Abstract
Objective
Physical and cognitive activities are associated with slower cognitive decline. However, few studies have examined racial differences in these associations.
Methods
Data came from a longitudinal study of 7742 (63% black and 61% female) participants assessed 3 times over an average of 9.5 years. At baseline, each participant reported number of hours of leisure time physical activity (categorized using quartiles) and rated frequency of participation in cognitively stimulating activities (range: 0 – 4). A standardized measure of cognition was derived from tests of memory, perceptual speed, and orientation.
Results
Of the 7742 participants, 2322 (30%) reported no physical activity. Cognitive decline was slower by about 20% (95% confidence interval: 13 – 27%) among whites with physical activity above 1.25 hours compared to those with no physical activity, but showed no significant decrease in cognitive decline relative to those with no physical activity among blacks. Further post-hoc analysis revealed cognitive decline to be slower by about 29% (95% CI: 20 – 38%) among blacks and whites with higher education and above average baseline cognition. A 1-point increase in cognitive activity frequency decreased cognitive decline by 8% (95% CI: 3 – 14%) among blacks and by 14% (95% CI: 7 – 20%) among whites.
Conclusions
The benefits of higher physical activity on cognitive decline was observed among whites, and among blacks with higher education and above average baseline cognitive function. However, the protective effect of cognitive activity seems to be independent of education and baseline cognitive function among both blacks and whites.
Keywords: Physical activity, cognitive activity, cognitive decline, minority health
INTRODUCTION
Late-life cognitive decline is a serious public health problem given the aging of the US population.Severe cognitive decline can lead to mild-cognitive impairment (MCI) and eventually to Alzheimer’s disease (AD) dementia in old age.1 and 2 Non-Hispanic blacks (blacks hereafter) are more likely to have a positive AD diagnosis and tend to perform more poorly on cognitive function tests compared to non-Hispanic whites (whites hereafter).3 and 4 Although reasons for these disparities are poorly understood, several explanations have been provided in the literature, including: racial differences in years or quality of education5 or reading level,6 lack of social resources for blacks,7 higher cognitive reserve among whites,8 and better cognition-preserving activities among whites over their lifespan.9
Despite substantial racial disparities in Alzheimer’s disease (AD) dementia, differences in cognitive decline between blacks and whites have been negligible.10, 11 and 12 Reasons for this discrepancy are unknown but the concept of cognitive reserve may partially account for this phenomenon. Cognitive reserve is defined as the ability of an individual to tolerate progressive brain pathology without demonstrating clinical cognitive symptoms.13 Examples of cognitive reserve include education and engagement in physical and cognitive activities.14, 15 and 16 Education is a well-established risk factor for AD dementia.17 and 18 Blacks have higher cognitive function for additional education beyond high school compared to whites with similar education,19 but engage less frequently in cognitive and physical activities.20 Given that higher cognitive reserve ensures that individuals can tolerate more neuropathology before reaching the threshold of dementia, if both racial groups have the same rate of cognitive decline, blacks may disproportionately develop dementia because of lower cognitive reserve,21 and this may help explain the lack of benefit of protective factors in blacks. If this hypothesis is correct, then intervening on cognitive decline may not be uniformly effective among blacks and whites. Blacks also have lower socioeconomic status than whites, which could explain poor psychosocial functioning and worse health outcomes,22 including the discrepancy in cognitive decline and AD dementia rates.
Physical activity can be broadly defined as any musculoskeletal movement that expends energy beyond the resting level. This broad definition may account in part for the heterogeneous findings with regard to the association between physical activity and cognitive functioning. Several prospective studies have reported no association between physical activity and cognitive decline23 and dementia,24, 25, 26, 27 and 28 while others suggest a significant association between physical activity and cognitive decline29, mostly in women,30 and 31 and persons with dementia,32, 33 and 34 depending on the type and intensity of physical activity.27, 28, 35 and 36 Prospective studies have also shown a significant association between late-life cognitive activity and cognitive decline.23, 37, 38 and 39 Frequent participation in cognitively stimulating activities is postulated to alter the structure and function of neural systems that support cognition such that a higher pathologic burden is required before functional impairment occurs.40
The primary aim of the present study is to examine the association of physical and cognitive activities with level and change in cognitive function among black and white participants. Our secondary aim was to explore observed differences between black and white participants using post-hoc analyses based on education and cognitive function levels.
METHODS
Setting
The Chicago Health and Aging Project (CHAP) is a population-based, longitudinal study of Alzheimer’s disease and other common health conditions among adults 65 years or older performed from 1993–2012, described previously.41 Assessments were conducted in three-year cycles in participants’ homes, which were geocoded for census tract and census block information. All CHAP participants came from a relatively small geographic area, thereby allowing us to use census block as the definition of neighborhood. Study participants who provided data for at least two cycles of data collection were included in this investigation.
Cognitive Function
Cognitive function was evaluated using a battery of four tests including two tests of episodic memory (immediate and delayed recall of the East Boston Story),42–43 a test of perceptual speed (Symbol Digits Modalities Test),44 and a test of general orientation and global cognition (Mini-Mental State Examination).45 In a factor analysis, these tests loaded on a single factor that accounted for about 75% of the variance.9 Hence, we constructed a composite measure of global cognitive function based on all four tests by averaging the four tests together after centering and scaling each to their baseline mean and standard deviation. Thus, a participant whose composite performance matches the average participant at baseline has a composite cognitive score of 0.
Physical Activity
Physical activity was assessed at baseline using an instrument from the US Health Interview Survey that was modified for use in older adults.46 Participants were asked whether they engaged in the following leisure activities during the past 2 weeks: walking for exercise, yard work, calisthenics or general exercise, bicycle riding, and swimming or water exercises. They were also asked to rate the number of times and the average number of minutes that they engaged in each of the activities. A composite index of physical activity was created by summing the products of the number of minutes for each activity.47 We found the distribution of the physical activity measure to be highly positively skewed with a large point mass at zero and significant differences between black and white participants. Hence, we created four groups of physical activities based on quartiles of the entire sample (0 hours (h), 0.01–1.24h, 1.25–3.99h, and above 4h) of physical activity per week.
Cognitive Activity
At the baseline interview, we asked about seven activities that mainly involve seeking or processing information and have minimal physical or social demands: viewing television; listening to radio; reading newspapers; reading magazines; reading books; playing games like cards, checkers, crosswords, or other puzzles; and going to a museum. Persons rated their current frequency of participation in each activity on a five-point scale: 4) every day or about every day; 3) several times a week; 2) several times a month; 1) several times a year; 0) once a year or less. The ratings were averaged to yield the composite measure of cognitive activity, which ranged from 0 to 4, with higher scores indicating more frequent participation.9
Covariate Measures
The study also consisted of gender (males or females), race (blacks or non-blacks), education (measured in number of years of schooling completed), and baseline measures of body mass index (kg/m2), and number of co-morbid conditions (cancer, heart condition, hypertension, stroke, diabetes, and hip fracture) that were included in our analysis, since they might influence the association between physical activity and cognitive decline. In a sensitivity analysis, we also adjusted for baseline physical function, activities of daily living, and depressive symptoms.
To capture various social and built environment features of the neighborhood that may influence patterns of physical activity,48 we constructed a measure of neighborhood-level socio-economic status (SES), using a previously published measure that is composed of four indicators of the census-level block groups: percent on public assistance, percent of households earning $25,000 per year or less, percent with a college degree or higher, and percent of owner-occupied dwellings valued at $200,000 or higher,49 in analyses. A z-score transformation was computed for each indicator across census block groups and the resulting four z-scores were averaged to create the neighborhood-level measure for each census block, with higher scores indicative of better neighborhood socioeconomic conditions.
Statistical Analysis
Descriptive statistics were computed using mean and standard deviation for continuous variables and percentages for categorical variables stratified by race. Linear mixed-effects regression models with a two-way interaction of time since baseline (in years) and baseline physical and cognitive activities, while adjusting for baseline covariates stratified by race, were used to study change in cognitive function over the follow-up period. Random effects for intercept and slope was also used to allow for heterogeneity in participant-level cognitive baseline cognitive function and slope of cognitive decline. Each model also included fixed effects for time, age, gender, education, number of chronic health conditions, and BMI. A second model with a three-way interaction of an indicator for physical activity, time since baseline, and an indicator for black participants was used to examine differences between coefficents for blacks and whites. In a separate analysis, we adjusted for neighborhood-level SES to examine the level of change in association of physical activity and change in cognitive function due to neighborhood-level SES. As a secondary analysis, we took two approaches: (1) restricted our sample to above average neighborhood-level SES (above −0.113 standardized score); and (2) restricted our sample to high SES (over 12 years of education) and above average cognitive function (above 0.2-standard deviation unit (SDU)). These post-hoc analyses were performed to explain race-related differences in our primary analysis. All analyses were performed using lme function in nlme library of R program.50
RESULTS
Participants with two or more cognitive assessments with an average follow-up time of 9.5 (SD=2.3) years (or about 3 cognitive assessments) were included in this investigation. The study sample consisted of 2322 (30%) participants with no physical activity, 1513 (20%) participants with 0.01 – 1.24h, 1883 (24%) participants with 1.25 – 3.99h, and 2024 (26%) participants with ≥4h of physical activity per week.
The baseline characteristics of participants stratified by race are shown in TABLE 1. Blacks were younger with less education and higher BMI, and had lower cognitive function scores, lower physical and cognitive activity, and a higher number of chronic health conditions, and lived in neighborhoods with lower SES compared to whites.
TABLE 1.
Sample Characteristics
| Black (N = 4976) | White (N = 2766) | Total (N = 7742) | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Age, years | 71.2 | 5.5 | 74.2 | 6.9 | 72.3 | 6.2 |
| Education, years | 11.5 | 3.4 | 13.9 | 3.2 | 12.4 | 3.5 |
| Neighborhood SES | −0.492 | 0.530 | 0.709 | 0.544 | −0.113 | 0.773 |
| Cognitive function | 0.126 | 0.736 | 0.553 | 0.623 | 0.279 | 0.727 |
| Symbol digits test score | 26.8 | 13.1 | 37.9 | 11.9 | 30.8 | 13.8 |
| Delayed recall test score | 7.8 | 2.9 | 8.9 | 2.5 | 8.2 | 2.8 |
| Immediate recall test score | 8.3 | 2.6 | 9.3 | 2.2 | 8.7 | 2.5 |
| Mini-mental state exam score | 26.1 | 4.3 | 27.9 | 3.1 | 26.8 | 4.0 |
| Physical activity, hours | 2.7 | 4.4 | 3.9 | 5.1 | 3.1 | 4.7 |
| Cognitive activity, frequency | 2.04 | 0.66 | 2.43 | 0.59 | 2.18 | 0.66 |
| Body mass index, kg/m2 | 28.8 | 6.1 | 26.8 | 5.2 | 28.1 | 5.9 |
| Number of chronic health conditions | 1.0 | 0.91 | 0.92 | 0.88 | 1.0 | 0.90 |
Notes: Cognitive function measured in standard deviation units (SDU).
Blacks reported less physical activity on average than whites (chi-square= 260, df=3, p<0.0001). Of the 4976 blacks, 1756 (35%) reported no physical activity, 1031 (21%) reported 0.01 – 1.24h, 1086 (22%) reported 1.25 – 3.99h, and 1103 (22%) reported ≥4h of physical activity. Of the 2766 whites, 566 (21%) reported no physical activity, 482 (17%) reported 0.01 – 1.24h, 797 (29%) reported 1.25 – 3.99h, and 921 (33%) reported ≥ 4h of physical activity.
Blacks also reported less cognitive activity compared to whites (chi-square=474, df=1, p<0.0001). Among blacks, 2828 (57%) reported cognitive activity below the overall mean of 2.18, and the remaining 2148 (43%) reported cognitive activity above the mean. Among whites, 858 (31%) reported cognitive activity below the overall mean and the remaining 1908 (69%) reported cognitive activity above the mean.
Physical activity of ≥4h was associated with baseline cognitive function in black and white participants (TABLE 2). Compared to participants with no physical activity, the average baseline cognitive function was higher by 0.061-standard deviation units (SDU) in black participants (SE=0.023, Wald t-statistic=2.62 , df=4967, p=0.009) and 0.078-SDU in white participants (SE=0.029, Wald t-statistic=2.67, df=2757, p=0.008) among those with more than 4h of physical activity. A similar trend was observed for physical activity of 1.25 – 3.99h with higher baseline cognitive function of 0.075-SDU among black participants (SE=0.023, Wald t-statistic=3.25, df=4967, p=0.001), but this level of physical activity was not significantly associated with baseline cognitive function (0.043-SDU) among white participants (Wald t-statistic=1.46, df=2757, p=0.14).
TABLE 2.
Regression Coefficients in the Race-Specific Mixed Effects Regression Model with Quartiles of Physical Activity Referenced to No Physical Activity
| Time | Variables | Blacks | Whites | ||||
|---|---|---|---|---|---|---|---|
| df | b | SE | df | b | SE | ||
| Baseline | Phy. Act. (0.01–1.24h) | 4967 | 0.023 | 0.024 | 2757 | 0.036 | 0.033 |
| Phy. Act. (1.25–3.99h) | 4967 | 0.075 | 0.023 | 2757 | 0.043 | 0.026 | |
| Phy. Act. (≥4h) | 4967 | 0.061 | 0.023 | 2757 | 0.078 | 0.029 | |
| Follow-up | Annual Cog. Dec. (CD) | 11748 | 0.063 | 0.002 | 6321 | 0.078 | 0.004 |
| Decrease in CD (0.01–1.24h) | 11748 | 0.002 | 0.003 | 6321 | 0.012 | 0.006 | |
| Decrease in CD (1.25–3.99h) | 11748 | 0.003 | 0.003 | 6321 | 0.015 | 0.005 | |
| Decrease in CD (≥4h) | 11748 | 0.004 | 0.003 | 6321 | 0.019 | 0.005 | |
Notes: Regression model adjusted for main effects of age, education, sex, body mass index, number of chronic health conditions, and interaction of time with age, education, and sex
The relative change in cognitive decline for each level of physical activity is also shown in TABLE 2. Cognitive function declined by 0.063-SDU per year among black participants with no physical activity (SE=0.002 , Wald t-statistic=31.2, df=11748, p<0.0001), a significantly slower rate of cognitive decline relative to white participants (Wald t-statistic=2.94, df=18064, p=0.003) with 0.078-SDU per year among white participants with no physical activity (SE=0.004, Wald t-statistic=19.5, df=6321, p<0.0001). A level of physical activity between 0.01 – 1.24h was not associated with slower cognitive decline among black (Wald t-statistic=0.74, df=11748, p=0.46) or white participants (Wald t-statistic=1.81, df=6321, p=0.07). A higher level of physical activity between 1.25 – 3.99h was not associated with slower cognitive decline among blacks (Wald t-statistic=0.89, df=11748, p=0.38), but slowed cognitive decline by 0.015-units per year among white participants (SE=0.005, Wald t-statistic=3.0, df=6321, p=0.003). A similar trend was observed for physical acitivty ≥4h; a higher level of physical activity was not associated with slower cognitive decline among black participants (Wald t-statistic=1.41, df=11748, p=0.16), but slowed cognitive decline by 0.019-units per year among white participants (SE=0.005, Wald t-statistic=3.8, df=6321, p=0.0001).
Using a linear contrast, slower cognitive decline among those who reported more than 1.25h of physical activity was significantly different between black and white participants (Wald t-statistic=2.11, df=18065, p=0.035). Therefore, for physical activity above the 50th percentile or 1.25h per week, cognitive decline decreased by about 20% (95% confidence interval [CI]: 13 – 27%) among white participants, but was not associated with decline among black participants. Even after controlling for physical function, activities of daily living, and depressive symptoms, physical activity above 1.25h was significantly associated with cognitive decline among white participants, but not among black participants, a difference that remained significant (Wald t-statistic=2.03, df=18062, p=0.042).
In our secondary analysis, we included neighborhood-level SES variable, and found that cognitive decline slowed by 0.004-SDU (SE=0.004, Wald t-statistic=1.09, df=10934, p=0.28) among blacks with over 4h of physical activity, similar to that reported in TABLE 2. In a second set of analyses, we restricted our sample to participants with above average neighborhood-level SES (above −0.112 standardized score), and found that cognitive decline slowed by 0.006-SDU (SE=0.010, Wald t-statistic=0.59, df=1636, p=0.55), which remained non-significant. Our analysis using neighborhood-level SES measure did not explain the lack of benefit of high physical activity (over 4h) among black participants.
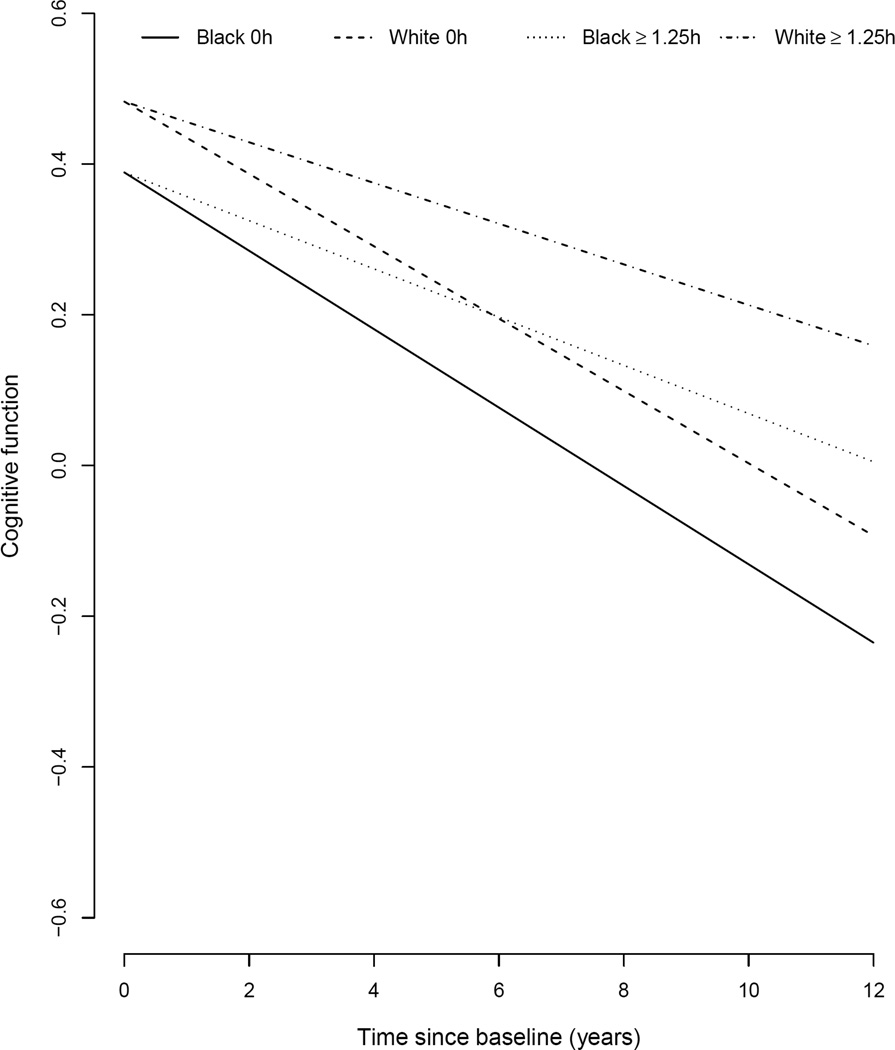
Given that considerable racial differences were observed in baseline cognitive function (0.126-SDU for black participants vs. 0.553-SDU for white participants) and education (11.5 years vs. 13.9 years), we restricted our sample to those with 12 or more years of education and above average cognitive function in our post-hoc analysis. In this subsample, the relative change in cognitive decline was similar for black and white participants across the four physical activity frequency groups. Most notably, participants above 1.25h of physical activity had slower cognitive decline by 0.020-SDU per year among white participants (SE=0.007, Wald t-statistic=2.85, df=5559, p=0.004) and 0.021-units per year among black participants (SE=0.007, Wald t-statistic=3.1, df=4942, p=0.002) relative to participants with no physical activity. This translated to about 29% (95% CI: 20 – 38%) slower cognitive decline for those above 1.25h of physical activity. The change in cognitive function relative to physical activity in this post-hoc analysis is shown in Figure 1. The graph shows that a higher level of physical activity was associated with slower cognitive decline in both black and white participants.
FIGURE 1.
Change in cognitive function relative to physical activity among black and white participants
Legend: Change in cognitive trajectories is depicted for physical activity above 1.25h per week among those with education higher than 12 years and standardized cognitive function above 0.2-SDU.
Translating the association of the four physical activity frequency groups with cognitive decline into effect sizes, an increase in physical activity between the four groups increases the effect size by roughly 0.6, which is considered to be an increase of medium effect size for each quartile increase (e.g., going from 0.01 – 1.24h to 1.25 – 3.99h). For black participants, this increase is roughly 0.28, which is a small effect size, and roughly half of that observed in white participants. However, restricting the analysis to participants with above population average baseline cognitive function and over 12 years of education yields an effect size of about 0.65 in both black and white participants.
Cognitive activity was positively associated with baseline cognitive function in both black and white participants. The average baseline cognitive function was higher by 0.255-units among black participants (SE=0.013, Wald t-statistic=19.6, df=4969, p<0.0001) and 0.278-units among white participants (SE=0.018, Wald t-statistic=16.3, df=2759, p<0.0001).
Among participants with low cognitive activity, cognitive function declined by 0.072-SDU per year among black participants (SE=0.004, Wald t-statistic=17.9, df=11750, p<0.0001), and by 0.096-units per year among white participants (SE=0.008, Wald t-statistic=11.8, df=6323, p<0.0001). For each unit of increase in the frequency of cognitive activity, the change in cognitive function decreased by 0.006-units per year among black participants (SE=0.002, Wald t-statistic=3.0, df=11750, p=0.003), and by 0.013-units per year among white participants (SE=0.003, Wald t-statistic=4.1, df=6323, p=0.0005). This translates to a roughly 8% (95% CI: 3 – 14%) decrease in cognitive decline among black participants and a 14% (95% CI: 7% – 20%) slower rate of cognitive decline among white participants for every one-point increase in cognitive activity. A three-way interaction of cognitive activity, time since baseline, and race was not statistically significant (Wald t-statistic=1.01, df= 18074, p=0.31).
Translating the association of cognitive activity with cognitive decline into effect sizes, a unit increase in frequency of cognitive activities provides an effect size of 1.52 in white participants and 1.34 in black participants, similarly large effect sizes.
DISCUSSION
Our findings demonstrate that physical activity was protective for cognitive decline among white participants, and the benefit of physical activity did not depend on white participants’ level of education or baseline cognitive functioning. In contrast, while physical activity did not appear to slow cognitive decline in the overall population of black participants, we did observe a benefit of physical activity in a subsample of blacks with higher education and higher baseline cognitive functioning. Cognitive activity, on the other hand, was associated with slower cognitive decline among both black and white participants, and resulted in large and meaningful effect sizes.
White participants had a moderate increase in effect size, with higher levels of physical activity resulting in slower cognitive decline. In contrast, benefits of physical activity among black participants yielded only a small effect size and did not impact cognitive decline. Further examination of the null association of physical activity on cognitive decline among black participants suggested an interaction of education and baseline cognitive function. Physical activity appeared to be protective against cognitive decline only among black participants with higher baseline cognitive function and higher education; and the effect size was moderate and similar to that of whites participants. The basis of this finding is not clear but the concept of cognitive reserve may help explain the findings. It has been proposed that individuals differ in their capacity to tolerate the pathology associated with AD dementia. This capacity is conceptualized as cognitive reserve and education is one of the factors thought to build cognitive reserve. It is possible that blacks in our study with higher education, and as a consequence, higher cognitive function, had higher cognitive reserve that allowed them to benefit from the effects of physical activity on cognitive decline. Extending this logic further, physical activity did not buffer the effects of pathology in blacks with lower education and lower cognitive reserve. Why this would be the case for blacks but not whites needs further exploration. But higher education is correlated with a number of experiential factors, resources, and skills that enable a healthier lifestyle and better health status. It is possible that blacks with higher education also tended to have healthier diets, lower BMI, were less likely to smoke, and had fewer health conditions, all factors that have been shown to be related to slower rates of cognitive decline.3 Further studies of physical activity and cognitive decline in older blacks are needed, particularly studies that can determine the mechanisms linking high education and physical activity to decline in this population.
We also accounted for the overall level of social disadvantage within neighborhood environments where participants lived that could conceivably influence participation in physical activities. Previous studies have identified neighborhood-level SES as a social determinant of health that is inversely related to a range of poor health outcomes and unhealthy behaviors, including cognitive decline and reduced physical activity.22,49 The broader social environment for older adults may be particularly important for participation in physical activities because of limited mobility and the tendency to rely more on resources in their immediate environment.48 However, neighborhood-SES did not explain the lack of benefit of physical activity among blacks. Nonetheless, while our findings for black participants are somewhat surprising and largely unreported in the literature, the results still fit within the existing cognitive reserve hypothesis.
The results of our study largely align with previous prospective studies that have found a positive association between physical activity and cognitive decline in white populations. 27 Importantly, the benefits of leisure time physical activity was not dependent on sex28–29 rather, on education and initial levels of cognition. Our study also suggests that leisure time physical activity over the duration of 1.25h is beneficial. However, our study did not measure intensity, such as those by Rovio et al.33 and Andel et al.34 A previous investigation based on 4055 participants from the CHAP study reported a null finding for the association between physical activity and cognitive decline.23 This previous null finding may be due to strong confounding by race, and the use of a linear predictor of physical activity which attenuates linear estimates towards the null due to a high mass of non-participation in physical activities among black participants. A previous report from CHAP on cognitive activities and decline reported a 19% reduction in cognitive decline for each unit increase in frequency of cognitive activities.15 However, our current estimates of 14% in white participants and 8% in black participants are based on a much larger sample with longer follow-up. Similar to our previous report, we found no significant difference in the association of cognitive activities and cognitive decline between black and white participants.
The main limitation of the study is that data was collected in 3-year cycles, making short-term changes less tractable. Given that CHAP collected data for six cycles with up to 18 years of follow-up and our analysis was restricted to those with two or more cognitive assessments, the estimates of cognitive decline are more reliable over a longer observation period. In addition, physical activity was measured using leisure time activities rather than aerobic exercise type activities, which might lead one to believe that the type and duration of physical activity might play an important role, especially among black participants. More research is needed to understand the relationship of physical activity with cognitive decline in black participants.
In summary, leisure time physical activity in old age was associated with decreased cognitive decline among white participants, but the benefits of physical activity were only observed in black participants with higher cognitive functioning and higher education. More frequent cognitive activities decreased cognitive decline in both black and white participants. A healthy lifestyle centered on physical and cognitive activities should help reduce the risk of cognitive decline in the general population.
TABLE 3.
Regression Coefficients in the Race-Specific Mixed Effects Regression Model with Cognitive Activity
| Time | Variables | Blacks | Whites | ||||
|---|---|---|---|---|---|---|---|
| df | b | SE | df | b | 95% CI | ||
| Baseline | Cognitive Activity (CA) | 4969 | 0.255 | 0.013 | 2759 | 0.278 | 0.018 |
| Follow-up | Annual Cog. Dec. (CD) | 11750 | 0.072 | 0.004 | 6323 | 0.096 | 0.008 |
| Decrease in CD (1 point CA) | 11750 | 0.006 | 0.002 | 6323 | 0.013 | 0.003 | |
Notes: Adjusted for main effects of age, education, sex, body mass index, number of chronic health conditions, and interaction of time with age, education, and sex
Acknowledgments
Acknowledgement & Funding: The authors were supported by International Alzheimer’s Association New Investigator Grant (2014-NIRG-302587). The CHAP data was collected by National Institutes of Health Grants (R01-AG11101 and R01-AG09966).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Dixon RA, de Frias CM. The Victoria Longitudinal Study: From characterizing cognitive aging to illustrating changes in memory compensation. Aging Neuropsych Cog. 2004;11:346–376. [Google Scholar]
- 2.MacDonald SW, Hultsch DF, Dixon RA. Aging and the shape of cognitive change before death: terminal decline or terminal drop? J Gerontol B Psychol Sci Soc Sci. 2011;66:292–301. doi: 10.1093/geronb/gbr001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manly JJ, Mayeux R. Ethnic differences in dementia and Alzheimer’s disease. In: Anderson N, Bulatao R, Cohen B, editors. Critical perspectives on racial and ethnic differentials in health in late life. Washington, D.C: National Academies Press; 2004. pp. 95–141. [PubMed] [Google Scholar]
- 4.Early DR, Widaman KF, Harvey D, et al. Demographic predictors of cognitive change in ethnically diverse older persons. Psychol Aging. 2013;28:633–645. doi: 10.1037/a0031645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sisco S, Gross AL, Shih RA, et al. The role of early-life educational quality and literacy in explaining racial disparities in cognition in late life. J Gerontol B Psychol Sci Soc Sci. 2014 Feb 28; doi: 10.1093/geronb/gbt133. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fyffe DC, Mukherjee S, Barnes LL, et al. Explaining differences in episodic memory performance among older African Americans and Whites: the role of factors related to cognitive reserve and test bias. JINS. 2011;17:625–638. doi: 10.1017/S1355617711000476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnes LL, Mendes de Leon CF, Bienias JL, Evans DA. A longitudinal study of black-white differences in social resources. J Gerontol B Psychol Sci Soc Sci. 2004;59:S146–S153. doi: 10.1093/geronb/59.3.s146. [DOI] [PubMed] [Google Scholar]
- 8.Sachs-Ericsson N, Blazer DG. Racial differences in cognitive decline in a sample of community-dwelling older adults: The mediating role of education and literacy. Am J Geriatr Psychiatry. 2005;13:968–975. doi: 10.1176/appi.ajgp.13.11.968. [DOI] [PubMed] [Google Scholar]
- 9.Wilson RS, Benette DA, Beckett LA, et al. Cognitive activity in older persons from a geographically defined population. J Gerontol B Psychol Sci Soc Sci. 1999;54:155–160. doi: 10.1093/geronb/54b.3.p155. [DOI] [PubMed] [Google Scholar]
- 10.Karlamangla AS, Miller-Martinez D, Aneshensel CS, et al. Trajectories of cognitive function in late life in the United States: demographic and socioeconomic predictors. Am J Epidemiol. 2009;170:331–342. doi: 10.1093/aje/kwp154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masel MC, Peek MK. Ethnic differences in cognitive function over time. Ann Epidemiol. 2009;19:778–783. doi: 10.1016/j.annepidem.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson RS, Aggarwal NT, Barnes LL, et al. Cognitive decline in incident Alzheimer disease in a community population. Neurology. 2010;74:951–955. doi: 10.1212/WNL.0b013e3181d64786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tucker AM, Stern Y. Cognitive Reserve in Aging. Curr Alzheimer Res. 2011;8:354–360. doi: 10.2174/156720511795745320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stern YP, Alexander GE, Prohovnik I, Mayeux R. Inverse relationship between education and parietotemporal perfusion deficit in Alzheimer’s disease. Ann Neurol. 1992;32:371–375. doi: 10.1002/ana.410320311. [DOI] [PubMed] [Google Scholar]
- 15.Scarmeas N, Zarahn E, Anderson KE, et al. Association of life activities with cerebral blood flow in Alzheimer disease: Implications for the cognitive reserve hypothesis. Arch Neurol. 2003;60:359–365. doi: 10.1001/archneur.60.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson RS, Mendes de Leon CF, Barnes LL, et al. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA. 2002;287:742–748. doi: 10.1001/jama.287.6.742. [DOI] [PubMed] [Google Scholar]
- 17.Stern Y, Gurland B, Tatemichi TK, et al. Influence of education and occupation on the incidence of Alzheimer’s disease. JAMA. 1994;271:1004–1010. [PubMed] [Google Scholar]
- 18.Evans DA, Hebert LE, Beckett LA, et al. Education and other measures of socioeconomic status and risk of incident Alzheimer disease in a defined population of older persons. Arch Neurol. 1997;54:1399–1405. doi: 10.1001/archneur.1997.00550230066019. [DOI] [PubMed] [Google Scholar]
- 19.Barnes LL, Wilson RS, Hebert LE, et al. Racial differences in the association of education with physical and cognitive function in older blacks and whites. J Gerontol B Psych Sci Soc Sci. 2011;66:354–363. doi: 10.1093/geronb/gbr016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crespo CJ, Keteyian SJ, Heath GW, Sempos CT. Leisure-time physical activity among US adults. Results from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 1996;156:93–98. [PubMed] [Google Scholar]
- 21.Meng X, D’Arcy C. Education and dementia in the context of the cognitive reserve hypothesis: a systematic review with meta-analyses and qualitative analyses. PLoS One. 2012;7:e38268. doi: 10.1371/journal.pone.0038268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harper S, Lynch J, Hsu WL, Everson SA, Hillemeier MM, Raghunathan TE, Salonen JT, Kaplan GA. Life course socioeconomic conditions and adult psychosocial functioning. Int J Epidemiol. 2002;31:395–403. [PubMed] [Google Scholar]
- 23.Verghese J, Lipton RB, Katz MJ, et al. Leisure activities and the risk of dementia in the elderly. N Eng J Med. 2003;348:2508–2516. doi: 10.1056/NEJMoa022252. [DOI] [PubMed] [Google Scholar]
- 24.Crowe M, Andel R, Pedersen NL, et al. Does participation in leisure activities lead to reduced risk of Alzheimer’s disease? A prospective study of Swedish twins. J Gerontol B Psych Sci Soc Sci. 2003;58:249–255. doi: 10.1093/geronb/58.5.p249. [DOI] [PubMed] [Google Scholar]
- 25.Wang HX, Karp A, Winblad B, Fratiglioni L. Late-life engagement in social and leisure activities is associated with a decreased risk of dementia: A longitudinal study from the Kungsholmen project. Am J Epidemiol. 2002;155:1081–1087. doi: 10.1093/aje/155.12.1081. [DOI] [PubMed] [Google Scholar]
- 26.Carlson MC, Helms MJ, Steffens DC, et al. Midlife activity predicts risk of dementia in older male twin pairs. Alzheimers Dement. 2008;4:324–331. doi: 10.1016/j.jalz.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verghese J, LeValley A, Derby C, et al. Leisure activities and the risk of amnestic mild cognitive impairment in the elderly. Neurology. 2006;66:821–827. doi: 10.1212/01.wnl.0000202520.68987.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCallum J, Simons LA, Simons J, et al. Delaying dementia and nursing home placement: The Dubbo study of elderly Australians over a 14-year follow-up. Ann N Y Acad Sci. 2007;1114:121–129. doi: 10.1196/annals.1396.049. [DOI] [PubMed] [Google Scholar]
- 29.Lytle ME, Vander Bilt J, Pandav RS, et al. Exercise level and cognitive decline: The MoVIES project. Alzheimer Dis Assoc Disord. 2004;18:57–64. doi: 10.1097/01.wad.0000126614.87955.79. [DOI] [PubMed] [Google Scholar]
- 30.Sumic A, Michael YL, Carlson NE, et al. Physical activity and the risk of dementia in oldest old. J Aging Health. 2007;19:242–259. doi: 10.1177/0898264307299299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Middleton L, Kirkland S, Rockwood K. Prevention of CIND by physical activity: Different impact on VCI-ND compared with MCI. J Neurol Sci. 2008;269:80–84. doi: 10.1016/j.jns.2007.04.054. [DOI] [PubMed] [Google Scholar]
- 32.Scarmeas N, Levy G, Tang MX, et al. Influence of leisure activity on the incidence of Alzheimer’s disease. Neurology. 2001;57:2236–2242. doi: 10.1212/wnl.57.12.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rovio S, Kareholt I, Helkala EL, et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurology. 2005;4:705–711. doi: 10.1016/S1474-4422(05)70198-8. [DOI] [PubMed] [Google Scholar]
- 34.Larson EB, Wang L, Bowen JD, et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006;144:73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- 35.Rovio S, Kareholt I, Viitanen M, et al. Work-related physical activity and the risk of dementia and Alzheimer’s disease. Int J Geriatr Psychiatry. 2007;22:874–882. doi: 10.1002/gps.1755. [DOI] [PubMed] [Google Scholar]
- 36.Andel R, Crowe M, Pedersen NL, et al. Physical exercise at midlife and risk of dementia three decades later: A population-based study of Swedish twins. J Gerontol A Biol Sci Med Sci. 2008;63:62–66. doi: 10.1093/gerona/63.1.62. [DOI] [PubMed] [Google Scholar]
- 37.Wilson RS, Barnes LL, Aggarwal NT, et al. Cognitive activity and the cognitive morbidity of Alzheimer disease. Neurology. 2010;75:990–996. doi: 10.1212/WNL.0b013e3181f25b5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marquine MJ, Segawa E, Wilson RS, et al. Association between cognitive activity and cognitive function in older Hispanics. J Int Neuropsychol Soc. 2012;18:1041–1051. doi: 10.1017/S135561771200080X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilson RS, Bennett DA, Bienias JL, et al. Cognitive activity and cognitive decline in a biracial community population. Neurology. 2003;61:812–816. doi: 10.1212/01.wnl.0000083989.44027.05. [DOI] [PubMed] [Google Scholar]
- 40.Wilson RS, Boyle PA, Yu L, et al. Life-span cognitive activity, neuropathologic burden, and cognitive aging. Neurology. 2013;81:314–321. doi: 10.1212/WNL.0b013e31829c5e8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bienias JL, Beckett LA, Bennett DA, et al. Design of the Chicago Health and Aging Project. J Alz Dis. 2003;5:349–355. doi: 10.3233/jad-2003-5501. [DOI] [PubMed] [Google Scholar]
- 42.Albert M, Smith LA, Scherr PA, et al. Use of brief cognitive tests to identify individuals in the community with clinically diagnosed Alzheimer’s disease. Int J of Neurosci. 1991;57:167–178. doi: 10.3109/00207459109150691. [DOI] [PubMed] [Google Scholar]
- 43.Wilson RS, Bennette DA, Bienias JL, et al. Cognitive activity and incident AD in a population-based sample of older adults. Neurology. 2002;59:1910–1914. doi: 10.1212/01.wnl.0000036905.59156.a1. [DOI] [PubMed] [Google Scholar]
- 44.Smith A. Symbol Digits Modalities Test. Los Angeles: Western Psychological Services; 1982. [Google Scholar]
- 45.Folstein MF, Folstein SE, McHugh TR“Mini-Mental State”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 46.McPhillips JB, Pellettara KM, Barrett-Connor E, et al. Exercise patterns in a population of older adults. Am J Prev Med. 1989;5:65–72. [PubMed] [Google Scholar]
- 47.Sturman MT, Morris MC, Mendes de Leon CF, et al. Physical activity, cognitive activity, and cognitive decline in a biracial community population. Arch Neurol. 2005;62:1750–1754. doi: 10.1001/archneur.62.11.1750. [DOI] [PubMed] [Google Scholar]
- 48.Sugiyama T, howard NJ, Pauget C, et al. Do relations between environmental attributes and recreational walking vary accordfing to area-level socio-economic status? J Urban Health. 2015;92:253–264. doi: 10.1007/s11524-014-9932-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Everson-Rose SA, Skarupski KA, Barnes LL, et al. Neighborhood socioeconomic conditions are associated with psychosocial functioning in older black and white adults. Health Place. 2011;17:793–800. doi: 10.1016/j.healthplace.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.R: A language and environment for statistical computing. Version 2.15.2. Vienna, Austria: R Foundation for Statistical Computing; 2010. [Google Scholar]