Abstract
The Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC) guidelines were last updated in 2007 (TASC II) and represented the collaboration of international vascular specialties involved in the management of patients with peripheral arterial disease (PAD). Since the publication of TASC II, there have been innovations in endovascular revascularization strategies for patients with PAD. The intent of this publication is to provide a complete anatomic lower limb TASC lesion classification, including the infrapopliteal segment, and an updated literature review of new endovascular techniques and practice patterns employed by vascular specialists today.
Keywords: angioplasty, arteries, claudication, critical limb ischemia, occlusion, peripheral artery disease, revascularization, stenosis, stents, surgery
Introduction
The TransAtlantic Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC I), which in the subsequent version was named the Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II), has previously provided expert recommendations on the diagnosis and treatment of peripheral arterial disease (PAD).1,2) One highly utilized aspect of the TASC guidelines, originally published in 2000, is the TASC anatomic artery lesion classification. This anatomic classification provides a characterization of the various patterns of disease and guidance on treatment decisions regarding the optimal revascularization strategy (endovascular vs. surgical) based on the complexity and location of the anatomic disease. The original classification stratified disease in the aortoiliac and femoropopliteal territories into categories, from A through to D. TASC A represented the least complex anatomic scenario (ie, focal stenosis) and TASC D reflected the most complex revascularization scenario (ie, diffuse, occlusive). TASC A lesions were originally designated as most appropriately treated with an endovascular strategy, while TASC D lesions were recommended for surgical revascularization based on available evidence at the time. The optimal management strategy for TASC B and TASC C lesions could favor either approach based on factors such as the technical resources available, patient status, and physician experience. These guidelines also provided a framework for future studies needed to support a robust body of data for subsequent recommendations.
The TASC II guidelines were published in 2007 and included a revision of the original TASC classification for PAD, with a focus on the aortoiliac and femoropopliteal territories. TASC II also aimed to provide guidance on treatment decisions relating to the optimal revascularization strategy (endovascular vs surgical) based on the anatomic and clinical status of the patient. In general, this revision resulted in the reclassification of more complex anatomies into less severe categories of the TASC classification (eg, TASC C lesions reclassified as TASC B lesions with an associated shift from surgical to endovascular management). TASC A and B lesions were still recommended for primary endovascular revascularization, TASC D lesions for surgical revascularization, and TASC C lesions for surgical revascularization in patients with appropriate perioperative risk and available conduit. The influence of patient comorbidity, patient preference, and operator experience on decision-making was highlighted. Importantly, TASC I and TASC II primarily utilized an anatomic classification to guide revascularization treatment decisions. At the time, there were few head-to-head comparative effectiveness trials of endovascular vs surgical revascularization to provide evidence-based specific recommendations for each strategy. The recommendations were, therefore, based on practice patterns, technical considerations, the ease and lower morbidity of the endovascular approach, and a consensus of experts. In addition, the TASC II lesion classification did not include an infrapopliteal classification, which was subsequently criticized as an important omission given the expanding technologies and techniques for catheter-based tibial intervention for critical limb ischemia (CLI).
Since the publication of the TASC II document in 2007, a number of scientific publications and observational reports have utilized regional and national databases to document the rapid adoption of endovascular therapy as a primary strategy for the treatment of symptomatic PAD.3–5) This rapid shift in clinical practice is presumably not related to a change in the patterns or natural history of arterial disease in the lower extremities. Rather, it appears to be due to the continued evolution of technology available for the endovascular treatment of PAD, including sheaths, catheters, wires, re-entry devices, balloons, stents, drug- device combinations, and debulking tools. In addition, improved vascular imaging techniques, the expanding skill level of many endovascular specialists across multiple disciplines, and the dissemination of these skills, have contributed significantly to this shift in treatment strategy. The overall result is that there has been an increase in adoption of the endovascular-first strategy for even the most complex anatomies (ie, TASC D) in the clinical practice of endovascular specialists, decreasing the number of anatomies that are primarily referred for open surgical revascularization.6) This shift was not clearly reflected in TASC II, a fact that has been criticized.7,8) However, trials evaluating surgical relative to endovascular treatment are difficult to perform and are uncommon; therefore, there remains a lack of evidence to support either approach having a clear advantage over the other. Importantly, key trials addressing this deficit in evidence are now ongoing in Europe and the United States.
Based on advances in technology and change in practice patterns, the TASC Steering Committee convened a multispecialty panel meeting in Örebro, Sweden, in May 2009. The aim of the meeting was to gain an updated consensus on revascularization strategies for PAD and to develop a TASC lesion classification for infrapopliteal artery disease. This was considered as an intermediate step to keep TASC current and bridge to a more comprehensive update in TASC III. Subsequent to that meeting, a consensus was not achieved, particularly on the concept of recommending an “endovascular-first” approach as has been documented in the recent literature. Based on this lack of consensus, the focus of the current publication is to provide a literature update on endovascular and surgical revascularization strategies and techniques and to add a TASC infrapopliteal anatomic classification. The evident importance of secondary preventive measures for all PAD patients, exercise, and other noninterventional treatment of intermittent claudication as highlighted in TASC II is not discussed in detail here but should precede invasive treatment.
This update does not provide treatment recommendations since there remains a paucity of well controlled randomized clinical trials to make treatment decisions, particularly in the specific choice of utilizing an endovascular vs open or hybrid surgical approach in a particular patient.
Literature Review
Prior to the meeting in Sweden, the TASC group embarked upon a review of all relevant published literature since the completion of the TASC II guidelines (in addition to pertinent literature prior to that date). Medline and EMBASE databases were searched, utilizing key words including “peripheral arterial (artery) (occlusive) disease” and “peripheral vascular disease.” A panel of 6 European and North American specialists in vascular surgery, vascular medicine, interventional radiology, and cardiovascular interventions evaluated the abstracts, and those pertaining to endovascular and surgical revascularization were selected. The review was divided into the 3 anatomic segments: aortoiliac, femoropopliteal, and the newly included infrapopliteal region. The literature review was subsequently updated by the TASC Steering Committee to include publications up to 2015. Since the intent is to provide an update and not formal treatment recommendations, no grading system was applied to the reviewed references.
Aortoiliac Disease
There have been several recent publications using mostly observational data on the treatment of aortoiliac disease. Data from the Healthcare Cost and Utilization Project Nationwide Inpatient Sample from 2004 to 2007 included 4119 patients with aortoiliac occlusive disease, 1100 of which were treated with an endovascular procedure. The type of TASC lesion or severity of disease was not presented. Clinical and economic outcomes were assessed, and endovascular procedures were associated with lower complication rates, shorter length of stay, and lower hospital costs than surgical management.9) A later systematic review and meta-analysis of a corresponding patient population demonstrated superior durability for open bypass compared with an endovascular approach, although surgery resulted in a longer hospital stay and increased risk for complications and mortality.10)
Open surgery vs endovascular intervention
No large-scale trials have compared surgical with endovascular intervention for the more complex TASC C and D lesions. Information is, however, available from small observational studies. In one series of 89 prospective TASC C–D aortoiliac lesions managed with percutaneous transluminal angioplasty (PTA) and stents, a 91% initial procedure success rate was demonstrated, with 3-year primary patency and limb salvage rates of 76% and 97%, respectively.11) However, 24% of procedures included an additional open surgical component to revascularize the common femoral artery or as a method to facilitate a percutaneous approach. Thus, a hybrid approach may be utilized in one quarter of cases.
Another case series of TASC C and D aortoiliac disease was described in a 2-center retrospective comparison of 40 patients who underwent endovascular therapy and 32 patients who underwent surgical revascularization from 1998 to 2007. In this series, primary patency at 48 months was significantly better with surgery, but at a risk of more pulmonary complications.12) Hospital length of stay was significantly longer in those patients treated with surgery (7 ± 2 days in the surgical group vs 1 ± 0.3 days in the stent group, p = 0.0001). In 10% of the endovascular procedures, intraprocedural complications required a hybrid surgical approach.
Endovascular intervention
A recent meta-analysis of aortoiliac endovascular interventions of TASC C–D lesions, including 19 nonrandomized cohort studies, demonstrated a 4- to 5-year primary patency ranging from 60% to 86%, while secondary patency was up to 80% to 98%.13) In another meta-analysis, the 12-month primary patency for TASC D lesions treated with stents was 87%.14)
Subsequently, a prospective multicenter registry of 125 patients from 28 centers in the United States treated with a self-expanding iliac artery stent demonstrated primary 12 month patency of 94.4%, with an excellent safety profile.15) Covered iliac stents have recently suggested improvement in the percutaneous management of complex aortoiliac bifurcation disease.16,17) In a randomized controlled trial (RCT) of polytetrafluoroethylene (PTFE) covered stents compared to bare metal stents, there were statistically reduced restenosis rates with covered stents, particularly in the more complex TASC C–D aortoiliac lesions.18) It should be noted that given the advances in percutaneous treatment of both abdominal and thoracic aortic aneurysms and aortic and mitral valve replacement, iliac artery stenosis may require treatment, even in asymptomatic patients, to facilitate passage of large bore catheters from the femoral artery to the target lesion. In a single center study of outcomes of balloon- expandable vs covered iliac artery stents, patency and target lesion revascularization (TLR) rates were superior for balloon-expandable stents.19)
Exercise and intervention
A prospective, multicenter randomized trial of optimal medical therapy, optimal medical therapy plus supervised exercise, and optimal medical therapy plus iliac artery stent placement sponsored by the United States National Institutes of Health demonstrated that walking time was superior in the group receiving supervised exercise at the 6-month primary endpoint.20) Interestingly, the same cohort followed prospectively to 18 months suggested that walking times were equivalent between the exercise and primary stent cohorts.21)
Choice of revascularization method
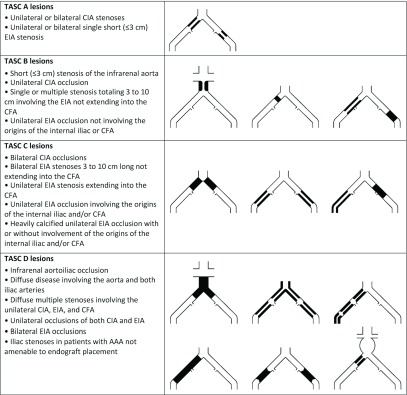
No contemporary RCTs have definitively established the magnitude and durability of the benefit of open surgical vs endovascular strategies. Comparisons within and between observational studies are prone to considerable bias. The choice of revascularization method must therefore to a great extent be based on each vascular center’s competence and experience with the anatomic complexity, considering patient comorbidity and overall prognosis. In complicated cases, primary surgical or hybrid endovascular and open strategies may be more appropriate in a medically fit patient, as the durability of an open approach over time may be superior to an endovascular procedure. The TASC lesion classification for aortoiliac disease is illustrated in Fig. 1.
Fig. 1.
Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC) classification of aortoiliac lesions. AAA: abdominal aortic aneurysm; CFA: common femoral artery; CIA: common iliac artery; EIA: external iliac artery
Femoropopliteal Disease
Surgical revascularization has been the principal strategy for femoropopliteal disease and was comprehensively reviewed in TASC II. This treatment is relevant in cases with extensive femoral artery disease, particularly in the common femoral artery with extension into the deep femoral (profunda) artery. Direct comparisons of endovascular therapy with surgical revascularization need to be performed to provide a basis for recommending a particular strategy but are limited owing to variation in vascular anatomy. In fact, enrollment in appropriate comparative trials is very difficult. In a series of 100 patients considered for enrollment in a trial of PTFE femoropopliteal bypass graft surgery, compared with an endovascular approach (femoropopliteal intraluminal thrupass), only 4% of screened patients were truly eligible for participation in the proposed RCT.22)
Open surgery vs endovascular intervention
The most current randomized trial, published in 2005, comparing infrainguinal saphenous vein bypass to the above-knee or below-knee segment with PTA (BASIL) found that endovascular therapy equaled the results with surgery based on amputation-free survival at 6 months. Endovascular therapy was a less morbid procedure with equivalent quality of life outcomes and was significantly less costly than surgery.23) At follow-up (3 to >5 years), there was a numerical trend for better overall survival and amputation-free survival for the surgical group compared to the endovascular group. A later subanalysis determined that in patients who lived for >2 years, there was a 7-month increase in overall survival for the bypass group without a significant difference in amputation-free survival.24) Surgery after a failed angioplasty had a worse prognosis than primary surgery, perhaps reflecting a greater disease severity of this patient population.25) It should be noted at the time the BASIL trial was performed, the angioplasty group did not include stents or other adjunctive procedures; as such, the primary comparison between surgery and angioplasty may not reflect modern endovascular strategies.
Comparing the results of endovascular therapy (balloon angioplasty without stent)5,26) with those of surgical revascularization is also difficult, as patients treated with endovascular interventions commonly present with symptoms of intermittent claudication, while those treated with surgical revascularization frequently have CLI. Patients with CLI have increased periprocedure morbidity and mortality and more diffuse arterial disease, including worse tibial runoff status.27,28) Therefore, it would be expected that the clinical outcomes for those patients undergoing surgical intervention would be significantly worse based more on the characteristics of the patient and anatomy than the specific choice of revascularization strategy. Only a few superficial femoral artery (SFA) occlusions are included in endovascular trials. The BASIL trial23) is the only study that has compared vein bypass and angioplasty in patients with severe ischemia. The PREVENT III trial29) is useful as a large cohort study of CLI patients followed after bypass surgery, but it did not compare this therapy with any other revascularization intervention. The randomized SUPERB trial, comparing heparin-bonded endoluminal vs surgical femoropopliteal bypass, is ongoing and results are awaited.30)
In a meta-analysis of observational studies, reviewing the literature from 1995 to 2012, no differences were found between endovascular and open surgical revascularization regarding all-cause mortality, amputation, or amputation-free survival at 2 years.31) In femoropopliteal bypass procedures, PTFE grafts as a surgical conduit are expected to have worse patency than autologous vein grafts. A recent Cochrane analysis included 13 RCTs (2313 patients) and demonstrated a significantly improved primary patency for vein bypass grafts compared with PTFE grafts in the femoral above-knee popliteal setting.32) This Cochrane report did not identify a trial comparing autologous vein grafts with a prosthetic graft in the femoral below-knee popliteal position. In a meta-analysis by Pereira et al.33) that included 49 retrospective and 24 prospective studies (6 of 24 were RCTs), the 5-year patency was similar for above-knee and below-knee vein bypasses in patients with CLI, while for claudicants, the above-knee position revealed a nonsignificant higher patency.
The PREVENT III trial did not report any difference in outcome for bypasses to the above-knee vs below-knee level. The analysis did find improved patency for shorter grafts, usually popliteal to distal bypass targets.29) One-year follow-up data from the Swedish Vascular Registry (1997–2007), including 2873 above-knee and 2960 below-knee femoropopliteal bypasses, demonstrated patency rates of 73.7% (above-knee) and 65.4% (below-knee) for vein bypass and 68.4% (above-knee) and 50% (below-knee) for prosthetic and composite grafts. Out of these 4833 procedures, 55% were performed for CLI and 45% for intermittent claudication.34) It is important to note these are observational data reflecting different disease anatomies and clinical settings.
Endovascular intervention
There have been multiple studies comparing nitinol stents with PTA or fabric-covered nitinol stents with bare nitinol stents35) in the SFA. Three-year follow-up of complex TASC C and D SFA lesions in a RCT did not confirm any advantage of the fabric covered stent compared to the bare metal stent.35) Technologic modifications, including coating the fabric with heparin, may offer some improvement in patency and outcomes; however, definitive data are still pending.36) A prospective multicenter trial comparing bare nitinol stents (mean lesion length 7.1 cm) to PTA (mean lesion length 6.4 cm) suggested a statistical advantage of improved patency with stent over PTA alone in femoropopliteal lesions.37) Three-year follow-up of this cohort demonstrated continued advantages of bare metal stents vs PTA.38) Additionally, in one large meta-analysis evaluating subintimal angioplasty as a potential alternative to surgical revascularization, the technical success and limb salvage rates were comparable, with inferior primary patency rates in the subintimal angioplasty group.39) However, the current evidence is insufficient to support a specific endovascular strategy, despite the current reports that 10- to 12-cm mean lesion lengths result in statistically superior patency and physical function outcomes with nitinol stents compared with PTA alone.40) These studies included patients with lesion lengths up to 17 cm. A recent midterm report on the use of an interwoven nitinol stent (Supera) claims high patency rates in the treatment of long lesions.41)
Literature has emerged regarding the role of primary atherectomy for femoropopliteal disease. Despite the different types of atherectomy devices (laser, directional, orbital),42–47) there remain concerns regarding the risk of distal embolic debris and restenosis with this strategy. Publications of larger prospective multicenter trials are needed to highlight the role of atherectomy in femoropopliteal arteryial disease.48)
With the advent of paclitaxel-coated self-expanding nitinol stents, recent 2-year data have demonstrated superiority of this strategy compared to standard PTA.49) Finally, considerable enthusiasm exists for the use of paclitaxel-coated balloon angioplasty catheters. Initial feasibility has been promising,5,26) and the results of randomized multicenter trials of drug-coated vs bare balloons have begun to emerge.50) Finally, the results of a large international prospective multicenter RCT of drug-coated balloon vs bare balloon angioplasty have been published, demonstrating a significant advantage of the drug-coated balloon when analyzing patency and TLR.51) It is anticipated that the results of the corresponding LEVANT 2 study will be published shortly.
Choice of revascularization method
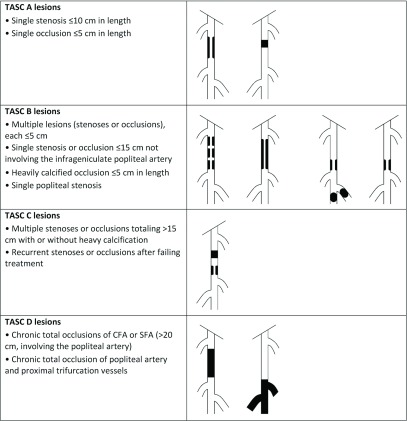
In practical terms, although the level of evidence is low, the initial revascularization strategy for femoropopliteal disease is commonly an endovascular approach. Depending on numerous factors, namely lesion complexity, availability of autologous conduit, patient condition, and center experience, hybrid procedures may reduce the surgical trauma, while bypass surgery is commonly reserved for complex, extensive lesions, provided that the patient’s health status suggests >2-year survival. This is supported by a recent meta-analysis of the published literature regarding endovascular vs surgical revascularization for femoropopliteal disease. In this analysis, only 10 published RCTs were included, with only one considered high quality. Accepting these limitations, an endovascular-first strategy was suggested by the authors, particularly for those patients with limited life expectancy.52) The TASC lesion classification for femoropopliteal disease is illustrated in Fig. 2.
Fig. 2.
Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC) classification of femoral popliteal lesions. CFA: common femoral artery; SFA: superficial femoral artery
Infrapopliteal Disease
Revascularization of infrapopliteal disease is almost exclusively utilized for patients with CLI, which occurs in <10% of all patients with PAD.53) Single-vessel tibial disease with patency of the other tibial arteries would not be expected to result in severe limb symptoms prompting the need for revascularization.
There is a significant body of literature pertaining to the surgical treatment of infrapopliteal disease, primarily using the greater saphenous vein as conduit, with various operative techniques (in situ, transposed, nonreversed, and reversed vein), or other autologous veins as conduit material. Less than ideal for the treatment of infrapopliteal disease is the use of spliced grafts, allograft material, or prosthetic material such as PTFE. Various inflow arteries have been used for the proximal anastomosis site of the grafts (ie, common femoral artery, SFA, popliteal artery) as well as multiple outflow targets (ie, tibial and pedal vessels), depending on the vascular anatomy and extent of atherosclerotic disease. One-year survival and limb salvage rates of 76% to 90% and 66% to 100%, respectively, have been reported.54–57) The PREVENT III trial did provide outcomes for surgical bypass in the setting of a randomized multicenter clinical study of edifoligide, with a large cohort of patients with CLI (n = 1404), in whom the distal anastomosis targets were at the tibial or pedal arteries in 65% of cases.29,58) At 1-year follow-up, the primary patency rate for surgery and the survival and limb salvage rates were 61%, 83.8%, and 88.5%, respectively.
Major morbidity related to surgical bypass for infrapopliteal disease and CLI is clinically important. Although the BASIL23) and PREVENT III29) trials included patients with infrainguinal rather than isolated infrapopliteal disease, they offer the most relevant data in this regard. Periprocedure mortality occurred in 5.5% and 2.7% of patients, myocardial infarction in 7% and 4.7% of patients, and stroke in 1.5% and 1.4% of patients in the BASIL and PREVENT III trials, respectively. In addition, surgical wound complications were reported in 22% and 4.8% of patients, respectively.23,29)
Objective performance goals in patients who present with CLI have been developed from large clinical trial databases and provide information on clinically important event rates. Objective performance goals have defined an important clinical outcome as the absence of 30-day mortality or major adverse limb events (amputation or reintervention).59) Using patient-level data from 3 RCTs totaling 838 patients, a threshold for the primary efficacy endpoint rate of 76.9% and amputation-free survival rate of 76.5% was established. This type of analysis may be helpful in putting noncomparative, nonrandomized data from clinical trials in perspective.
A Japanese CLI registry reported data describing risk-adjusted outcomes for endovascular procedures.60) It was determined that heart failure, wound infections, and being underweight (body mass index <18.5 kg/m2) put patients at increased risk for a worse outcome, including a lower amputation-free survival. Patients with no adverse criteria were at low risk, those with one criterion were at intermediate risk, and those with 2 or 3 criteria were at high risk for an event.60)
Percutaneous revascularization of infrapopliteal disease has been reported since the early 1990s,61) and there has been widespread temporal adoption of an endovascular-first approach for CLI patients following the publication of the BASIL trial.23) Technical success rates approaching 100% have been reported but likely suffer from case selection and reporting bias. In a large meta-analysis of series using PTA as the primary treatment modality that included many older series, the 3-year limb salvage rate was 82.4%.62) This is comparable to open surgical results of 82.3% reported in a meta-analysis of popliteal-to-distal bypass using similar technique.55) This comparative clinical efficacy was achieved despite significantly lower primary and secondary patency rates in the endovascular series.
New technologies
There has been an evolution of newer technologies, specifically patency-enhancing drug coating for balloons and stents. In addition, several adjunctive endovascular devices, including atherectomy, cryoplasty, cutting balloons, and laser, have been shown to be feasible and safe in the infrapopliteal vessels but have failed to show superior efficacy when compared to conventional, less expensive therapies.45,63–65) These devices add cost to the basic endovascular procedure, and therefore their added expense needs to be justified.66) The data suffer from many of the limitations of the observational surgical series in that they largely represent the retrospective reports or uncontrolled registries, subject to selection bias, at individual centers.
Drug-eluting stents
In contrast to the adjunctive therapies mentioned above, there have been 4 RCTs (Table 1) and 4 meta-analyses analyzing the results of infrapopliteal drug-eluting stent (DES) vs either PTA or bare metal stents (BMS).67–75) These studies are summarized below. Following BASIL, the preferred endovascular approach was PTA with BMS reserved to preserve patency in case of a dissection as a “bailout” technique. Clinical data suggested that BMS had little clinical advantage over successful balloon angioplasty in patients with CLI.76,77) As DES platforms became established in the coronary bed, this encouraged acquisition of preliminary infrapopliteal data regarding primary DES.78,79) These data led to the performance of 4 RCTs testing DES in infrapopliteal lesions.
Table 1.
Randomized controlled trials of drug-eluting stents in infrapopliteal disease
| Study/stent type | N | CLI/IC | Control arm | Follow-up, mo | Outcome | p |
|---|---|---|---|---|---|---|
| ACHILLES Sirolimus-eluting |
200 | CLI + IC | PTA | 12 | Primary patency 75% vs 57% |
0.025 |
| DESTINY Everolimus-eluting |
140 | CLI | BMS | 12 | Primary patency 85% vs 54% |
<0.001 |
| YUKON-BTX Sirolimus-eluting |
161 | CLI + IC | BMS | 12 | Primary patency 81% vs 56% |
0.004 |
| IDEAS Drug-eluting |
50 | CLI + IC | PCB | 6 | Restenosis 28% vs 58% |
0.046 |
BMS: bare metal stent; CLI: critical limb ischemia; IC: intermittent claudication; PCB: paclitaxel-coated balloon; PTA: percutaneous transluminal angioplasty
The ACHILLES trial randomized 200 patients with infrapopliteal disease to PTA or DES (Cypher Select Sirolimus Eluting Stent; Cordis Corporation, Bridgewater, NJ, USA) and found superior patency rates at 1 year for the DES group (DES 75% vs PTA 57.1%, p = 0.025).73) There was no difference between the PTA or DES groups for death, amputation rates, or improved clinical status.
The Drug-Eluting Stents in the Critically Ischemic Lower Leg (DESTINY) study randomized 140 CLI patients with infrapopliteal disease to either a BMS (Multi-LinkVision; Abbott Vascular, Abbott Park, IL, USA) or a DES (Xience V; Abbott Vascular).68) Over 12 months of follow-up, the DES group showed superior patency (DES 85% vs BMS 54%, p <0.001) and freedom from reintervention (DES 91% vs BMS 66%, p = 0.001). They found no difference between groups for clinical Rutherford class improvement, major amputations, or mortality.
The YUKON-BTX trial randomized 161 patients to infrapopliteal treatment with BMS or DES (sirolimus- eluting YUKON stent; Translumina, Hechingen, Germany) and found superior patency at 12 months for the DES group but no difference in event-free survival.72) Significant clinical benefit for the DES group was demonstrated when the trial follow-up was extended to 3 years, with an event-free survival for DES of 65.8% compared to 44.6% for BMS (p = 0.02) and reduced amputation rates (DES 2.6% vs BMS 12.2%, p = 0.03).71)
The Paclitaxel-Coated Balloon Angioplasty vs Drug-Eluting Stenting for the Treatment of Infrapopliteal Long-Segment Arterial Occlusive Disease: The IDEAS Randomized Controlled Trial compared paclitaxel-coated balloons (PCB) vs DES in long (>70 mm) infrapopliteal lesions in patients with Rutherford categories 3 to 6. Fifty patients were randomized to infrapopliteal PCB angioplasty (25 arteries in 25 limbs) or primary DES placement (30 arteries in 27 limbs). At 6 months, 5 patients died (2 in PCB vs 3 in DES, p = 1.0), and 3 suffered a major amputation (1 in PCB vs 2 in DES, p = 1.0); the angiographic restenosis rate was significantly lower in DES [7 (28%) of 25 vs 11 (57.9%) of 19 in PCB, p = 0.046]. There were no significant differences with regard to TLR [2 (7.7%) of 26 in DES vs 3 (13.6%) of 22 in PCB, p = 0.65]. Compared with PCB in long infrapopliteal lesions, DES are associated with significantly lower residual immediate postprocedure stenosis and significantly reduced restenosis at 6 months.75)
The 4 published meta-analyses of infrapopliteal DES compared to PTA and/or BMS demonstrated no benefit for primary infrapopliteal BMS over PTA, suggesting BMS be reserved for bailout use to salvage patency in patients treated with PTA.67,69,70,74) The preponderance of the evidence for infrapopliteal DES, however, has demonstrated significant benefit over both BMS and PTA for (1) patency, (2) reduced reinterventions, (3) reduced amputation, and (4) improved event-free survival. These results are not specific to CLI, as most trials have included severe claudicants into their populations. It is also likely that the lesions selected for randomized trials do not reflect “real world” infrapopliteal lesions (eg, fewer lesions, more discrete lesions, less calcified lesions, and fewer occlusions). Despite these limitations, there is now Level 1 evidence from 4 randomized trials71–73,75) and 4 meta-analyses67,69,70,74) to support primary DES use in anatomically suitable infrapopliteal lesions causing symptomatic lower extremity ischemia.
There are only limited clinical data available on the below-knee applications of drug-eluting balloons (DEB). A registry of 104 patients treated with infrapopliteal angioplasty using a paclitaxel-eluting balloon (In.Pact Amphirion; Medtronic Cardiovascular, Santa Rosa, CA, USA) demonstrated favorable results compared to historical controls for restenosis reduction, and there were no safety issues noted.80) The Drug-Eluting Balloon Evaluation for Lower Limb MUltilevel TreatMent (DEBELLUM) was a RCT of femoropopliteal (92, 75.4%) or below-the-knee (BTK) arteries (30, 24.6%). Patients were randomly assigned to the DEB (25 patients with 57 lesions; In.Pact Amphirion, Medtronic Cardiovascular) or PTA (25 patients with 65 lesions) group.81,82) Overall, ankle-brachial index improved more in the DEB group: 0.81 ± 0.3 vs 0.68 ± 0.13 (p = 0.02), and the Fontaine stage improved (from IIb to I): 80% DEB vs 56% PTA (p <0.05). For the BTK lesions, the overall late lumen loss was 0.66 < 0.9 mm in DEB patients vs 1.69 < 0.5 mm in the PTA arm (p = 0.03).
The DEBATE-BTK trial randomized DEB (In.Pact Amphirion, Medtronic Cardiovascular) vs PTA in 132 diabetic patients with CLI and 158 infrapopliteal lesions.83) Notably, the mean lesion length was 129 ± 83 mm, which is dramatically (∼100 mm) longer than lesions treated in the infrapopliteal DES randomized trials. The primary endpoint, restenosis at 1 year, occurred in 20 (27%) and 55 (74.3%) lesions in the DEB and PTA groups, respectively (p <0.001). Target vessel occlusion occurred in 13 (17.6%) DEB-treated vs 41 (55.4%) PTA-treated vessels (p <0.001). Twelve-month major adverse events occurred less frequently in the DEB (31%) than in the PTA (51%) group (p = 0.02), driven mainly by a reduction in TLR and better ulcer healing. However, there was no difference in the rates of amputation, limb salvage, or mortality between the groups.
The In.Pact Deep CLI trial outcomes resulted in the DEB (In.Pact Amphirion, Medtronic Cardiovascular) being withdrawn from the market worldwide by the sponsor.84) The trial enrolled 358 CLI patients with infrapopliteal lesions and randomized them 2:1 to DEB and PTA, respectively. The primary efficacy endpoints were no different for (1) 12-month late lumen loss for the DEB (0.61 < 0.78 mm) group or the PTA (0.62 ± 0.78, p = 0.95) group and (2) the clinically driven TLR for the DEB (17.7%) group or the PTA (15.8%, p = 0.66) group. There was a nonsignificant trend toward higher amputation rates in the DEB (8.8%) compared to the PTA group (3.6%, p = 0.08). It seems clear that more data and more experience are needed to understand the relative benefits of DES vs DEB for infrapopliteal lesions.84)
It is too early to recommend that infrapopliteal DEB should be used for infrapopliteal lesions, particularly given the superior comparative results of the DES. The DEB may have an advantage in very long lesions (including foot lesions), with demonstrated superiority over PTA in terms of restenosis and reintervention rates. Adverse periprocedure events with endovascular therapy for the treatment of infrapopliteal disease appear to be few, with mortality rates in observational series approaching <1%. However, in the PTA arm of the BASIL trial,23) the periprocedure mortality was 3% compared with 5.5% in the surgical arm. Similarly, stroke and myocardial infarction are rarely reported in observational series but were reported in 0.4% and 2.5% of patients, respectively, in the PTA arm of the BASIL trial. In addition to these point estimates of risk, the confidence interval around these estimates needs to be considered to understand the potential risks when these procedures are performed in larger populations.
Choice of revascularization method
In practical terms, an “endovascular-first” approach is the current standard of care for symptomatic infrainguinal atherosclerotic disease strengthened by the recent technological advances of DES and DEBs. The Best Endovascular vs Best Surgical Therapy in patients with CLI (BEST-CLI) trial has just been launched and will answer the question of whether optimal surgery for selected patients with CLI and good quality saphenous vein available for bypass is a better choice than endovascular therapy.85) With a corresponding aim, the BASIL 2 trial has recently been launched.86)
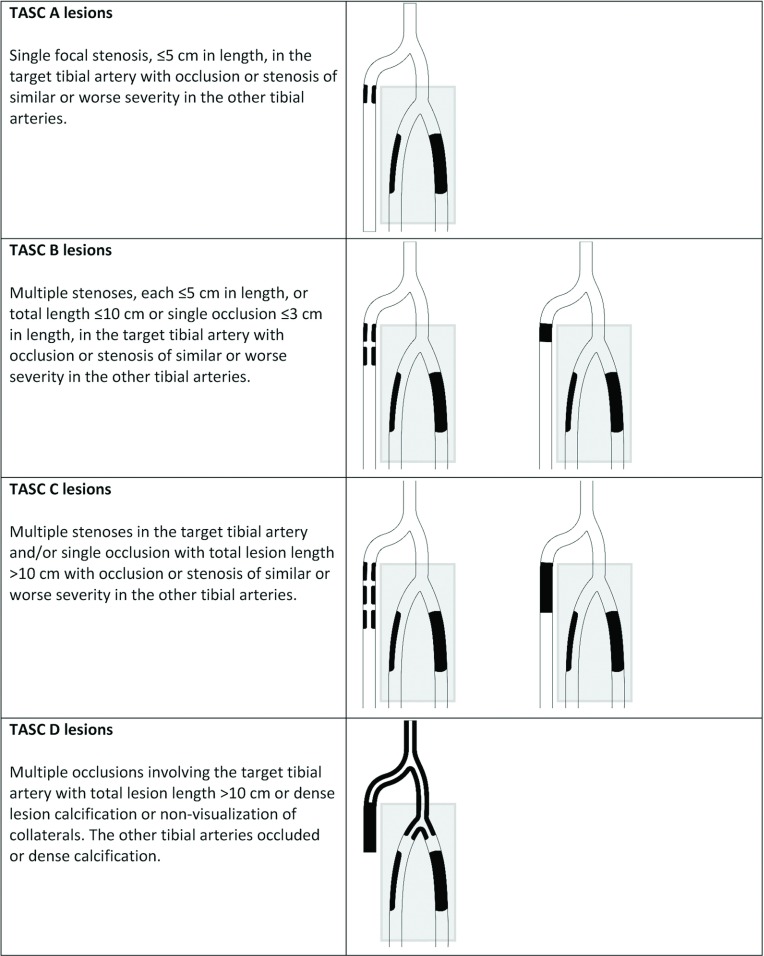
The TASC infrapopliteal classification is illustrated in Fig. 3. This classification has been developed to maintain consistency with the anatomic descriptions of the other vascular beds (aortoiliac, femoropopliteal). The classification acknowledges that patients with CLI have lesions in other tibial and inflow arteries.
Fig. 3.
Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC) classification of infrapopliteal lesions. The unshaded area represents the target lesion; area inside the shaded rectangle represents typical background disease (see text for further explanation).
Hybrid Endovascular and Surgical Approaches
Given the advances in endovascular technology and techniques, the adoption of endovascular skills by vascular surgeons,6) and the close cooperation between vascular surgeons and interventionists, revascularization strategies that employ both endovascular and surgical procedures are commonplace and may reduce the magnitude of the surgical trauma and systemic complications. The classic scenario is the patient with both inflow aortoiliac disease and infrainguinal disease.87,88) A combined strategy might offer advantages, as inflow procedures may be managed by endovascular techniques, followed by infrainguinal surgical revascularization when appropriate. This scenario is usually found in patients with CLI, implying a more fragile patient.
Skill and Experience
Considering endovascular revascularization as the primary mode of therapy, the interventionist (regardless of specialty) has to be trained and thereafter possess appropriate technical expertise in the performance of complex endovascular revascularizations. Additionally, the interventionist must have access to all appropriate devices with adequate technology and up-to-date imaging modalities to achieve an optimal outcome. Likewise, the vascular surgeon must be trained and thereafter possess the technical skill and experience required to complete the surgical procedure efficiently and must have adequate conduit in order to achieve a successful outcome. The principal strategy must be to employ the least complex and most cost-effective procedure, thereby reducing the risk of complications and costs as much as possible. A fundamental caveat is that only experienced operators capable of efficient management of potential complications should employ the revascularization procedure.89) Results of vascular and endovascular procedures should be recorded and be benchmarked in national databases to improve quality of care.
TASC Classifications
Together with the new infrapopliteal classification, the TASC II classifications, including the aortoiliac and femoropopliteal segments, are reproduced in this publication. Of note is that these 2 classifications are not changed from the TASC II version, except for a correction of an aortoiliac figure and clarification of some figure text. All 3 vascular territory classifications should be regarded as anatomic descriptions of the lesion patterns to enable comparison between various grades of complexity of lesions, but they are not sufficient on their own to guide clinical decisions regarding revascularization treatment strategies. Additional considerations to determine specific revascularization approaches include the hemodynamic condition in the limb, the overall health of the patient and the desired outcome, limb preservation and symptom relief in CLI, and improved limb function in all patients with PAD, which is a ‘patient-limb-lesion’ approach.
The new infrapopliteal lesion classification incorporates several features that attempt to address the multivessel nature of possible infrapopliteal anatomies. Occlusive disease in a single tibial artery rarely leads to clinical signs or symptoms. Thus, a clinically significant reduction in distal arterial perfusion requires multivessel disease that can occur from multiple anatomic patterns of arterial occlusions. The figures include a shaded portion encompassing the peroneal and posterior tibial arteries that represent typical “background” disease, but this representation is not comprehensive and other patterns of 2- and 3-vessel disease that are associated with clinical consequences may occur. Based on these considerations, the figures provide a TASC A–D classification for the anterior tibial artery as the selected example, but similar patterns in various combinations would generally apply if the target vessel were the peroneal artery, posterior tibial artery, or the tibial-peroneal trunk. Recently, the Society for Vascular Surgery proposed a lower extremity threatened limb classification in CLI based on wound, ischemia, and foot infection (WIfI).90) This approach lends to evaluating the risks and benefits of a positive outcome for revascularization and the risks of amputation based on the severity of the components of the disease classification. TASC recommends the design of studies focusing on the interaction of the following key factors in making revascularization decisions: patient clinical stage and desired treatment outcome, lesion anatomy, limb hemodynamics, and systemic comorbidities.
Conclusion
Except for the BASIL trial, there is an absence of meaningful data comparing surgical to endovascular strategies for key outcomes, such as limb viability, wound healing, quality of life, survival, and costs in patients with CLI. Similar limitations exist for comparative effectiveness trials in patients with intermittent claudication. Most information on outcome is from observational studies, not infrequently in small series from single centers and industry sponsored, therefore resulting in an inferior level of evidence to make firm recommendations. With improving techniques and technologies, however, an increasing number of cases may now be managed with endovascular procedures. An important issue is that both for endovascular and open surgical procedures, there is a difference between what can be done in centers of excellence involved in the development of refined and simplified procedures and what should be completed in everyday practice. Appropriate training and benchmarking of outcomes is mandatory to improve the quality of care. TASC continues to highlight the limitations of the current endovascular and comparative surgical literature. It is critical that future clinical trials with appropriate design, inclusive of patients with common clinical and anatomic patterns of PAD, measure meaningful functional outcomes in addition to presenting information regarding anatomic patency.
Acknowledgments
The TASC Steering Committee acknowledges the cooperation and input from representatives of the following societies:
Society for Vascular Medicine (Michael Jaff)
Society for Cardiovascular Angiography and Intervention (Christopher J. White)
European Society for Cardiology (Victor Aboyans)
Japanese College of Angiology (Hiroshi Shigematsu)
Chinese Society of Surgery (Chang Shu)
Cardiovascular and Interventional Radiology Society of Europe (Jim Reekers)
Society of Interventional Radiology (Mahmood Razavi)
International Diabetes Federation (Nicolaas Schaper)
The Israeli Society for Vascular and Endovascular Surgery (Yehuda Wolf)
Interventional Radiology Society for Australasia (William Clark, Gerard Goh)
Swiss Society of Angiology (Iris Baumgartner, Beatrice Amann-Vesti)
Vascular Independent Research and Education European Organization (Mariella Catalano)
Emirates Cardiac Society (Wael Al Mahmeed)
Colegio Argentino de Cirujanos Cardiovasculares y Endovasculares (Aldo Paganini, Emilio Turco)
Cirujanos Endovasculares de Latinoamerica (Luis Bechara-Zamudio)
Vascular Interventional Advances, VIVA Physicians (Krishna Rocha-Singh)
European Society for Vascular Medicine (Sigrid Nikol)
Secretarial, logistical, and editorial support was provided by Discovery London
The Committee acknowledges the international society of endovascular specialists for endorsing this document.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article:
Gerry Fowkes: co-chair of the Steering Committee for the EUCLID trial sponsored by AstraZeneca and ad hoc advisor to Bayer.
William Hiatt: research grants received by CPC Clinical Research (a clinical trials research organization and affiliate of the University of Colorado) include: AstraZeneca, Bayer, NIH, CSI, Kyushu University, Merck, Pluristem, ReNeuron, and Takeda.
Michael Jaff: noncompensated advisor for Abbott Vascular, Boston Scientific, Cordis Corporation, Covidien Vascular, Medtronic Vascular. Equity Investor in PQ Bypass. Consultant for Cardinal Health. Board member of VIVA Physicians [a 501(c)(3) not-for-profit education and research organization].
Lars Norgren: Steering Committee/Advisory Board member for AnGes, AstraZeneca, Novartis, and Mitsubishi Tanabe.
Mahmood Razavi: consultant to Abbott Vascular, Bard Peripheral Vascular, Boston Scientific, Covidien, Codman, Microvention/Terumo, Neuravi, TriVascular, Veniti, and 480 Biomedical.
Christopher White: Noncompensated clinical investigator for CR Bard (Lutonics).
Funding
The author(s) report receiving the following financial support for the research, authorship, and/or publication of this article: VIVA Physicians [a 501(c)(3) education and research multispecialty organization] provided an unrestricted grant to the TASC organization. The development of this TASC II Supplement was supported by unrestricted educational grants awarded to Discovery London from (in alphabetical order): Aastrom Biosciences, Abbott Vascular, AnGes MG Inc, Bayer Schering Pharma, Biomedix, Cook, ev3, Medtronic, Mitsubishi Tanabe, Otsuka Pharmaceutical Co, Sanofi-Aventis, and Toray Industries. The companies did not participate in the discussions or in the preparation, review, or approval of the document.
Footnotes
This article, published online in the Journal of Endovascular Therapy on 3 August 2015 (J Endovasc Ther. 2015; 22: 663-677. DOI: 10.1177/1526602815592206), is being co-published with permission of the authors and SAGE Publications, Inc., in the following journals: Vascular Medicine, Catheterization and Cardiovascular Interventions, Annals of Vascular Diseases, The Journal of Japanese College of Angiology (Japanese translation), and Técnicas Endovasculares (Spanish translation).
References
- Dormandy JA, Rutherford RB. Management of peripheral arterial disease (PAD). TASC Working Group. TransAtlantic Inter-Society Consensus (TASC). J Vasc Surg 2000; 31: S1-S296. [PubMed] [Google Scholar]
- Norgren L, Hiatt WR, Dormandy JA, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg 2007; 45 Suppl S: S5-67. [DOI] [PubMed] [Google Scholar]
- Krankenberg H, Schlüter M, Steinkamp HJ, et al. Nitinol stent implantation versus percutaneous transluminal angioplasty in superficial femoral artery lesions up to 10 cm in length: the femoral artery stenting trial (FAST). Circulation 2007; 116: 285-92. [DOI] [PubMed] [Google Scholar]
- Saxon RR, Dake MD, Volgelzang RL, et al. Randomized, multicenter study comparing expanded polytetrafluoroethylene-covered endoprosthesis placement with percutaneous transluminal angioplasty in the treatment of superficial femoral artery occlusive disease. J Vasc Interv Radiol 2008; 19: 823-32. [DOI] [PubMed] [Google Scholar]
- Werk M, Langner S, Reinkensmeier B, et al. Inhibition of restenosis in femoropopliteal arteries: paclitaxel-coated versus uncoated balloon: femoral paclitaxel randomized pilot trial. Circulation 2008; 118: 1358-65. [DOI] [PubMed] [Google Scholar]
- Goodney PP, Beck AW, Nagle J, et al. National trends in lower extremity bypass surgery, endovascular interventions, and major amputations. J Vasc Surg 2009; 50: 54-60. [DOI] [PubMed] [Google Scholar]
- Diehm N, Schillinger M, Minar E, et al. TASC II section E3 on the treatment of acute limb ischemia: commentary from European interventionists. J Endovasc Ther 2008; 15: 126-8. [DOI] [PubMed] [Google Scholar]
- Schillinger M, Diehm N, Baumgartner I, et al. TASC II section F on revascularization: commentary from an interventionist’s point of view. J Endovasc Ther 2007; 14: 734-42. [DOI] [PubMed] [Google Scholar]
- Indes JE, Mandawat A, Tuggle CT, et al. Endovascular procedures for aorto-iliac occlusive disease are associated with superior short-term clinical and economic outcomes compared with open surgery in the inpatient population. J Vasc Surg 2010; 52: 1173-9, 1179.e1. [DOI] [PubMed] [Google Scholar]
- Indes JE, Pfaff MJ, Farrokhyar F, et al. Clinical outcomes of 5358 patients undergoing direct open bypass or endovascular treatment for aortoiliac occlusive disease: a systematic review and meta-analysis. J Endovasc Ther 2013; 20: 443-55. [DOI] [PubMed] [Google Scholar]
- Leville CD, Kashyap VS, Clair DG, et al. Endovascular management of iliac artery occlusions: extending treatment to TransAtlantic Inter-Society Consensus class C and D patients. J Vasc Surg 2006; 43: 32-9. [DOI] [PubMed] [Google Scholar]
- Hans SS, DeSantis D, Siddiqui R, et al. Results of endovascular therapy and aortobifemoral grafting for Transatlantic Inter-Society type C and D aortoiliac occlusive disease. Surgery 2008; 144: 583-9; discussion 589-90. [DOI] [PubMed] [Google Scholar]
- Jongkind V, Akkersdijk GJ, Yeung KK, et al. A systematic review of endovascular treatment of extensive aortoiliac occlusive disease. J Vasc Surg 2010; 52: 1376-83. [DOI] [PubMed] [Google Scholar]
- Ye W, Liu CW, Ricco JB, et al. Early and late outcomes of percutaneous treatment of TransAtlantic Inter-Society Consensus class C and D aorto-iliac lesions. J Vasc Surg 2011; 53: 1728-37. [DOI] [PubMed] [Google Scholar]
- Clair DG, Adams J, Reen B, et al. The EPIC nitinol stent system in the treatment of iliac artery lesions: one-year results from the ORION clinical trial. J Endovasc Ther 2014; 21: 213-22. [DOI] [PubMed] [Google Scholar]
- Mwipatayi BP, Thomas S, Wong J, et al. A comparison of covered vs bare expandable stents for the treatment of aortoiliac occlusive disease. J Vasc Surg 2011; 54: 1561-70. [DOI] [PubMed] [Google Scholar]
- Sabri SS, Choudhri A, Orgera G, et al. Outcomes of covered kissing stent placement compared with bare metal stent placement in the treatment of atherosclerotic occlusive disease at the aortic bifurcation. J Vasc Interv Radiol 2010; 21: 995-1003. [DOI] [PubMed] [Google Scholar]
- Grimme FA, Goverde PA, Van Oostayen JA, et al. Covered stents for aortoiliac reconstruction of chronic occlusive lesions. J Cardiovasc Surg (Torino) 2012; 53: 279-89. [PubMed] [Google Scholar]
- Humphries MD, Armstrong E, Laird J, et al. Outcomes of covered versus bare-metal balloon-expandable stents for aortoiliac occlusive disease. J Vasc Surg 2014; 60: 337-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TP, Cutlip DE, Regensteiner JG, et al. Supervised exercise versus primary stenting for claudication resulting from aortoiliac peripheral artery disease: six-month outcomes from the claudication: exercise versus endoluminal revascularization (CLEVER) study. Circulation 2012; 125: 130-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TP, Cutlip DE, Regensteiner JG, et al. Supervised exercise, stent revascularization, or medical therapy for claudication due to aortoiliac peripheral artery disease: the CLEVER study. J Am Coll Cardiol 2015; 65: 999-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepantalo M, Laurila K, Roth WD, et al. PTFE bypass or thrupass for superficial femoral artery occlusion? A randomised controlled trial. Eur J Vasc Endovasc Surg 2009; 37: 578-84. [DOI] [PubMed] [Google Scholar]
- Adam DJ, Beard JD, Cleveland T, et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet 2005; 366: 1925-34. [DOI] [PubMed] [Google Scholar]
- Bradbury AW, Adam DJ, Bell J, et al. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: An intention-to-treat analysis of amputation-free and overall survival in patients randomized to a bypass surgery-first or a balloon angioplasty-first revascularization strategy. J Vasc Surg 2010; 51: 5S-17S. [DOI] [PubMed] [Google Scholar]
- Bradbury AW, Adam DJ, Bell J, et al. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: Analysis of amputation free and overall survival by treatment received. J Vasc Surg 2010; 51: 18S-31S. [DOI] [PubMed] [Google Scholar]
- Tepe G, Zeller T, Albrecht T, et al. Local delivery of paclitaxel to inhibit restenosis during angioplasty of the leg. N Engl J Med 2008; 358: 689-99. [DOI] [PubMed] [Google Scholar]
- Faglia E, Clerici G, Airoldi F, et al. Revascularization by angioplasty of type D femoropopliteal and long infrapopliteal lesion in diabetic patients with critical limb ischemia: are TASC II recommendations suitable? A population-based cohort study. Int J Low Extrem Wounds 2012; 11: 277-85. [DOI] [PubMed] [Google Scholar]
- Gargiulo M, Giovanetti F, Bianchini Massoni C, et al. Bypass to the ankle and foot in the era of endovascular therapy of tibial disease. Results and factors influencing the outcome. J Cardiovasc Surg (Torino) 2014; 55: 367-74. [PubMed] [Google Scholar]
- Conte MS, Bandyk DF, Clowes AW, et al. Results of PREVENT III: a multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J Vasc Surg 2006; 43: 742-51; discussion 751. [DOI] [PubMed] [Google Scholar]
- Lensvelt MM, Holewijn S, Fritschy WM, et al. SUrgical versus PERcutaneous Bypass: SUPERB-trial; Heparin-bonded endoluminal versus surgical femoro-popliteal bypass: study protocol for a randomized controlled trial. Trials 2011; 12: 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones WS, Dolor RJ, Hasselblad V, et al. Comparative effectiveness of endovascular and surgical revascularization for patients with peripheral artery disease and critical limb ischemia: systematic review of revascularization in critical limb ischemia. Am Heart J 2014; 167: 489-98.e7. [DOI] [PubMed] [Google Scholar]
- Twine CP, McLain AD. Graft type for femoro-popliteal bypass surgery. Cochrane Database Syst Rev 2010: CD001487. [DOI] [PubMed] [Google Scholar]
- Pereira CE, Albers M, Romiti M, et al. Meta-analysis of femoropopliteal bypass grafts for lower extremity arterial insufficiency. J Vasc Surg 2006; 44: 510-7. [DOI] [PubMed] [Google Scholar]
- Norgren L, Troeng T. Personal communication on behalf of the Swedish Vascular Registry (SWEDVASC). 2010.
- Geraghty PJ, Mewissen MW, Jaff MR, et al. Three-year results of the VIBRANT trial of VIABAHN endoprosthesis versus bare nitinol stent implantation for complex superficial femoral artery occlusive disease. J Vasc Surg 2013; 58: 386-95.e4. [DOI] [PubMed] [Google Scholar]
- Saxon RR, Chervu A, Jones PA, et al. Heparin-bonded, expanded polytetrafluoroethylene-lined stent graft in the treatment of femoropopliteal artery disease: 1-year results of the VIPER (Viabahn Endoprosthesis with Heparin Bioactive Surface in the Treatment of Superficial Femoral Artery Obstructive Disease) trial. J Vasc Interv Radiol 2013; 24: 165-73. [DOI] [PubMed] [Google Scholar]
- Laird JR, Katzen BT, Scheinert D, et al. Nitinol stent implantation versus balloon angioplasty for lesions in the superficial femoral artery and proximal popliteal artery: twelve-month results from the RESILIENT randomized trial. Circ Cardiovasc Interv 2010; 3: 267-76. [DOI] [PubMed] [Google Scholar]
- Laird JR, Katzen BT, Scheinert D, et al. Nitinol stent implantation vs. balloon angioplasty for lesions in the superficial femoral and proximal popliteal arteries of patients with claudication: three-year follow-up from the RESILIENT randomized trial. J Endovasc Ther 2012; 19: 1-9. [DOI] [PubMed] [Google Scholar]
- Bown MJ, Bolia A, Sutton AJ. Subintimal angioplasty: meta- analytical evidence of clinical utility. Eur J Vasc Endovasc Surg 2009; 38: 323-37. [DOI] [PubMed] [Google Scholar]
- Schillinger M, Sabeti S, Loewe C, et al. Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery. N Engl J Med 2006; 354: 1879-88. [DOI] [PubMed] [Google Scholar]
- Brescia AA, Wickers BM, Correa JC, et al. Stenting of femoropopliteal lesions using interwoven nitinol stents. J Vasc Surg 2015; 61: 1472-8. [DOI] [PubMed] [Google Scholar]
- Das T, Mustapha J, Indes J, et al. Technique optimization of orbital atherectomy in calcified peripheral lesions of the lower extremities: the CONFIRM series, a prospective multicenter registry. Catheter Cardiovasc Interv 2014; 83: 115-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave RM, Patlola R, Kollmeyer K, et al. Excimer laser recanalization of femoropopliteal lesions and 1-year patency: results of the CELLO registry. J Endovasc Ther 2009; 16: 665-75. [DOI] [PubMed] [Google Scholar]
- Korabathina R, Mody KP, Yu J, et al. Orbital atherectomy for symptomatic lower extremity disease. Catheter Cardiovasc Interv 2010; 76: 326-32. [DOI] [PubMed] [Google Scholar]
- Laird JR, Zeller T, Gray BH, et al. Limb salvage following laser-assisted angioplasty for critical limb ischemia: results of the LACI multicenter trial. J Endovasc Ther 2006; 13: 1-11. [DOI] [PubMed] [Google Scholar]
- Zeller T, Krankenberg H, Steinkamp H, et al. One-year outcome of percutaneous rotational atherectomy with aspiration in infrainguinal peripheral arterial occlusive disease: the multicenter pathway PVD trial. J Endovasc Ther 2009; 16: 653-62. [DOI] [PubMed] [Google Scholar]
- Zeller T, Rastan A, Sixt S, et al. Long-term results after directional atherectomy of femoro-popliteal lesions. J Am Coll Cardiol 2006; 48: 1573-8. [DOI] [PubMed] [Google Scholar]
- McKinsey JF, Zeller T, Rocha-Singh KJ, et al. Lower extremity revascularization using directional atherectomy: 12-month prospective results of the DEFINITIVE LE study. JACC Cardiovasc Interv 2014; 7: 923-33. [DOI] [PubMed] [Google Scholar]
- Dake MD, Ansel GM, Jaff MR, et al. Sustained safety and effectiveness of paclitaxel-eluting stents for femoropopliteal lesions: 2-year follow-up from the Zilver PTX randomized and single-arm clinical studies. J Am Coll Cardiol 2013; 61: 2417-27. [DOI] [PubMed] [Google Scholar]
- Scheinert D, Duda S, Zeller T, et al. The LEVANT I (Lutonix paclitaxel-coated balloon for the prevention of femoropopliteal restenosis) trial for femoropopliteal revascularization: first-in-human randomized trial of low-dose drug-coated balloon versus uncoated balloon angioplasty. JACC Cardiovasc Interv 2014; 7: 10-9. [DOI] [PubMed] [Google Scholar]
- Tepe G, Laird J, Schneider P, et al. Drug-coated balloon versus standard percutaneous transluminal angioplasty for the treatment of superficial femoral and popliteal peripheral artery disease: 12-month results from the IN. PACT SFA randomized trial. Circulation 2015; 131: 495-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniou GA, Chalmers N, Georgiadis GS, et al. A meta- analysis of endovascular versus surgical reconstruction of femoropopliteal arterial disease. J Vasc Surg 2013; 57: 242-53. [DOI] [PubMed] [Google Scholar]
- Hiatt WR. Medical treatment of peripheral arterial disease and claudication. N Engl J Med 2001; 344: 1608-21. [DOI] [PubMed] [Google Scholar]
- Albers M, Romiti M, Brochado-Neto FC, et al. Meta- analysis of alternate autologous vein bypass grafts to infrapopliteal arteries. J Vasc Surg 2005; 42: 449-55. [DOI] [PubMed] [Google Scholar]
- Albers M, Romiti M, Pereira CA, et al. Meta-analysis of allograft bypass grafting to infrapopliteal arteries. Eur J Vasc Endovasc Surg 2004; 28: 462-72. [DOI] [PubMed] [Google Scholar]
- Dorweiler B, Neufang A, Schmiedt W, et al. Pedal arterial bypass for limb salvage in patients with diabetes mellitus. Eur J Vasc Endovasc Surg 2002; 24: 309-13. [DOI] [PubMed] [Google Scholar]
- Kashyap VS, Ahn SS, Quinones-Baldrich WJ, et al. Infrapopliteal-lower extremity revascularization with prosthetic conduit: a 20-year experience. Vasc Endovascular Surg 2002; 36: 255-62. [DOI] [PubMed] [Google Scholar]
- Schanzer A, Hevelone N, Owens CD, et al. Technical factors affecting autogenous vein graft failure: observations from a large multicenter trial. J Vasc Surg 2007; 46: 1180-90; discussion 1190. [DOI] [PubMed] [Google Scholar]
- Conte MS, Geraghty PJ, Bradbury AW, et al. Suggested objective performance goals and clinical trial design for evaluating catheter-based treatment of critical limb ischemia. J Vasc Surg 2009; 50: 1462-73.e1-3. [DOI] [PubMed] [Google Scholar]
- Iida O, Nakamura M, Yamauchi Y, et al. Endovascular treatment for infrainguinal vessels in patients with critical limb ischemia: OLIVE registry, a prospective, multicenter study in Japan with 12-month follow-up. Circ Cardiovasc Interv 2013; 6: 68-76. [DOI] [PubMed] [Google Scholar]
- Iyer SS, Dorros G, Zaitoun R, et al. Retrograde recanalization of an occluded posterior tibial artery by using a posterior tibial cutdown: two case reports. Cathet Cardiovasc Diagn 1990; 20: 251-3. [DOI] [PubMed] [Google Scholar]
- Romiti M, Albers M, Brochado-Neto FC, et al. Meta- analysis of infrapopliteal angioplasty for chronic critical limb ischemia. J Vasc Surg 2008; 47: 975-81. [DOI] [PubMed] [Google Scholar]
- Ansel GM, Sample NS, Botti III CF, et al. Cutting balloon angioplasty of the popliteal and infrapopliteal vessels for symptomatic limb ischemia. Catheter Cardiovasc Interv 2004; 61: 1-4. [DOI] [PubMed] [Google Scholar]
- Das T, McNamara T, Gray B, et al. Cryoplasty therapy for limb salvage in patients with critical limb ischemia. J Endovasc Ther 2007; 14: 753-62. [DOI] [PubMed] [Google Scholar]
- Zeller T, Rastan A, Schwarzwälder U, et al. Midterm results after atherectomy-assisted angioplasty of below- knee arteries with use of the Silverhawk device. J Vasc Interv Radiol 2004; 15: 1391-7. [DOI] [PubMed] [Google Scholar]
- White CJ. Brave new world: value-based purchasing for peripheral vascular stents is coming to a hospital near you. Circulation 2013; 127: 2475-6. [DOI] [PubMed] [Google Scholar]
- Antoniou GA, Chalmers N, Kanesalingham K, et al. Meta-analysis of outcomes of endovascular treatment of infrapopliteal occlusive disease with drug-eluting stents. J Endovasc Ther 2013; 20: 131-44. [DOI] [PubMed] [Google Scholar]
- Bosiers M, Scheinert D, Peeters P, et al. Randomized comparison of everolimus-eluting versus bare-metal stents in patients with critical limb ischemia and infrapopliteal arterial occlusive disease. J Vasc Surg 2012; 55: 390-8. [DOI] [PubMed] [Google Scholar]
- Fusaro M, Cassese S, Ndrepepa G, et al. Drug-eluting stents for revascularization of infrapopliteal arteries: updated meta-analysis of randomized trials. JACC Cardiovasc Interv 2013; 6: 1284-93. [DOI] [PubMed] [Google Scholar]
- Katsanos K, Spiliopoulos S, Diamantopoulos A, et al. Systematic review of infrapopliteal drug-eluting stents: a meta-analysis of randomized controlled trials. Cardiovasc Intervent Radiol 2013; 36: 645-58. [DOI] [PubMed] [Google Scholar]
- Rastan A, Brechtel K, Krankenberg H, et al. Sirolimus-eluting stents for treatment of infrapopliteal arteries reduce clinical event rate compared to bare-metal stents: long-term results from a randomized trial. J Am Coll Cardiol 2012; 60: 587-91. [DOI] [PubMed] [Google Scholar]
- Rastan A, Tepe G, Krankenberg H, et al. Sirolimus- eluting stents vs. bare-metal stents for treatment of focal lesions in infrapopliteal arteries: a double-blind, multi- centre, randomized clinical trial. Eur Heart J 2011; 32: 2274-81. [DOI] [PubMed] [Google Scholar]
- Scheinert D, Katsanos K, Zeller T, et al. A prospective randomized multicenter comparison of balloon angioplasty and infrapopliteal stenting with the sirolimus-eluting stent in patients with ischemic peripheral arterial disease: 1-year results from the ACHILLES trial. J Am Coll Cardiol 2012; 60: 2290-5. [DOI] [PubMed] [Google Scholar]
- Yang X, Lu X, Ye K, et al. Systematic review and meta- analysis of balloon angioplasty versus primary stenting in the infrapopliteal disease. Vasc Endovascular Surg 2014; 48: 18-26. [DOI] [PubMed] [Google Scholar]
- Siablis D, Kitrou PM, Spiliopoulos S, et al. Paclitaxel- coated balloon angioplasty versus drug-eluting stenting for the treatment of infrapopliteal long-segment arterial occlusive disease: the IDEAS randomized controlled trial. JACC Cardiovasc Interv 2014; 7: 1048-56. [DOI] [PubMed] [Google Scholar]
- Rand T, Lammer J, Rabbia C, et al. Percutaneous transluminal angioplasty versus turbostatic carbon-coated stents in infrapopliteal arteries: InPeria II trial. Radiology 2011; 261: 634-42. [DOI] [PubMed] [Google Scholar]
- Randon C, Jacobs B, De Ryck F, et al. Angioplasty or primary stenting for infrapopliteal lesions: results of a prospective randomized trial. Cardiovasc Intervent Radiol 2010; 33: 260-9. [DOI] [PubMed] [Google Scholar]
- Feiring AJ, Krahn M, Nelson L, et al. Preventing leg amputations in critical limb ischemia with below-the- knee drug-eluting stents: the PaRADISE (PReventing Amputations using Drug eluting StEnts) trial. J Am Coll Cardiol 2010; 55: 1580-9. [DOI] [PubMed] [Google Scholar]
- Siablis D, Karnabatidis D, Katsanos K, et al. Infrapopliteal application of sirolimus-eluting versus bare metal stents for critical limb ischemia: analysis of long-term angiographic and clinical outcome. J Vasc Interv Radiol 2009; 20: 1141-50. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Piorkowski M, Werner M, et al. First experience with drug-eluting balloons in infrapopliteal arteries: restenosis rate and clinical outcome. J Am Coll Cardiol 2011; 58: 1105-9. [DOI] [PubMed] [Google Scholar]
- Fanelli F, Cannavale A, Boatta E, et al. Lower limb multilevel treatment with drug-eluting balloons: 6-month results from the DEBELLUM randomized trial. J Endovasc Ther 2012; 19: 571-80. [DOI] [PubMed] [Google Scholar]
- Fanelli F, Cannavale A, Corona M, et al. The “DEBELLUM” — lower limb multilevel treatment with drug eluting balloon — randomized trial: 1-year results. J Cardiovasc Surg (Torino) 2014; 55: 207-16. [PubMed] [Google Scholar]
- Liistro F, Porto I, Angioli P, et al. Drug-eluting balloon in peripheral intervention for below the knee angioplasty evaluation (DEBATE-BTK): a randomized trial in diabetic patients with critical limb ischemia. Circulation 2013; 128: 615-21. [DOI] [PubMed] [Google Scholar]
- Zeller T, Baumgartner I, Scheinert D, et al. Drug-eluting balloon versus standard balloon angioplasty for infrapopliteal arterial revascularization in critical limb ischemia: 12-month results from the IN. PACT DEEP randomized trial. J Am Coll Cardiol 2014; 64: 1568-76. [DOI] [PubMed] [Google Scholar]
- Farber A, Rosenfield K, Menard M. The BEST-CLI trial: a multidisciplinary effort to assess which therapy is best for patients with critical limb ischemia. Tech Vasc Interv Radiol 2014; 17: 221-4. [DOI] [PubMed] [Google Scholar]
- University of Birmingham Clinical Trials Unit BASIL-2 trial. http://www.birmingham.ac.uk/research/activity/mds/trials/bctu/trials/portfolio-v/Basil-2/index.aspx. Accessed May 31, 2015.
- Chang RW, Goodney PP, Baek JH, et al. Long-term results of combined common femoral endarterectomy and iliac stenting/stent grafting for occlusive disease. J Vasc Surg 2008; 48: 362-7. [DOI] [PubMed] [Google Scholar]
- Timaran CH, Stevens SL, Freeman MB, et al. Infrainguinal arterial reconstructions in patients with aortoiliac occlusive disease: the influence of iliac stenting. J Vasc Surg 2001; 34: 971-8. [DOI] [PubMed] [Google Scholar]
- Creager MA, Goldstone J, Hirshfeld JW, et al. ACC/ACP/SCAI/SVMB/SVS clinical competence statement on vascular medicine and catheter-based peripheral vascular interventions: a report of the American College of Cardiology/American Heart Association/American College of Physician Task Force on Clinical Competence (ACC/ACP/SCAI/SVMB/SVS Writing Committee to develop a clinical competence statement on peripheral vascular disease). J Am Coll Cardiol 2004; 44: 941-57. [DOI] [PubMed] [Google Scholar]
- Mills JL, Sr, Conte MS, Armstrong DG, et al. The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: risk stratification based on wound, ischemia, and foot infection (WIfI). J Vasc Surg 2014; 59: 220-34.e1-2. [DOI] [PubMed] [Google Scholar]