Abstract
Background and Aims
For more than 4 decades endoscopists have relied on ulcer stigmata for risk stratification and as a guide to hemostasis. None used arterial blood flow underneath stigmata to predict outcomes. For patients with severe peptic ulcer bleeding (PUBs), we used Doppler endoscopic probe (DEP) for: 1. detection of blood flow underlying stigmata of recent hemorrhage (SRH), 2. quantitating rates of residual arterial blood flow under SRH after visually directed standard endoscopic treatment, and 3. comparing risks of rebleeding and actual 30 day rebleed rates for spurting arterial bleeding (Forrest – FIA) and oozing bleeding (FIB).
Methods
Prospective cohort study of 163 consecutive patients with severe PUBs and different SRH.
Results
All blood flow detected by DEP was arterial. Detection rates were 87.4% in major SRH - spurting arterial bleeding (FIA), non bleeding visible vessel (FIIA), clot (FIIB) - and significantly lower at 42.3% (p<0.0001) for intermediate group of oozing bleeding (FIB) or flat spot (FIIC). For spurting bleeding (FIA) vs. oozing (FIB), baseline DEP arterial flow was 100% vs. 46.7%; residual blood flow detected after endoscopic hemostasis was 35.7% vs. 0%; and 30 day rebleed rates were 28.6% vs. 0% (all p<0.05).
Conclusions
1. For major SRH vs. oozing or spot, the arterial blood flow detection rates by DEP was significantly higher, indicating a higher rebleed risk. 2. Before and after endoscopic treatment, spurting FIA PUB’s had significantly higher rates of blood flow detection than oozing FIB PUB’s and a significantly higher 30 rebleed rate. 3. DEP is recommended as a new endoscopic guide with SRH to improve risk stratification and potentially definitive hemostasis for PUBs.
Keywords: Doppler probe, endoscopy, ulcer bleeding, stigmata of hemorrhage, risk stratification, GI hemostasis
INTRODUCTION
Peptic ulcer bleeding (PUB) is still the most common cause of severe upper gastrointestinal hemorrhage (UGIH) worldwide, with significant morbidity and mortality related to rebleeding (1–5). Experts in current GI clinical and endoscopic guidelines recommend using stigmata of ulcer hemorrhage (SRH) as a visual guide to risk stratification and also for endoscopic hemostasis (1, 2, 6–8). Initially, successful PUB hemostasis has been reported when either the bleeding stops; the visible vessel or the adherent clot is flattened, well coagulated, or firmly hemoclipped; or in experimental studies or extrapolations from them, the underlying artery is coapted or closed (9–12).
Visible vessels have been used as a visual endoscopic risk factor for the last four decades since PUB experts described the association of the visible vessel with a high risk of PUB rebleeding and surgery (1–5, 12 -14). Early investigators in histopathology studies of surgically resected ulcers reported that a high risk for rebleeding of visible vessels actually related to the invisible, underlying artery with a disruption in its wall, underneath the endoscopically detected visible vessel or sentinel clot in the ulcer base (13–17). Since then, visible vessels and other visually detected SRH have served as guide to both classification and PUB risk stratification for rebleeding and as endoscopic landmarks for hemostasis (1–17). However, the underlying artery and its blood flow have rarely been used as a potential indicator of PUB rebleeding either before or after endoscopic hemostasis (12, 18).
One significant problem about using visual SRHs for risk stratification is the lack of agreement and reproducibility in classification by endoscopists for different ulcer stigmata, particularly in large groups or international studies (19–22). For example, among experienced endoscopists and international experts, there is often disagreement about classification of different SRH when endoscopic pictures or video clips were reviewed in blinded studies (19, 20). In contrast, there are reports that training about visual SRH, their classifications (descriptive vs. Forrest types), and learning sessions for pre-study standardizations can improve accuracy and agreement (21–23). In addition, the classifications of SRH themselves may have contributed to potential mislabeling or inaccurate interpretations of the rebleed risks of different SRH in PUBs, particularly in Europe, the United Kingdom, and Asia where the Forrest classification is used (4, 6, 12, 17, 24–26). For example, based upon the Forrest classification of “active bleeding”, Forrest group I patients are often grouped together – those with Forrest (F) IA (spurting or pulsatile arterial bleeding) and FIB (oozing bleeding). Consequently, some PUB experts by combining both Forrest I subgroups have interpreted them as having a similar high risk of rebleeding (3, 27–30). However, others have questioned whether patients with oozing PUB bleeding (without another SRH, such as a visible vessel or clot associated with the oozing) ought to be considered as a major SRH (4, 5, 12, 18, 26, 31).
Before Doppler endoscopic probes (DEP’s) for blood flow detection during endoscopy, there was no accurate or feasible way to detect underlying blood flow except by angiography or surgery (4, 12, 18). Different types of DEP probes and their control units were previously used in the United Kingdom and Europe for the study of PUBs, but they are not often used there now as a guide to risk stratification or definitive endoscopic hemostasis of ulcers. There are very few prior reports of risk stratification for patients with SRH and none using DEP as a guide to definitive hemostasis of PUBs with major SRH and current endoscopic hemostasis techniques (12, 18, 32–35). Since the development of a disposable endoscopic Doppler probe in the United States and FDA clearance for endoscopic detection of blood flow, there also have been a limited number of reports with this device for the study of bleeding peptic ulcers and risk stratification (12, 18, 36).
Our hypothesis for this study was that DEP would be a more accurate method for determining PUB rebleeding risk (e.g. risk stratification) and in evaluating complete endoscopic hemostasis than SRH and visual cues alone without DEP monitoring. Furthermore, we hypothesized that PUB patients with a high risk stigmata such as spurting or pulsative arterial bleeding (FIA) who had residual blood flow detected by DEP after visually directed endoscopic hemostasis would have a significantly higher risk of rebleeding on standard medical therapy than other low risk patients with oozing bleeding (FIB) and negative residual blood flow.
For patients with endoscopically documented PUBs, our purposes were to use DEP for the following: (1) detection and characterization of blood flow underlying SRH (as arterial vs. venous) and as a potential guide to risk stratification for rebleeding before and after endoscopic treatment, (2) quantitating rates of residual blood flow of different SRH after standard visually guided endoscopic treatment, as a potential future guide for more treatment and definitive hemostasis, and (3) comparison of prospectively determined potential risks of rebleeding (based upon DEP detected arterial bleed flow under SRH) and actual rates of rebleeding for PUB patients with spurting arterial bleeding (FIA) vs. oozing bleeding after visually guided standard endoscopic hemostasis and medical therapy.
METHODS
This was a prospective cohort study of consecutive patients hospitalized with severe UGI hemorrhage who were evaluated by the CURE Hemostasis Research Group and who gave written informed consent before urgent endoscopy to use the DEP for study of blood flow underlying PUBs with different SRH before and after standard endoscopic hemostasis. The study was approved by the IRBs of both Ronald Reagan UCLA and VA West Los Angeles Medical Centers and conducted between January 2008 and December 2013.
All patients had severe UGI hemorrhage as evidenced by clinical signs of UGI bleeding (e. g. hematemesis, melena, or hematochezia with a positive nasogastric aspirate), hemodynamic instability and transfusions (e.g. hypotension, shock or syncope; tachycardia and orthostatic changes in blood pressure and pulse; and low hemoglobins - hemoglobin decrease from pre-bleed baseline of 2 or more grams; or hemoglobin after IV hydration of 8 grams or less with RBC’s transfused). Patients who did not meet these criteria for “severity” were not included in this study. Benign appearing ulcers (5 or more millimeters in diameter as judged on endoscopy by an accessory of known size) of the stomach, duodenum, or UGI surgical anastomosis were included. Therapeutic endoscopes with 3.7 mm channels and a separate channel for target jet irrigation were used in both hospitals for all patients. After target jet irrigation and suctioning of blood and clots, PUB patients were included in this cohort study if they had ulcers that were either clean based (Forrest III), or had what we subgrouped as “major SRH” (spurting or pulsatile arterial bleeding- Forrest IA, non-bleeding visible vessel – VV-FIIA, or adherent clot – FIIB,) or intermediate SRH (oozing bleeding from the ulcer base without other SRH -FIB, or a flat spot - FIIC). Before starting this study, the investigators had tutorial sessions and study cases of different SRH to standardize the classification of SRH and also on utilization of the DEP control unit and probes. Teaching materials included 3–5 cases of each SRH for PUB’s presented in several formats. These were atlas materials with cases and endoscopic pictures and diagrams, case presentations with endoscopic videos before and after treatment, and techniques for application of the Doppler probe were also shown by diagrams and video cases. Dry lab workshops of Doppler technical applications were also conducted before the start of the study for investigators. For this very experienced group of CURE Hemostasis investigators, there was good to excellent agreement for classifying major SRH and good agreement for spots and clots.
Patients with severe PUBs included 139 with different SRH and 24 patients with clean ulcer bases who were prospectively studied. DEP was performed with 2 mm diameter single use Doppler endoscopic probe (Vascular Technology Inc, Nashua, NH). See Figure 1. This was done at baseline on and adjacent to the SRH in the ulcer base before endoscopic treatment or on clean based ulcers to interrogate for underlying blood flow and determine its direction. See figures 2 and 3. The two superficial settings (up to 4 mm deep) were used as set on the control unit (18). The deepest setting (up to 7 mm) was not used to classify ulcer blood flow because of potential interference with adjacent large vessels outside the wall of the stomach or duodenum (18). All positive auditory signals were verified and confirmed by two or more observers at the bedside during the urgent EGD - the investigator endoscopist and 1 or more endoscopy nurses or technicians.
Figure 1.
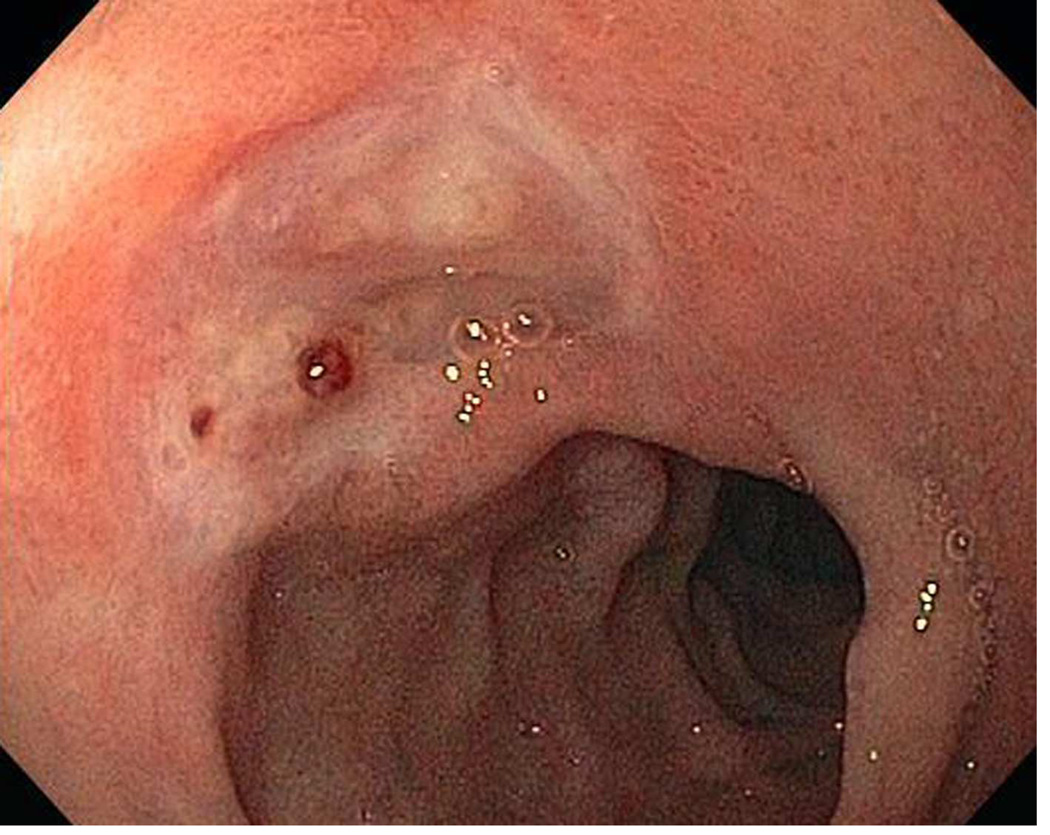
Doppler ultrasound unit and disposable probe used in this study. (From Vascular Technology Inc., Nashua, New Hampshire).
Figure 2.
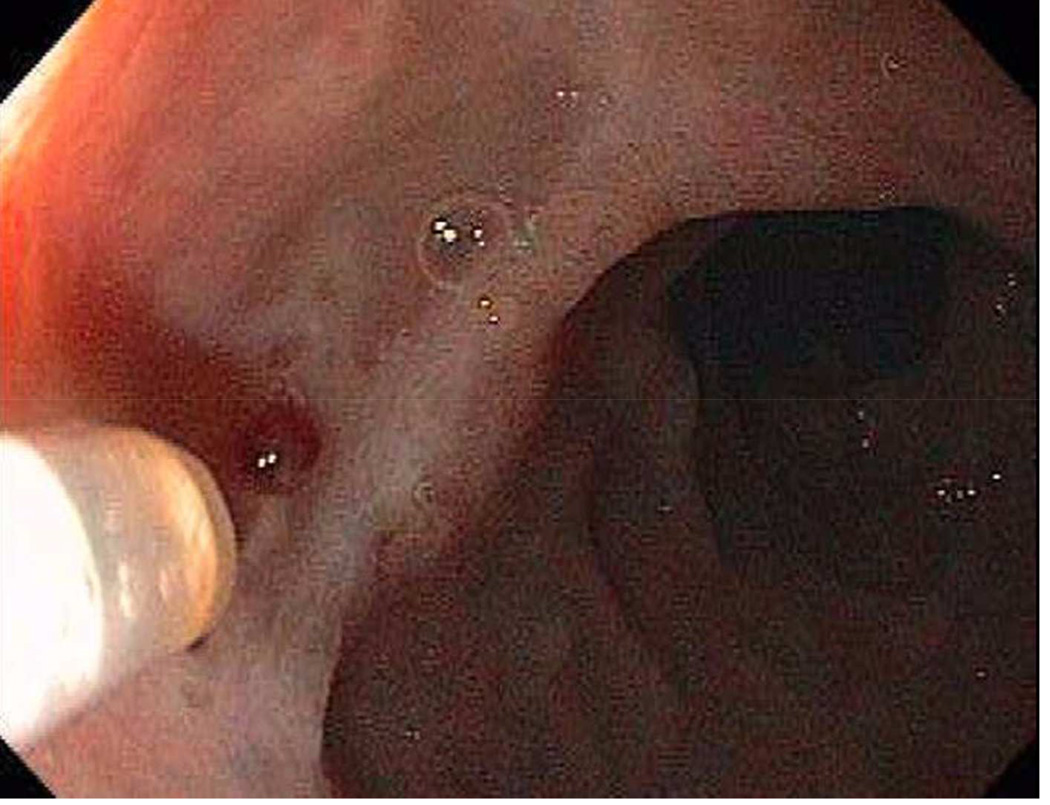
A duodenal ulcer with a non-bleeding visible vessel (Forrest IIA) in the base.
Figure 3.
Doppler probe placement next to the NBVV (FIIA) to interrogate it for underlying blood flow.
As a description of the endoscopic DEP method, the probe tip was lubricated and lightly contacted the SRH in the ulcer base in a tangential orientation and then moved away from the SRH for 5 to 10 mm in a straight line. This was repeated in 4 different quadrants to detect the direction of blood flow and location of the vessel. First the superficial Doppler depth setting (< 1.5 mm) was used and then the middle setting (< 4 mm) and results were recorded. If an arterial signal was positive on one side of the SRH, this was repeated with the same depth setting on the opposite side of the SRH, 180° away. If there was a faint arterial signal or none was heard, the procedure was repeated with the middle depth setting (< 4 mm). The auditory sound from arterial flow was like a blood pressure “swish-swish” whereas venous flow is a low pitched venous hum. Intensity was recorded as soft, medium, or loud.
Standard endoscopic treatment (e.g. multipolar probe electrocoagulation –MPEC- or hemoclips with or without epinephrine pre-injection – 1:20,000 in saline solution in 1 mL aliquots) was applied to PUBs with major SRH and oozing until bleeding stopped and/or the SRH was flattened, firmly hemoclipped or well coagulated with MPEC (4, 5). The type of endoscopic treatment for these SRH was at the discretion of the investigator/endoscopist. Flat spots with negative DEP or clean ulcer bases were not treated endoscopically. However, flat spots with positive DEP at baseline in this study were treated with epinephrine injection (1:20,000) as monotherapy.
For those patients with SRH who were treated endoscopically, DEP was repeated within 5 minutes after the endoscopic treatment to detect residual blood flow as a potential indicator of rebleed risk. In this non-interventional cohort study, the second DEP result (after initial endoscopic hemostasis) was not used as a guide to apply more endoscopic treatment. Instead standard medical treatment according to current published guidelines was used after the index endoscopy. After successful endoscopic hemostasis, patients with major SRH received high dose proton pump inhibitor (PPI) infusion (80 mg bolus and 8 mg/hr for 72 hours), followed by twice-daily oral PPI for 30 days. Those with oozing bleeding (FIB), flat spots (FIIC), or clean-based ulcers (FIII) received PPI in standard doses orally (or intravenously if not eating) twice daily and not high-dose IV PPI infusion, in accordance with other recent results (12, 31).
To track clinical outcomes of the two Forrest I subgroups, 29 patients with FIA (active arterial bleeding) or FIB (oozing bleeding without another SRH- clot or VV) were treated with visually directed hemostasis at emergency endoscopy, as described above. These subgroups had prospective follow-up for 30 days to assess rebleeding rates on medical therapy. Rebleeding was defined as clinical evidence of UGI bleeding (hematemesis, melena, hematochezia, or red blood via NG tube) with a hemoglobin drop of 2 or more grams, or transfusion of 2 or more units RBCs, 6 or more hours after the index endoscopy and treatment.
Baseline clinical, demographic, laboratory, and endoscopic data were prospectively collected and data management was with SAS. The rates of DEP positivity for arterial blood flow for all SRH (at baseline and after standard endoscopic treatment) and the 30 day rebleeding rates for Forrest I (A and B) stigmata were compared by Fisher exact tests. P < 0.05 was considered statistically significant.
RESULTS
For the study period, we retrospectively estimated that approximately 800 patients with evidence of severe UGI bleeding were assessed. This included 350 with ulcers, 200 with varices or portal hypertensive lesions, 200 patients with other lesions (e.g. erosions, esophagitis, cancers, angioma syndromes, Dieulafoys lesions, or Mallory Weiss tears) and 50 other with different diagnosis (epistaxis, no source found, or small-bowel lesions). 163 patients with severe PUB’s who gave written informed consent after meeting entry criteria and lacking exclusions were included in this study. Baseline data are shown in table 1. There were no significant differences detected.
Table 1.
Baseline Characteristics of Patients Studied
| Forrest I | |||
|---|---|---|---|
| All Patients | IA (Spurt) | IB (Ooze) | |
| Number of Patients | 163 | 14 | 15 |
| Age (years-mean-range) | 65.7 (19.93) | 65.2 | 66.3 |
| Male/Female (%) | 80%/20% | 80%/20% | 79%/21% |
| Ethnicity-#’s White/Black/Hispanic/Asia |
96/31/19/17 | 7/3/2/2 | 8/3/2/2 |
| Co-Morbidity Scores - #’s 1 ASA (1,2,3,4) |
11/49/88/15 | 1/4/7/2 | 1/4/8/2 |
| 2 CURE Prog-Median (Range) | 3 (1–6) | 2.9 (2–6) | 3.0 (1–5) |
| Either Hypotension or Shock (%) | 47% | 50% | 46.7% |
| 3 Inpatient Bleeder (%) | 20% | 21.4% | 20% |
| Hemoglobin-Mean (Range) | 7.8 (3.5–12.5) | 7.9 (4.5–8.8) | 8.0 (6.8–12) |
| NSAID’s (% yes) | 26% | 28.6% | 26.6% |
| Aspirin (% yes) | 45% | 50% | 33% |
| Other Anti-platelet drugs (% yes) | 12% | 14.3% | 6.7% |
| Anti-coagulants | 21% | 21.4% | 20% |
| Smoking (%) | 4% | 7.1% | 6.6% |
| Drinking (%) | 3% | 7.1% | 0% |
| H.pylori (% +) | 37% | 35.7% | 40% |
| DU/GU/Anastomotic Ulcer (#’s) | 88/62/13 | 8/5/2 | 8/6/1 |
| Ulcer Size – mm (Range) | 11.5 (6–35) | 11.8 (7–25) | 10.5 (6–18) |
| Size ≥ 20 mm | 17.7% | 21.4% | 6.7% |
| Baseline Transfusions – Mean (Range) | |||
| 4 U Red Cells | 3.3 (0–14) | 3.4 (2–10) | 3.1 (2–6) |
| 4 U Fresh Frozen Plasma | 0.8 (0–11) | 0.6 (0–6) | 1.0 (0–5) |
| Platelet Packs | 0.5 (0–10) | 0.2 (0.3) | 0.9 (0–3) |
Legend:
ASA = American Society of Anesthesiology.
CURE prognosis score- range 0–6 based upon age, co-morbid severity, hypotension-shock & RBC’s to resuscitate.
Inpatient bleeder = started UGI bleed as an inpatient after hospitalization for another non-bleeding diagnosis such as surgery, medical, or other reason.
U = units of blood products transfused for resuscitation or correction of coagulopathies.
All blood flow detected by DEP was arterial, none was venous, and findings were reproducible. For those with SRH, the intensity (loudness) of the arterial signal and the location of the artery and arterial flow in the ulcer base were detected on the SRH and for about 3 to 5 mm straight out from it, rather than serpiginous. The localization of the artery detected before treatment was used as a target for the visually directed endoscopic hemostasis.
For PUBs with “major SRH” the rates of arterial blood flow detection at baseline before endoscopic hemostasis were very high (87.4%). See table 2 for different SRH. The overall and individual subgroup rates were significantly different than the intermediate risk groups of oozing (FIB) or flat spots (FIIC) with combined DEP positivity rate of 42.3%- p<0.00001.
Table 2.
Doppler Probe Results at Index Endoscopy before Hemostasis
| Stigma | N | + DEP Before Rx | % + Before | |
|---|---|---|---|---|
| Active Arterial | 14 | 14 | 100.0% | 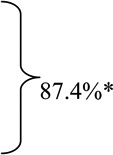 |
| NBVV | 54 | 49 | 90.7% | |
| Adherent Clot | 19 | 13 | 68.4% | |
| Oozing Alone | 15 | 7 | 46.7% | 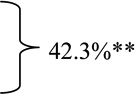 |
| Flat Spot Alone | 37 | 15 | 40.5% | |
| Subtotal | 139 | 98 | 70.5% | |
| Clean base | 24 | 2 | 8.3% |
DEP= Doppler endoscopic probe. Rx= Endoscopic hemostasis.
+= Positive DEP for arterial blood flow.
P value of * major stigmata (Active arterial, NBVV, or clot vs. ** intermediate group (oozing alone and flat spot) by the Fisher exact test was p=0.0000000276.
With more target irrigation and careful re-inspection of the two clean-based ulcer with positive DEP at baseline, the endoscopist in each case reclassified these as having a translucent visible vessel and treated them endoscopically. This is because of the high rebleeding rate reported for this subgroup of VVs (37).
For PUB patients with major SRH and positive DEP (for arterial blood flow) at baseline, 27.4% still had residual arterial blood flow detected after standard visually guided endoscopic hemostasis (4, 5, 9–12). This rate was significantly higher than the 0% residual blood flow rate for oozing patients (p<0.005). No patient with negative arterial flow at baseline developed positive DEP after endoscopic treatment. Refer to table 3 for different SRH.
Table 3.
Comparison of Doppler Probe Results before and after Endoscopic Hemostasis for Different Stigmata.
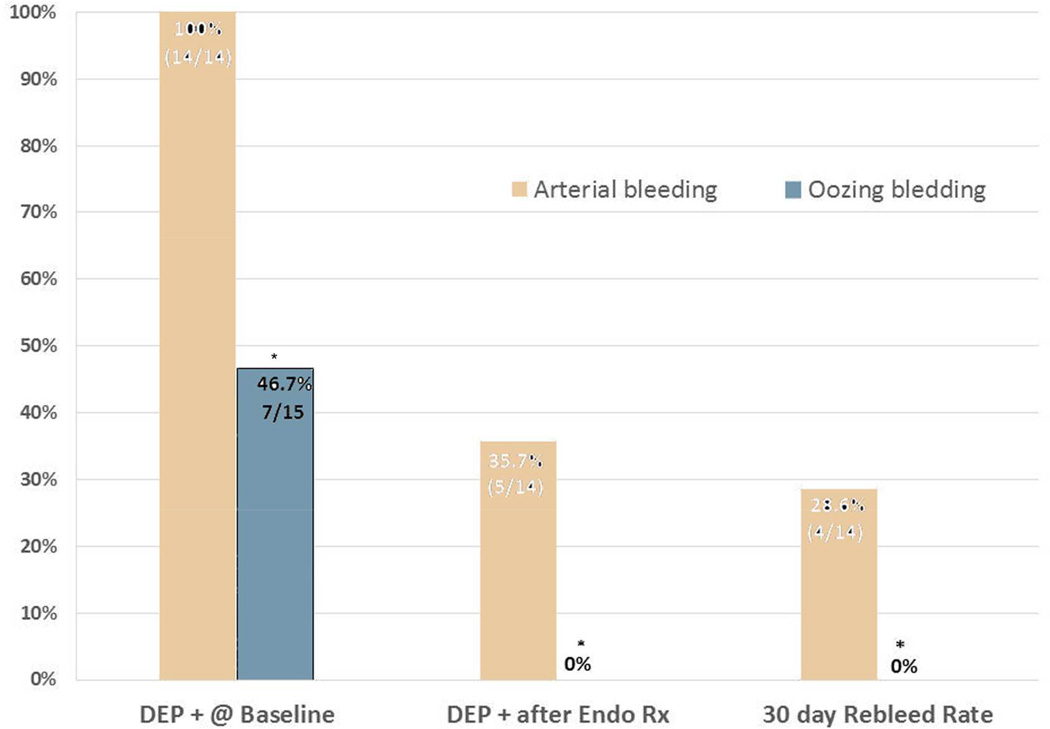
In the statistical comparisons of patients in the two subgroups with “active bleeding” - Forrest I subgroups- A and B- there were significant differences in all comparisons. For FIA (spurting or pulsatile arterial bleeding) vs FIB (oozing) PUBs respectively, these included baseline DEP arterial flow detection (100% vs. 46.7%- p=0.0022), residual arterial flow after visually directed endoscopic hemostasis (35.7% vs 0%- p=0.02), and in 30 day rebleeding rates on standard medical treatment (28.6% vs 0%- p=0.042). Refer to table 4 and figure 4 for details. The endoscopists also reported that PUBs with oozing bleeding (as classified by us without another SRH such as a clot or NBVV) were faster and easier to treat endoscopically and to achieve visually guided hemostasis than PUB’s with spurting arterial bleeding (FIA).
Table 4.
A comparison of oozing (FIB) and spurting (FIA) PUB’s in Doppler Probe Results and 30 day Rebleed Rates
| Oozing bleeding (FIB) |
Spurting bleeding (FIA) |
p Value** | |
|---|---|---|---|
| Patients | 15 | 14 | |
| DEP + @ baseline* | 46.7% (7/15) | 100% (14/14) | 0.0022 |
| DEP + after Endo Rx*** | 0% (0/7) | 35.7% (5/14) | 0. 02 |
| 30 day Rebleed Rate | 0% (0/15) | 28.6% (4/14) | 0.0422 |
All blood flow detected in PUBs was arterial, none was venous
p values by the Fisher exact test
For those PUBs with + DEP @ baseline who had repeat DEP after endoscopic treatment
Figure 4.
PUB active arterial bleeding (Forrest IA) has a significantly higher risk and rate of rebleeding than oozing bleeding (Forrest IB).
Legend. DEP is Doppler endoscopic probe. + is positive signal for arterial blood flow detected underneath the stigma in ulcers. * p < 0.05 for oozing PUBs (Forrest IB) compared to active arterial bleeding (Forrest IA).
DISCUSSION
This is the first large study of PUBs that includes different SRH and Doppler endoscopic probe interrogation of them to quantify the prevalence of arterial blood flow underneath both major and minor SRH. It is also the first study to use the Doppler endoscopic probe to report residual arterial blood flow rates after currently recommended, visually guided endoscopic hemostasis techniques are applied. The results confirm our hypothesis that the DEP combined with visual SRH is more accurate than SRH alone for risk stratification for ulcer rebleeding at baseline and as a new potential guide for definitive endoscopic hemostasis of bleeding ulcers. We documented that the blood flow for PUBs with SRH and identified by DEP was always arterial and none was venous.
The results have major clinical implications for endoscopic risk stratification at baseline during urgent endoscopy and to monitor whether underlying arterial blood flow has been obliterated with the visually directed endoscopic hemostasis techniques currently recommended by guidelines and used by most endoscopists. Our results are directly related to clinical risk stratification for PUB rebleeding of patients based upon endoscopic SRH and could also lead to changes in risk classification of PUB’s and to different ways of reporting results for several different SRH.
One important result in terms of potential numbers of PUB patients involved worldwide is for oozing bleeding (Forrest IB). This subgroup represented over 40% of patients enrolled in a previously reported large international randomized therapeutic PPI trial (22). We think there is a major problem for interpretation when some investigators and PUB experts combine oozing bleeding PUBs (Forrest IB) together with spurting arterial bleeding (Forrest IA) and report their results as “active bleeding” PUBs and use this for sample size determinations, analyses, and final reports (3, 4, 27–30). This conclusion is based upon our current results with DEP where patients with oozing bleeding PUBs had a significantly lower rate of detection of underlying arterial blood flow at baseline than spurting arterial bleeders (46.7% vs. 100%- p=0.0022), were easier to treat endoscopically than major SRH, and none had residual arterial blood flow by DEP detected after standard, visually directed endoscopic hemostasis compared to spurting arterial bleeders (0% vs. 35.7%). Furthermore, as an important clinical outcomes result, there was a significantly lower 30 day rebleeding rate after successful initial endoscopic hemostasis for PUB patients with oozing vs. spurting arterial bleeding (0% vs 28.6%- p=0.042). After successful standard endoscopic hemostasis, this PUB rebleed difference was seen in spite of the medical treatment that was different in these two subgroups, with oral BID PPI’s in oozing patients (FIB) versus high dose IV PPI infusion in those with spurting arterial bleeding (FIA). With this difference in acid suppression, the opposite PUB rebleeding rates might have been anticipated. However, the difference between oozing bleeding (F1B) and spurting arterial bleeding (F1A) appears to be dependent not on acid suppression levels but instead upon smaller arteries underlying oozing PUBs and lower arterial blood flow detection rates compared to spurting arterial bleeders (FIA).
Our results confirm earlier reports that PUBs with spurting arterial bleeding (Forrest IA) and oozing bleeding PUBs (Forrest IB) are significantly different in risks for important clinical outcomes of rebleeding and surgery and should be separated by clinicians and investigators in endoscopic and risk classifications (4, 5, 11–16, 24–26, 31, 36). Based upon earlier reports and our current DEP results, oozing bleeding PUBs (without an associated clot or a visible vessel) are thought to have smaller underlying arteries than PUBs with major SRH- spurting arterial bleeding (FIA), visible vessel (FIIA), or adherent clot (FIIB) (4, 11, 12, 14–17, 24–26).
These results are also very relevant to planning of future PUB studies, particularly for sample size calculations, reporting of prospective studies, and grouping of rebleed risks by SRH for meta-analyses of PUBs. Grouping oozing (FIB) and spurting bleeding (FIA) PUBs together will potentially result in inaccuracies, underestimation of sample size, and can confound interpretation of PUB study results.
One other interesting result on adherent clots could change risk stratification and the decision about endoscopic hemostasis. Adherent clots had a 66.7% prevalence of DEP detected underlying arterial blood flow at baseline and 18.8% had residual blood flow after combination endoscopic hemostasis. These DEP results, in conjunction with our previously reported prospective studies and a randomized controlled trial of medical vs. endoscopic therapy of adherent clots in chronic peptic ulcers where the rebleeding rate of the medical group was 35%, confirm the high risk for rebleeding of this SRH and provide new evidence about underlying arterial blood flow that supports our recommendation to endoscopically treat patients with adherent clots on PUBs (4, 5, 10, 12, 21).
This study has several limitations. One is that we relied upon the endoscopic visual SRH and a descriptive classification by PUB investigators to stratify lesions. Although the investigators were very experienced and tutorials were conducted before this study started, some of the SRH may have been misclassified. We think this is much less of a problem with spurting arterial bleeding that is a more reproducibly classified SRH than other SRH in hemostasis studies and by international experts (19, 20). However, misclassification could have been the case, particularly with flat spots or non-bleeding visible vessels, where there often is less agreement about classifying these two SRH (19, 20). Second, are the potential limitations of the pattern of application of the DEP probe for detection of arterial flow as described in our methods. However, since arteries on ulcer histopathology are known to dive deeper as one moves away from the SRH and there is a small field effect, the method described for detection of arterial blood flow underneath SRH has proven to be reproducible and efficient. The limitations of DEP application are that with perpendicular placement of the DEP blood flow will not be detected as expected from the physics of the Doppler principle nor will it be if the artery is too small and blood flow too low. And arterial blood flow will be missed if it is out of field, temporarily interrupted by treatment, or too deep (>4mm). While interrogating the ulcer base with DEP probe after hemoclipping, the endoscopist may be presented with some challenges and potential limitations. However, if an adaptation is made to interrogate between and on either side of the hemoclips with both superficial (<1.5 mm) and middle settings (<4 mm) in multiple areas this limitation can be overcome. Third, this was a cohort study and not an interventional nor outcomes study of all patients (except for spurting arterial bleeding – F1A and oozing bleeding – F1B - where 30 day rebleed rates are reported). Therefore the rebleed risk of other SRH (FIIA, IIB, IIC) and additional 30 day clinical outcomes should be confirmed in an interventional type RCT for all major SRH for PUB’s before DEP is recommended for other SRH without active bleeding. Specifically, such a RCT is warranted to evaluate whether use of DEP during urgent endoscopy for risk stratification and as a guide to definite endoscopic hemostasis of PUB’s with different SRH significantly improves 30 day clinical outcomes such as rebleeding rates, surgery, adverse events, or mortality.
We came to the following conclusions: (1) With spurting arterial bleeding (Forrest IA), visible vessels (Forrest IIA), or adherent clots (Forrest IIB) as major SRH for PUBs, arterial blood flow underlying the SRH in the ulcer base was detected in 87.4% of these patients which is similar to surgical pathology studies of arteries underlying visible vessels 4 decades ago. (2) Such major SRH were harder to treat endoscopically than PUBs without these SRH and had residual arterial blood flow detected in 27.4% of PUBs after standard, visually guided endoscopic treatment. (3) Oozing bleeding PUBs (Forrest IB), without other SRH such as a clot or VV, had significantly lower rates than PUBs with spurting arterial bleeding (FIA) in detection of underlying arterial blood flow by DEP at baseline, lower rates of residual blood flow detection after standard endoscopic treatment, and significantly lower 30 day PUB rebleeding rates after successful endoscopic hemostasis on standard medical therapy, probably indicating smaller underlying arteries. (4) Based upon these results, DEP is now recommended as a new endoscopic guide along with visual endoscopic SRH to potentially improve risk stratification, to guide endoscopic treatment for definitive hemostasis, and to improve the clinical outcomes for high-risk patients with PUBs.
Acknowledgments
This study was partially supported by NIH CURE DDRC Grant 41301 (Human Studies Core), a VA Clinical Merit Review Grant (to Dr. Jensen, PI), an Investigator Initiated Research Grant from AstraZeneca USA, and an equipment grant from Vascular Technology Inc (VTI).
Disclosures: Dr. Jensen is a consultant for VTI and received an equipment grant for endoscopic Doppler control units to partially support this study. Dr. Jensen previously was a consultant and also received an investigator initiated research grant from AstraZeneca which partially supported a preliminary study.
ACRONYMS USED
- CURE:DDRC
CURE Digestive Diseases Research Center
- DEP
Doppler endoscopic probe
- EGD
Esophogogastric duodenoscopy = Panendoscopy
- F
Forrest (classification)
- PPI
Proton pump inhibitor
- PUB
Peptic ulcer bleeding
- SRH
Stigmata of recent hemorrhage
- RBC’s
Red blood cells
- RCT
Randomized Controlled Trial
- UGIH
Upper Gastrointestinal Hemorrhage
- VTI
Vascular Technology Incorporated
- VV
Visible vessel
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No other co-authors have disclosures.
Authors Contribution
Dr. Jensen designed the study, secured the funding, and was the principal investigator. He enrolled study patients, supervised study personnel, analyzed the data, wrote and revised the manuscript. All co-authors were co-investigators, enrolled patients into the study, helped supervise research staff, and reviewed the compiled data. All authors edited drafts of the manuscript and approved the final manuscript including tables and figures which is being submitted to GIE.
REFERENCES
- 1.Barkun A, Bardou M, Kuipers EJ, Sung J, Hunt R, Martel M, Sinclair P for the International Consensus Upper Gastrointestinal Bleeding Conference Group. International consensus recommendations on the management of patients with non-variceal upper gastrointestinal bleeding (ICON-UGIB) Ann Intern Med. 2010;152:101–113. doi: 10.7326/0003-4819-152-2-201001190-00009. [DOI] [PubMed] [Google Scholar]
- 2.Laine L, Jensen DM. Management of patients with ulcer bleeding. Am J Gastroenterol. 2012;107:345–360. doi: 10.1038/ajg.2011.480. [DOI] [PubMed] [Google Scholar]
- 3.Gralnek IM, Barkun AN, Bardou M. Management of acute bleeding from a peptic ulcer. N Engl J Med. 2008;359:928–937. doi: 10.1056/NEJMra0706113. [DOI] [PubMed] [Google Scholar]
- 4.Savides TS, Jensen DM. GI Bleeding. In: Feldman M, Friedman LS, Brandt LJ, Sleisenger, Fordtran’s, editors. Gastrointestinal and Liver Disease. Pathophysiology/Diagnosis/Management. Eighth Edition. Philadelphia: Saunders Elsevier; 2010. pp. 285–322. [Google Scholar]
- 5.Jensen DM, Machicado GA. Endoscopic hemostasis of ulcer hemorrhage with injection, thermal, or combination methods. Techniques in Gastrointestinal Endoscopy. 2005;7:124–131. [Google Scholar]
- 6.Forrest JA, Finlayson ND, Shearman DJ. Endoscopy in gastrointestinal bleeding. Lancet. 1974;2:394–397. doi: 10.1016/s0140-6736(74)91770-x. [DOI] [PubMed] [Google Scholar]
- 7.Sung JJ, Chan FK, Chen M, Ching JY, Ho KY, Kachintorn U, et al. Asia-Pacific Working Group consensus on non-variceal upper gastrointestinal bleeding. Gut. 2011;60:1170–1177. doi: 10.1136/gut.2010.230292. [DOI] [PubMed] [Google Scholar]
- 8.Laine L, Peterson WL. Bleeding peptic ulcer. N Engl J Med. 1994;331:717–727. doi: 10.1056/NEJM199409153311107. [DOI] [PubMed] [Google Scholar]
- 9.Johnston JH, Jensen DM, Auth D. Experimental comparison of endoscopic Yttrium-aluminum- Garnet laser, electrosurgery, and heater probe for canine gut arterial coagulation: The importance of vessel compression and avoidance of tissue erosion. Gastroenterology. 1987;92:1101–1108. doi: 10.1016/s0016-5085(87)91065-1. [DOI] [PubMed] [Google Scholar]
- 10.Jensen DM. Heat probe for hemostasis of bleeding peptic ulcers: Techniques and results of randomized controlled trials. Gastrointest Endosc. 1990 Supplement to 36;S42-S49. [PubMed] [Google Scholar]
- 11.Chung SCS, Leung JWC, Stelle RJC, et al. Endoscopic injection of adrenaline for actively bleeding ulcers: a randomized trail. Br Med J. 1988;296:1631–1633. doi: 10.1136/bmj.296.6637.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jensen DM. Where next with endoscopic ulcer hemostasis? Am J Gastroenterology. 2002;97:2161–2165. doi: 10.1111/j.1572-0241.2002.05966.x. [DOI] [PubMed] [Google Scholar]
- 13.Storey DW, Bown SG, Swain CP, Salmon PR, Kirkham JS, Northfield TC. Endoscopic prediction of recurrent bleeding in peptic ulcers. N Engl J Med. 1981:305, 915–916. doi: 10.1056/NEJM198110153051603. [DOI] [PubMed] [Google Scholar]
- 14.Swain CP, Storey DW, Bown SG, et al. Nature of bleeding vessel in recurrently bleeding gastric ulcers. Gastroenterology. 1986;90:595–608. doi: 10.1016/0016-5085(86)91113-3. [DOI] [PubMed] [Google Scholar]
- 15.Johnston JH. The sentinel clot and invisible vessel: pathologic anatomy of bleeding peptic ulcer. Gastrointest Endosc. 1984;30:313–315. doi: 10.1016/s0016-5107(84)72431-x. [DOI] [PubMed] [Google Scholar]
- 16.Swain P. Perception and interpretation: The problem of the visible vessel. Endoscopy. 1998;30:570–574. doi: 10.1055/s-2007-1001346. [DOI] [PubMed] [Google Scholar]
- 17.Chen JJ, Changchien CS, Lin CC, Chang WC. The visible vessel on the bleeding gastric ulcer: An endoscopicpathological study. Endoscopy. 1997;29:821–826. doi: 10.1055/s-2007-1004315. [DOI] [PubMed] [Google Scholar]
- 18.Wong RC. Endoscopic Doppler US probe for acute peptic ulcer hemorrhage. Gastrointest Endosc. 2004;60:804–812. doi: 10.1016/s0016-5107(04)02046-2. [DOI] [PubMed] [Google Scholar]
- 19.Lau JY, Sung JJ, Chan AC, et al. Stigmata of hemorrhage in bleeding peptic ulcers: an interobserver agreement study among international experts. Gastrointest Endosc. 1997;46:33–36. doi: 10.1016/s0016-5107(97)70206-2. [DOI] [PubMed] [Google Scholar]
- 20.Laine L, Friedman M, Cohen H. Lack of uniformity in evaluation of endoscopic prognostic features of bleeding ulcers. Gastrointest Endosc. 1994;40:411–417. doi: 10.1016/s0016-5107(94)70202-0. [DOI] [PubMed] [Google Scholar]
- 21.Jensen DM, Kovacs TOG, Jutabha R, Machicado GA, Gralnek IM, Savides TJ, Smith J, Jensen ME, Alofaituli G, Gornbein J. Randomized, Controlled trial of Medical Therapy Compared to Endoscopic Therapy for Prevention of Recurrent Ulcer Hemorrhage in Patients with Non-bleeding Adherent Clots. Gastroenterol. 2002;123:407–413. doi: 10.1053/gast.2002.34782. [DOI] [PubMed] [Google Scholar]
- 22.Sung JJ, Barkun A, Kuipers EJ, Mössner J, Jensen DM, Stuart R, Lau JY, Ahlbom H, Kilhamn J, Lind T the Peptic Ulcer Bleed Study Group. Intravenous esomeprazole for prevention of recurrent peptic ulcer bleeding: A randomized trial. Ann Intern Med. 2009;50:455–464. doi: 10.7326/0003-4819-150-7-200904070-00105. [DOI] [PubMed] [Google Scholar]
- 23.Sung JJ, Mössner J, Barkun A, Kuipers EJ, Lau J, Jensen D, Stuart R, Junghard O, Olsson G on behalf of the PUB Study Group. Intravenous esomeprazole for prevention of peptic ulcer re-bleeding: Rationale/design of the peptic ulcer bleed study. Aliment Pharmacol Ther. 2008;27:666–677. doi: 10.1111/j.1365-2036.2008.03631.x. [DOI] [PubMed] [Google Scholar]
- 24.Swain CP, Bown SG, Storey DW, et al. Controlled trial of argon laser photocoagulation in bleeding peptic ulcers. Lancet. 1981:1313–1316. doi: 10.1016/s0140-6736(81)91340-4. [DOI] [PubMed] [Google Scholar]
- 25.Swain CP, Kirkham JS, Salmon PR, et al. Controlled trial of Nd-YAG laser photocoagulation in bleeding peptic ulcers. Lancet. 1986;1:1113–1117. doi: 10.1016/s0140-6736(86)91835-0. [DOI] [PubMed] [Google Scholar]
- 26.Chung SCS, Lau JY, Sung JJ, et al. Randomized comparison between adrenaline injection alone and adrenaline injection plus heat probe treatment for actively bleeding ulcers. BMJ. 1997;314:1307–1311. doi: 10.1136/bmj.314.7090.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sung JJ, Tsoi KK, Lai LH, Wu JC, Lau JY. Endoscopic clipping versus injection and thermo-coagulation in the treatment of non-variceal upper gastrointestinal bleeding: a meta-analysis. Gut. 2007;56:1364–1373. doi: 10.1136/gut.2007.123976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cipoletta L, Bianco MA, Rotondado G, et al. Prospective comparison of argon plasma coagulator and heater probe in the endoscopic treatment of major peptic ulcer bleeding. Gastrointest Endosc. 1998;48:191–195. doi: 10.1016/s0016-5107(98)70163-4. [DOI] [PubMed] [Google Scholar]
- 29.Lin HJ, Tseng GY, Perng CL, Lee FY, Chang FY, Lee SD. Comparison of adrenaline injection and bipolar electrocoagulation for the arrest of peptic ulcer bleeding. Gut. 1999;44:715–719. doi: 10.1136/gut.44.5.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barkun A, Herba K, Adam V, Kennedy W, Fallone CA, Bardou M. High-dose intravenous proton pump inhibition following endoscopic therapy in the acute management of patients with bleeding peptic ulcers in the USA and Canada: a cost-effectiveness analysis. Aliment Pharmacol Ther. 2004;19:591–600. doi: 10.1046/j.1365-2036.2004.01808.x. [DOI] [PubMed] [Google Scholar]
- 31.Jensen DM, Ahlbom H, Eklund S, Stuart R, Barkun AN, Kuipers EJ, Mössner J, Lau JY, Sung JJ, Lind T, Kilhamn J. Rebleeding risk for oozing peptic ulcer bleeding (PUB) in a large international study- A reassessment based upon a multivariate analysis. Gastrointest Endosc. 2010;71:356. AB117. [Google Scholar]
- 32.Kohler B, Riemann JF. Endoscopic Doppler Ultrasound. Gastrointest Endosc. 2004;60:493–495. doi: 10.1016/s0016-5107(04)01717-1. [DOI] [PubMed] [Google Scholar]
- 33.Jakobs R, Zoepf T, Schilling D, Siegel EG, Riemann JF. Endoscopic Doppler ultrasound after injection therapy for peptic ulcer hemorrhage. Hepatogastroenterology. 2004;51:1206–1209. [PubMed] [Google Scholar]
- 34.Kohler B, Maier M, Benz C, Riemann JR. Acute ulcer bleeding: A prospective randomized trial to compare Doppler and Forrest classifications in endoscopic diagnosis and therapy. Dig Dis Sci. 1997;42:1370–1374. doi: 10.1023/a:1018877602113. [DOI] [PubMed] [Google Scholar]
- 35.Van Leerdam ME, Breeberg EM, Rauws EAJ, et al. Acute upper GI bleeding: Did anything change? Am J Gastroenterol. 2003;98:1494–1499. doi: 10.1111/j.1572-0241.2003.07517.x. [DOI] [PubMed] [Google Scholar]
- 36.Jensen D, Ohning G, Singh B, Kovacs T, Jutabha R, Machicado G. For severe UGI hemorrhage, Doppler ultrasound probe is more accurate for risk stratification and helpful for complete endoscopic hemostasis than lesion stigmata alone. Gastrointest Endosc. 2008;67:264. AB81. [Google Scholar]
- 37.Friedman ML, Cass OW, Peine CJ, Onstad GR. The non-bleeding visible vessel versus the sentinel clot: natural history and risk of rebleeding. Gastrointest Endosc. 1993;39:359–366. doi: 10.1016/s0016-5107(93)70106-6. [DOI] [PubMed] [Google Scholar]