stefanovic T, Tscharke DC, Heath WR, Inouye M, Carbone FR, Gebhardt T. The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nature immunology. 2013;14:1294–301. doi: 10.1038/ni.2744. [DOI] [PubMed] [Google Scholar]
- 7.Xie J, Lin YK, Wang K, Che B, Li JQ, Xu X, Han F, Liang DH. Induced immune tolerance of autoantigen loaded immature dendritic cells in homogenic lupus mice. Genetics and molecular research : GMR. 2014;13:1251–62. doi: 10.4238/2014.February.27.10. [DOI] [PubMed] [Google Scholar]
- 8.Mbongue J, Nicholas D, Firek A, Langridge W. The role of dendritic cells in tissue-specific autoimmunity. Journal of immunology research. 2014;2014:857143. doi: 10.1155/2014/857143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ganguly D, Haak S, Sisirak V, Reizis B. The role of dendritic cells in autoimmunity. Nature reviews Immunology. 2013;13:566–77. doi: 10.1038/nri3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hassan I, Brendel C, Zielke A, Burchert A, Danila R. Immune regulatory plasmacytoid dendritic cells selectively accumulate in perithyroidal lymph nodes of patients with Graves disease: implications for the understanding of autoimmunity. Revista medico-chirurgicala a Societatii de Medici si Naturalisti din Iasi. 2013;117:46–51. [PubMed] [Google Scholar]
- 11.Manh TP, Alexandre Y, Baranek T, Crozat K, Dalod M. Plasmacytoid, conventional, and monocyte-derived dendritic cells undergo a profound and convergent genetic reprogramming during their maturation. European journal of immunology. 2013;43:1706–15. doi: 10.1002/eji.201243106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Froidure A, Shen C, Gras D, Van Snick J, Chanez P, Pilette C. Myeloid dendritic cells are primed in allergic asthma for thymic stromal lymphopoietin-mediated induction of Th2 and Th9 responses. Allergy. 2014;69:1068–76. doi: 10.1111/all.12435. [DOI] [PubMed] [Google Scholar]
- 13.Kim SJ, Diamond B. Modulation of tolerogenic dendritic cells and autoimmunity. Seminars in cell & developmental biology. 2014;S1084-9521(14):00091–3. doi: 10.1016/j.semcdb.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alonso MN, Gregorio JG, Davidson MG, Gonzalez JC, Engleman EG. Depletion of inflammatory dendritic cells with anti-CD209 conjugated to saporin toxin. Immunologic research. 2014;58:374–7. doi: 10.1007/s12026-014-8511-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torres-Aguilar H, Sanchez-Torres C, Jara LJ, Blank M, Shoenfeld Y. IL-10/TGF-beta-treated dendritic cells, pulsed with insulin, specifically reduce the response to insulin of CD4+ effector/memory T cells from type 1 diabetic individuals. Journal of clinical immunology. 2010;30:659–68. doi: 10.1007/s10875-010-9430-5. [DOI] [PubMed] [Google Scholar]
- 16.Nikolic T, Roep BO. Regulatory multitasking of tolerogenic dendritic cells - lessons taken from vitamin d3-treated tolerogenic dendritic cells. Frontiers in immunology. 2013;4:113. doi: 10.3389/fimmu.2013.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu C, Zhang Y, Jiang Y, Wang Q, Long Y, Wang C, Cao X, Chen G. Apoptotic cell administration enhances pancreatic islet engraftment by induction of regulatory T cells and tolerogenic dendritic cells. Cellular & molecular immunology. 2013;10:393–402. doi: 10.1038/cmi.2013.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annual review of immunology. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. 2003. [DOI] [PubMed] [Google Scholar]
- 19.Mastorodemos V, Ioannou M, Verginis P. Cell-Based Modulation of Autoimmune Responses in Multiple Sclerosis and Experimental Autoimmmune Encephalomyelitis: Therapeutic Implications. Neuroimmunomodulation. 2015;22(3):181–95. doi: 10.1159/000362370. [DOI] [PubMed] [Google Scholar]
- 20.Yeh WI, McWilliams IL, Harrington LE. Autoreactive Tbet-positive CD4 T cells develop independent of classic Th1 cytokine signaling during experimental autoimmune encephalomyelitis. Journal of immunology. 2011;187:4998–5006. doi: 10.4049/jimmunol.1100031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou F, Lauretti E, di Meco A, Ciric B, Gonnella P, Zhang GX, Rostami A. Intravenous transfer of apoptotic cell-treated dendritic cells leads to immune tolerance by blocking Th17 cell activity. Immunobiology. 2013;218:1069–76. doi: 10.1016/j.imbio.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou F, Ciric B, Zhang GX, Rostami A. Immune tolerance induced by intravenous transfer of immature dendritic cells via up-regulating numbers of suppressive IL-10(+) IFN-gamma(+)-producing CD4(+) T cells. Immunologic research. 2013;56:1–8. doi: 10.1007/s12026-012-8382-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou F, Ciric B, Li H, Yan Y, Li K, Cullimore M, Lauretti E, Gonnella P, Zhang GX, Rostami A. IL-10 deficiency blocks the ability of LPS to regulate expression of tolerance-related molecules on dendritic cells. European journal of immunology. 2012;42:1449–58. doi: 10.1002/eji.201141733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou F, Ciric B, Zhang GX, Rostami A. Immunotherapy using lipopolysaccharide-stimulated bone marrow-derived dendritic cells to treat experimental autoimmune encephalomyelitis. Clinical and experimental immunology. 2014;178:447–58. doi: 10.1111/cei.12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Torres-Aguilar H, Aguilar-Ruiz SR, Gonzalez-Perez G, Munguia R, Bajana S, Meraz-Rios MA, Sanchez-Torres C. Tolerogenic dendritic cells generated with different immunosuppressive cytokines induce antigen-specific anergy and regulatory properties in memory CD4+ T cells. Journal of immunology. 2010;184:1765–75. doi: 10.4049/jimmunol.0902133. [DOI] [PubMed] [Google Scholar]
- 26.Torres-Aguilar H, Blank M, Kivity S, Misgav M, Luboshitz J, Pierangeli SS, Shoenfeld Y. Tolerogenic dendritic cells inhibit antiphospholipid syndrome derived effector/memory CD4(+) T cell response to beta2GPI. Annals of the rheumatic diseases. 2012;71:120–8. doi: 10.1136/annrheumdis-2011-200063. [DOI] [PubMed] [Google Scholar]
- 27.Nasreen M, Waldie TM, Dixon CM, Steptoe RJ. Steady-state antigen-expressing dendritic cells terminate CD4+ memory T-cell responses. European journal of immunology. 2010;40:2016–25. doi: 10.1002/eji.200940085. [DOI] [PubMed] [Google Scholar]
- 28.Zhou F. Molecular mechanisms of IFN-gamma to up-regulate MHC class I antigen processing and presentation. International reviews of immunology. 2009;28:239–60. doi: 10.1080/08830180902978120. [DOI] [PubMed] [Google Scholar]
- 29.Perry LL, Feilzer K, Caldwell HD. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-gamma-dependent and -independent pathways. Journal of immunology. 1997;158:3344–52. [PubMed] [Google Scholar]
- 30.Olson NC, Doyle MF, Jenny NS, Huber SA, Psaty BM, Kronmal RA, Tracy RP. Decreased naive and increased memory CD4(+) T cells are associated with subclinical atherosclerosis: the multi-ethnic study of atherosclerosis. PloS one. 2013;8:e71498. doi: 10.1371/journal.pone.0071498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oh HM, Yu CR, Lee Y, Chan CC, Maminishkis A, Egwuagu CE. Autoreactive memory CD4+ T lymphocytes that mediate chronic uveitis reside in the bone marrow through STAT3-dependent mechanisms. Journal of immunology. 2011;187:3338–46. doi: 10.4049/jimmunol.1004019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haines CJ, Chen Y, Blumenschein WM, Jain R, Chang C, Joyce-Shaikh B, Porth K, Boniface K, Mattson J, Basham B, Anderton SM, McClanahan TK, Sadekova S, Cua DJ, McGeachy MJ. Autoimmune memory T helper 17 cell function and expansion are dependent on interleukin-23. Cell reports. 2013;3:1378–88. doi: 10.1016/j.celrep.2013.03.035. [DOI] [PubMed] [Google Scholar]
- 33.Elyaman W, Kivisakk P, Reddy J, Chitnis T, Raddassi K, Imitola J, Bradshaw E, Kuchroo VK, Yagita H, Sayegh MH, Khoury SJ. Distinct functions of autoreactive memory and effector CD4+ T cells in experimental autoimmune encephalomyelitis. The American journal of pathology. 2008;173:411–22. doi: 10.2353/ajpath.2008.080142. [DOI] [PMC free article] [PubMed] [Google Scholar]