Abstract
Background
Children with somatic complaints are at increased risk for emotional disorders during childhood. Whether this elevated risk extends into young adulthood—and to which specific disorders—has rarely been tested with long-term prospective-longitudinal community samples. Here we test whether frequent and recurring stomach aches, headaches, and muscle aches during childhood predict emotional disorders in adulthood after accounting for childhood psychiatric and physical health status and psychosocial adversity.
Methods
The Great Smoky Mountains Study is a community-representative sample with 1,420 participants. Children/adolescents were assessed 4–7 times between ages 9 to 16. They were assessed again up to three times between ages 19 to 26. Childhood somatic complaints were coded when subjects or their parents reported frequent and recurrent headaches, stomach aches, or muscular/joint aches at some point when children were ages 9 to 16 years old. Psychiatric disorders were assessed with the Child and Adolescent Psychiatric Assessment and the Young Adult Psychiatric Assessment.
Results
Frequent and recurrent somatic complaints in childhood predicted adulthood emotional disorders. After controlling for potential confounders, predictions from childhood somatic complaints were specific to later depression and generalized anxiety disorder. Long-term predictions did not differ by sex. Somatic complaints that persisted across developmental periods were associated with the highest risk for young adult emotional distress disorders.
Conclusions
Children from the community with frequent and recurrent physical distress are at substantially increased risk for emotional distress disorders during young adulthood. Preventions and interventions for somatic complaints could help alleviate this risk.
Keywords: depression, generalized anxiety, headaches, somatic complaints, stomach aches
Depression and anxiety pose a costly burden for families, workplaces, and society (Collins et al., 2011). In order to reduce this burden, early identification of individuals at risk is key. Great strides have been made in characterizing childhood psychiatric, psychosocial, and biological risk factors for later emotional disorders (e.g., Rohde et al., 2013). Nevertheless, insufficient identification of at-risk individuals remains a chief barrier to prevention and services use, especially during the early life course (Farmer et al., 2003, Green et al., 2013, Costello et al., 2014). Identifying easy-to-detect and relatively stigma-free early markers of long-term risk could be key in ultimately reducing burden from depression and anxiety.
Here we examine childhood somatic complaints—including frequent and recurrent headaches, stomach aches, and muscle aches—as a marker of risk for depression and anxiety during young adulthood. Somatic complaints during childhood and adolescence are frequently reported to parents, teachers, school nurses/counselors, pediatricians, family doctors, and general practitioners—who are not only children’s primary caregivers, but also their de-facto first responders to mental health symptoms (Farmer et al., 2003). Somatic symptoms predict anxiety disorders years later in pediatric specialty patient samples (e.g., Shelby et al., 2013), but no prospective long-term U.S. community study has examined childhood somatic complaints as markers of long-term risk for emotional disorders.
Previous Work on Childhood Somatic Symptoms and Psychopathology
Cross-sectional and short-term longitudinal community- and clinic-based research suggests that childhood somatic complaints often co-occur with and/or predict anxiety and depression (e.g., Egger et al., 1998, Egger et al., 1999, Saps et al., 2009, Dunn et al., 2011, Garralda, 2011), and also behavioral disorders that tend to precede adulthood emotional disorders (e.g., oppositional defiant disorder, Copeland et al., 2009). Few studies have tested long-term predictions from childhood somatic symptoms to later psychopathology. In two British cohort studies, children with frequent stomach aches and headaches at ages 7, 11, and 15 were at increased risk for higher severity of global psychiatric symptoms at ages 33 and 36 (Hotopf et al., 1998; Fearon & Hotopf, 2001), but predictions toward specific disorders were not tested. In a small U.S. patient sample, young adults (average age 24) who had been clinically evaluated for recurrent abdominal pain during childhood had higher rates of adult anxiety disorders and also family history of generalized anxiety disorder compared to matched controls (Campo et al., 2001), but prediction toward subjects’ specific anxiety disorders was not possible.
Recent work has raised renewed interest in somatic complaints as a long-term marker of later emotional disorder. Eight-year old Finish children with frequent headaches or abdominal pain were at higher risk for severe suicidality by age 24 (Luntamo et al., 2014). However, predictions toward other psychiatric disorders were not tested. Pediatric specialty clinic patients with functional abdominal pain in the U.S., aged 8–17 years, had higher current and lifetime rates of anxiety diagnoses (i.e., generalized anxiety and social phobia) at approximately age 20 compared to matched-community controls (Shelby et al., 2013). They also were at increased risk for lifetime diagnoses of depression. However, the pediatric pain patients in this study likely had more severe and impairing pain compared to the “average child” with somatic complaints in the community. Replication and extension of Shelby and colleagues’ important findings in community samples is needed in order to firmly establish childhood somatic complaints as a marker of risk for later emotional disorders.
Clarification of Long-Term Predictions
When testing predictions from childhood somatic complaints to later psychiatric disorders several questions need to be clarified. First, do somatic complaints predict later emotional disorders independent of potential confounders of this association? Known correlates of both somatic complaints and later emotional disorders include childhood maltreatment, bullying, parental psychopathology, and obesity (for a review, see Garralda, 2011). Whether somatic complaints uniquely contribute to predictions of adult emotional disorders above and beyond these potential confounders (and/or potential mediators) needs to be firmly established.
Second, are there sex differences in predictions from childhood somatic complaints to later emotional disorders? A female preponderance for both somatic complaints and select emotional disorders (e.g., depression) emerges during adolescence (e.g., Angold et al., 1999a, Stanford et al., 2008). Furthermore, some cross-sectional studies reported stronger associations between childhood somatic complaints and emotional disorders in girls than in boys (e.g., Egger et al., 1999). In contrast, one longer-term longitudinal study reported predictions from childhood somatic complaints to later severe suicidality in males only (Luntamo et al., 2014). Other long-term prediction studies have not reported (or tested) sex differences (e.g., Shelby et al., 2013). Clarification of potential sex differences in long-term predictions from somatic complaints to later emotional disorders is needed.
Finally, what mechanisms account for long-term predictions from childhood somatic complaints to emotional disorders many years later? Persistence and/or escalation of pain is one potential mechanism, and with our long-term longitudinal design, we can test whether children whose somatic complaints persist across relatively distinct developmental periods are at greatest risk for later emotional disorders. Support for the persistence hypothesis comes from work showing that children with somatic complaints later report higher rates of medication and general medical services use (Hotopf et al., 1998, Luntamo et al., 2012)—which tend to be associated with emotional disorders. Nevertheless, persistence of abdominal pain into adulthood did not account for increased risk for later psychiatric disorder in a few studies (Hotopf et al., 1998, Shelby et al., 2013). However, headaches—which constitute a common somatic complaint in adults (Kalaydjian & Merikangas, 2008)—have rarely been considered when testing whether persistence of somatic complaints is associated with psychopathology during young adulthood.
The present study uses a community-representative U.S. sample to test which specific emotional disorders at ages 19–26 are predicted by childhood somatic complaints (experienced from ages 9–16) in children from the community, whether there are sex differences in these predictions, and whether persistence of somatic complaints across developmental periods is a marker for the highest long-term risk.
Methods
Participants
The Great Smoky Mountains Study is a representative sample of three cohorts of children, age 9, 11, and 13 at intake, recruited from 11 counties in Western North Carolina in 1993 using a multi-stage household equal probability, accelerated cohort design (Costello et al., 1996). Participants were recruited from a pool of some 12,000 children using a two-stage sampling design (see also Copeland et al., 2014). The first stage involved screening parents (N=3,896) for child behavior problems. All non-American Indian/Native American children scoring in the top 25% on a behavioral problems screener, plus a 1-in-10 random sample of the rest, were recruited for detailed interviews. About 8% of the area residents and the sample are African American, and fewer than 1% are Hispanic. American Indians constituted only about 3% of the population of the study area, but, because they are an understudied group, they were oversampled to constitute 25% of the study sample. More detailed information about the American Indian subsample is available in Copeland and colleagues (2014) and Costello and colleagues (2003); race/ethnic differences were, however, not a primary focus of the present analyses. Of all subjects recruited, 80% (N = 1,420) agreed to participate. The weighted sample was 49.0% female. All subjects were given a weight inversely proportional to their probability of selection in our statistical analyses; therefore results are representative of the population from which the sample was drawn.
Annual assessments were completed 4–7 times with the child and primary caregiver until age 16 and then again up to three times with the subject only at ages 19, 21, and 24–26 years, as recently described by Copeland and colleagues (2014) and also shown in Supplement 1. An average of 83% of possible interviews was completed across assessments (range: 74% to 94%). Before interviews began, participants signed informed consent forms approved by the Duke University Medical Center Institutional Review Board.
Assessment of Adult Outcomes
Outcome status was coded positive when subjects met criteria for a psychiatric disorder at age 19, 21, or 24–26. All adult psychiatric disorders were assessed through self-report interviews with the Young Adult Psychiatric Assessment (YAPA, Angold et al., 1999b). The timeframe for the YAPA was the 3 months immediately preceding the interview. Scoring programs, written in SAS (SAS Institute Inc), combined information about the date of onset, duration, and intensity of each symptom to create diagnoses according to the DSM-IV (American Psychiatric Association, 2000). Two-week test-retest reliability of the YAPA is comparable to that of other highly structured interviews (kappas for individual disorders range from .56 to 1.0, Angold & Costello, 1995). Validity is well-established using multiple indices of construct validity (Angold & Costello, 2000). The interviews, glossaries, and diagnostic codebooks used are available at http://devepi.duhs.duke.edu/instruments.html.
Emotional disorder diagnoses included any DSM-IV anxiety disorder (generalized anxiety, agoraphobia, panic disorder, social phobia, obsessive-compulsive disorder, posttraumatic stress disorder) and depressive disorders (major depression, minor depression, dysthymia). Note that obsessive-compulsive disorder and posttraumatic stress disorder had a very low prevalence, and, thus, were not analyzed separately. In past research, generalized anxiety disorder and depression were highly correlated; some have suggested combining these two categories (e.g., Clark & Watson, 2006). Therefore, in addition to examining separate generalized anxiety and depression categories, we created a single “emotional distress disorders” category that grouped participants with either generalized anxiety disorder or depression into one category (e.g., Lahey et al., 2008). We did not expect childhood somatic complaints to predict adult substance abuse/dependence, but included such a variable to test whether findings for the emotional disorders would generalize to other adult disorders.
Assessment of Somatic Complaints
At each assessment between ages 9 and 16 with the Child and Adolescent Psychiatric Assessment (CAPA, Angold & Costello, 1995, Angold & Costello, 2000), the child and their parent reported whether the child had headaches, abdominal, or muscular/joint pain (assessed beginning at wave 2) in the 3 months immediately prior to the interview. Somatic complaints in a particular domain were coded if the pain occurred at least once a week in the past three months and if each pain episode lasted for at least one hour. Given the frequency threshold of “at least once a week for 3 months,” monthly peri-menstrual pains were effectively excluded. Pains due to sports involvement were not counted. Extensive medical assessments to determine organic causes of somatic symptoms were not possible, but final multivariate analyses were adjusted for parent-reported child medical conditions, medication use, and interviewer-assessed obesity.
During young adulthood, there was no parallel assessment of somatic complaints, but the YAPA assessed whether participants had experienced headaches that lasted at least one hour in the past 3 months. Participants were coded positive if they endorsed headaches during any of the young adult assessments.
Assessment of Control Variables
All childhood psychiatric and family hardships variables were assessed by parent and/or self-report using the CAPA (Angold et al., 1995, Angold & Costello, 2000), using its 3-months timeframe, unless otherwise noted. Childhood psychiatric status included the same anxiety and depressive disorders as in adulthood, and also separation anxiety disorder. We also coded childhood behavioral disorders (conduct disorder, attention-deficit hyperactivity disorder, oppositional defiant disorder, substance use disorder). Subjects were coded positive for a diagnosis if they met full DSM-IV criteria for the disorder at any childhood assessment.
The following psychosocial adversity domains were coded. (1) Low socioeconomic status (SES) was positive if the child’s family met two or more of the following conditions: below the federal poverty line for family income, parental high school education only, or low parental occupational prestige. (2) Unstable family structure was positive if the child’s family met two or more of the following conditions: single parent structure, step-parent in household, divorce, parental separation, or change in parent structure. (3) Family dysfunction was positive if the child’s family met five or more of the following conditions during at least one childhood assessment: lax parental supervision, parental over-involvement, physical violence between parents, frequent parental arguments, parental apathy, involvement of the child in parental arguments, current maternal depression, high conflict between subject and parent, and parental activities being the source of tension or worry for the child. (5) Maltreatment was coded if child or parent reported lifetime physical or sexual abuse of the child or if interviewers noted neglect of the child. (6) Being a victim of bullying was coded if the child had been bullied/teased at school, home, or in other settings. The coding of all individual risk factors is described at: http://devepi.duhs.duke.edu/library/pdf/RiskfactorsCodebook.pdf. These summary risk factor scales have been used in previous work with the GSMS (Copeland et al., 2013); additional details are also available from the first author.
Child physical illness in the past 12 months was reported by parents using a survey adapted from the CDC National Health Interview Survey (NHIS) Child Supplement (1988). This questionnaire assessed 40+ medical conditions ranging from infections to childhood allergic disease to other chronic diseases (e.g., diabetes, epilepsy, cancer). A dichotomous “any medical condition in the past 12 months” variable was created. Results remained unchanged when more specific disease categories (e.g., allergic disease, other chronic disease, injury, recent infections) were accounted for in the analyses. Children’s medication use in the past 12 months was assessed using the Child and Adolescent Services Assessment (Ascher et al., 1996). BMI was calculated from weight and height measurements obtained by trained interviewers at each assessment. Obesity was coded consistent with CDC guidelines.
Statistical Analyses
All childhood data (ages 9 to 16) were aggregated into one observation per participant, and all young adulthood data (ages 19 to 26) were aggregated into one observation per participant. For example, if childhood somatic complaints were endorsed at any point between ages 9 and 16, the child was coded as having experienced childhood somatic symptoms. Predictions from childhood somatic complaints to later psychopathology were tested using weighted logistic regression models in a generalized estimating equations framework implemented by SAS PROC GENMOD. Robust variance (sandwich type) estimates were used to adjust the standard errors of the parameter estimates for the stratified design effects. Of the 1,420 subjects assessed in childhood, 1,273 (89.7%) were followed up in young adulthood. Participation versus non-participation during adulthood was not significantly predicted by childhood somatic complaints status, overall childhood psychiatric case status, or the psychosocial risk domains examined here.
Results
Four hundred and sixty-four children (34.4%: all percentages weighted) in the analytic sample reported somatic complaints during at least one assessment between ages 9–16. Consistent with previous reports (e.g., Garber et al., 1991, Campo & Fritsch, 1994, Egger et al., 1999, Dunn et al., 2011), headaches were the most common somatic complaints (24.7%), followed by stomach aches (9.6%), and muscle aches (8.4%). The proportion of somatic complaints was higher in females than in males (39.9% vs. 29.03%, p = 0.01). The proportion of somatic complaints did not differ during childhood (9–13 years) versus adolescence (14–16; 23.3% vs. 22.6%, respectively). Children with one type of somatic complaints had an increased likelihood of also displaying other types of somatic complaints at some point during childhood (OR = 2.81; CI: 1.55–5.11, p < .001 for the headaches-stomach aches association; OR = 2.86; CI: 1.29–6.33, p < .01 for the stomach aches-muscle aches association; and OR = 1.91; CI: 0.99–3.69, p = .05 for the headaches-muscle aches association). During young adulthood, 51% of participants reported having had a headache lasting more than one hour at some point during the last 3 months; this percentage was reduced to 23% when only headaches that impaired daily activities were coded.
We characterized childhood somatic complaints in terms of their diagnostic, psychosocial adversity, and health correlates during childhood (Table 1). Given our aggregation of data across ages 9 to 16, these factors could have occurred at earlier, later, or the same assessment(s) as childhood somatic complaints. As expected, children with somatic complaints had higher proportions of childhood depression and anxiety, and also behavioral disorders. Among the childhood adversities, only maltreatment and having been bullied and family instability (in females only) were associated with somatic complaints. In terms of health status, children with somatic complaints were more likely to have been ill, to have used medications, and to be obese (at the statistical trend level). Generally, there were no sex differences in these associations (see p-values in last column on Table 1).
Table 1.
Bivariate associations of childhood psychiatric problems and adversity with childhood somatic complaints.
| No Somatic Complaints N = 809 (65.6%) | Somatic Complaints N = 464 (34.4%) | Comparing the Somatic Complaints Group with the No Somatic Complaints Group | Testing Sex Differences in Bivariate Associations | ||
|---|---|---|---|---|---|
| Weighted % | Weighted % | OR (95%CI) | p value | p value | |
| Demographics | |||||
| Male | 55.1 | 43.1 | 0.62 (0.42–0.91) | 0.014 | – |
| American Indian1 | 4.0 | 3.5 | 0.87 (0.64–1.18) | 0.372 | 0.384 |
| Child Psychiatric | |||||
| Depressive Disorders | 3.1 | 18.2 | 6.90 (3.57–13.34) | <.001 | 0.280 |
| Anxiety Disorders | 6.1 | 16.3 | 2.75 (1.55–4.89) | <.001 | 0.157 |
| Distress Disorders | 6.9 | 25.7 | 4.70 (2.77–7.97) | <.001 | 0.439 |
| Behavioral Disorder | 18.1 | 29.2 | 1.86 (1.22–2.84) | 0.004 | 0.489 |
| Psychosocial Adversity | |||||
| Low SES | 28.4 | 28.4 | 1.00 (0.66–1.50) | 0.996 | 0.191 |
| Family instability | 23.1 | 28.5 | 1.32 (0.87–2.02) F: 2.40 (1.32–4.34) M: 0.64 (0.35–1.19) |
0.196 F: 0.004 M: 0.162 |
0.003 |
| Family dysfunction | 12.4 | 13.9 | 1.34 (0.80–2.24) | 0.271 | 0.479 |
| Maltreatment | 18.0 | 26.7 | 1.65 (1.08–2.53) | 0.020 | 0.918 |
| Bullied | 26.4 | 38.6 | 1.76 (1.18–2.62) | 0.006 | 0.138 |
| Health | |||||
| Obesity | 25.4 | 33.5 | 1.48 (0.98–2.23) | 0.061 | 0.799 |
| Illness | 54.7 | 81.4 | 3.61 (2.32–5.64) | <.001 | 0.825 |
| Medication Use | 53.3 | 71.9 | 2.24 (1.49–3.29) | <.001 | 0.495 |
Bolded ORs significant at p < 0.05;
all others = reference category
Table 2 shows associations between somatic complaints during childhood and psychopathology outcomes in adulthood derived in bivariate and in multivariate models. Significant bivariate associations were identified in the prediction of depression, any anxiety, generalized anxiety disorder, panic disorder, and the combined emotional distress disorders category (i.e., depression and/or generalized anxiety), with the largest effect sizes in the prediction of depression, generalized anxiety and the combined distress disorders. Significant sex differences in predictions were not identified.
Table 2.
Bivariate associations between childhood somatic complaints and young adult psychopathology, and odds ratios from multivariate analyses after adjusting for sex, race, cohort, and potential confounders/mediators (N = 1,261 for multivariate models).
| No Somatic Complaints N = 809 (65.6%) | Somatic Complaints N = 464 (34.4%) | Bivariate Models Comparing the Somatic Complaints Group with the No Somatic Complaints Group | Multivariate Models Comparing the Somatic Complaints Group with the No Somatic Complaints Group | |||
|---|---|---|---|---|---|---|
| Weighted % | Weighted % | OR (95% CI) | p value | OR (95% CI) | p value | |
| Depressive Disorders | 3.4 | 10.0 | 3.21 (1.54–6.70) | 0.002 | 2.69 (1.18–6.14) | 0.019 |
| Any Anxiety Disorder | 8.5 | 17.7 | 2.32 (1.30–4.14) | 0.005 | 1.24 (0.69–2.23) | 0.469 |
| Generalized | 4.5 | 15.6 | 3.93 (1.96–7.91) | <.001 | 2.35 (1.07–5.16) | 0.034 |
| Panic | 5.5 | 12.5 | 2.46 (1.30–4.67) | 0.006 | 1.39 (0.65–2.98) | 0.383 |
| Agoraphobia | 3.6 | 6.5 | 1.88 (0.78–4.55) | 0.159 | 1.59 (0.66–3.80) | 0.727 |
| Social Phobia | 1.2 | 0.7 | 0.54 (0.14–2.13) | 0.377 | 0.26 (0.07–1.02) | 0.054 |
| Distress Disorders | 6.8 | 18.9 | 3.21 (1.80–5.73) | <.001 | 2.38 (1.21–4.67) | 0.012 |
| Substance Abuse/Dependence | 19.2 | 18.6 | 0.96 (0.59–1.55) | 0.859 | 0.97 (0.57–1.65) | 0.919 |
Bolded ORs significant at p < 0.05.
In multivariate analyses (last columns in Table 2), we adjusted for all childhood covariates in the prediction of young adult psychopathology to test whether somatic complaints were uniquely predictive of young adult psychopathology or whether these complaints were merely markers of other conditions (e.g., adversity, poor health) that are associated with later psychopathology. All models were also adjusted for race, sex, and cohort.
Results revealed that after adjusting for the broad set of potential confounders/potential mediators, somatic complaints during childhood remained a significant predictor of depressive disorders, generalized anxiety, and the combined distress disorders category. The odds ratios for any anxiety disorder and panic disorder attenuated to non-significance. Follow-up analyses suggested that, in both cases, the childhood somatic complaints variables attenuated to non-significance with the inclusion of sex and the childhood depression variables. Taken together, direct predictions from childhood somatic complaints to young adult emotional disorders are specific to the emotional distress disorders: depression and generalized anxiety. Supplement 2 shows which covariates were significant in the prediction of adult outcomes in multivariate models.
Are Children with Persistent Somatic Complaints at the Greatest Risk?
For all cohorts, we could distinguish between broad childhood (ages 9–16) and young adulthood (ages 19–26) developmental periods. For the two youngest cohorts (i.e., cohorts A and B in the table shown in Supplement 1), we could further break down the broad childhood category into late middle childhood (ages 9–13) and adolescence (ages 14–16). Consequently, in persistence analyses with all cohorts, we created a variable that coded whether any somatic complaint had been experienced never, during one developmental period or during two developmental periods. In separate analyses with the two youngest cohorts only, we also coded whether somatic complaints had been experienced during three developmental periods.
Figure 1 shows that experiencing somatic symptoms just once significantly increased risk for later emotional distress disorders. However, as somatic symptoms (i.e., headaches, stomach aches, muscle aches during childhood/adolescence and headaches during young adulthood) stretched across different developmental periods, risk for later emotional distress disorders further increased. Indeed, although only 6% of the two youngest cohorts reported somatic complaints across three developmental periods, the prevalence of adult emotional distress disorders in this subgroup was at 35%. These persistence predictions replicated when using the more constrained “adult headaches with impairment” category.
Figure 1.
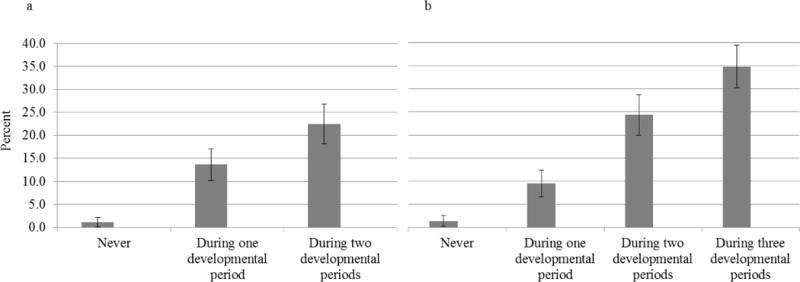
Prevalence of distress disorders in young adulthood by number of developmental periods with somatic complaints.
a) All cohorts. Somatic complaints counted during childhood (9–16 years) and young adulthood (19–26 yrs).
OR = 3.07, CI: 2.21-4.43, p < .001 for persistence term
b) The two youngest cohorts—recruited at ages 9 and 11—only. Somatic complaints counted during late middle childhood (9–13 yrs), adolescence (14–16 yrs), and young adulthood (19–26 yrs).
OR = 2.92, CI: 2.11-4.05, p < .001 for persistence term
Follow-up Analyses
A series of additional analyses tested the robustness of effects identified here. First, prediction toward later distress disorders remained significant when the average number of childhood symptoms (rather than childhood diagnoses) of depression and generalized anxiety were accounted for. Thus, predictions toward later distress disorders were not simply accounted for by subthreshold depression and generalized anxiety cases that later met full diagnostic criteria. Second, when including suicidality as an outcome, we identified significant bivariate predictions from somatic symptoms (OR = 2.19, CI: 1.02–4.72, p = .04); but these predictions attenuated in multivariate models (OR = 1.37, CI: 0.62–3.05), and sex differences were not observed. Third, because only few young adults met diagnostic criteria for social phobia, we also used Poisson regressions to predict the average number of symptoms of social phobia in young adulthood. These analyses also showed that social phobia symptoms were not predicted by childhood somatic complaints (means ratio = 0.91, CI: 0.46–1.79).
Fourth, although our measurements of adult somatic complaints were limited, we tested alternative directions of effects: from childhood emotional distress disorders to later headaches. Childhood distress disorders predicted adult headaches in a bivariate model (OR = 2.02, CI: 1.20–3.41, p = .008), but when adjusting for childhood somatic complaints, this effect was no longer significant (OR = 1.47, CI: 0.84–2.58). Fifth, we replicated patterns of predictions using the two youngest cohorts, which had the highest numbers of childhood observations. When using these two cohorts only, predictions from childhood somatic complaints to later emotional distress disorders further strengthened. Finally, follow-up analyses by type of somatic complaints were conducted (see Supplement 3). Bivariately, each type of somatic complaints was associated with both generalized anxiety disorders and the overall distress disorders category. In the multivariate models, it was the combined somatic complaints that predicted later distress disorders.
Discussion
Young adulthood sets the stage for long-term interpersonal, educational, and economic growth throughout adulthood. Preventing depression and anxiety disorders during this important developmental period may be key for reducing overall burden from mental illness. In our community sample, children’s frequent and recurrent somatic symptoms predicted a substantially increased risk for emotional distress disorders during young adulthood, suggesting that childhood somatic complaints constitute an easy-to-detect marker for long-term risk. Predictions were (1) specific toward depressive and generalized anxiety disorders (i.e., the emotional “distress disorders”), (2) universal for both sexes, and (3) strongest among children for whom somatic complaints persisted across developmental periods.
Generalized anxiety and depression are highly comorbid and share many correlates. Indeed, a large proportion of depression and generalized anxiety symptoms are physical; thus, long-term pathways from somatic symptoms to these two disorders are not surprising. Predictions toward anxiety replicated previous work with abdominal pain patients, which had reported associations with subjects’ generalized anxiety disorder (Ramchandani et al., 2007, Shelby et al., 2013) and also a family history of generalized anxiety (Campo et al., 2001). However, long-term predictions toward depression had been less consistent in previous work. For example, abdominal pain was not associated with current late adolescent/young adulthood depression in a pediatric abdominal pain patients sample—although it was associated with lifetime depression (Shelby et al., 2013). Considering the episodic nature of depression, our study with repeated young adult assessments may have better captured links with depression compared to studies with one-time follow-ups. It is also possible, that pharmacological interventions with pediatric abdominal pain patients (e.g., use of SSRIs) could have reduced long-term risk for depression.
Previous work with patient samples had reported that “any anxiety” and specifically social anxiety was one of the most common late adolescent/young adult outcomes in pediatric abdominal pain patients (Shelby et al., 2013). In the current community sample, it was only young adult generalized anxiety disorder—not social phobia or other anxiety disorders—that was independently predicted by childhood somatic complaints in final models that adjusted for potential confounders and mediators. Pediatric pain patients likely experience more medical testing, days away from home and school, and also family financial burden compared to children with somatic complaints in the community. It is also possible that their parents score higher on anxiety and somatic symptoms compared to other parents. Taken together, it is possible that these potential differences between pediatric pain and community samples, in addition to differences in measurement of social anxiety, could have contributed to differences in findings with these different types of samples.
Mechanisms involved in long-term predictions need to be illuminated. The highest risk for later distress disorders occurred among children whose somatic symptoms persisted across several developmental periods. Chronic pain may extract cumulative psychosocial consequences that increase risk for later emotional distress (Walker et al., 2002): decreased academic achievement; restriction of mental health-enhancing activities (e.g., exercise); maladaptive interoception (Hyams & Hyman, 1998), including increased attentional bias to/perception of pain (Dimsdale & Dantzer, 2007, Beck et al., 2011); and periods of irritability and social withdrawal.
Biological mechanisms are also likely. Correlates of both somatic complaints and emotional distress disorders include dysregulation of the HPA axis and serotonergic pathways (e.g., for a review, see Rief et al., 2010). Somatic symptoms could also indicate sensitization of the brain cytokine system to minor stressors, which could underlie inflammatory-based pathways to emotional distress disorders (Raison et al., 2006, Dantzer et al., 2008). Furthermore, somatic complaints could be indicative of maladaptive function of gut microbiota, which, in turn, may be involved in regulating physiological systems that have been implicated in emotional distress disorders (Dinan & Cryan, 2012). Indeed, although many somatic complaints occur without a distinct, diagnosable physical disease, work on animals and adults suggest that they do have wide-ranging physiological correlates that are also markers of increased vulnerability to emotional distress disorders.
Limitations and Future Directions
Although we assessed three common somatic complaints during childhood, other complaints, including back, facial, and chest pains were not assessed (Stanford et al., 2008, Dunn et al., 2011). Indeed, conclusions about somatoform/somatic symptoms disorders cannot be drawn considering the assessment of only three somatic complaints (Egger et al., 1999). Furthermore, we aggregated data across all childhood observations, which has important advantages (e.g., parsimony in long-term predictions). However, disadvantages include a less fine-grained analysis during childhood. Zooming in on shorter time-frames that clearly illuminate the unfolding of long-term predictions is important (Walker et al., 2001). Moreover, we controlled for children’s medical conditions and medication use, but it is possible that predictions from childhood somatic complaints to later disorder were carried by diagnosable organic causes in some children. In addition, we had only limited assessment of adult somatic complaints. Future research should aim to assess a more complete range of adult somatic symptoms, and test additional mechanisms in long-term predictions to emotional distress disorders, including extent of medical testing/experiences, social and educational consequences of somatic complaints, and encouragement of illness behaviors, parental somatic complaints, and parental physical illness (Walker et al., 1993, Hotopf et al., 1998, Hoftun et al., 2013). Finally, given associations between adult chronic pain and abuse of opioids, future work should also zoom in on links between childhood somatic complaints and later abuse/dependence of substances involved in pain management.
Conclusions
Somatic complaints in children have been hailed as a transdiagnostic tool (Weersing et al., 2012) and an internalizing disorder of itself (Dufton et al., 2009). At a minimum, the presence of frequent and recurrent physical distress during childhood is an early risk marker of risk for significant emotional distress in both females and males in adulthood. The presence of somatic complaints in childhood is an early marker that is much easier to detect and carries less stigma compared to other markers of risk for emotional distress, including parental psychopathology, previous psychiatric disorder, and maltreatment (e.g., Rohde et al., 2013). Guidelines for how to respond to and appropriately refer children with frequent and recurrent somatic complaints are needed, and recommendations for dual physical and mental health checkups are likely warranted. Successful cognitive behavioral and pharmacological treatments for somatic complaints in children have been and are being developed (for reviews, see Garralda, 2011, Weersing et al., 2012) and could help prevent risk for later emotional distress disorders.
Supplementary Material
Acknowledgments
We thank the participants and their parents for their cooperation, and the GSMS staff for their help in conducting this study.
Financial Support
The work presented here was supported by the National Institute of Mental Health (MH094605, MH63970, MH63671, MH48085), the National Institute on Drug Abuse (DA/MH11301), and the William T. Grant Foundation. No authors have any conflicts of interest to report.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: Fourth Edition Text Revision. American Psychiatric Press; Washington D.C: 2000. [Google Scholar]
- Angold A, Costello EJ. A test-retest reliability study of child-reported psychiatric symptoms and diagnoses using the Child and Adolescent Psychiatric Assessment (CAPA-C) Psychological Medicine. 1995;25:755–762. doi: 10.1017/s0033291700034991. [DOI] [PubMed] [Google Scholar]
- Angold A, Costello EJ. The Child and Adolescent Psychiatric Assessment (CAPA) Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:39–48. doi: 10.1097/00004583-200001000-00015. [DOI] [PubMed] [Google Scholar]
- Angold A, Costello EJ, Worthman CM. Pubertal changes in hormone levels and depression in girls. Psychological Medicine. 1999a;29:1043–1053. doi: 10.1017/s0033291799008946. [DOI] [PubMed] [Google Scholar]
- Angold A, Cox A, Prendergast M, Rutter M, Simonoff E, Costello EJ, Ascher BH. The Young Adult Psychiatric Assessment (YAPA) Duke University Medical Center; Durham, NC: 1999b. [Google Scholar]
- Angold A, Prendergast M, Cox A, Harrington R, Simonoff E, Rutter M. The Child and Adolescent Psychiatric Assessment (CAPA) Psychological Medicine. 1995;25:739–753. doi: 10.1017/s003329170003498x. [DOI] [PubMed] [Google Scholar]
- Ascher BH, Farmer EMZ, Burns BJ, Angold A. The Child and Adolescent Services Assessment (CASA): Description and psychometrics. Journal of Emotional and Behavioral Disorders. 1996;4:12–20. [Google Scholar]
- Beck JE, Lipani TA, Baber KF, Dufton L, Garber J, Smith CA, Walker LS. Attentional bias to pain and social threat in pediatric patients with functional abdominal pain and pain-free youth before and after performance evaluation. Pain. 2011;152:1061–7. doi: 10.1016/j.pain.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campo JV, Di Lorenzo C, Chiappetta L, Bridge J, Colborn DK, Gartner JCJ, Gaffney P, Kocoshis S, Brent D. Adult outcomes of pediatric recurrent abdominal pain: Do they just grow out of it? Pediatrics. 2001;108:E1. doi: 10.1542/peds.108.1.e1. [DOI] [PubMed] [Google Scholar]
- Campo JV, Fritsch SL. Somatization in children and adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 1994;33:1223–1235. doi: 10.1097/00004583-199411000-00003. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D. Distress and fear disorders: An alternative empirically based taxonomy of the “mood” and “anxiety” disorders. British Journal of Psychiatry. 2006;189:481–3. doi: 10.1192/bjp.bp.106.03825. [DOI] [PubMed] [Google Scholar]
- Collins PY, Patel V, Joestl SS, March D, Insel TR, Daar AS, Scientific Advisory Board and the Executive Committee of the Grand Challenges on Global Mental Health Grand challenges in global mental health. Nature. 2011;475:27–30. doi: 10.1038/475027a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland WE, Angold A, Shanahan L, Costello EJ. Longitudinal patterns of anxiety from childhood to adulthood: The Great Smoky Mountains Study. Journal of the American Academy of Child and Adolescent Psychiatry. 2014;53:21–33. doi: 10.1016/j.jaac.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland WE, Shanahan L, Costello EJ, Angold A. Childhood and adolescent psychiatric disorders as predictors of young adult disorders. Archives of General Psychiatry. 2009;66:764–772. doi: 10.1001/archgenpsychiatry.2009.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland WE, Wolke D, Angold A, Costello EJ. Adult psychiatric outcomes of bullying and being bullied by peers in childhood and adolescence. The Journal of the American Medical Association: Psychiatry. 2013;70:419–26. doi: 10.1001/jamapsychiatry.2013.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello EJ, Angold A, Burns BJ, Stangl DK, Tweed DL, Erkanli A, Worthman CM. The Great Smoky Mountains Study of Youth: Goals, designs, methods, and the prevalence of DSM-III-R disorders. Archives of General Psychiatry. 1996;53:1129–1136. doi: 10.1001/archpsyc.1996.01830120067012. [DOI] [PubMed] [Google Scholar]
- Costello EJ, Compton SN, Keeler G, Angold A. Relationships between poverty and psychopathology: A natural experiment. The Journal of the American Medical Association. 2003;290:2023–2029. doi: 10.1001/jama.290.15.2023. [DOI] [PubMed] [Google Scholar]
- Costello EJ, He JP, Sampson NA, Kessler RC, Merikangas KR. Services for adolescents with psychiatric disorders: 12-month data from the National Comorbidity Survey-Adolescent Supplement. Psychiatric Services. 2014;65:359–66. doi: 10.1176/appi.ps.201100518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: When the immune system subjugates the brain. Nature Reviews Neuroscience. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimsdale JE, Dantzer R. A biological substrate for somatoform disorders: Importance of pathophysiology. Psychosomatic Medicine. 2007;69:850–4. doi: 10.1097/PSY.0b013e31815b00e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinan TG, Cryan JF. Regulation of the stress response by the gut microbiota: Implications for Psychoneuroendocrinology. Psychoneuroendocrinology. 2012;37 doi: 10.1016/j.psyneuen.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Dufton LM, Dunn MJ, Compas BE. Anxiety and somatic complaints in children with recurrent abdominal pain and anxiety disorders. Journal of Pediatric Psychology. 2009;34:176–86. doi: 10.1093/jpepsy/jsn064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KM, Jordan KP, Mancl L, Drangsholt MT, Le Resche L. Trajectories of pain in adolescents: A prospective cohort study. Pain. 2011;152:66–73. doi: 10.1016/j.pain.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger HL, Angold A, Costello EJ. Headaches and psychopathology in children and adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 1998;37:951–958. doi: 10.1097/00004583-199809000-00015. [DOI] [PubMed] [Google Scholar]
- Egger HL, Angold A, Costello EJ. Somatic complaints and psychopathology in children and adolescents: Stomach aches, musculoskeletal pains and headaches. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38:852–860. doi: 10.1097/00004583-199907000-00015. [DOI] [PubMed] [Google Scholar]
- Farmer E, Burns B, Phillips S, Angold A, Costello EJ. Pathways into and through mental health services for children and adolescents. Psychiatric Services. 2003;54:60–66. doi: 10.1176/appi.ps.54.1.60. [DOI] [PubMed] [Google Scholar]
- Fearon P, Hotopf M. Relation between headache in childhood and physical and psychiatric symptoms in adulthood: National birth cohort study. British Medical Journal. 2001;322:1145. doi: 10.1136/bmj.322.7295.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber J, Walker LS, Zeman J. Somatization symptoms in a community sample of children and adolescents: Further validation of the children’s somatization inventory. Psychological Assessment. 1991;3:588–595. [Google Scholar]
- Garralda ME. Unexplained physical complaints. Pediatric Clinics of North America. 2011;58:803–13. doi: 10.1016/j.pcl.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Green JG, McLaughlin KA, Alegría M, Costello EJ, Gruber MJ, Hoagwood K, Leaf PJ, Olin S, Sampson NA, Kessler RC. School mental health resources and adolescent mental health service use. Journal of the American Academy of Child & Adolescent Psychiatry. 2013;52:501–10. doi: 10.1016/j.jaac.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoftun GB, Romundstad PR, Rygg M. Association of parental chronic pain with chronic pain in the adolescent and young adult: family linkage data from the HUNT Study. The Journal of the American Medical Association: Pediatrics. 2013;167:61–9. doi: 10.1001/jamapediatrics.2013.422. [DOI] [PubMed] [Google Scholar]
- Hotopf M, Carr S, Mayou R, Wadsworth M, Wessely S. Why do children have chronic abdominal pain, and what happens to them when they grow up? Population based cohort study. British Medical Journal. 1998;316:1196–1200. doi: 10.1136/bmj.316.7139.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyams JS, Hyman PE. Recurrent abdominal pain and the biopsychosocial model of medical practice. Journal of Pediatrics. 1998;133:473–8. doi: 10.1016/s0022-3476(98)70053-8. [DOI] [PubMed] [Google Scholar]
- Kalaydjian A, Merikangas K. Physical and mental comorbidity of headache in a nationally representative sample of US adults. Psychosomatic Medicine. 2008;70:773–80. doi: 10.1097/PSY.0b013e31817f9e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey B, Rathouz P, Van Hulle C, Urbano R, Krueger R, Applegate B, Garriock H, Chapman D, Waldman I. Testing structural models of DSM-IV symptoms of common forms of child and adolescent psychopathology. Journal of Abnormal Child Psychology. 2008;36:187–206. doi: 10.1007/s10802-007-9169-5. [DOI] [PubMed] [Google Scholar]
- Luntamo T, Sourander A, Gyllenberg D, Sillanmäki L, Aromaa M, Tamminen T, Kumpulainen K, Moilanen I, Piha J. Do headache and abdominal pain in childhood predict suicides and severe suicide attempts? Finnish nationwide 1981 birth cohort study. Child Psychiatry & Human Development. 2014;45:110–8. doi: 10.1007/s10578-013-0382-x. [DOI] [PubMed] [Google Scholar]
- Luntamo T, Sourander A, Sillanmäki L, Gyllenberg D, Aromaa M, Kumpulainen K, Moilanen I, Almqvist F, Tamminen T, Piha J. Pain at age eight as a predictor of antidepressant medication use by age 24: findings from the Finnish nationwide 1981 birth cohort study. Journal of Affective Disorders. 2012;138:153–9. doi: 10.1016/j.jad.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: Inflammation and the pathogenesis of depression. Trends in Immunology. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramchandani PG, Fazel M, Stein A, Wiles N, Hotopf M. The impact of recurrent abdominal pain: Predictors of outcome in a large population cohort. Acta Paediatrica. 2007;96:697–701. doi: 10.1111/j.1651-2227.2007.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rief W, Hennings A, Riemer S, Euteneuer F. Psychobiological differences between depression and somatization. Journal of Psychosomatic Research. 2010;68:495–502. doi: 10.1016/j.jpsychores.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Rohde P, Lewinsohn PM, Klein DN, Seeley JR, Gau JM. Key characteristics of Major Depressive Disorder occurring in childhood, adolescence, emerging adulthood, and adulthood. Clinical Psychological Science. 2013;1:41–53. doi: 10.1177/2167702612457599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saps M, Seshadri R, Sztainberg M, Schaffer G, Marshall BM, Di Lorenzo C. A prospective school-based study of abdominal pain and other common somatic complaints in children. Journal of Pediatrics. 2009;154:322–6. doi: 10.1016/j.jpeds.2008.09.047. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc. SAS/STAT® Software: Version 9.2. SAS Institute, Inc; Cary, NC: 2008. [Google Scholar]
- Shelby GD, Shirkey KC, Sherman AL, Beck JE, Haman K, Shears AR, Horst SN, Smith CA, Garber J, Walker LS. Functional abdominal pain in childhood and long-term vulnerability to anxiety disorders. Pediatrics. 2013;132:475–82. doi: 10.1542/peds.2012-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford EA, Chambers CT, Biesanz JC, Chen E. The frequency, trajectories and predictors of adolescent recurrent pain: A population-based approach. Pain. 2008;138:11–21. doi: 10.1016/j.pain.2007.10.032. [DOI] [PubMed] [Google Scholar]
- Walker LS, Claar RL, Garber J. Social consequences of children’s pain: When do they encourage symptom maintenance? Journal of Pediatric Psychology. 2002;27:689–98. doi: 10.1093/jpepsy/27.8.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LS, Garber J, Greene JW. Psychosocial correlates of recurrent childhood pain: A comparison of pediatric patients with recurrent abdominal pain, organic illness, and psychiatric disorders. Journal of Abnormal Psychology. 1993;102:248–258. doi: 10.1037//0021-843x.102.2.248. [DOI] [PubMed] [Google Scholar]
- Walker LS, Garber J, Smith CA, Van Slyke DA, Claar RL. The relation of daily stressors to somatic and emotional symptoms in children with and without recurrent abdominal pain. Journal of Consulting and Clinical Psychology. 2001;69:85–91. [PMC free article] [PubMed] [Google Scholar]
- Weersing VR, Rozenman MS, Maher-Bridge M, Campo JV. Anxiety, depression, and somatic distress: Developing a transdiagnostic internalizing toolbox for pediatric practice. Cognitive and Behavioral Practice. 2012;19:68–82. doi: 10.1016/j.cbpra.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


