Abstract
Introduction
Unhealthy diets, often low in potassium, likely contribute to racial disparities in blood pressure. We tested the effectiveness of providing weekly dietary advice, assistance with selection of higher potassium grocery items, and a $30 per week food allowance on blood pressure and other outcomes in African American adults with hypertension.
Design
We conducted an 8-week RCT with two parallel arms between May 2012 and November 2013.
Setting/participants
We randomized 123 African Americans with controlled hypertension from an urban primary care clinic in Baltimore, Maryland and implemented the trial in partnership with a community supermarket and the Baltimore City Health Department. Mean (SD) age was 58.6 (9.5) years, 71% were female, blood pressure was 131.3 (14.7)/77.2 (10.5) mmHg, BMI was 34.5 (8.2) kg/m2, and 28% had diabetes.
Intervention
Participants randomized to the active intervention group (Dietary Approaches to Stop Hypertension [DASH]-Plus) received coach-directed dietary advice and assistance with weekly online ordering and purchasing of high-potassium foods ($30/week) delivered by a community supermarket to a neighborhood library. Participants in the control group received a printed DASH diet brochure along with debit account of equivalent value to that of the DASH-Plus group.
Main outcome measures
The primary outcome was blood pressure change. Analyses were conducted in January to October 2014.
Results
Compared with the control group, the DASH-Plus group increased self-reported consumption of fruits and vegetables (mean=1.4, 95% CI=0.7, 2.1 servings/day), estimated intake of potassium (mean=0.4, 95% CI=0.1, 0.7 grams/day), and urine potassium excretion (mean=19%, 95% CI=1%, 38%). There was no significant effect on blood pressure.
Conclusions
A program providing dietary advice, assistance with grocery ordering, and $30/week of high-potassium foods in African American patients with controlled hypertension in a community-based clinic did not reduce BP. However, the intervention increased consumption of fruits, vegetables, and urinary excretion of potassium.
Introduction
Elevated blood pressure (BP) is an extraordinarily common and important risk factor for cardiovascular disease and stroke, particularly among African Americans.1,2 Recommendations to adopt the Dietary Approaches to Stop Hypertension (DASH) diet are recognized as integral to medical management of hypertension; yet, suboptimal diets persist among patients with hypertension and likely contribute to racial disparities in BP control.3 African American race and low income are strongly associated with unhealthy food intake.4,5 There is a markedly lower availability of components of DASH diet foods such as fruits, vegetables, low-fat dairy, and nuts in predominantly African American and lower-income neighborhoods compared with Caucasian and higher-income neighborhoods.5,6
Unhealthy dietary patterns may contribute to risk of hypertension, in part because deficiencies in potassium, a micronutrient with independent BP-lowering effects, and from excess sodium intake, which elevates BP. Even moderate potassium deficiencies are associated with increased BP and salt sensitivity.7 Furthermore, use of thiazide-based antihypertensive therapy, which is otherwise highly effective as an antihypertensive agent, worsens potassium deficiencies via increased urinary potassium excretion.8 Inadequate intake and accelerated loss of potassium not only affect BP control, but have also been directly linked to development of glucose intolerance.9
Strategies, such as tailored dietary advice, to improve adherence to dietary recommendations and reverse micronutrient deficiencies in African American adults with hypertension are needed.4,10 We designed the trial to test the hypothesis that in African American adults with controlled hypertension on stable doses of antihypertensive medications, an intervention delivered by a health coach, with weekly provision of $30 worth of high potassium foods consistent with key elements of the DASH diet, will lower BP; increase consumption of fruits, vegetables, nuts, and beans; increase urine potassium excretion; and improve cardiovascular disease risk factors, such as fasting glucose and low-density lipoprotein (LDL) cholesterol.
Methods
Study Design
We conducted a single-center RCT with two parallel arms as one project in the Johns Hopkins Center to Eliminate Cardiovascular Health Disparities.11 Our center used the principles of community-based participatory research to build strong ties between the researchers, healthcare provider networks, community members, and policymakers. The Center’s Data and Safety Monitoring Board and Community Advisory Board participated in finalizing the design and in oversight throughout implementation.12 Study visits occurred at an urban community-based clinic within Johns Hopkins Community Physicians in Baltimore, Maryland, serving a predominately low-income and African American patient population. Participants were randomized to one of the two intervention arms: (1) those receiving minimal intervention (control); and (2) those receiving coach-directed, tailored DASH diet advice (DASH-Plus). It was not possible to mask the participant or interventionist to the randomization assignment, but study personnel who performed the outcome follow-up assessments were masked. The total intervention period was 8 weeks, and study visits occurred between May 2012 and November 2013. The study was approved by the Johns Hopkins Medicine IRB. All participants provided written informed consent. The study was registered at clinicaltrials.gov (NCT01689844).
Study Sample
Inclusion criteria were an electronic medical record diagnosis of hypertension [ICD-9 code 401.1], age ≥21 years, self-reported African American race, average systolic BP (SBP) of 120–140 mmHg or diastolic BP (DBP) of 80–90 mmHg at the two most recent clinic visits, and stable doses of antihypertensive medications for a minimum of 2 months prior to randomization. Major exclusion criteria were self-report of a cardiovascular event within 6 months, a chronic disease that might interfere with trial participation (e.g., chronic kidney disease defined as an estimated glomerular filtration rate <60 cc/minute), unwillingness or inability to adopt a DASH-like diet, consumption of >14 alcoholic drinks per week, poorly controlled diabetes (hemoglobin A1c >9%), or use of insulin. Those using potassium supplements could enroll if they were willing to stop supplements 1 month prior to randomization and refrain from the supplements during the study.
We mailed brochures inviting potentially eligible participants identified through the clinic electronic medical record database to participate in the study. We recruited exclusively from those who responded with an interest in participating. Each invitation included a letter signed by the person’s physician endorsing participation in the trial.
After informed consent and screening visits, eligible participants were randomly assigned in a 1:1 ratio. The study biostatistician generated the assignments using a pseudorandom number generator program with permuted block sizes of two, four, and six. Assignments were placed in consecutively numbered manila envelopes. Two participants lived together and a single randomization allocated both to the same treatment group.
Interventions
Control Group
Those assigned to the control group received printed materials on improving BP control by adoption of the DASH diet at their first visit with the study coordinator. This visit lasted 15 minutes and the coordinator gave no advice during or after this initial visit. Participants in this arm received a total of $240 in a debit account to purchase foods at the same community supermarket. The account had $90 credit for the first 3 weeks and $150 for the last 5 weeks. This amount is the same dollar amount that was available to the DASH-Plus group over the 8-week study period. The supermarket provided us with an electronic, itemized receipt of all purchases made on each individual in the control group.
Dietary Approaches to Stop Hypertension–Plus Group
Participants in the DASH-Plus group received a single, 1-hour, in-person, one-on-one session with the study coach who delivered a dietitian-developed module on adoption of the DASH diet. This was followed by weekly 15-minute calls with the participant during the 8-week study period. Key aspects of the intervention were adapted from those used in lifestyle intervention trials12,13 and also included a $30/week allowance for the purchase of high-potassium foods. The coach used literacy-sensitive modules about hypertension and barriers to self-management and individual food preferences, including the DASH diet, to guide the discussion. The participants chose from a list of foods that were available for online purchase and delivery each week from our partnering community supermarket (Appendix Table 1). The selections included fruits (11 fresh, six canned, four dried, two frozen, and three juices), vegetables (13 fresh, six canned (no added salt), or six frozen), nuts (four), or dried beans (six). We pre-selected these specific items because of the high per-serving content of potassium. Purchases targeted a goal of 17,000 mg/week of potassium in order to increase dietary intake of potassium to 4.7 grams/day (DASH diet target) based on an estimated daily baseline intake of 2.4 grams/day in this population.5 The coach provided education and recommendations specific to the participant’s needs, preferences, and local context, and assisted with online or phone ordering from the supermarket.
The community supermarket provided free packaging of food orders and delivery by a supermarket employee to a public library in close proximity to the clinic for the participants to pick-up. This process built upon an ongoing partnership between the supermarket and the public library (for food pick-up) that had been established by the Baltimore City Health Department “Virtual Supermarket” Program. Weekly food pick-up by participants occurred on the same day of the week between the hours of 4:00PM–5:00PM over the course of the study.
Data Collection Visits
Baseline data collection occurred over two screening visits and a randomization visit. The average BP from the two screening visits determined BP eligibility prior to randomization. Participant follow-up visits occurred at 3 and 8 weeks following randomization and were conducted by study personnel blinded to intervention status. The total intervention period was 8 weeks. All visits (screening, randomization, and follow-ups) occurred at the primary care clinic at times other than the participants’ scheduled visits with their physicians.
Measures
Blood pressure was measured at the two screening visits, the randomization visit, and at both follow-up visits using an OMRON 907-XL automated BP machine programmed with a 5-minute delay followed by three measurements separated by 30 seconds. Trained certified staff performed and recorded all three measures and the average at each visit. The average BP of the Screening Visits 1 and 2 established baseline BP, and the average BPs measured at Weeks 3 and 8 determined intervention effects.
Following an overnight fast, a fasting blood specimen was collected at baseline and at the 8-week follow-up visit. Assays included fasting glucose, total cholesterol, high-density lipoprotein (HDL), and triglycerides. LDL was estimated by the Friedewald equation (LDL = total cholesterol − HDL − triglycerides/5).14
Fasting, second-void urine collections were also collected at the baseline and 8-week follow-up visits and urinary potassium and creatinine excretion were assayed in order to estimate group compliance with dietary aspects of the intervention (i.e., potassium excretion for fruit and vegetable intake). To ease participants’ burden and to alleviate some of the expected difficulties that might occur in trying to collect complete 24-hour urines in the primary care setting, we chose to use spot fasting urine. Although spot urines may be used to estimate 24-hour potassium consumption by the Kawasaki equation,15 accurately quantifying 24-hour urine excretion is not possible. Hence, our primary comparisons report the percentage change from baseline, pre–post intervention effects using creatinine-normalized measures as an indication of adherence to the dietary intervention.
The Block Fruit/Vegetable/Fiber Screener and Dietary Fat Screener are self-reported assessments that provide validated16 measures of fruit, vegetable, fiber, and fat intake. Both the screeners were administered at Screening Visit 1, the randomization visit, and the 8-week follow-up. Respondents were asked to indicate how often they ate a variety of specific foods and food groups in the prior 8 weeks.
The screener estimates average 24-hour intake of potassium, magnesium and vitamin C, and number of servings of fruits and vegetables per day. The fat screener estimates daily cholesterol and percent fat intake.
Weight was measured at Screening Visit 1 and the 8-week follow-up visit. Height was measured at Screening Visit 1 along with self-reported characteristics including age, gender, tobacco use, health insurance status, post-menopausal status, income and employment, education level, and current health status (presence of diabetes and cardiovascular disease) through demographic and medical history questionnaires. Self-reported medication use was collected at each visit. Of primary interest were medications used to treat hypertension, diabetes, and hypercholesterolemia.
Participants were asked to remain on stable doses of their current BP-lowering medications for the duration of the study if possible. Medication use was reviewed by questionnaire at each visit to assess for changes in BP medications and for use of new over-the-counter or prescription medications that might affect BP.
Measures of Intervention Adherence
In order to track purchases for the control group, we collected individual electronic account receipts supplied by the supermarket. We categorized the control group food purchases into those that were consistent with the DASH dietary pattern versus those that were processed foods. For the DASH-Plus group, the coach tracked weekly purchases and we monitored self-reported consumption of foods provided by the intervention. Each week, participants in the DASH-Plus group were asked whether they consumed the foods provided the prior week. An adherence score was developed based on the potassium content of the foods multiplied by the self-reported consumption score. The consumption scores were assigned as 0%, 25%, 50%, 75%, or 100% corresponding to none, less than half, half, more than half, or all of the foods provided the prior week.
Statistical Analysis
The analysis was conducted under the intent-to-treat principle for all outcomes. Demographic and clinical characteristics of the participants by randomization group were summarized using mean (SD), median (interquartile range [IQR]), or n (%). Distributions of data were checked and transformed where appropriate. We calculated within-participant change in the outcome measures between baseline and follow-up. For the urine markers, we calculated the within-participant percentage change. Differences in mean change of outcomes between the DASH-Plus group and the control group were tested using two-sample t-tests or Wilcoxon rank-sum tests. Ninety-five percent CIs of the estimated mean between-group differences were constructed for each outcome.
The trial had sufficient resources to enroll 120 participants. For power calculations on SBP, we used an SD of 6.7 mmHg, which was the within-group SD of change in the DASH trial,17 and estimated 90% power to detect a treatment effect of ≥2.5 mmHg at a two-sided α=0.05. Secondary analyses were evaluated at the nominal α=0.05 level and fixed sample size of N=120. Based on these and the SD of change of 9.9 mg/dL observed in the DASH study, the minimal detectable difference for glucose was 5.6 mg/dL.18
Statistical analyses were performed between March 2014 and April 2015 using SAS, version 9.3 and STATA, version 11.1. Missing data were assumed to be missing at random and no imputation was performed. All reported p-values are two-sided with significance set at p<0.05.
Results
Screening, Eligibility, Randomization
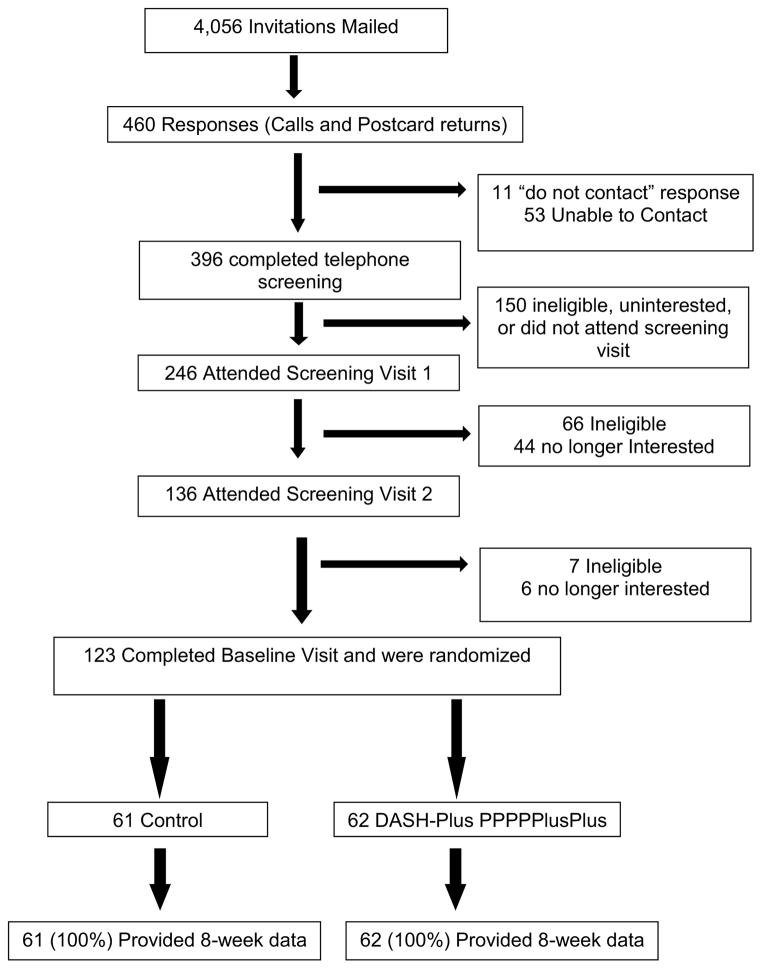
Invitations were mailed to 4,056 potential participants, based on BP eligibility determined through the clinic electronic medical record (Figure 1) and we received 460 responses (11.3% response rate). There were 246 in-person screening visits from which 123 participants were randomized. The most common reasons for exclusion at the time of the telephone prescreening was being uninterested in participation in the trial owing to trial obligations (n=150).
Figure 1.
Recruitment flow-chart of participants leading to randomization.
Participant Characteristics
Baseline characteristics are reported in Table 1. Mean (SD) age was 58.6 (9.5) years, 71% were women, 100% were African American, mean BMI was 34.5 (8.2) kg/m2, and 28% had self-reported diabetes. The mean number of antihypertensive medications was 1.7 (0.9) and 1.9 (0.9) in the control and DASH-plus groups, respectively. Income, educational attainment, marital status, and insurance status were similar between groups. Few participants self-reported a history of heart failure (n=3), angina (n=1), or coronary bypass or angioplasty (n=1).
Table 1.
Baseline Demographic and Clinical Characteristics by Randomized Group
| Characteristic | DASH-Plus | Control | p-value |
|---|---|---|---|
| Number of participants | 62 | 61 | |
| Sex: | 0.32 | ||
| Female | 41 (66) | 46 (75) | |
| Male | 21 (34) | 15 (25) | |
| Age, years, M (SD) | 58.8 (8.7) | 58.5 (10.4) | 0.85 |
| Marital Status: | 0.85 | ||
| Married or living with partner | 22 (35) | 23 (38) | |
| Single, divorced or widowed | 40 (65) | 38 (62) | |
| Education: | 0.40 | ||
| Less than high school degree | 11 (18) | 14 (23) | |
| High school degree or GED | 31 (50) | 35 (57) | |
| Some college | 18 (29) | 10 (16) | |
| Unknown | 2 (3) | 2 (3) | |
| Household Income: | 0.80 | ||
| Under $10,000 | 16 (26) | 11 (18) | |
| $10,000–29,999 | 21 (34) | 24 (39) | |
| $30,000–49,999 | 14 (23) | 12 (20) | |
| $50,000+ | 6 (10) | 7 (11) | |
| Don’t know or refused | 5 (8) | 7 (11) | |
| Health Insurance a | 56 (92) | 54 (92) | 0.99 |
| Diabetes | 21 (34) | 13 (21) | 0.16 |
| Antihypertensive Medications, M (SD) | 1.9 (0.9) | 1.7 (0.9) | 0.38 |
| History of stroke | 5 (8) | 2 (3) | 0.31 |
| History of heart attack | 2 (3) | 2 (3) | 0.60 |
| Weight, kg, M (SD) b | 97.8 (25.0) | 92.9 (21.6) | 0.25 |
| BMI, kg/m2, M (SD) b | 34.9 (8.4) | 34.1 (8.0) | 0.59 |
Note: Values represent n (%) unless otherwise noted.
Missing data: 1 in DASH-Plus group, 2 in Control group.
Missing data: 1 in each randomization group.
Measures of Adherence and Process Variables
Baseline and change from baseline at 8 weeks in daily servings of fruits and vegetables, minerals, dietary fat, and cholesterol intake are reported in Table 2. Overall, the DASH-Plus group reported consuming a mean (SD) of 5.9 (1.8) servings of fruits and vegetables per day at 8 weeks, an increase of 3.4 (1.9) servings per day from baseline. There were significant increases in potassium, magnesium, vitamin C, and fiber in the DASH-Plus group compared with the control group, but no change in self-reported consumption of daily dietary fat or cholesterol.
Table 2.
Dietary Adherence Measures Between the DASH-Plus and Control Groups
| DASH-Plus | Control | |||||
|---|---|---|---|---|---|---|
| Dietary Intake | Baseline | Change from baseline | Baseline | Change from baseline | Difference (95% CI) in change | p-value |
| Servings of fruits and vegetables/day | 3.7 (1.9) | 2.3 (2.2) | 3.4 (1.9) | 0.9 (1.8) | 1.4 (0.7, 2.1) | <0.001 |
| Potassium, g/day | 2.84 (0.81) | 0.86 (0.84) | 2.67 (0.93) | 0.44 (0.83) | 0.42 (0.12, 0.71) | 0.007 |
| Magnesium, mg/day | 293 (86) | 87 (85) | 275 (96) | 45 (83) | 42 (12, 72) | 0.007 |
| Vitamin C, mg/day | 116 (44) | 49 (48) | 108 (51) | 26 (47) | 24 (7, 41) | 0.007 |
| Fiber, g/day | 11.9 (5.5) | 5.8 (5.6) | 10.8 (6.5) | 3.0 (5.5) | 2.8 (0.8, 4.8) | 0.007 |
| Dietary fat, % of calories | 32.6 (5.0) | −2.5 (6.7) | 33.6 (6.0) | −3.9 (6.7) | 1.0 (−0.7, 2.6) | 0.25 |
| Cholesterol, mg/day | 267 (70) | −23 (59) | 272 (82) | −35 (60) | 12 (−9, 34) | 0.25 |
| Urine Potassium mmol/g creatinine | 32.9 (26.1, 47.4) | 21.1 (−13.8,71.2) | 34.3 (29.6, 49.3) | −1.4 (−18.8,34.8) | 19.0 (0.8, 37.5) | 0.04 |
Note: Change was calculated as follow-up – baseline and difference was calculated as the change for DASH-Plus – change for control. P-values correspond to between-group differences using the two-sample t-test. Values presented are M (SD) unless otherwise noted. Boldface indicates statistical significance (p<0.05).
Electronic purchase receipts of the control group were tracked. Median (IQR) purchases were $234 ($211, $246). An analysis of purchases made by the control group revealed that 53% of the dollars were spent on foods that would be considered part of the DASH diet (e.g., fruits, vegetables, lean meats, and low-fat dairy), and 47% of the dollars were spent on non-DASH foods (e.g., processed foods or sweetened beverages). All foods purchased in the DASH-Plus group were consistent with the DASH diet plan.
Over the 8-week study period, participants in the DASH-Plus group spent median (IQR) of $232 ($209, $237) on study-recommend food. On average, most participants consumed >75% of foods provided each week. Weekly milligrams of potassium provided in purchased foods were estimated. Based on self-reported consumption of foods, weekly milligrams of consumed potassium from the supplemented foods were median (IQR) potassium 18,366 (17,055, 20,422) per week (target was 17,000 mg/week).
Change in Urine Biomarkers
Urinary potassium/creatinine ratio (mmol/grams creatinine), a biomarker of consumption of dietary potassium, increased by approximately 19% (p=0.04) in the DASH-Plus compared with the control group (Table 2).
Cardiovascular Disease Risk Factors
Mean SBP at baseline was 130.7 (11.2) and 132.0 (11.7) mmHg in the DASH-Plus and control group, respectively. There were modest reductions from baseline in SBP in both the DASH-Plus and control group (−1.1 [11.9] and −2.6 [11.1], respectively). There was no significant difference in change in BP between the DASH-Plus compared to the control group mean (95% CI): SBP, 1.5 (−2.6, 5.6 mmHg, p=0.48); DBP, 1.3 (−1.3, 3.9, p=0.33). There were non-significant differences in change between the DASH-Plus and control groups in serum cholesterol, triglycerides, HDL, LDL, and weight, but an unexpected and significant increase in fasting glucose of 10.9 mg/dL (p=0.002) (Table 3). In a subgroup analysis stratified by self-reported diabetes status, this effect in the DASH-Plus group compared with the control group on glucose was significant in those with diabetes (31.5 mg/dL, p=0.002), but not among those without diabetes at baseline (3.9 mg/dL, p=0.20) (Table 3).
Table 3.
Blood Pressure, Weight, Lipids, and Glucose Outcomes Between the DASH-Plus and Control Groups
| DASH-Plus | Control | |||||
|---|---|---|---|---|---|---|
| Dietary Intake | Baseline | Change from baseline | Baseline | Change from baseline | Difference (95% CI) in change | p-value |
| Systolic BP, mm Hg | 130.7 (11.2) | −1.1 (11.9) | 132.0 (11.7) | −2.6 (11.1) | 1.5 (−2.6, 5.6) | 0.48 |
| Diastolic BP, mm Hg | 77.0 (9.4) | 0.3 (7.5) | 77.4 (9.3) | −1.0 (7.2) | 1.3 (−1.3, 3.9) | 0.33 |
| Cholesterol, mg/dL a | 185.7 (39.8) | −2.2 (26.9) | 193.1 (58.1) | −3.4 (25.5) | 1.2 (−8.2, 10.7) | 0.80 |
| Triglycerides, mg/dL a | 117.7 (56.2) | 13.4 (92.5) | 104.4 (62.2) | −0.0 (32.9) | 13.4 (−11.7, 38.5) | 0.29 |
| LDL, mg/dL a,b | 109.8 (34.6) | −4.2 (29.1) | 113.9 (53.7) | −3.1 (24.6) | −1.1 (−10.8, 8.6) | 0.82 |
| HDL, mg/dL a | 52.3 (11.7) | −0.6 (7.7) | 58.3 (19.8) | −0.3 (7.7) | −0.3 (−3.1, 2.5) | 0.84 |
| Weight, kgs a | 97.8 (25.0) | 0.3 (2.8) | 92.9 (21.6) | 0.0 (2.2) | 0.3 (−0.6, 1.2) | 0.51 |
| Glucose, mg/dL: a | ||||||
| All participants | 104.2 (26.4) | 7.6 (18.0) | 104.0 (44.9) | −3.3 (19.8) | 10.9 (4.1, 17.7) | 0.002 |
| With diabetes | 124.0 (35.1) | 14.9 (21.1) | 136.5 (77.8) | −16.6 (33.8) | 31.5 (12.4, 50.6) | 0.002 |
| Without diabetes | 94.1 (11.6) | 3.9 (15.1) | 95.5 (26.4) | 0.0 (12.0) | 3.9 (−2.1, 9.8) | 0.20 |
Note: Change was calculated as follow-up – baseline and difference was calculated as the change for DASH-Plus – change for control. P-values are from two-sample t-test. Values represent M (SD) unless otherwise noted. Boldface indicates statistical significance (p<0.05).
BP, blood pressure; LDL, low-density lipoprotein; HDL, high-density lipoprotein
Missing data: 1 in each randomization group.
One additional LDL in the DASH-Plus group could not be estimated because of high triglycerides.
Although we recommended that participants not change their BP medications during the trial, change in BP medication, often initiated by participants, occurred in eight of 61 (13%) and nine of 62 (15%) participants in the control and DASH-Plus groups, respectively. In addition, 16 of 61 (26%) in the control and eight of 62 (13%) in the DASH-Plus group took other medications that may affect BP (non-steroidal anti-inflammatory drugs, steroids, opiates) or skipped their morning dose of antihypertensive medications on the day of the final BP measurements. Hence, the observed SD of change in the control group was 11.5 mmHg, which greatly exceeded the SD of 6.7 mmHg we used in our power calculations.
Discussion
This RCT of a dietary intervention for urban African American adults with controlled hypertension compared a program of tailored dietary advice, assistance with selecting and ordering food purchases, and weekly delivery of $30 of high-potassium foods including fruits, vegetables, nuts, and beans (DASH-Plus) to a minimum intervention of dietary advice in a printed brochure and self-directed food purchases at the supermarket’s physical location (control). The DASH-Plus intervention resulted in: increased self-reported consumption of fruits and vegetables; increased estimated intake of potassium, magnesium and vitamin C; and increased urine potassium excretion. However, there was no significant effect on SBP or DBP.
The DASH diet contains a mean of nine servings of fruits and vegetables and 4.7 grams of potassium per day.18 Our goal was to increase potassium intake through consumption of fruits, vegetables, nuts, and beans selected for high potassium content in order to increase potassium over baseline by 2.4 grams per day. Participation in this intervention increased estimated potassium intake to 3.7 (0.8) grams per day, a significant increase, yet still below recommended levels.19 Participants in the control group had non-significant improvements in fruits and vegetables, total cholesterol, LDL, and SBP, perhaps reflecting the observed improvement in dietary purchases. This change likely reflects that a participant’s motivation to make dietary changes, and ability to make changes in diet in spite of being randomized to the control group. The observed significant increase in intake from diet of magnesium and vitamin C intake would expectantly have modest and potentially independent BP-lowering effects.20,21
Limitations
There are several limitations of this trial, which may, in part, contribute to the non-significant BP effects. First, the study participants had well-controlled BP at baseline. A low baseline BP limits the ability to detect effects of an intervention on BP. Secondly, participants were on an average of 1.8 BP medications (range, 0–5), and although we had hoped to measure intervention effects on stable doses of medications, a large percentage of participants had medication changes. In addition, even in those who remained on stable doses of antihypertensive medications, at the 8-week visit, many took medications that may affect BP (e.g., non-steroidal anti-inflammatory medications or “cold medications”) or skipped their BP medication dose. Though the randomized trial approach should assure that these non-adherence measures would be equally distributed between the two groups, these patterns may explain the higher-than-expected variances of BP measurements, which would cause a reduction in the power of the study to see differences (expected SD used in power calculations was 6.7 mmHg, whereas observed SD was 11.5 mmHg). In future trials, to increase statistical power, the variance of the difference in BP could be reduced by increasing the number of daily BP measurements or using 24-hour ambulatory BP. Finally, only 11.3% of potentially eligible participants responded and expressed interest in screening for the trial. It is possible that the responders were healthier than the non-responders given the low prevalence of cardiovascular disease in those randomized.
An unexpected finding was the adverse effects of the dietary intervention on fasting glucose. Contrary to our hypothesis, the DASH-Plus intervention significantly increased fasting glucose, with the greatest effects noted in the subgroup analysis stratified by diabetes status. Although these results may be spurious as a result of a subgroup analysis that includes an imbalance at baseline in the number of participants with diabetes in the control group (n=13) versus in the DASH-Plus group (n=21), these results may also reflect a less-ideal dietary intervention for those with diabetes. The foods selected and purchased by the DASH-Plus participants included many foods that were high in potassium yet were also high in natural sugars (e.g., fruits and fruit juices) or high in starches (e.g., yams and potatoes). A future improvement to the intervention could be to provide a selection of foods that are high in potassium yet appropriate for those with or at risk for diabetes (e.g., food choices reduced in carbohydrates, calories, or carbohydrates with a low glycemic index).22,23 In a trial subsequent to publication of the DASH diet, replacement of 10% of carbohydrates with unsaturated fat, similar to a “Mediterranean” diet, resulted in increased insulin sensitivity.24 Hence, modification of a dietary approach is appropriate for those with diabetes (e.g., high potassium content yet reduced in carbohydrate amount).
Several important strengths of this trial should be noted. First, the clinic where the study took place is a community-based clinic where the majority of patients seen live in the surrounding neighborhoods. This provided an important link for recruitment and successful implementation of intervention. The advantage of this approach is that participants were familiar with the clinic location and sensed a partnership among the intervention, the clinic, and provider. Also, with the study coordinator utilizing a collaborative approach to provide disease-specific (hypertension) dietary instructions may enhance motivation for participation and adherence. Furthermore, by partnering with the community supermarket and the Baltimore City Health Department’s “Virtual Supermarket” program, we took advantage of established community-based programs that provided infrastructure and an opportunity for sustainability once the trial was completed. Finally, the randomized trial design and 100% follow-up on the primary endpoint strengthen the internal validity of our findings.
It is important to note several features of our intervention were uniquely different from the “Virtual Supermarket” program. Instead of participants being able to order all available foods at the supermarket, we limited purchases to a selection of 60 high-potassium foods and a weekly budget allotment of $30. We also provided individuals with nutritional advice at the time of placing their orders. For example, if study participants’ preferred foods did not contain adequate micronutrient content, our coach suggested alternatives to preferred foods and if study participants expressed an interest, different methods of food preparation were shared. The $30 per week amount was chosen in order to demonstrate that daily consumption of high potassium foods is possible with a limited amount of money. The weekly amount replicates the average weekly allocations per individual for those who participate in the federal “food stamp” program or Supplemental Nutrition Assistance Program (SNAP). This approach, which adheres to a simple strategy of enhanced education, restriction of purchases of nutrient-poor foods, and increasing access to fresh foods, enhances the generalizability of the program to other low-income populations.10,25,26 The partnership with a community supermarket offers an innovative model that can be replicated in other communities with poor access to nutritious and affordable foods and supermarkets, that is, “food deserts.”27,28
From the viewpoint of determining the feasibly and acceptability of an inexpensive community-based dietary intervention, this trial was successful. Our community-based participatory research approach built strong ties among participants, a community-based healthcare provider network, the Baltimore City Health Department, a community advisory board, and university-based researchers. Our study was a success based on the high participation and retention rates. Future research building on this approach should include modifying the intervention to include DASH-Plus food choices for patients with diabetes and testing the intervention among those with uncontrolled BP or newly diagnosed patients who wish to avoid starting antihypertensive medication, to determine whether effects on BP can be demonstrated in these populations.
Conclusions
In this dietary intervention RCT, in African American patients with hypertension recruited from a community clinic, we were not able to demonstrate a significant effect on BP, likely as a result of an insufficient number of BP measurements used for outcome assessment and the potential BP medication changes post-randomization. However, the intervention increased consumption of fruits and vegetables, potassium, vitamin C, and magnesium. Our results in the context of prior trials, and with the observed benefits in dietary consumption patterns on BP, provide a strong rationale for larger trials that are adequately powered and designed to detect BP effects.
Supplementary Material
Acknowledgments
This work was supported by a grant from the National Heart, Lung, and Blood Institute (P50HL0105187). The National Heart, Lung, and Blood Institute did not have a role in study design; collection, analysis or interpretaion of the data; writing the manuscript; or the decision to submit the manuscript for publication.
Footnotes
This trial was registered at clinicaltrials.gov as NCT01689844.
No financial disclosures were reported by the authors of this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cutler JA, Sorlie PD, Wolz M, Thom T, Fields LE, Roccella EJ. Trends in hypertension prevalence, awareness, treatment, and control rates in united states adults between 1988–1994 and 1999–2004. Hypertension. 2008;52(5):818–827. doi: 10.1161/HYPERTENSIONAHA.108.113357. http://dx.doi.org/10.1161/HYPERTENSIONAHA.108.113357. [DOI] [PubMed] [Google Scholar]
- 2.Gutierrez J, Williams OA. A decade of racial and ethnic stroke disparities in the United States. Neurology. 2014;82(12):1080–1082. doi: 10.1212/WNL.0000000000000237. http://dx.doi.org/10.1212/WNL.0000000000000237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thorpe RJ, Jr, Bowie JV, Smolen JR, et al. Racial disparities in hypertension awareness and management: Are there differences among African Americans and whites living under similar social conditions? Ethn Dis. 2014;24(3):269–275. [PMC free article] [PubMed] [Google Scholar]
- 4.Ammerman AS, Lindquist CH, Lohr KN, Hersey J. The efficacy of behavioral interventions to modify dietary fat and fruit and vegetable intake: A review of the evidence. Prev Med. 2002;35(1):25–41. doi: 10.1006/pmed.2002.1028. http://dx.doi.org/10.1006/pmed.2002.1028. [DOI] [PubMed] [Google Scholar]
- 5.Gary TL, Baptiste-Roberts K, Gregg EW, et al. Fruit, vegetable and fat intake in a population-based sample of African Americans. J Natl Med Assoc. 2004;96(12):1599–1605. [PMC free article] [PubMed] [Google Scholar]
- 6.Millstein RA, Yeh HC, Brancati FL, Batts-Turner M, Gary TL. Food availability, neighborhood socioeconomic status, and dietary patterns among blacks with type 2 diabetes mellitus. Medscape J Med. 2009;11(1):15. [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao Q, Gu D, Chen J, et al. Blood pressure responses to dietary sodium and potassium interventions and the cold pressor test: The GenSalt replication study in rural north China. Am J Hypertens. 2014;27(1):72–80. doi: 10.1093/ajh/hpt163. http://dx.doi.org/10.1093/ajh/hpt163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shafi T, Appel LJ, Miller ER, 3rd, Klag MJ, Parekh RS. Changes in serum potassium mediate thiazide-induced diabetes. Hypertension. 2008;52(6):1022–1029. doi: 10.1161/HYPERTENSIONAHA.108.119438. http://dx.doi.org/10.1161/HYPERTENSIONAHA.108.119438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chatterjee R, Yeh HC, Shafi T, et al. Serum potassium and the racial disparity in diabetes risk: The atherosclerosis risk in communities (ARIC) study. Am J Clin Nutr. 2011;93(5):1087–1091. doi: 10.3945/ajcn.110.007286. http://dx.doi.org/10.3945/ajcn.110.007286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leung CW, Hoffnagle EE, Lindsay AC, et al. A qualitative study of diverse experts’ views about barriers and strategies to improve the diets and health of supplemental nutrition assistance program (SNAP) beneficiaries. J Acad Nutr Diet. 2013;113(1):70–76. doi: 10.1016/j.jand.2012.09.018. http://dx.doi.org/10.1016/j.jand.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooper LA, Boulware LE, Miller ER, 3rd, et al. Creating a transdisciplinary research center to reduce cardiovascular health disparities in Baltimore, Maryland: Lessons learned. Am J Public Health. 2013;103(11):e26–38. doi: 10.2105/AJPH.2013.301297. http://dx.doi.org/10.2105/AJPH.2013.301297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper LA, Marsteller JA, Noronha GJ, et al. A multi-level system quality improvement intervention to reduce racial disparities in hypertension care and control: Study protocol. Implement Sci. 2013;8:60-5908-8-60. doi: 10.1186/1748-5908-8-60. http://dx.doi.org/10.1186/1748-5908-8-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin PH, Appel LJ, Funk K, et al. The PREMIER intervention helps participants follow the dietary approaches to stop hypertension dietary pattern and the current dietary reference intakes recommendations. J Am Diet Assoc. 2007;107(9):1541–1551. doi: 10.1016/j.jada.2007.06.019. http://dx.doi.org/10.1016/j.jada.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 14.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 15.Kawasaki T, Itoh K, Uezono K, Sasaki H. A simple method for estimating 24 h urinary sodium and potassium excretion from second morning voiding urine specimen in adults. Clin Exp Pharmacol Physiol. 1993;20(1):7–14. doi: 10.1111/j.1440-1681.1993.tb01496.x. http://dx.doi.org/10.1111/j.1440-1681.1993.tb01496.x. [DOI] [PubMed] [Google Scholar]
- 16.Block G, Gillespie C, Rosenbaum EH, Jenson C. A rapid food screener to assess fat and fruit and vegetable intake. Am J Prev Med. 2000;18(4):284–288. doi: 10.1016/s0749-3797(00)00119-7. http://dx.doi.org/10.1016/S0749-3797(00)00119-7. [DOI] [PubMed] [Google Scholar]
- 17.Vollmer WM, Appel LJ, Svetkey LP, et al. Comparing office-based and ambulatory blood pressure monitoring in clinical trials. J Hum Hypertens. 2005;19(1):77–82. doi: 10.1038/sj.jhh.1001772. http://dx.doi.org/10.1038/sj.jhh.1001772. [DOI] [PubMed] [Google Scholar]
- 18.Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH collaborative research group. N Engl J Med. 1997;336(16):1117–1124. doi: 10.1056/NEJM199704173361601. http://dx.doi.org/10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 19.Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the dietary approaches to stop hypertension (DASH) diet. DASH-sodium collaborative research group. N Engl J Med. 2001;344(1):3–10. doi: 10.1056/NEJM200101043440101. http://dx.doi.org/10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 20.Jee SH, Miller ER, 3rd, Guallar E, Singh VK, Appel LJ, Klag MJ. The effect of magnesium supplementation on blood pressure: A meta-analysis of randomized clinical trials. Am J Hypertens. 2002;15(8):691–696. doi: 10.1016/s0895-7061(02)02964-3. http://dx.doi.org/10.1016/S0895-7061(02)02964-3. [DOI] [PubMed] [Google Scholar]
- 21.Juraschek SP, Guallar E, Appel LJ, Miller ER., 3rd Effects of vitamin C supplementation on blood pressure: A meta-analysis of randomized controlled trials. Am J Clin Nutr. 2012;95(5):1079–1088. doi: 10.3945/ajcn.111.027995. http://dx.doi.org/10.3945/ajcn.111.027995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wallace IR, McEvoy CT, Hunter SJ, et al. Dose-response effect of fruit and vegetables on insulin resistance in people at high risk of cardiovascular disease: A randomized controlled trial. Diabetes Care. 2013;36(12):3888–3896. doi: 10.2337/dc13-0718. http://dx.doi.org/10.2337/dc13-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evert AB, Boucher JL, Cypress M, et al. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care. 2013;36(11):3821–3842. doi: 10.2337/dc13-2042. http://dx.doi.org/10.2337/dc13-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gadgil MD, Appel LJ, Yeung E, Anderson CA, Sacks FM, Miller ER., 3rd The effects of carbohydrate, unsaturated fat, and protein intake on measures of insulin sensitivity: Results from the OmniHeart trial. Diabetes Care. 2013;36(5):1132–1137. doi: 10.2337/dc12-0869. http://dx.doi.org/10.2337/dc12-0869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monsivais P, Rehm CD, Drewnowski A. The DASH diet and diet costs among ethnic and racial groups in the United States. JAMA Intern Med. 2013;173(20):1922–1924. doi: 10.1001/jamainternmed.2013.9479. http://dx.doi.org/10.1001/jamainternmed.2013.9479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nguyen BT, Shuval K, Njike VY, Katz DL. The supplemental nutrition assistance program and dietary quality among U.S. adults: Findings from a nationally representative survey. Mayo Clin Proc. 2014;89(9):1211–1219. doi: 10.1016/j.mayocp.2014.05.010. http://dx.doi.org/10.1016/j.mayocp.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 27.Thomson CA, Ravia J. A systematic review of behavioral interventions to promote intake of fruit and vegetables. J Am Diet Assoc. 2011;111(10):1523–1535. doi: 10.1016/j.jada.2011.07.013. http://dx.doi.org/10.1016/j.jada.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 28.Wedick NM, Ma Y, Olendzki BC, et al. Access to healthy food stores modifies effect of a dietary intervention. Am J Prev Med. 2015;48(3):309–317. doi: 10.1016/j.amepre.2014.08.020. http://dx.doi.org/10.1016/j.amepre.2014.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.