Abstract
Myocardial infarction (MI) affects millions of people worldwide. MI causes massive cardiac cell death and heart function decrease. However, heart tissue cannot effectively regenerate by itself. While stem cell therapy has been considered an effective approach for regeneration, the efficacy of cardiac stem cell therapy remains low due to inferior cell engraftment in the infarcted region. This is mainly a result of low cell retention in the tissue and poor cell survival under ischemic, immune rejection and inflammatory conditions. Various approaches have been explored to improve cell engraftment: increase of cell retention using biomaterials as cell carriers; augmentation of cell survival under ischemic conditions by preconditioning cells, genetic modification of cells, and controlled release of growth factors and oxygen; and enhancement of cell survival by protecting cells from excessive inflammation and immune surveillance. In this paper, we review current progress, advantages, disadvantages, and potential solutions of these approaches.
1. Introduction
Heart disease has a high rate of morbidity and mortality [1]. Myocardial infarction (MI) is a major heart disease that causes massive cardiac cell death and partial loss of heart function. The infarcted heart tissue cannot effectively regenerate by itself because adult cardiomyocytes are unable to proliferate, and cardiac stem cells spontaneously generate only a limited number of cardiomyocytes [2]. Heart function thus cannot be restored. Following MI, the left ventricular wall progressively becomes thinner, and heart function gradually decreases. This adverse remodeling process leads to heart failure [3]. Heart transplantation is the only solution for patients with end-stage heart failure, but the number of donors available for transplantation is extremely limited, and the recipients require long-term immune suppressants to prevent organ rejection. Stem cell therapy is an alternate strategy. It aims to regenerate the infarcted heart tissue and/or improve heart function.
2. Stem Cells for Cardiac Therapy
Multiple cell types have been tested in animal models and clinical trials for cardiac therapy. Some stem cell types are capable of differentiating into cardiomyocytes to regenerate the heart tissue, leading to the restoration of heart function. These cells include cardiac stem cells [4–8] and pluripotent stem cell-derived cardiovascular progenitor cells [9, 10]. Some stem cell types cannot differentiate into functional cardiomyocytes but provide paracrine effects to augment the survival of resident cardiac cells, vascularize infarcted heart tissue, modulate immune response, recruit endogenous stem cells, and facilitate beneficial remodeling [11–17], resulting in an overall improvement of heart function. These stem cells include bone marrow-derived stem cells [18–23], adipose-derived stem cells [24–27], and cardiosphere-derived cells (CDCs) [28–35].
In the majority of current animal studies and clinical trials, stem cells are injected directly into the infarcted heart. However approximately 90% of cells are lost to the circulation, leaked, or squeezed out of the injection site [36]. For those cells retained in the infarcted tissue, most of them die within the first few weeks [37]. Overall, cell engraftment of current stem cell therapy is low, and its therapeutic efficacy is limited.
3. Major Causes of Low Cell Engraftment in Infarcted Hearts
As discussed above, the major causes of the low cell engraftment are inferior cell retention and survival in the infarcted heart tissue. The commonly used saline solution has very low viscosity and cannot efficiently hold the cells in tissue. Transplanted cell death is mainly a result of inadequate cell attachment to the host tissue, severe ischemia, and excessive inflammation. Anoikis is a form of programmed cell death of adherent cells induced by poor or weak interaction between cell and extracellular matrix (ECM) [38]. In normal heart tissue, adherent cells attach strongly to the surrounding ECM. In the infarcted tissue, however, the ECM does not allow strong cell attachment [39]. Moreover, the saline used for cell transplantation does not provide cells with a matrix for attachment. These events cause anoikis [40].
Another factor is oxygen tension in the tissue. After MI, an extremely low oxygen and nutrient ischemic environment exists in the infarcted region. Although hypoxia is considered necessary to preserve the stem cell properties [41], the harsh ischemic environment activates cell death pathways, resulting in death of the transplanted cells [42].
Following MI, acute inflammation ensues with recruitment of inflammatory cells (neutrophils and monocytes) into the infarcted heart tissue. These recruited inflammatory cells are engaged in production of various inflammatory cytokines and chemokines to recruit more inflammatory cells, secretion of various proteolytic enzymes and reactive oxygen species (ROS), and phagocytosis to remove dead cells and tissue debris [43–45]. Both ROS and proinflammatory cytokines, such as tumor necrosis factor-α (TNF-α), can compromise survival of transplanted cells.
4. Approaches to Improve Stem Cell Engraftment
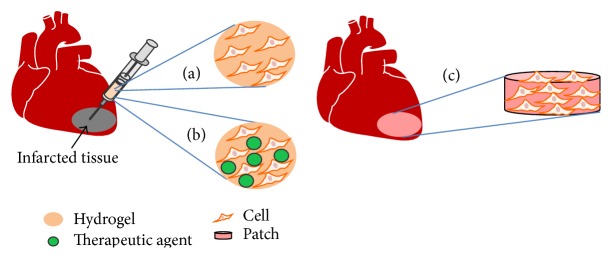
To increase cell engraftment in infarcted hearts, improving both cell retention and survival is necessary. The former may be achieved by using viscous, injectable hydrogels as cell carriers since low viscosity saline cannot efficiently hold cells in tissue. An injectable hydrogel can be delivered into the infarcted hearts through a minimal invasive injection approach (Figure 1) [49]. Seeding cells into scaffolds and patching them onto the heart may also increase cell retention (Figure 1). Both injectable hydrogels and scaffolds can augment cell survival. They provide an environment for cell attachment, which is required for cell survival. They can also be modified to promote stem cell survival under ischemic and inflammatory conditions [50]. In addition, the hydrogels and scaffolds offer mechanical support to the infarcted tissue to improve cardiac function.
Figure 1.
Strategies to improve cell retention in infarcted hearts. An injectable hydrogel can be used as a delivery vehicle for cells (a), or cells and therapeutic agents such as genes or proteins (b). A scaffold can be seeded with cells in vitro and then implanted to the infarcted region (c).
To address the issue of cell survival under ischemic conditions, approaches including ischemic preexposure of cells, genetic modulation of cells, and delivery of growth factors and oxygen to cells have been used. To promote cell survival under inflammatory conditions, biomaterials have been modified to prevent immune proteins and proinflammatory cytokines from penetrating inside to attack the encapsulated stem cells.
4.1. Using Biomaterials and Cell Adhesion Molecules for Stem Cell Delivery
Biomaterials used for stem cell transplantation should be biodegradable and biocompatible [51]. Specifically, they should have a controlled biodegradation rate, which ideally coincides with the rate of new tissue regeneration [52]. The degradation products should be nontoxic. The biomaterials should ideally mimic mechanical properties of the heart tissue, for example, stiffness. This will decrease the elevated wall stress to improve cardiac function [53]. Both natural and synthetic polymers have been employed for stem cell transplantation. Natural polymers are biologically derived materials. Some of them, like fibrin [49, 54], alginate [55–57], collagen [58, 59], Matrigel [60], hyaluronic acid [61], and chitosan [62], have been used to deliver stem cells into infarcted hearts.
Synthetic polymers are generated via chemical method to pursue desired properties and functions. The properties can be controlled by composition and chemistry. The capability of endowing synthetic polymers with functional groups and tunable properties are advantages of using these polymers for stem cell transplantation [63]. Commonly used synthetic polymers include polyesters, such as polyglycolide (PGA), polylactide (PLA), poly(lactide-co-glycolide) (PLGA), polycaprolactone (PCL), and their copolymers [64]. These polymers are often used in the form of scaffold. PLGA scaffolds loaded with bFGF have been used to promote cardiac angiogenesis [65]. Porous PCL scaffolds have been used to deliver endothelial progenitor cells into heart tissue to promote vascularization [66].
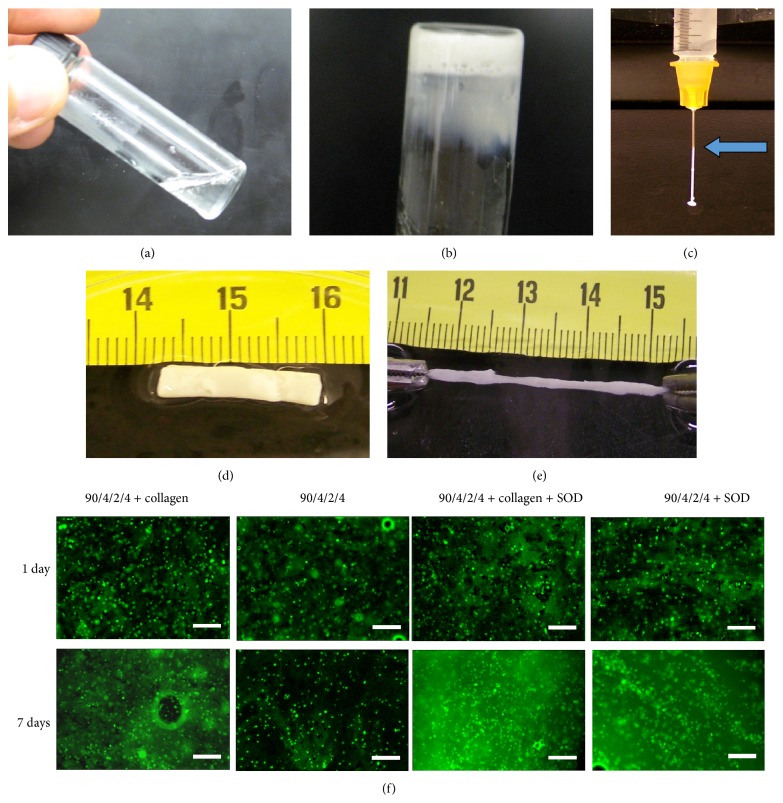
Some synthetic polymers can be used in the form of hydrogel. For example, Li et al. generated thermosensitive hydrogels based on N-isopropylacrylamide (NIPAAm), acrylic acid (AAc), dimethyl-gamma-butyrolactone acrylate (DBA), and 2-hydroxyethyl methacrylate-poly(trimethylene carbonate) (HEMAPTMC) [46]. The hydrogels are injectable at room temperature and solidify at body temperature within 10 seconds. They can therefore quickly solidify to efficiently hold cells in the tissue. Interestingly, mesenchymal stem cells (MSCs) were able to proliferate inside (Figure 2). Other synthetic hydrogels developed for cardiac repair include poly(ethylene oxide)-b-poly(propylene oxide)-b-poly(ethylene oxide) (PEO-PPO-PEO) [67], poly(D-lysine) (PDL) [68], and MPEG-PCL-MPEG [69, 70].
Figure 2.
Macroscopic images of hydrogel and cells. The copolymer solution is flowable at 4°C (a) and forms gel after gelation at 37°C (b). The copolymer solution can be injected through a 26-gauge needle (c). At 37°C, the formed gel is flexible and can be stretched: (d) before stretching; (e) after stretching; (f) fluorescence images of MSCs encapsulated in hydrogels with or without collagen and superoxide dismutase (SOD, 4 mg/mL) after 1 and 7 days of culture. The cells were stained with live cell stain CMFDA before encapsulation. Scale bar = 100 μm. This figure is adopted from [46].
Biomaterials can be modified with cell adhesive molecules to improve cell attachment, thus decreasing anoikis-induced stem cell death during transplantation [71]. Cell adhesive molecules are often mixed with or conjugated to the biomaterials. Karoubi et al. studied MSC survival in agarose with and without the addition of fibronectin and fibrinogen [72]. The results showed that cell survival was significantly increased after addition of fibronectin and fibrinogen. Similarly, fibrin glue remarkably improved cell survival in infarcted hearts [54]. Cooke et al. investigated cell adhesion on surfaces modified with several cell adhesive molecules, collagen I, collagen IV, fibronectin, and laminin, and found that cell attachment was increased [71]. Peptides YIGSR/IKVAV and RGD derived from laminin and fibronectin, respectively, have also been used to modify biomaterials to improve cell affinity [73].
4.2. Preexposure of Stem Cells for Enhanced Cell Survival
Preexposure of stem cells with ischemia or cytokines for cytoprotection is an alternate strategy to alleviate cell death. It enhances the cell tolerance to the harsh ischemic conditions. Murry et al. showed that cyclic exposure of stem cells to ischemia improved cell survival in ischemic myocardium [74]. Maulik et al. further demonstrated that ischemic preexposure allowed cells to adapt to ischemia, thus attenuating cell death under ischemia [75]. Grund et al. found that ischemically preexposed cells had reduced oxygen consumption [76]. In addition, ischemic preexposure enhanced cell secretion of growth factors [77, 78].
Cytokine preexposure can also improve cell survival [79–81]. MSCs pretreated with SDF-1α released antiapoptotic and angiogenic cytokines to improve cell survival and augment tissue angiogenesis [79]. Preexposure of endothelial progenitor cells with VEGF and bFGF enhanced cell paracrine effects, resulting in enhanced cell survival, angiogenesis, and reduced infarct size and left ventricle remodeling [80, 81]. Cells pretreated with IGF-1 showed cytoprotection effect both in vitro and in vivo [82, 83]. PI3K/Akt and MAPK/Erk1/2 pathways are responsible for the prosurvival effect.
4.3. Release of Growth Factors to Improve Cell Survival
High rates of both short-term and long-term cell survival are necessary for cardiac stem cell therapy. These may be achieved by using growth factors. Prosurvival growth factors allow for short-term cell survival, while angiogenic growth factors stimulate angiogenesis for long-term cell survival [84–86]. IGF-1 and HGF are two commonly used prosurvival growth factors. FGF [87], PDGF [88], and VEGF [89] are angiogenic growth factors used to promote angiogenesis in various tissues. bFGF also enhances cell survival under ischemic conditions [47]. In addition, specific growth factors can be used with prosurvival and angiogenic growth factors to promote stem cell differentiation into functional cells such as cardiomyocytes [90–92].
However, a concern for using growth factors in stem cell transplantation is that most of them have a relatively short half-life [93, 94]. Genetic modification of stem cells to promote the cells to secrete prosurvival and proangiogenic growth factors and sustained release of growth factors using biomaterials are commonly used approaches to address this concern.
Stem cells transfected with encoded genes of angiogenic growth factors like VEGF and FGF, and antiapoptotic factors like Akt and heme oxygenase-1, were able to secrete autocrine and paracrine growth factors [95, 96]. After transplanting these cells into infarcted hearts, cell survival, angiogenesis, and heart function were improved [97–99]. Matsumoto et al. transfected VEGF gene to MSCs and injected the modified cells into MI rat hearts [100]. High expression of VEGF was detected. This not only reduced cell death and increased capillary density, but also decreased the infarct size.
The growth factors are often encapsulated in biomaterials for controlled release [101–103]. Li et al. developed a bFGF release system based on a thermoresponsive and degradable hydrogel [47]. bFGF can be gradually released from the hydrogel for more than 2 weeks. The bFGF releasing system significantly increased MSC survival under low oxygen and nutrient conditions (Figure 3). It is expected that transplantation of stem cells using this bFGF release system will augment cell survival in the infarcted hearts. The release system may also promote angiogenesis due to the angiogenic effect of bFGF.
Figure 3.
(a) Release kinetics of bFGF loaded in the hydrogels. bFGF loading was 10 and 50 μg/mL, respectively. The error bars are small. (b) VEGF expression of MSCs in the hydrogels under different culture conditions. Cells were cultured under conditions of 10% FBS and 21% oxygen, 1% FBS and 21% oxygen, 1% FBS and 5% oxygen, and 1% FBS and 1% oxygen, respectively. The expression was normalized by that under 10% FBS with 21% oxygen culture condition. (c) MSC survival in hydrogels cultured under different conditions. Culture conditions: 10% FBS and 21% oxygen, 1% FBS and 21% oxygen, 1% FBS and 5% oxygen, and 1% FBS and 1% oxygen. Double stranded DNA (dsDNA) content was used to quantify live cell number in the hydrogels. The dsDNA content at day 1 (100%) was used for normalization. This figure is adopted from [47].
Padin-Iruega et al. tethered IGF-1 to self-assembling peptide nanofibers and used them for delivery of cardiac progenitor cells (CPCs) into infarcted hearts [104]. The IGF-1 was found to continuously release from the nanofibers to the myocardium. The released IGF-1 not only augmented CPC survival but also promoted cardiac differentiation, leading to enhanced cardiac regeneration.
4.4. Augmentation of Cell Survival by Releasing Oxygen to Transplanted Cells
Oxygen is critical for cell survival. The extremely low oxygen concentration in the infarcted heart results in significant cell death [105, 106]. Transplantation of stem cells with an oxygen release system is considered a feasible strategy to improve cell survival [107].
An oxygen release system can be generated using inorganic peroxide. Oh et al. developed a calcium peroxide-based oxygen release system by incorporating calcium peroxide into PLGA scaffold [108]. The system can continuously release oxygen for 10 days. The released oxygen enhanced cell survival under hypoxic conditions in vitro. However, this approach is not well suited for cardiac application since the Ca2+ generated together with oxygen may lead to abnormal Ca2+ transient in cardiomyocytes [109]. To avoid the ion effect, organic molecules-based oxygen release systems, such as pyridine endoperoxide oxygen release system [110], hydrogen peroxide/poly(methyl methacrylate) microcapsule oxygen release system [111], and porphyrin-hemoprotein (rHSA(FeP-Glu)) oxygen release system [112], were developed and found to enhance cell survival. However, these oxygen release systems can release oxygen for less than 24 hours [108, 110–112].
To achieve longer term oxygen release, Abdi et al. encapsulated hydrogen peroxide into PLGA microspheres and obtained oxygen release for 7 days [113]. Li et al. encapsulated hydrogen peroxide/PVP complex in the PLGA microspheres and achieved sustained oxygen release at a relatively high oxygen level for 2 weeks (Figure 4) [48]. This oxygen release system significantly improved cell survival under hypoxic conditions in vitro. It has a great potential to augment cell survival in infarcted hearts for an extended period of time. Yet the concentration of released oxygen needs to be controlled so as not to overproduce ROS.
Figure 4.
(a) Oxygen release kinetics of the H2O2 -releasing microspheres with different H2O2/VP ratio; (b) dsDNA content of live CDCs encapsulated in hydrogels with or without oxygen release. The higher dsDNA content represents higher live cell number. Cells were cultured under 1% oxygen condition. Hydrogels with oxygen release had microspheres with H2O2/VP ratio of 6/1, 4.5/1, and 3/1, respectively. This figure is adopted from [48].
4.5. Modification of Biomaterials to Enhance Cell Survival under Immune Rejection and Inflammation Conditions
Immune rejection and excessive inflammation also decrease the survival rate of transplanted cells. Proinflammatory cytokines like TNF-α and IL-1 induce excessive inflammation and create a noxious microenvironment, in addition to causing apoptosis of the cells [114]. Optimization of biomaterial properties and introduction of anti-inflammatory molecules into biomaterials may provide protection for the transplanted cells. By controlling pore size of the biomaterials, transplanted cells can be immunoisolated, leading to better cell survival of the transplanted cells [115–119]. However, small cytotoxic molecules such as TNF-α and IL-1β can still diffuse into the biomaterials [120, 121]. To address this issue, approaches like increasing degree of cross-linking and matrix concentration were used. However, these approaches may impede nutrient transport to cells. An alternate approach is to modify the biomaterials with anti-inflammatory molecules. For example, anti-TNF-peptide WP9QY (YCWSQYLCY) may be conjugated into hydrogel to prevent TNF from penetrating inside [122].
After MI, ROS content in the failing heart is upregulated [123], which can be cytotoxic against the transplanted cells. To decrease the cytotoxic effects of ROS, Hume and Anseth incorporated superoxide dismutase mimetic (SODm) into PEG hydrogel. This largely protected cells from oxidative stress damage and improved cell survival [124].
5. Conclusions and Prospects
Stem cell therapy is considered a potent and promising approach for cardiac therapy. However, the efficacy is limited as only a small percentage of transplanted cells engrafted in the infarcted tissue. Low cell retention and inferior cell survival are mainly responsible for the limited cell engraftment. Hydrogels and scaffolds can be utilized to improve cell retention. Injectable hydrogels may be more convenient for cell delivery than scaffolds as they can be delivered by a minimally invasive injection approach. Injectable hydrogels increase cell retention because of their high viscosity. Yet, long gelation time may not allow the hydrogels to largely increase cell retention, as they may be squeezed out of heart tissue or washed into circulation before gelation. Some hydrogels require UV radiation, pH changing, or ion addition to solidify, which may cause potential harm to cells. Thermosensitive and biodegradable hydrogels that have a fast gelation rate (in seconds) may address this issue.
Different approaches have been explored to enhance cell survival in infarcted hearts. While they can improve cell survival to some extent, different types of stem cells may require dissimilar optimization approaches for preparation, activation, transplantation procedures, and maintenance in vivo. There are also disadvantages associated with these approaches. Ischemic preexposure may damage cells in the process, and the transplanted cells may not survive under ischemic conditions for a long enough period. Genetic modification of cells may raise safety concerns. Controlled release of growth factors and oxygen and immune protection appear to be more effective to promote cell survival. However, further studies on the long-term effect of these approaches on cell survival, functioning, and differentiation are needed.
From a clinical point of view, safety and efficacy are still paramount issues. More studies on animals are required in order to develop a reliable cell-biomaterial delivery system with long-term safety and efficacy. Biomaterial type, degradation product toxicity, dose, and timing must be well studied before clinical application.
Acknowledgments
This work was supported by US National Science Foundation (1006734 and 1160122), American Heart Association (15GRNT25830058 and 13GRNT17150041), National Science Foundation of China (81471788), Institute for Materials Research Seed Grant at The Ohio State University, and Competitive Medical Research Fund from University of Pittsburgh Medical Center Health System.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Mozaffarian D., Benjamin E. J., Go A. S., et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Etzion S., Kedes L. H., Kloner R. A., Leor J. Myocardial regeneration: present and future trends. American Journal of Cardiovascular Drugs. 2001;1(4):233–244. doi: 10.2165/00129784-200101040-00002. [DOI] [PubMed] [Google Scholar]
- 3.Cohn J. N., Ferrari R., Sharpe N. Cardiac remodeling—concepts and clinical implications: a consensus paper from an International Forum on Cardiac Remodeling. Journal of the American College of Cardiology. 2000;35(3):569–582. doi: 10.1016/s0735-1097(99)00630-0. [DOI] [PubMed] [Google Scholar]
- 4.Hosoda T., Zheng H., Cabral-Da-Silva M., et al. Human cardiac stem cell differentiation is regulated by a mircrine mechanism. Circulation. 2011;123(12):1287–1296. doi: 10.1161/CIRCULATIONAHA.110.982918. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Chugh A. R., Beache G. M., Loughran J. H., et al. Administration of cardiac stem cells in patients with ischemic cardiomyopathy: the SCIPIO trial: surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation. 2012;126(11):S54–S64. doi: 10.1161/circulationaha.112.092627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolli R., Tang X.-L., Sanganalmath S. K., et al. Intracoronary delivery of autologous cardiac stem cells improves cardiac function in a porcine model of chronic ischemic cardiomyopathy. Circulation. 2013;128(2):122–131. doi: 10.1161/circulationaha.112.001075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Latham N., Ye B., Jackson R., et al. Human blood and cardiac stem cells synergize to enhance cardiac repair when cotransplanted into ischemic myocardium. Circulation. 2013;128(1) supplement 1:S105–S112. doi: 10.1161/circulationaha.112.000374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams A. R., Hatzistergos K. E., Addicott B., et al. Enhanced effect of combining human cardiac stem cells and bone marrow mesenchymal stem cells to reduce infarct size and to restore cardiac function after myocardial infarction. Circulation. 2013;127(2):213–223. doi: 10.1161/circulationaha.112.131110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Später D., Abramczuk M. K., Buac K., et al. A HCN4+ cardiomyogenic progenitor derived from the first heart field and human pluripotent stem cells. Nature Cell Biology. 2013;15(9):1098–1106. doi: 10.1038/ncb2824. [DOI] [PubMed] [Google Scholar]
- 10.Nsair A., Schenke-Layland K., van Handel B., et al. haracterization and therapeutic potential of induced pluripotent stem cell-derived cardiovascular progenitor cells. PLoS ONE. 2012;7(10) doi: 10.1371/journal.pone.0045603.e45603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forrester J. S., Makkar R. R., Marbán E. Long-term outcome of stem cell therapy for acute myocardial infarction: right results, wrong reasons. Journal of the American College of Cardiology. 2009;53(24):2270–2272. doi: 10.1016/j.jacc.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 12.Wang F., Guan J. Cellular cardiomyoplasty and cardiac tissue engineering for myocardial therapy. Advanced Drug Delivery Reviews. 2010;62(7-8):784–797. doi: 10.1016/j.addr.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Don C. W., Murry C. E. Improving survival and efficacy of pluripotent stem cell-derived cardiac grafts. Journal of Cellular and Molecular Medicine. 2013;17(11):1355–1362. doi: 10.1111/jcmm.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang Y. L., Wang Y. J., Chen L. J., Pan Y. H., Zhang L., Weintraub N. L. Cardiac-derived stem cell-based therapy for heart failure: Progress and clinical applications. Experimental Biology and Medicine. 2013;238(3):294–300. doi: 10.1177/1535370213477982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garbern J. C., Lee R. T. Cardiac stem cell therapy and the promise of heart regeneration. Cell Stem Cell. 2013;12(6):689–698. doi: 10.1016/j.stem.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosen M. R., Myerburg R. J., Francis D. P., Cole G. D., Marbán E. Translating stem cell research to cardiac disease therapies: pitfalls and prospects for improvement. Journal of the American College of Cardiology. 2014;64(9):922–937. doi: 10.1016/j.jacc.2014.06.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Berlo J. H., Molkentin J. D. An emerging consensus on cardiac regeneration. Nature Medicine. 2014;20(12):1386–1393. doi: 10.1038/nm.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagaya N., Kangawa K., Itoh T., et al. Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy. Circulation. 2005;112(8):1128–1135. doi: 10.1161/circulationaha.104.500447. [DOI] [PubMed] [Google Scholar]
- 19.Gnecchi M., He H., Noiseux N., et al. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. The FASEB Journal. 2006;20(6):661–669. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 20.Ripa R. S., Haack-Sørensen M., Wang Y., et al. Bone marrow-derived mesenchymal cell mobilization by granulocyte-colony stimulating factor after acute myocardial infarction: results from the Stem Cells in Myocardial Infarction (STEMMI) trial. Circulation. 2007;116(11):I24–I30. doi: 10.1161/circulationaha.106.678649. [DOI] [PubMed] [Google Scholar]
- 21.Hare J. M., Traverse J. H., Henry T. D., et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (Prochymal) after acute myocardial infarction. Journal of the American College of Cardiology. 2009;54(24):2277–2286. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Traverse J. H., McKenna D. H., Harvey K., et al. Results of a phase 1, randomized, double-blind, placebo-controlled trial of bone marrow mononuclear stem cell administration in patients following ST-elevation myocardial infarction. American Heart Journal. 2010;160(3):428–434. doi: 10.1016/j.ahj.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duran J. M., Makarewich C. A., Sharp T. E., et al. Bone-derived stem cells repair the heart after myocardial infarction through transdifferentiation and paracrine signaling mechanisms. Circulation Research. 2013;113(5):539–552. doi: 10.1161/circresaha.113.301202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mazo M., Planat-Bénard V., Abizanda G., et al. Transplantation of adipose derived stromal cells is associated with functional improvement in a rat model of chronic myocardial infarction. European Journal of Heart Failure. 2008;10(5):454–462. doi: 10.1016/j.ejheart.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 25.Mazo M., Hernández S., Gavira J. J., et al. Treatment of reperfused ischemia with adipose-derived stem cells in a preclinical Swine model of myocardial infarction. Cell Transplantation. 2012;21(12):2723–2733. doi: 10.3727/096368912x638847. [DOI] [PubMed] [Google Scholar]
- 26.Shevchenko E. K., Makarevich P. I., Tsokolaeva Z. I., et al. Transplantation of modified human adipose derived stromal cells expressing VEGF165 results in more efficient angiogenic response in ischemic skeletal muscle. Journal of Translational Medicine. 2013;11(1, article 138) doi: 10.1186/1479-5876-11-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rigol M., Solanes N., Roura S., et al. Allogeneic adipose stem cell therapy in acute myocardial infarction. European Journal of Clinical Investigation. 2014;44(1):83–92. doi: 10.1111/eci.12195. [DOI] [PubMed] [Google Scholar]
- 28.Smith R. R., Barile L., Cho H. C., et al. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115(7):896–908. doi: 10.1161/circulationaha.106.655209. [DOI] [PubMed] [Google Scholar]
- 29.Davis D. R., Zhang Y., Smith R. R., et al. Validation of the cardiosphere method to culture cardiac progenitor cells from myocardial tissue. PLoS ONE. 2009;4(9) doi: 10.1371/journal.pone.0007195.e7195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnston P. V., Sasano T., Mills K., et al. Engraftment, differentiation, and functional benefits of autologous cardiosphere-derived cells in porcine ischemic cardiomyopathy. Circulation. 2009;120(12):1075–1083. doi: 10.1161/CIRCULATIONAHA.108.816058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chimenti I., Smith R. R., Li T.-S., et al. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circulation Research. 2010;106(5):971–980. doi: 10.1161/circresaha.109.210682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mishra R., Vijayan K., Colletti E. J., et al. Characterization and functionality of cardiac progenitor cells in congenital heart patients. Circulation. 2011;123(4):364–373. doi: 10.1161/circulationaha.110.971622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li T.-S., Cheng K., Malliaras K., et al. Direct comparison of different stem cell types and subpopulations reveals superior paracrine potency and myocardial repair efficacy with cardiosphere-derived cells. Journal of the American College of Cardiology. 2012;59(10):942–953. doi: 10.1016/j.jacc.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maxeiner H., Mufti S., Krehbiehl N., et al. Interleukin-6 contributes to the paracrine effects of cardiospheres cultured from human, murine and rat hearts. Journal of Cellular Physiology. 2014;229(11):1681–1689. doi: 10.1002/jcp.24613. [DOI] [PubMed] [Google Scholar]
- 35.Xie Y., Ibrahim A., Cheng K., et al. Importance of cell-cell contact in the therapeutic benefits of cardiosphere-derived cells. Stem Cells. 2014;32(9):2397–2406. doi: 10.1002/stem.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leor J., Aboulafia-Etzion S., Dar A., et al. Bioengineered cardiac grafts: a new approach to repair the infarcted myocardium? Circulation. 2000;102(19):III56–III61. doi: 10.1161/01.cir.102.suppl_3.iii-56. [DOI] [PubMed] [Google Scholar]
- 37.Reinecke H., Murry C. E. Taking the death toll after cardiomyocyte grafting: a reminder of the importance of quantitative biology. Journal of Molecular and Cellular Cardiology. 2002;34(3):251–253. doi: 10.1006/jmcc.2001.1494. [DOI] [PubMed] [Google Scholar]
- 38.Frisch S. M., Screaton R. A. Anoikis mechanisms. Current Opinion in Cell Biology. 2001;13(5):555–562. doi: 10.1016/S0955-0674(00)00251-9. [DOI] [PubMed] [Google Scholar]
- 39.Imanaka-Yoshida K., Hiroe M., Yoshida T. Interaction between cell and extracellular matrix in heart disease: multiple roles of tenascin-C in tissue remodeling. Histology and Histopathology. 2004;19(2):517–525. doi: 10.14670/HH-19.517. [DOI] [PubMed] [Google Scholar]
- 40.Frisch S. M., Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. Journal of Cell Biology. 1994;124(4):619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Csete M. Oxygen in the cultivation of stem cells. Annals of the New York Academy of Sciences. 2005;1049:1–8. doi: 10.1196/annals.1334.001. [DOI] [PubMed] [Google Scholar]
- 42.Haider H. K., Ashraf M. Strategies to promote donor cell survival: combining preconditioning approach with stem cell transplantation. Journal of Molecular and Cellular Cardiology. 2008;45(4):554–566. doi: 10.1016/j.yjmcc.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mehta J. L., Nichols W. W., Mehta P. Neutrophils as potential participants in acute myocardial ischemia: relevance to reperfusion. Journal of the American College of Cardiology. 1988;11(6):1309–1316. doi: 10.1016/0735-1097(88)90297-5. [DOI] [PubMed] [Google Scholar]
- 44.Nahrendorf M., Swirski F. K., Aikawa E., et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. The Journal of Experimental Medicine. 2007;204(12):3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nahrendorf M., Frantz S., Swirski F. K., et al. Imaging systemic inflammatory networks in ischemic heart disease. Journal of the American College of Cardiology. 2015;65(15):1583–1591. doi: 10.1016/j.jacc.2015.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Z., Wang F., Roy S., Sen C. K., Guan J. Injectable, highly flexible, and thermosensitive hydrogels capable of delivering superoxide dismutase. Biomacromolecules. 2009;10(12):3306–3316. doi: 10.1021/bm900900e. [DOI] [PubMed] [Google Scholar]
- 47.Li Z., Guo X., Guan J. A thermosensitive hydrogel capable of releasing bFGF for enhanced differentiation of mesenchymal stem cell into cardiomyocyte-like cells under ischemic conditions. Biomacromolecules. 2012;13(6):1956–1964. doi: 10.1021/bm300574j. [DOI] [PubMed] [Google Scholar]
- 48.Li Z., Guo X., Guan J. An oxygen release system to augment cardiac progenitor cell survival and differentiation under hypoxic condition. Biomaterials. 2012;33(25):5914–5923. doi: 10.1016/j.biomaterials.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 49.Christman K. L., Lee R. J. Biomaterials for the treatment of myocardial infarction. Journal of the American College of Cardiology. 2006;48(5):907–913. doi: 10.1016/j.jacc.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 50.Shoichet M. S. Polymer scaffolds for biomaterials applications. Macromolecules. 2009;43(2):581–591. doi: 10.1021/ma901530r. [DOI] [Google Scholar]
- 51.Seal B. L., Otero T. C., Panitch A. Polymeric biomaterials for tissue and organ regeneration. Materials Science & Engineering: R: Reports. 2001;34(4-5):147–230. doi: 10.1016/s0927-796x(01)00035-3. [DOI] [Google Scholar]
- 52.Shin H., Jo S., Mikos A. G. Biomimetic materials for tissue engineering. Biomaterials. 2003;24(24):4353–4364. doi: 10.1016/S0142-9612(03)00339-9. [DOI] [PubMed] [Google Scholar]
- 53.Christman K. L., Fok H. H., Sievers R. E., Fang Q., Lee R. J. Fibrin glue alone and skeletal myoblasts in a fibrin scaffold preserve cardiac function after myocardial infarction. Tissue Engineering. 2004;10(3-4):403–409. doi: 10.1089/107632704323061762. [DOI] [PubMed] [Google Scholar]
- 54.Christman K. L., Vardanian A. J., Fang Q., Sievers R. E., Fok H. H., Lee R. J. Injectable fibrin scaffold improves cell transplant survival, reduces infarct expansion, and induces neovasculature formation in ischemic myocardium. Journal of the American College of Cardiology. 2004;44(3):654–660. doi: 10.1016/j.jacc.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 55.Landa N., Miller L., Feinberg M. S., et al. Effect of injectable alginate implant on cardiac remodeling and function after recent and old infarcts in rat. Circulation. 2008;117(11):1388–1396. doi: 10.1161/CIRCULATIONAHA.107.727420. [DOI] [PubMed] [Google Scholar]
- 56.Gomez-Mauricio R. G., Acarregui A., Sánchez-Margallo F. M., et al. A preliminary approach to the repair of myocardial infarction using adipose tissue-derived stem cells encapsulated in magnetic resonance-labelled alginate microspheres in a porcine model. European Journal of Pharmaceutics and Biopharmaceutics. 2013;84(1):29–39. doi: 10.1016/j.ejpb.2012.11.028. [DOI] [PubMed] [Google Scholar]
- 57.Roche E. T., Hastings C. L., Lewin S. A., et al. Comparison of biomaterial delivery vehicles for improving acute retention of stem cells in the infarcted heart. Biomaterials. 2014;35(25):6850–6858. doi: 10.1016/j.biomaterials.2014.04.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dai W., Wold L. E., Dow J. S., Kloner R. A. Thickening of the infarcted wall by collagen injection improves left ventricular function in rats: a novel approach to preserve cardiac function after myocardial infarction. Journal of the American College of Cardiology. 2005;46(4):714–719. doi: 10.1016/j.jacc.2005.04.056. [DOI] [PubMed] [Google Scholar]
- 59.Suuronen E. J., Veinot J. P., Wong S., et al. Tissue-engineered injectable collagen-based matrices for improved cell delivery and vascularization of ischemic tissue using CD133+ progenitors expanded from the peripheral blood. Circulation. 2006;114(1) supplement:I138–I144. doi: 10.1161/circulationaha.105.001081. [DOI] [PubMed] [Google Scholar]
- 60.Xu C., Inokuma M. S., Denham J., et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nature Biotechnology. 2001;19(10):971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- 61.Chang C. Y., Chan A. T., Armstrong P. A., et al. Hyaluronic acid-human blood hydrogels for stem cell transplantation. Biomaterials. 2012;33(32):8026–8033. doi: 10.1016/j.biomaterials.2012.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ishihara M., Nakanishi K., Ono K., et al. Photocrosslinkable chitosan as a dressing for wound occlusion and accelerator in healing process. Biomaterials. 2002;23(3):833–840. doi: 10.1016/S0142-9612(01)00189-2. [DOI] [PubMed] [Google Scholar]
- 63.Zhang N., Kohn D. H. Using polymeric materials to control stem cell behavior for tissue regeneration. Birth Defects Research, Part C—Embryo Today: Reviews. 2012;96(1):63–81. doi: 10.1002/bdrc.21003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thomson R. C., Wake M. C., Yaszemski M. J., et al. Biodegradable polymer scaffolds to regenerate organs. In: Peppas N., Langer R., editors. Biopolymers II. Berlin, Germany: Springer; 1995. pp. 245–274. [Google Scholar]
- 65.Wang Y., Liu X.-C., Zhao J., et al. Degradable PLGA scaffolds with basic fibroblast growth factor: experimental studies in myocardial revascularization. Texas Heart Institute Journal. 2009;36(2):89–97. [PMC free article] [PubMed] [Google Scholar]
- 66.Singh S., Wu B. M., Dunn J. C. Y. Accelerating vascularization in polycaprolactone scaffolds by endothelial progenitor cells. Tissue Engineering Part: A. 2011;17(13-14):1819–1830. doi: 10.1089/ten.tea.2010.0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bawa P., Pillay V., Choonara Y. E., Toit L. C. D. Stimuli-responsive polymers and their applications in drug delivery. Biomedical Materials. 2009;4(2) doi: 10.1088/1748-6041/4/2/022001.022001 [DOI] [PubMed] [Google Scholar]
- 68.Crompton K. E., Goud J. D., Bellamkonda R. V., et al. Polylysine-functionalised thermoresponsive chitosan hydrogel for neural tissue engineering. Biomaterials. 2007;28(3):441–449. doi: 10.1016/j.biomaterials.2006.08.044. [DOI] [PubMed] [Google Scholar]
- 69.Jiang X.-J., Wang T., Li X.-Y., et al. Injection of a novel synthetic hydrogel preserves left ventricle function after myocardial infarction. Journal of Biomedical Materials Research A. 2009;90(2):472–477. doi: 10.1002/jbm.a.32118. [DOI] [PubMed] [Google Scholar]
- 70.Tseng H., Puperi D. S., Kim E. J., et al. Anisotropic poly(ethylene glycol)/polycaprolactone hydrogel–fiber composites for heart valve tissue engineering. Tissue Engineering Part A. 2014;20(19-20):2634–2645. doi: 10.1089/ten.tea.2013.0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cooke M. J., Phillips S. R., Shah D. S. H., Athey D., Lakey J. H., Przyborski S. A. Enhanced cell attachment using a novel cell culture surface presenting functional domains from extracellular matrix proteins. Cytotechnology. 2008;56(2):71–79. doi: 10.1007/s10616-007-9119-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Karoubi G., Ormiston M. L., Stewart D. J., Courtman D. W. Single-cell hydrogel encapsulation for enhanced survival of human marrow stromal cells. Biomaterials. 2009;30(29):5445–5455. doi: 10.1016/j.biomaterials.2009.06.035. [DOI] [PubMed] [Google Scholar]
- 73.Jongpaiboonkit L., King W. J., Murphy W. L. Screening for 3D environments that support human mesenchymal stem cell viability using hydrogel arrays. Tissue Engineering Part A. 2009;15(2):343–353. doi: 10.1089/ten.tea.2008.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Murry C. E., Jennings R. B., Reimer K. A. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74(5):1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 75.Maulik N., Yoshida T., Engelman R. M., et al. Ischemic preconditioning attenuates apoptotic cell death associated with ischemia/reperfusion. Molecular and Cellular Biochemistry. 1998;186(1-2):139–145. doi: 10.1023/A:1006883717174. [DOI] [PubMed] [Google Scholar]
- 76.Grund F., Sommerschild H. T., Kirkebøen K. A., Ilebekk A. Preconditioning with ischaemia reduces both myocardial oxygen consumption and infarct size in a graded pattern. Journal of Molecular and Cellular Cardiology. 1997;29(11):3067–3079. doi: 10.1006/jmcc.1997.0521. [DOI] [PubMed] [Google Scholar]
- 77.Ii M., Nishimura H., Iwakura A., et al. Endothelial progenitor cells are rapidly recruited to myocardium and mediate protective effect of ischemic preconditioning via ‘imported’ nitric oxide synthase activity. Circulation. 2005;111(9):1114–1120. doi: 10.1161/01.cir.0000157144.24888.7e. [DOI] [PubMed] [Google Scholar]
- 78.Addya S., Shiroto K., Turoczi T., et al. Ischemic preconditioning-mediated cardioprotection is disrupted in heterozygous Flt-1 (VEGFR-1) knockout mice. Journal of Molecular and Cellular Cardiology. 2005;38(2):345–351. doi: 10.1016/j.yjmcc.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 79.Vandervelde S., van Luyn M. J. A., Tio R. A., Harmsen M. C. Signaling factors in stem cell-mediated repair of infarcted myocardium. Journal of Molecular and Cellular Cardiology. 2005;39(2):363–376. doi: 10.1016/j.yjmcc.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 80.Shintani S., Kusano K., Ii M., et al. Synergistic effect of combined intramyocardial CD34+ cells and VEGF2 gene therapy after MI. Nature Clinical Practice Cardiovascular Medicine. 2006;3, supplement 1:S123–S128. doi: 10.1038/ncpcardio0430. [DOI] [PubMed] [Google Scholar]
- 81.Pasha Z., Wang Y., Sheikh R., Zhang D., Zhao T., Ashraf M. Preconditioning enhances cell survival and differentiation of stem cells during transplantation in infarcted myocardium. Cardiovascular Research. 2008;77(1):134–142. doi: 10.1093/cvr/cvm025. [DOI] [PubMed] [Google Scholar]
- 82.Párrizas M., Saltiel A. R., LeRoith D. Insulin-like growth factor 1 inhibits apoptosis using the phosphatidylinositol 3'-kinase and mitogen-activated protein kinase pathways. The Journal of Biological Chemistry. 1997;272(1):154–161. doi: 10.1074/jbc.272.1.154. [DOI] [PubMed] [Google Scholar]
- 83.Humbert S., Bryson E. A., Cordelières F. P., et al. The IGF-1/Akt pathway is neuroprotective in Huntington's disease and involves huntingtin phosphorylation by Akt. Developmental Cell. 2002;2(6):831–837. doi: 10.1016/s1534-5807(02)00188-0. [DOI] [PubMed] [Google Scholar]
- 84.Nör J. E., Christensen J., Mooney D. J., Polverini P. J. Vascular endothelial growth factor (VEGF)-mediated angiogenesis is associated with enhanced endothelial cell survival and induction of Bcl-2 expression. The American Journal of Pathology. 1999;154(2):375–384. doi: 10.1016/s0002-9440(10)65284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Deuse T., Peter C., Fedak P. W. M., et al. Hepatocyte growth factor or vascular endothelial growth factor gene transfer maximizes mesenchymal stem cell-based myocardial salvage after acute myocardial infarction. Circulation. 2009;120(1):S247–S254. doi: 10.1161/circulationaha.108.843680. [DOI] [PubMed] [Google Scholar]
- 86.Lee T.-J., Bhang S. H., Yang H. S., et al. Enhancement of long-term angiogenic efficacy of adipose stem cells by delivery of FGF2. Microvascular Research. 2012;84(1):1–8. doi: 10.1016/j.mvr.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 87.Ma F., Xiao Z., Chen B., et al. Accelerating proliferation of neural stem/progenitor cells in collagen sponges immobilized with engineered basic fibroblast growth factor for nervous system tissue engineering. Biomacromolecules. 2014;15(3):1062–1068. doi: 10.1021/bm500062n. [DOI] [PubMed] [Google Scholar]
- 88.Chen Y., Xu H., Liu J., Zhang C., Leutz A., Mo X. The c-Myb functions as a downstream target of PDGF-mediated survival signal in vascular smooth muscle cells. Biochemical and Biophysical Research Communications. 2007;360(2):433–436. doi: 10.1016/j.bbrc.2007.06.078. [DOI] [PubMed] [Google Scholar]
- 89.Haider H. K., Ye L., Jiang S., et al. Angiomyogenesis for cardiac repair using human myoblasts as carriers of human vascular endothelial growth factor. Journal of Molecular Medicine. 2004;82(8):539–549. doi: 10.1007/s00109-004-0546-z. [DOI] [PubMed] [Google Scholar]
- 90.Choi K.-C., Yoo D.-S., Cho K.-S., Huh P.-W., Kim D.-S., Park C.-K. Effect of single growth factor and growth factor combinations on differentiation of neural stem cells. Journal of Korean Neurosurgical Society. 2008;44(6):375–381. doi: 10.3340/jkns.2008.44.6.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Minato A., Ise H., Goto M., Akaike T. Cardiac differentiation of embryonic stem cells by substrate immobilization of insulin-like growth factor binding protein 4 with elastin-like polypeptides. Biomaterials. 2012;33(2):515–523. doi: 10.1016/j.biomaterials.2011.09.070. [DOI] [PubMed] [Google Scholar]
- 92.Xue Y., Yan Y., Gong H., et al. Insulin-like growth factor binding protein 4 enhances cardiomyocytes induction in murine-induced pluripotent stem cells. Journal of Cellular Biochemistry. 2014;115(9):1495–1504. doi: 10.1002/jcb.24804. [DOI] [PubMed] [Google Scholar]
- 93.Lynch S. E., de Castilla G. R., Williams R. C., et al. The effects of short-term application of a combination of platelet-derived and insulin-like growth factors on periodontal wound healing. Journal of Periodontology. 1991;62(7):458–467. doi: 10.1902/jop.1991.62.7.458. [DOI] [PubMed] [Google Scholar]
- 94.Nixon A. J., Brower-Toland B. D., Bent S. J., et al. Insulinlike growth factor-I gene therapy applications for cartilage repair. Clinical Orthopaedics and Related Research. 2000;(379):S201–S213. doi: 10.1097/00003086-200010001-00026. [DOI] [PubMed] [Google Scholar]
- 95.Dzau V. J., Gnecchi M., Pachori A. S. Enhancing stem cell therapy through genetic modification. Journal of the American College of Cardiology. 2005;46(7):1351–1353. doi: 10.1016/j.jacc.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 96.Yau T. M., Kim C., Ng D., et al. Increasing transplanted cell survival with cell-based angiogenic gene therapy. Annals of Thoracic Surgery. 2005;80(5):1779–1786. doi: 10.1016/j.athoracsur.2005.04.079. [DOI] [PubMed] [Google Scholar]
- 97.Kofidis T., de Bruin J. L., Yamane T., et al. Insulin-like growth factor promotes engraftment, differentiation, and functional improvement after transfer of embryonic stem cells for myocardial restoration. Stem Cells. 2004;22(7):1239–1245. doi: 10.1634/stemcells.2004-0127. [DOI] [PubMed] [Google Scholar]
- 98.Kanemitsu N., Tambara K., Premaratne G. U., et al. Insulin-like growth factor-1 enhances the efficacy of myoblast transplantation with its multiple functions in the chronic myocardial infarction rat model. Journal of Heart and Lung Transplantation. 2006;25(10):1253–1262. doi: 10.1016/j.healun.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 99.Ye L., Haider H. K., Jiang S., et al. Improved angiogenic response in pig heart following ischaemic injury using human skeletal myoblast simultaneously expressing VEGF165 and angiopoietin-1. European Journal of Heart Failure. 2007;9(1):15–22. doi: 10.1016/j.ejheart.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 100.Matsumoto R., Omura T., Yoshiyama M., et al. Vascular endothelial growth factor-expressing mesenchymal stem cell transplantation for the treatment of acute myocardial infarction. Arteriosclerosis, Thrombosis, and Vascular Biology. 2005;25(6):1168–1173. doi: 10.1161/01.atv.0000165696.25680.ce. [DOI] [PubMed] [Google Scholar]
- 101.Babensee J. E., McIntire L. V., Mikos A. G. Growth factor delivery for tissue engineering. Pharmaceutical Research. 2000;17(5):497–504. doi: 10.1023/a:1007502828372. [DOI] [PubMed] [Google Scholar]
- 102.Martens T. P., Godier A. F. G., Parks J. J., et al. Percutaneous cell delivery into the heart using hydrogels polymerizing in situ. Cell Transplantation. 2009;18(3):297–304. doi: 10.3727/096368909788534915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li Z., Guan J. Thermosensitive hydrogels for drug delivery. Expert Opinion on Drug Delivery. 2011;8(8):991–1007. doi: 10.1517/17425247.2011.581656. [DOI] [PubMed] [Google Scholar]
- 104.Padin-Iruega M. E., Misao Y., Davis M. E., et al. Cardiac progenitor cells and biotinylated insulin-like growth factor-1 nanofibers improve endogenous and exogenous myocardial regeneration after infarction. Circulation. 2009;120(10):876–887. doi: 10.1161/CIRCULATIONAHA.109.852285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Egred M., Al-Mohammad A., Waiter G. D., et al. Detection of scarred and viable myocardium using a new magnetic resonance imaging technique: blood oxygen level dependent (BOLD) MRI. Heart. 2003;89(7):738–744. doi: 10.1136/heart.89.7.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Robey T. E., Saiget M. K., Reinecke H., Murry C. E. Systems approaches to preventing transplanted cell death in cardiac repair. Journal of Molecular and Cellular Cardiology. 2008;45(4):567–581. doi: 10.1016/j.yjmcc.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Camci-Unal G., Alemdar N., Annabi N., Khademhosseini A. Oxygen releasing biomaterials for tissue engineering. Polymer International. 2013;62(6):843–848. doi: 10.1002/pi.4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Oh S. H., Ward C. L., Atala A., Yoo J. J., Harrison B. S. Oxygen generating scaffolds for enhancing engineered tissue survival. Biomaterials. 2009;30(5):757–762. doi: 10.1016/j.biomaterials.2008.09.065. [DOI] [PubMed] [Google Scholar]
- 109.Ai X. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circulation Research. 2005;97(12):1314–1322. doi: 10.1161/01.res.0000194329.41863.89. [DOI] [PubMed] [Google Scholar]
- 110.Benz S., Nötzli S., Siegel J. S., Eberli D., Jessen H. J. Controlled oxygen release from pyridone endoperoxides promotes cell survival under anoxic conditions. Journal of Medicinal Chemistry. 2013;56(24):10171–10182. doi: 10.1021/jm4016137. [DOI] [PubMed] [Google Scholar]
- 111.Mallepally R. R., Parrish C. C., Mc Hugh M. A. M., Ward K. R. Hydrogen peroxide filled poly(methyl methacrylate) microcapsules: potential oxygen delivery materials. International Journal of Pharmaceutics. 2014;475(1-2):e130–e137. doi: 10.1016/j.ijpharm.2014.08.052. [DOI] [PubMed] [Google Scholar]
- 112.Wang R.-M., Komatsu T., Nakagawa A., Tsuchida E. Human serum albumin bearing covalently attached iron(II) porphyrins as O2-coordination sites. Bioconjugate Chemistry. 2005;16(1):23–26. doi: 10.1021/bc049859m. [DOI] [PubMed] [Google Scholar]
- 113.Abdi S. I. H., Ng S. M., Lim J. O. An enzyme-modulated oxygen-producing micro-system for regenerative therapeutics. International Journal of Pharmaceutics. 2011;409(1-2):203–205. doi: 10.1016/j.ijpharm.2011.02.041. [DOI] [PubMed] [Google Scholar]
- 114.Hedayat M., Mahmoudi M. J., Rose N. R., Rezaei N. Proinflammatory cytokines in heart failure: double-edged swords. Heart Failure Reviews. 2010;15(6):543–562. doi: 10.1007/s10741-010-9168-4. [DOI] [PubMed] [Google Scholar]
- 115.Li R. H. Materials for immunoisolated cell transplantation. Advanced Drug Delivery Reviews. 1998;33(1-2):87–109. doi: 10.1016/S0169-409X(98)00022-2. [DOI] [PubMed] [Google Scholar]
- 116.Wilson J. T., Chaikof E. L. Challenges and emerging technologies in the immunoisolation of cells and tissues. Advanced Drug Delivery Reviews. 2008;60(2):124–145. doi: 10.1016/j.addr.2007.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Weber L. M., Hayda K. N., Anseth K. S. Cell-matrix interactions improve beta-cell survival and insulin secretion in three-dimensional culture. Tissue Engineering—Part A. 2008;14(12):1959–1968. doi: 10.1089/ten.tea.2007.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Weber L. M., Anseth K. S. Hydrogel encapsulation environments functionalized with extracellular matrix interactions increase islet insulin secretion. Matrix Biology. 2008;27(8):667–673. doi: 10.1016/j.matbio.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lin C.-C., Anseth K. S. Glucagon-like peptide-1 functionalized PEG hydrogels promote survival and function of encapsulated pancreatic β-cells. Biomacromolecules. 2009;10(9):2460–2467. doi: 10.1021/bm900420f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.de Vos P., Marchetti P. Encapsulation of pancreatic islets for transplantation in diabetes: the untouchable islets. Trends in Molecular Medicine. 2002;8(8):363–366. doi: 10.1016/s1471-4914(02)02381-x. [DOI] [PubMed] [Google Scholar]
- 121.Jang J. Y., Lee D. Y., Park S. J., Byun Y. Immune reactions of lymphocytes and macrophages against PEG-grafted pancreatic islets. Biomaterials. 2004;25(17):3663–3669. doi: 10.1016/j.biomaterials.2003.10.062. [DOI] [PubMed] [Google Scholar]
- 122.Lin C.-C., Metters A. T., Anseth K. S. Functional PEG-peptide hydrogels to modulate local inflammation induced by the pro-inflammatory cytokine TNFα . Biomaterials. 2009;30(28):4907–4914. doi: 10.1016/j.biomaterials.2009.05.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Inoue T., Ide T., Yamato M., et al. Time-dependent changes of myocardial and systemic oxidative stress are dissociated after myocardial infarction. Free Radical Research. 2009;43(1):37–46. doi: 10.1080/10715760802534820. [DOI] [PubMed] [Google Scholar]
- 124.Hume P. S., Anseth K. S. Polymerizable superoxide dismutase mimetic protects cells encapsulated in poly(ethylene glycol) hydrogels from reactive oxygen species-mediated damage. Journal of Biomedical Materials Research - Part A. 2011;99(1):29–37. doi: 10.1002/jbm.a.33160. [DOI] [PMC free article] [PubMed] [Google Scholar]